Opposite Self-Folding Behavior of Polymeric Photoresponsive Actuators Enabled by a Molecular Approach
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Material Preparation
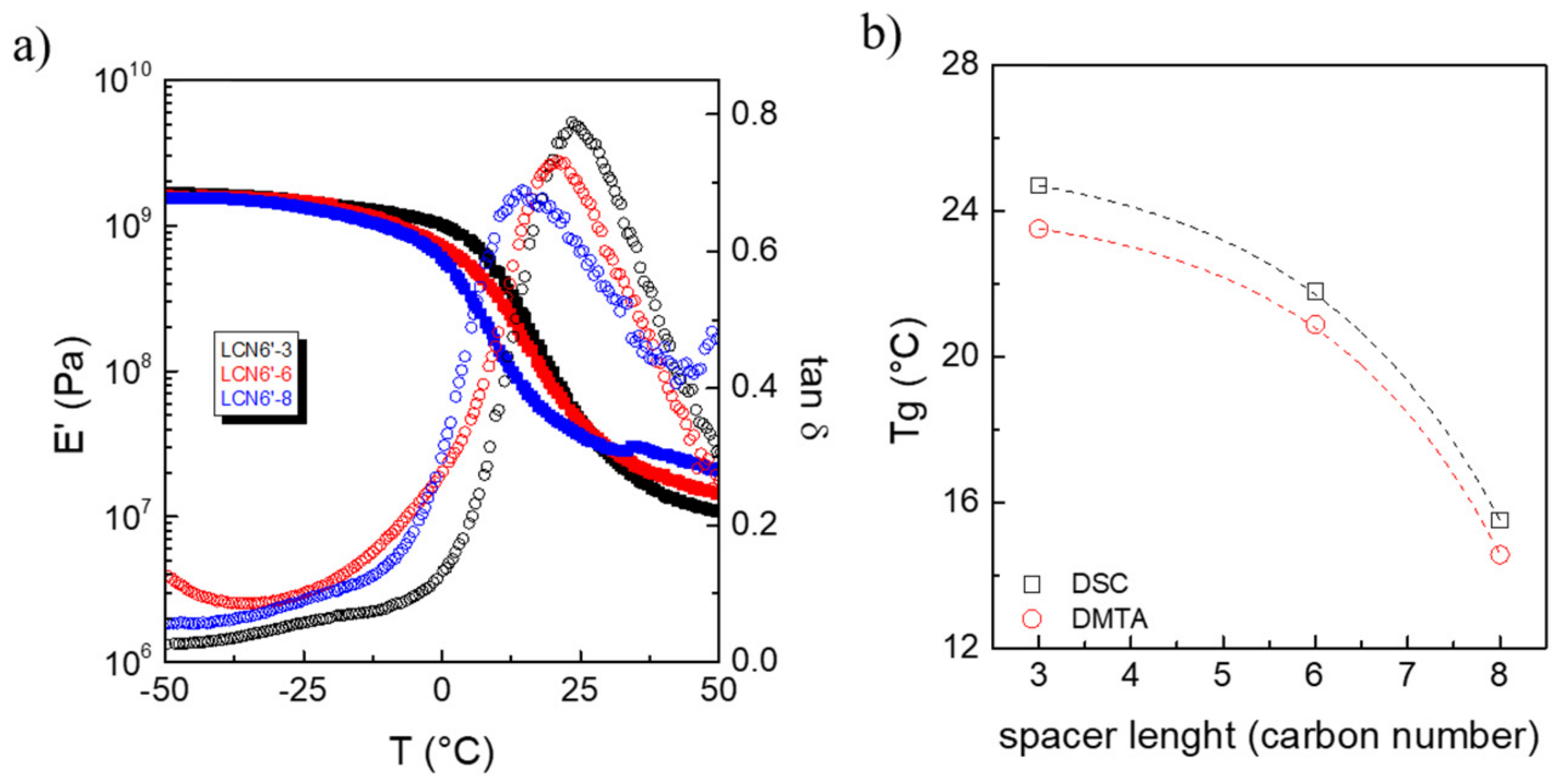
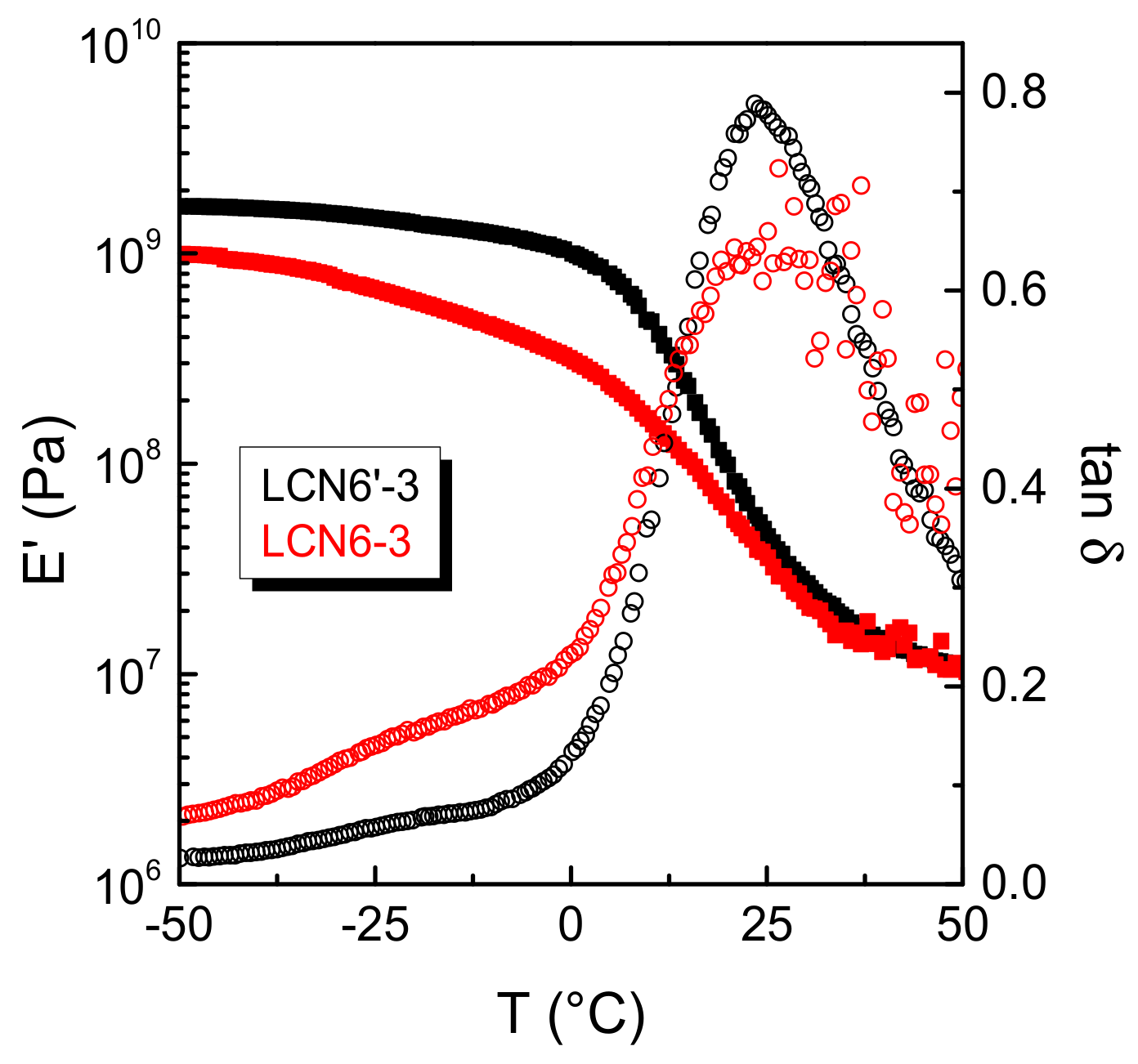
3.2. Thermo-Mechanical Analysis of the Polymers
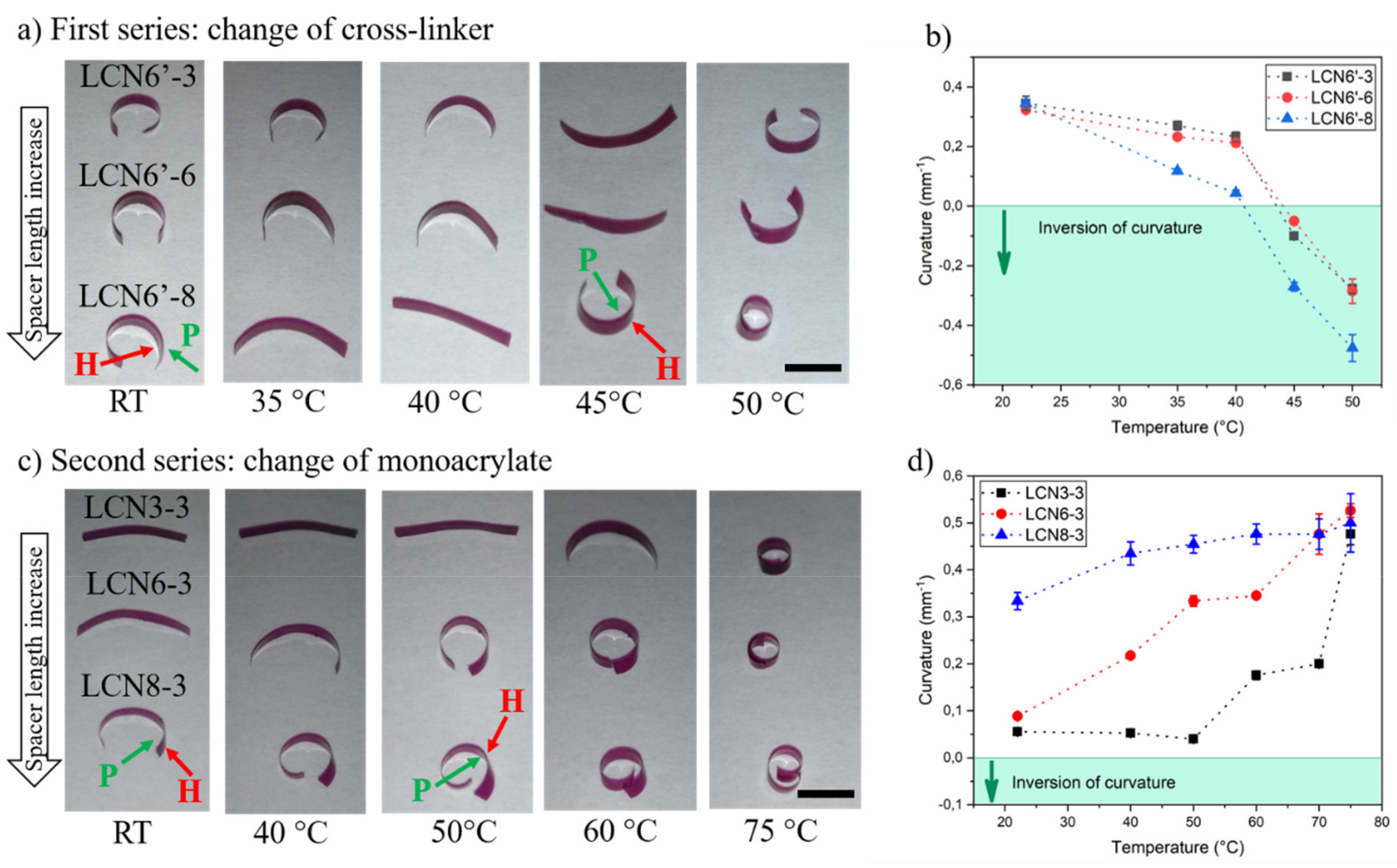
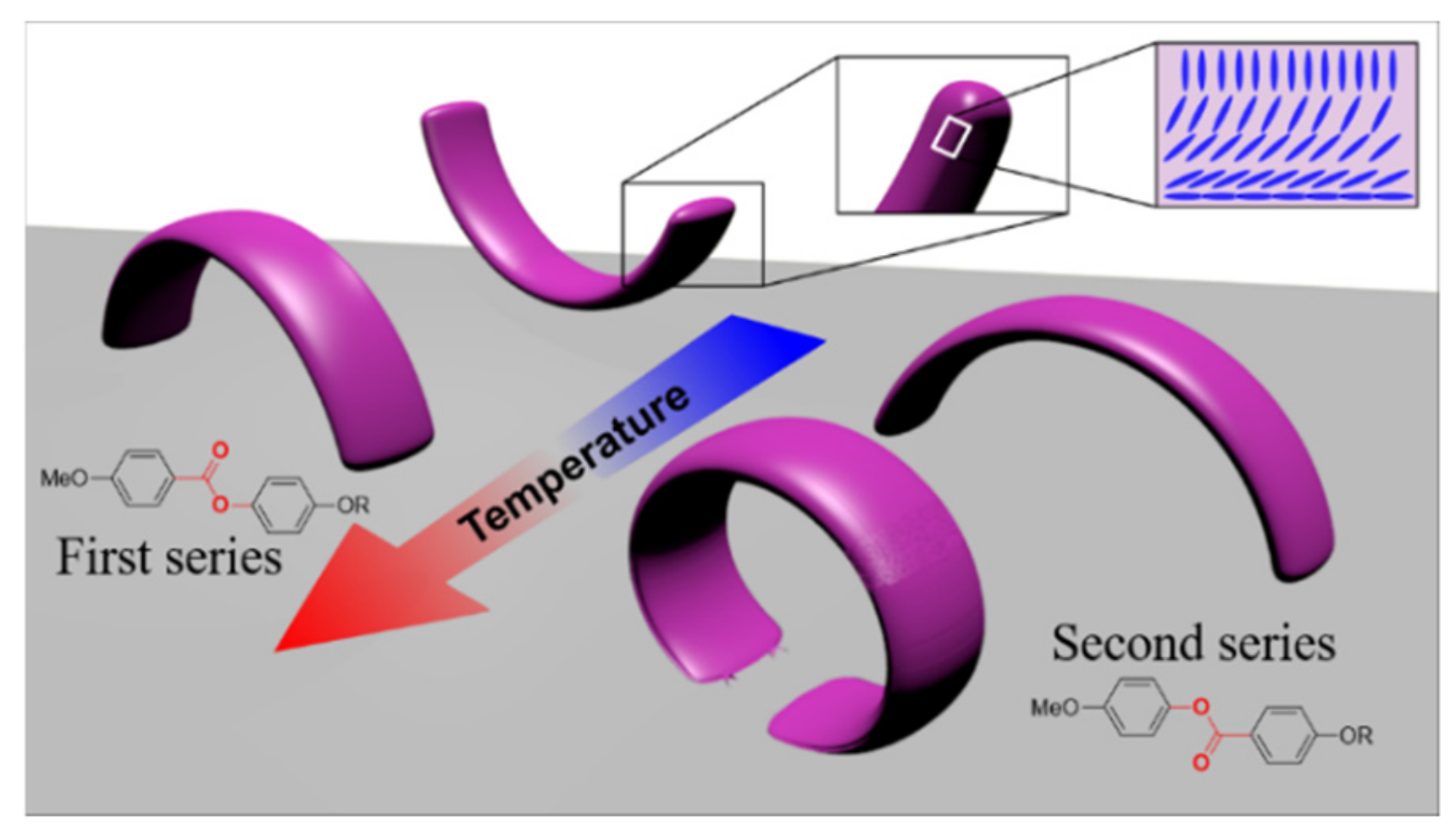
3.3. Self-Curving of LCN Films and Shape-Change in Response to Heating
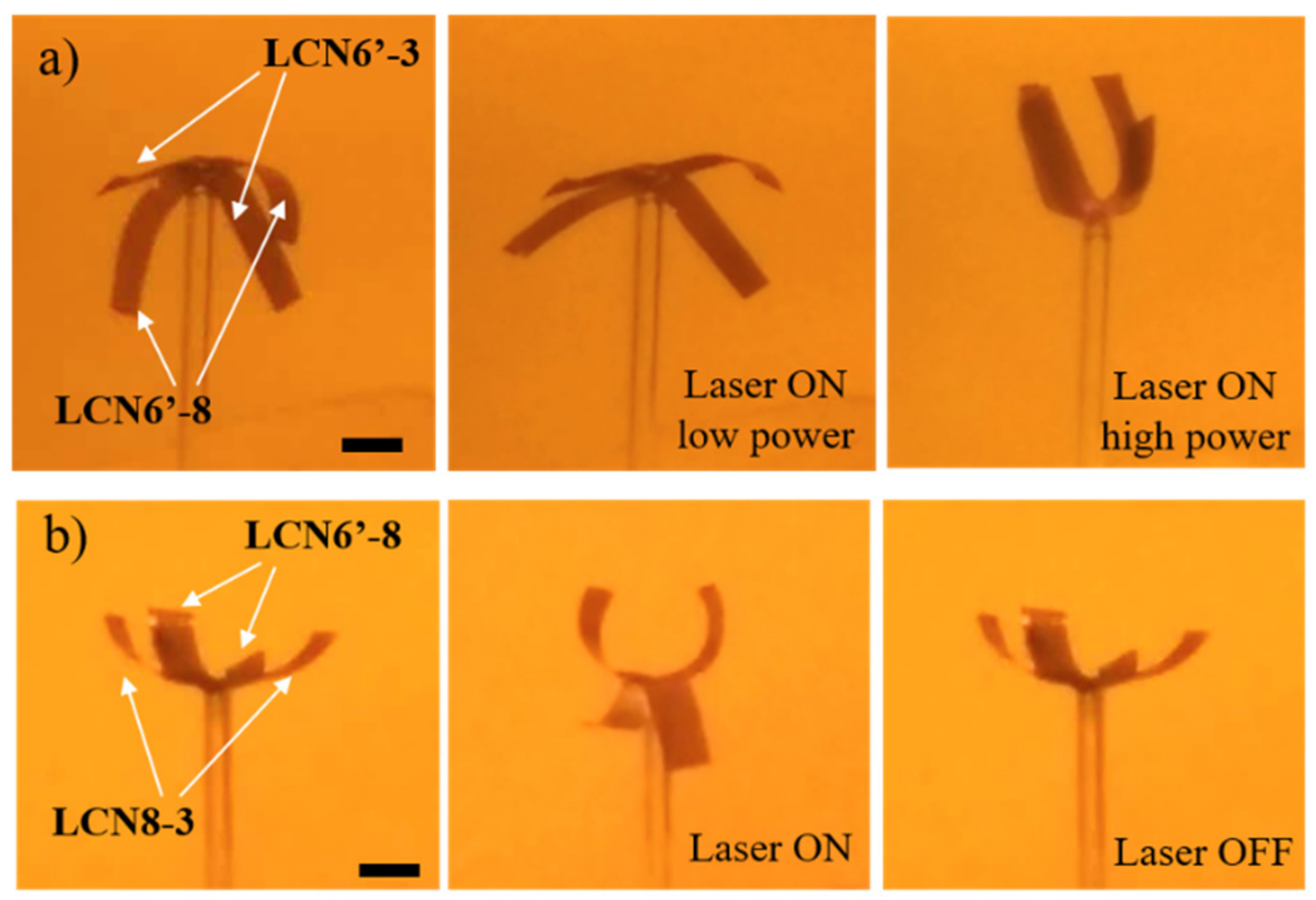
3.4. Shape-Change of LCN Films in Response to Light Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.-Y.; An, J.; Chua, C.K. Fundamental and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Bastmeyer, M.; Blasco, E.; Delaittre, G.; Müller, P.; Richter, B.; Wegener, M. 3D laser micro-and nanoprinting: Challenges for chemistry. Angew. Chem. Int. Ed. 2017, 56, 15828–15845. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, Y. Micro/Nanoscale 3D Assembly by Rolling, Folding, Curving, and Buckling Approaches. Adv. Mater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Jin, Y.; Sun, Y.; Wang, D.; Sun, J.; Wang, Z.; Zhang, W.; Jiang, X. A strategy for depositing different types of cells in three dimensions to mimic tubular structures in tissues. Adv. Mater. 2012, 24, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.C.; Stoykovich, M.P.; Song, J.; Malyarchuk, V.; Choi, W.M.; Yu, C.J.; Geddes, J.B., III; Xiao, J.; Wang, S.; Huang, Y.; et al. A hemispherical electronic eye camera based on compressible silicon optoelectronics. Nature 2008, 454, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Zarafshar, A.M.; Gracias, D.H. Differentially photo-crosslinked polymers enable self-assembling microfluidics. Nat. Commun. 2011, 2, 527. [Google Scholar] [CrossRef]
- Hippler, M.; Blasco, E.; Qu, J.; Tanaka, M.; Barner-Kowollik, C.; Wegener, M.; Bastmeyer, M. Controlling the shape of 3D microstructures by temperature and light. Nat. Commun. 2019, 10, 232. [Google Scholar] [CrossRef]
- Zeng, H.; Wani, O.M.; Wasylczyk, P.; Kaczmarek, R.; Priimagi, A. Self-regulating iris based on light-actuated liquid crystal elastomer. Adv. Mater. 2017, 29, 1701814. [Google Scholar] [CrossRef]
- Anglaret, E.; Brunet, M.; Desbat, B.; Keller, P.; Buffeteau, T. Molecular orientation in liquid crystal elastomers. Macromolecules 2005, 38, 4799–4810. [Google Scholar] [CrossRef]
- Küpfer, J.; Finkelmann, H. Nematic liquid single crystal elastomers. Die Makromol. Chemie Rapid Commun. 1991, 12, 717–726. [Google Scholar] [CrossRef]
- Ohm, C.; Brehmer, M.; Zentel, R. Liquid crystalline elastomers as actuators and sensors. Adv. Mater. 2010, 22, 3366–3387. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Ware, T.H.; McConney, M.E.; Wie, J.J.; Tondiglia, V.P.; White, T.J. Voxelated liquid crystal elastomers. Science 2015, 347, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Mostajeran, C.; Warner, M.; Modes, C.D. Frame, metric and geodesic evolution in shape-changing nematic shells. Soft Matter 2017, 13, 8858–8863. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, S.; Martella, D.; Wiersma, D.S.; Parmeggiani, C. Beam steering by liquid crystal elastomer fibres. Soft Matter 2017, 13, 8590–8596. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Broer, D.J. Liquid crystal polymer networks: Preparation, properties, and applications of films with patterned molecular alignment. Langmuir 2014, 30, 13499–13509. [Google Scholar] [CrossRef] [PubMed]
- Hikmet, R.A.M.; Broer, D.J. Dynamic mechanical properties of anisotropic networks formed by liquid crystalline acrylates. Polymer 1991, 32, 1627–1632. [Google Scholar] [CrossRef]
- Iamsaard, S.; Aßhoff, S.J.; Matt, B.; Kudernac, T.; Cornelissen, J.J.; Fletcher, S.P.; Katsonis, N. Conversion of light into macroscopic helical motion. Nat. Chem. 2014, 6, 229–235. [Google Scholar] [CrossRef]
- Kim, H.; Gibson, J.; Maeng, J.; Saed, M.; Pimentel, K.; Rihani, R.; Pancrazio, J.J.; Georgakopoulos, S.; Ware, T.H. Responsive, 3D Electronics Enabled by Liquid Crystal Elastomer Substrates. ACS Appl. Mater. Interfaces 2019, 11, 19506–19513. [Google Scholar] [CrossRef]
- Sawa, Y.; Ye, F.; Urayama, K.; Takigawa, T.; Gimenez-Pinto, V.; Selinger, R.L.; Selinger, J.V. Shape selection of twist-nematic-elastomer ribbons. Proc. Natl. Acad. Sci. USA 2011, 108, 6364–6368. [Google Scholar] [CrossRef]
- Broer, D.J.; Mol, G.N. In-situ photopolymerization of oriented liquid-crystalline acrylates, 5. Influence of the alkylene spacer on the properties of the mesogenic monomers and the formation and properties of oriented polymer networks. Makromol. Chem. 1991, 192, 59–74. [Google Scholar] [CrossRef]
- Renbo, W.; Zhou, L.; He, Y.; Wang, X.; Keller, P. Effect of molecular parameters on thermomechanical behavior of side-on nematic liquid crystal elastomers. Polymer 2013, 54, 5321–5329. [Google Scholar]
- Mol, G.N.; Harris, K.D.; Bastiaansen, C.W.; Broer, D.J. Thermo-mechanical responses of liquid-crystal networks with a splayed molecular organization. Adv. Funct. Mater. 2005, 15, 1155–1159. [Google Scholar] [CrossRef]
- Hikmet, R.A.M.; Lub, J.; Tol, A.J.W. Effect of the orientation of the ester bonds on the properties of three isomeric liquid crystal diacrylates before and after polymerization. Macromolecules 1995, 28, 3313–3327. [Google Scholar] [CrossRef]
- Zeng, H.; Martella, D.; Wasylczyk, P.; Cerretti, G.; Lavocat, J.C.G.; Ho, C.H.; Parmeggiani, C.; Wiersma, D.S. High-resolution 3D direct laser writing for liquid-crystalline elastomer microstructures. Adv. Mater. 2014, 26, 2319–2322. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, S.; Martella, D.; Parmeggiani, C.; Wiersma, D. Photoresist design for elastomeric light tunable photonic devices. Materials 2016, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Martella, D.; Antonioli, D.; Nocentini, S.; Wiersma, D.S.; Galli, G.; Laus, M.; Parmeggiani, C. Light activated non-reciprocal motion in liquid crystalline networks by designed microactuator architecture. RSC Adv. 2017, 7, 19940–19947. [Google Scholar] [CrossRef]
- Lam, J.W.; Kong, X.; Dong, Y.; Cheuk, K.K.; Xu, K.; Tang, B.Z. Synthesis and properties of liquid crystalline polyacetylenes with different spacer lengths and bridge orientations. Macromolecules 2000, 33, 5027–5040. [Google Scholar] [CrossRef]
- Braun, L.; Linder, T.; Hessberger, T.; Zentel, R. Influence of a crosslinker containing an Azo group on the actuation properties of a photoactuating LCE system. Polymers 2016, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Skandani, A.; Clement, J.A.; Tristam-Nagie, S.; Shankar, M.R. Aliphatic flexible spacer length controls photomechanical response in compact, ordered liquid crystalline polymer networks. Polymer 2017, 133, 30–39. [Google Scholar] [CrossRef]
- Harris, K.D.; Cuypers, R.; Scheibe, P.; Van Oosten, C.L.; Bastiaansen, C.W.M.; Lub, J.; Broer, D.J. Large amplitude light-induced motion in high elastic modulus polymer actuators. J. Mater. Chem. 2005, 15, 5043–5048. [Google Scholar] [CrossRef]
- Van Oosten, C.L.; Harris, K.D.; Bastiaansen, C.W.M.; Broer, D.J. Glassy photomechanical liquid-crystal network actuators for microscale devices. Eur. Phys. J. 2007, E23, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Hikmet, R.A.M.; Zwerver, B.H.; Broer, D.J. Anisotropic polymerization shrinkage behaviour of liquid-crystalline diacrylates. Polymer 1992, 33, 89–95. [Google Scholar] [CrossRef]
- Martella, D.; Paoli, P.; Pioner, J.M.; Sacconi, L.; Coppini, R.; Santini, L.; Lulli, M.; Cerbai, E.; Wiersma, D.S.; Poggesi, C.; et al. Liquid crystalline networks toward regenerative medicine and tissue repair. Small 2017, 13, 1702677. [Google Scholar] [CrossRef] [PubMed]
- Martella, D.; Pattelli, L.; Matassini, C.; Ridi, F.; Bonini, M.; Paoli, P.; Baglioni, P.; Wiersma, D.S.; Parmeggiani, C. Liquid Crystal-Induced Myoblast Alignment. Adv. Health. Mater. 2019, 8, 1801489. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Mamiya, J.I.; Yu, Y. Photomechanics of liquid-crystalline elastomers and other polymers. Angew. Chem. Int. Ed. 2007, 46, 506–528. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ikeda, T. Photocontrollable liquid-crystalline actuators. Adv. Mater. 2011, 23, 2149–2180. [Google Scholar] [CrossRef] [PubMed]
- Ferrantini, C.; Pioner, J.M.; Martella, D.; Coppini, R.; Piroddi, N.; Paoli, P.; Calamai, M.; Pavone, F.S.; Wiersma, D.S.; Tesi, C.; et al. Development of Light-Responsive Liquid Crystalline Elastomers to Assist Cardiac Contraction. Cir. Res. 2019, 124, e44–e54. [Google Scholar] [CrossRef] [PubMed]
- Martella, D.; Nocentini, S.; Micheletti, F.; Wiersma, D.S.; Parmeggiani, C. Polarization-dependent deformation in light responsive polymers doped by dichroic dyes. Soft Matter 2019, 15, 1312–1318. [Google Scholar] [CrossRef]
- Martella, D.; Nocentini, S.; Parmeggiani, C.; Wiersma, D.S. Self-Regulating Capabilities in Photonic Robotics. Adv. Mater. Technol. 2019, 4, 1800571. [Google Scholar] [CrossRef]
- Gelebart, A.H.; Mulder, D.J.; Vantomme, G.; Schenning, A.P.; Broer, D.J. A Rewritable, Reprogrammable, Dual Light-Responsive Polymer Actuator. Angew. Chem. Int. Ed. 2017, 56, 13436–13439. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Sugimoto, M.; Yamada, M.; Naka, Y.; Mamiya, J.I.; Kinoshita, M.; Shishido, A.; Yu, Y.; Ikeda, T. Effect of concentration of photoactive chromophores on photomechanical properties of crosslinked azobenzene liquid-crystalline polymers. J. Mater. Chem. 2010, 20, 117–122. [Google Scholar] [CrossRef]
- Wani, O.M.; Zeng, H.; Wasylczyk, P.; Priimagi, A. Programming photoresponse in liquid crystal polymer actuators with laser projector. Adv. Opt. Mater. 2018, 6, 1700949. [Google Scholar] [CrossRef]
- Nocentini, S.; Parmeggiani, C.; Martella, D.; Wiersma, D.S. Optically driven soft micro robotics. Adv. Opt. Mater. 2018, 6, 1800207. [Google Scholar] [CrossRef]
- Palagi, S.; Fischer, P. Bioinspired microrobots. Nat. Rev. Mater. 2018, 3, 113–124. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martella, D.; Nocentini, S.; Antonioli, D.; Laus, M.; Wiersma, D.S.; Parmeggiani, C. Opposite Self-Folding Behavior of Polymeric Photoresponsive Actuators Enabled by a Molecular Approach. Polymers 2019, 11, 1644. https://doi.org/10.3390/polym11101644
Martella D, Nocentini S, Antonioli D, Laus M, Wiersma DS, Parmeggiani C. Opposite Self-Folding Behavior of Polymeric Photoresponsive Actuators Enabled by a Molecular Approach. Polymers. 2019; 11(10):1644. https://doi.org/10.3390/polym11101644
Chicago/Turabian StyleMartella, Daniele, Sara Nocentini, Diego Antonioli, Michele Laus, Diederik S. Wiersma, and Camilla Parmeggiani. 2019. "Opposite Self-Folding Behavior of Polymeric Photoresponsive Actuators Enabled by a Molecular Approach" Polymers 11, no. 10: 1644. https://doi.org/10.3390/polym11101644
APA StyleMartella, D., Nocentini, S., Antonioli, D., Laus, M., Wiersma, D. S., & Parmeggiani, C. (2019). Opposite Self-Folding Behavior of Polymeric Photoresponsive Actuators Enabled by a Molecular Approach. Polymers, 11(10), 1644. https://doi.org/10.3390/polym11101644









