New Kind of Polymer Materials Based on Selected Complexing Star-Shaped Polyethers
Abstract
1. Introduction
2. Materials and Methods
- ∆f = frequency change of a quartz resonator (MHz)
- ∆m = mass change of a quartz resonator(g)
- μQ = shear modulus of quartz (2.947 × 1011 dyn∙cm−2)
- ρQ = quartz density (2.648 g∙cm−3)
- A = acoustically active area of a quartz resonator (πr2 = 3.14 × 0.252 = 0.1963 cm2)
- f0 = resonant frequency of a quartz resonator (5 or 10 MHz for EQCM 5710)
3. Results and Discussion
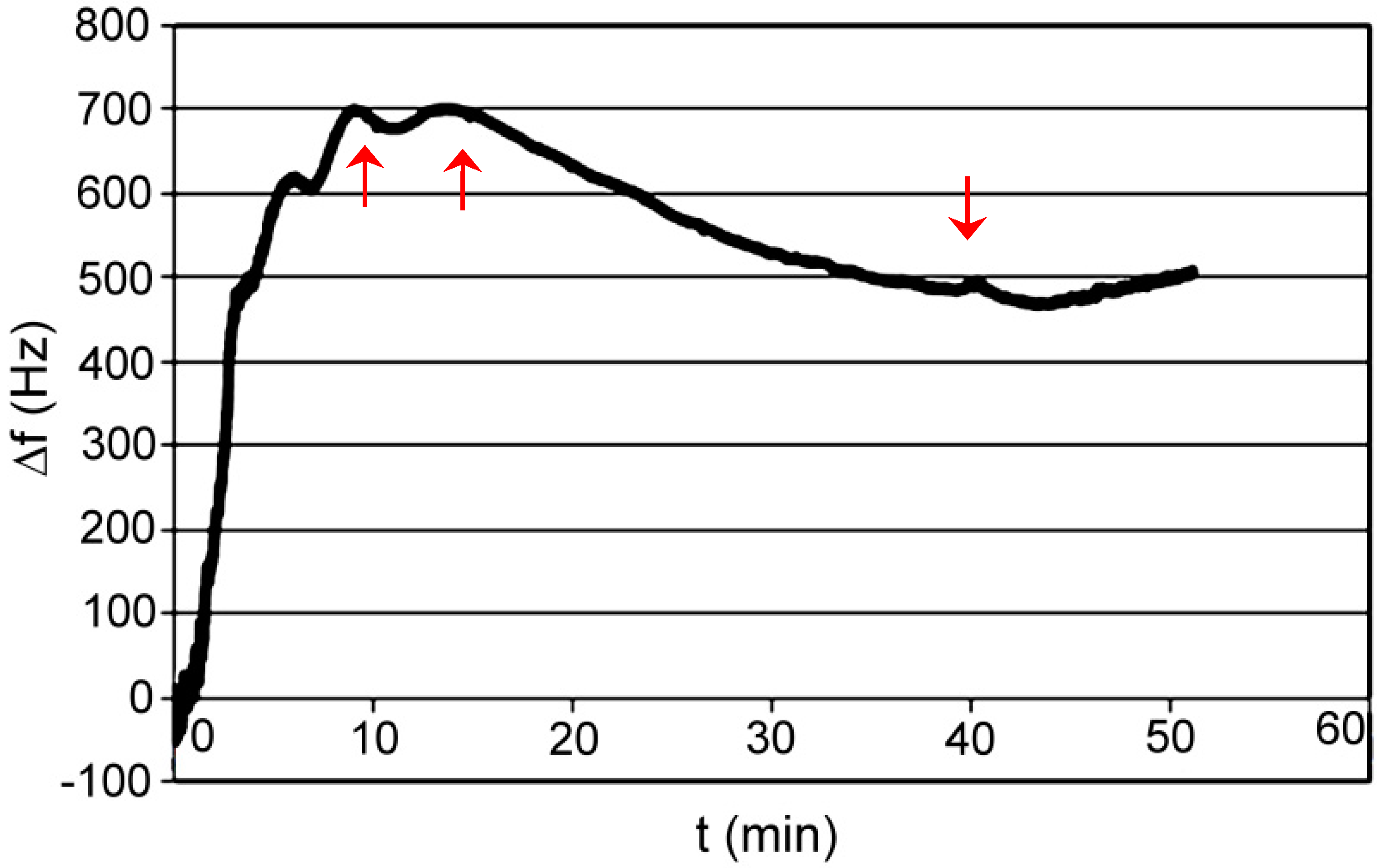
3.1. Polymer AP I
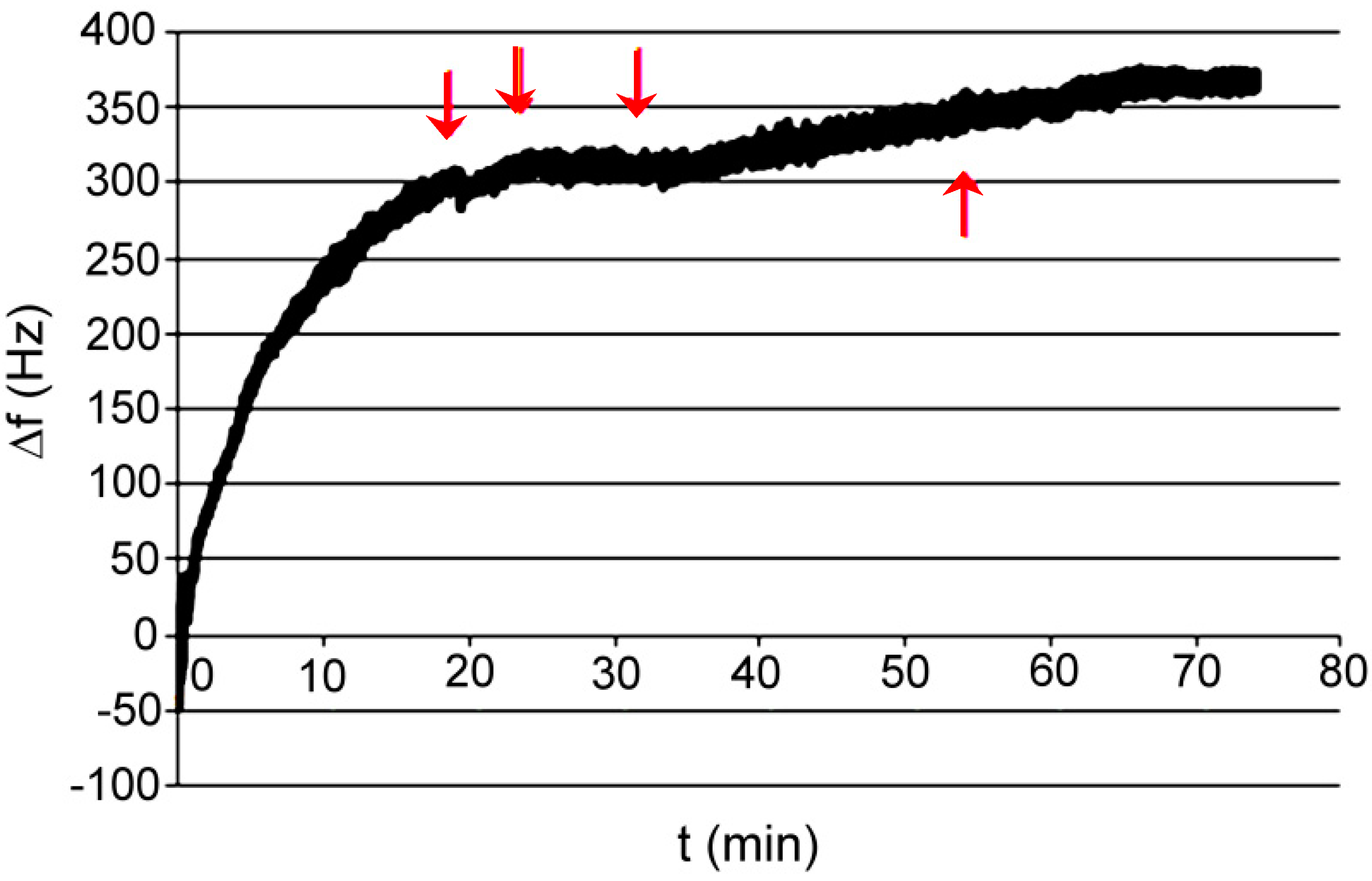
3.2. Polymer XVI
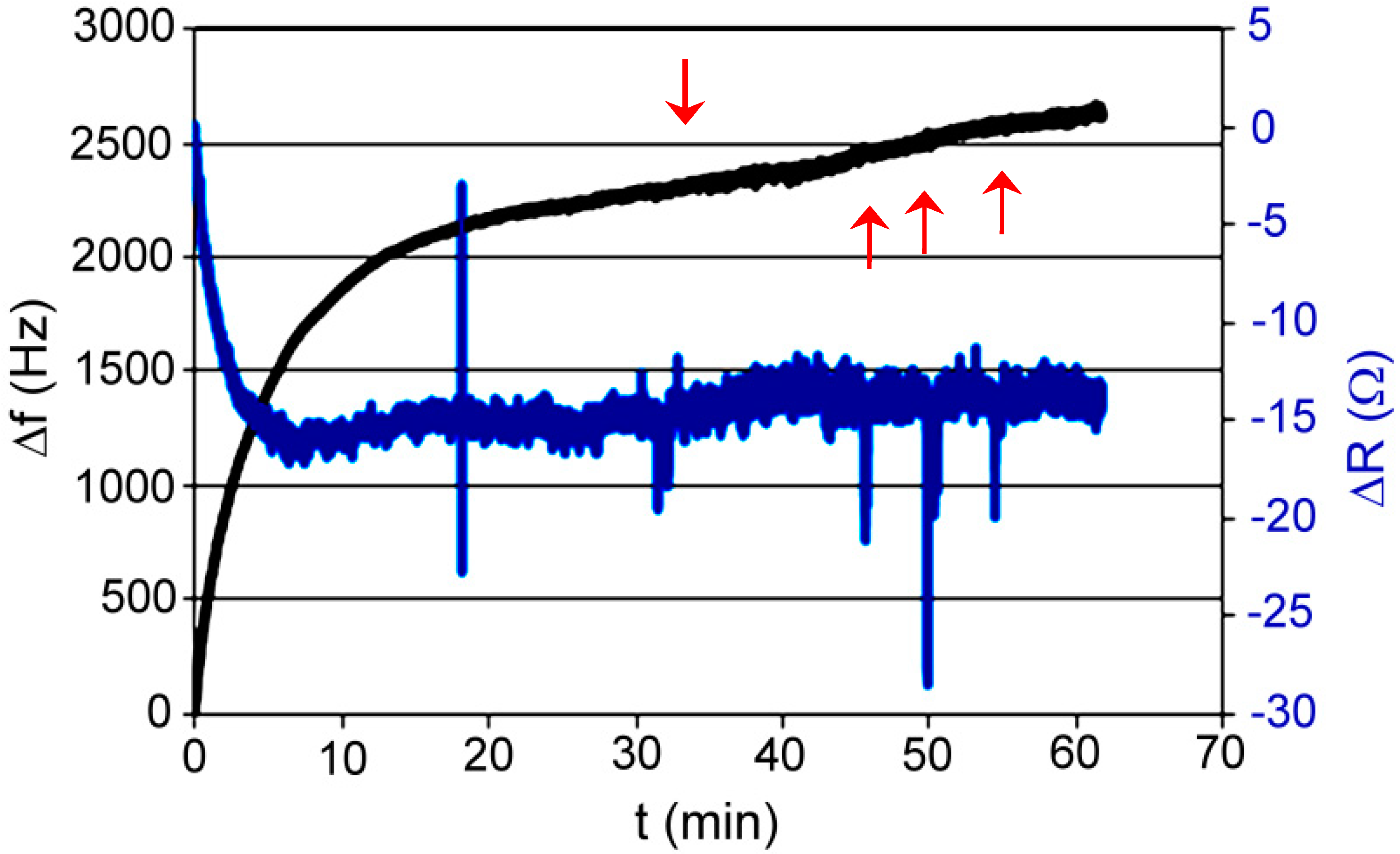
3.3. Polymer XVII
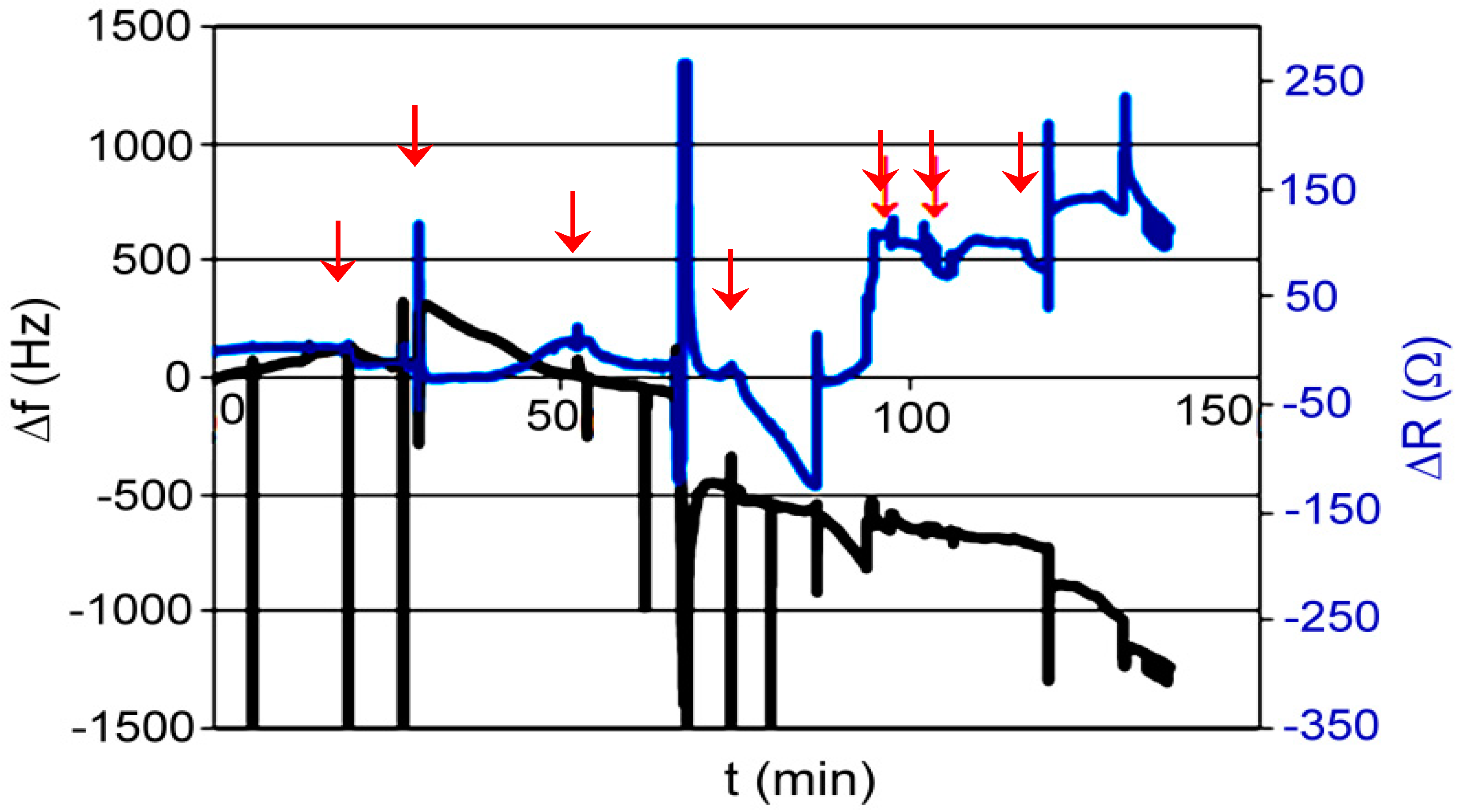
3.4. Polymer XVIII
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| THF | tetrahydrofurane |
| macroinitiator | cyclic oligo potassium glycidoxide |
| AP I | a polymer with photoluminescent properties, and a carbazol group terminated with CH3 groups, soluble in nonpolar solvents (THF, hexane) |
| S XVI | poliglycidol soluble in polar solvents (water, methanol), terminated with OH groups |
| S XVII | poliglycidol soluble in polar solvents (water, methanol), terminated with OH groups |
| S XVII | poly (propylene oxide) soluble in nonpolar solvents (THF, hexane), terminated with CH3 groups |
| S XIX | poly (propylene oxide) soluble in nonpolar solvents (THF, hexane), terminated with CH3 groups |
References
- Lüttringhaus, A.; Ziegler, K. Über vielgliedrige Ringsysteme: VIII. Über eine neue Anwendung des Verdünnungsprinzips. Justus Liebig’s Ann. Chem. 1937, 528, 155–161. [Google Scholar] [CrossRef]
- Pedersen, C.J. Crystalline Salt Complexes of Macrocyclic Polyethers. J. Am. Chem. Soc. 1970, 92, 386–391. [Google Scholar] [CrossRef]
- Sahoo, S.K.; kanta Bera, R.; Kanungo, B.K.; Baral, M. Spectroscopic and pH-metric studies on the complexation of a novel tripodal amine-phenol ligand towards Al(III), Ga(III) and In(III). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 89, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Failli, P.; Bani, D.; Bencini, A.; Cantore, M.; Di Cesare Mannelli, L.; Ghelardini, C.; Giorgi, C.; Innocenti, M.; Rugi, F.; Spepi, A.; et al. A Novel Manganese Complex Effective as Superoxide Anion Scavenger and Therapeutic Agent against Cell and Tissue Oxidative Injury. J. Med. Chem. 2009, 52, 7273–7283. [Google Scholar] [CrossRef] [PubMed]
- Zobnina, V.G.; Kosevich, M.V.; Chagovets, V.V.; Boryak, O.A.; Vékey, K.; Gömöry, Á.; Kulyk, A.N. Interactions of oligomers of organic polyethers with histidine amino acid. Rapid Commun. Mass Spectrom. 2012, 26, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Bakó, T.; Bakó, P.; Keglevich, G.; Báthori, N.; Czugler, M.; Tatai, J.; Novák, T.; Parlagh, G.; Tőke, L. Enantioselective Michael addition of 2-nitropropane to chalcone analogues catalyzed by chiral azacrown ethers based on α-d-glucose and d-mannitol. Tetrahedron Asymmetry 2003, 14, 1917–1923. [Google Scholar]
- Bakó, P.; Makó, A.; Keglevich, G.; Kubinyi, M.; Pál, K. Synthesis of d-mannose-based azacrown ethers and their application in enantioselective reactions. Tetrahedron Asymmetry 2005, 16, 1861–1871. [Google Scholar] [CrossRef]
- Yokozawa, T.; Suzuki, R.; Nojima, M.; Ohta, Y.; Yokoyama, A. Precision Synthesis of Poly(3-hexylthiophene) from Catalyst-Transfer Suzuki−Miyaura Coupling Polymerization. Macromol. Rapid Commun. 2011, 32, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, Y.; Shibata, R.; Miyakoshi, R.; Yokoyama, A.; Yokozawa, T. Investigation of catalyst-transfer condensation polymerization for the synthesis of n-type π-conjugated polymer, poly(2-dioxaalkylpyridine-3,6-diyl). J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3628–3640. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kohno, H.; Ohta, Y.; Yokoyama, A. Catalyst-Transfer Suzuki−Miyaura Coupling Polymerization for Precision Synthesis of Poly( p-phenylene). Macromolecules 2010, 43, 7095–7100. [Google Scholar] [CrossRef]
- Su, R.; Qin, Y.; Qiao, L.; Li, J.; Zhao, X.; Wang, P.; Wang, X.; Wang, F. Synthesis of poly(2-furyloxirane) with high molecular weight and improved regioregularity using macrocyclic ether as a cocatalyst to potassium tert-butoxide. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1434–1442. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Swinarew, A.S.; Czaja, M.; Piekarnik, B.; Grobelny, Z.; Getautis, V.; Gražulevičius, J.V.; Niedziela, T.; Trzebicka, B.; Stolarzewicz, A. Optically induced carbazolyl containing polyethers: Concentration effects. J. Mol. Struct. 2008, 887, 205–208. [Google Scholar] [CrossRef]
- Anselment, T.M.J.; Anderson, C.E.; Rieger, B.; Boeddinghaus, M.B.; Fässler, T.F. Synthesis of non-symmetrically sulphonated phosphine sulphonate based Pd(ii) catalyst salts for olefin polymerisation reactions. Dalt. Trans. 2011, 40, 8304. [Google Scholar] [CrossRef]
- Song, Y.; Jing, H.; Li, B.; Bai, D. Crown Ether Complex Cation Ionic Liquids: Preparation and Applications in Organic Reactions. Chem. A Eur. J. 2011, 17, 8731–8738. [Google Scholar] [CrossRef] [PubMed]
- Marin, T.W.; Shkrob, I.A.; Dietz, M.L. Hydrogen-Bonding Interactions and Protic Equilibria in Room-Temperature Ionic Liquids Containing Crown Ethers. J. Phys. Chem. B 2011, 115, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Baker, G.A.; Zhao, H. Ether- and alcohol-functionalized task-specific ionic liquids: Attractive properties and applications. Chem. Soc. Rev. 2012, 41, 4030. [Google Scholar] [CrossRef] [PubMed]
- Swinarew, A.; Stolarzewicz, A.; Grobelny, Z.; Swinarew, B.; Grazulevicius, J.V.; Simokaitiene, J.; Andrikaityte, E. Star-shaped poly(2-(9-carbazolyl)methylthiirane): Synthesis, analysis and photoluminescencent properties. J. Mol. Struct. 2011, 1005, 129–133. [Google Scholar] [CrossRef]
- Penso, M.; Maia, A.; Lupi, V.; Tricarico, G. Selective O-functionalization of phenolic α-amino acids with crown ethers bearing cyclophosphazene sub-units. Tetrahedron 2011, 67, 2096–2102. [Google Scholar] [CrossRef]
- Wild, G.P.; Wiles, C.; Watts, P.; Haswell, S.J. The use of immobilized crown ethers as in-situ N-protecting groups in organic synthesis and their application under continuous flow. Tetrahedron 2009, 65, 1618–1629. [Google Scholar] [CrossRef]
- Gong, X.Y.; Kubáň, P.; Tanyanyiwa, J.; Hauser, P.C. Separation of enantiomers in capillary electrophoresis with contactless conductivity detection. J. Chromatogr. A 2005, 1082, 230–234. [Google Scholar] [CrossRef]
- Anouti, S.; Vandenabeele-Trambouze, O.; Koval, D.; Cottet, H. Heart-cutting 2-D CE using multiple detection points for chiral analysis of native amino acids. Electrophoresis 2009, 30, 2–10. [Google Scholar] [CrossRef]
- Anouti, S.; Vandenabeele-Trambouze, O.; Cottet, H. Heart-cutting 2D-CE with on-line preconcentration for the chiral analysis of native amino acids. Electrophoresis 2010, 31, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, F.; Otsuka, K. Recent progress in capillary electrophoretic analysis of amino acid enantiomers. J. Chromatogr. B 2011, 879, 3078–3095. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.N.R.; Li, Y.; Kim, H.J.; Hyun, M.H. A colorimetric chiral sensor based on chiral crown ether for the recognition of the two enantiomers of primary amino alcohols and amines. Chirality 2011, 23, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.H.; Rahman, H.; Davis, J.J.; Beer, P.D. Surface-attached sensors for cation and anion recognition. Anal. Bioanal. Chem. 2012, 402, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Chailap, B.; Tuntulani, T. Optical and electrochemical properties of heteroditopic ion receptors derived from crown ether-based calix[4]arene with amido-anthraquinone pendants. Org. Biomol. Chem. 2012, 10, 3617. [Google Scholar] [CrossRef]
- Elmosallamy, M.A.F. Novel Sensors for Batch and Flow Injection Analysis of Histamine Based on Crown Ethers. Electroanalysis 2012, 24, 1226–1235. [Google Scholar] [CrossRef]
- Wysocka-Żołopa, M.; Winkler, K.; Gadde, S.; D’Souza, F. Two-component polymer films of palladium and fullerene with covalently linked crown ether voids: Effect of cation binding on the redox behavior. J. Solid State Electrochem. 2012, 16, 65–74. [Google Scholar] [CrossRef]
- Li, Y.; Huszthy, P.; Kunsági-Máté, S. Effect of molecular vibrations on the selectivity character of pyridino-18-crown-6 derivatives towards potassium ion. Chem. Phys. Lett. 2012, 533, 45–49. [Google Scholar] [CrossRef]
- Kumar, A.; Mittal, S. PVC based dibenzo-18-crown-6 electrode for Ca(II) ions. Sensors Actuators B Chem. 2004, 99, 340–343. [Google Scholar] [CrossRef]
- Aghaie, H.; Giahi, M.; Monajjemi, M.; Arvand, M.; Nafissi, G.H.; Aghaie, M. Tin(II)-selective membrane potentiometric sensor using a crown ether as neutral carrier. Sensors Actuators B Chem. 2005, 107, 756–761. [Google Scholar] [CrossRef]
- Yakshin, V.V.; Tsarenko, N.A.; Koshcheev, A.M.; Tananaev, I.G.; Myasoedov, B.F. Extraction of metal ions from aqueous solutions of mineral acids with crown ether-ionic liquid systems. Radiochemistry 2012, 54, 54–58. [Google Scholar] [CrossRef]
- Aydogan, A.; Coady, D.J.; Kim, S.K.; Akar, A.; Bielawski, C.W.; Marquez, M.; Sessler, J.L. Poly(methyl methacrylate)s with Pendant Calixpyrroles and Crown Ethers: Polymeric Extractants for Potassium Halides. Angew. Chem. Int. Ed. 2008, 47, 9648–9652. [Google Scholar] [CrossRef]
- Valente, M.; Sousa, S.F.; Lopes Magalhães, A.; Freire, C. Crown-Ether Type Podands as Alkali Metal Cation Extractants: Influence of the Number of Oxygens in the Chain. J. Solution Chem. 2010, 39, 1230–1242. [Google Scholar] [CrossRef]
- Yakshin, V.V.; Vilkova, O.M.; Kotlyar, S.A.; Tsivadze, A.Y. Dibromo and diiodo derivatives of [4.4]dibenzo-24-crown-8 as novel efficient sorbents of elements from mineral acid solutions. Russ. J. Coord. Chem. 2012, 38, 295–299. [Google Scholar] [CrossRef]
- Kang, S.O.; Day, V.W.; Bowman-James, K. The Influence of Amine Functionalities on Anion Binding in Polyamide-Containing Macrocycles. Org. Lett. 2009, 11, 3654–3657. [Google Scholar] [CrossRef]
- Gokel, G.W.; Daschbach, M.M. Coordination and transport of alkali metal cations through phospholipid bilayer membranes by hydraphile channels. Coord. Chem. Rev. 2008, 252, 886–902. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, M.; Alpaydin, S.; Sirit, A.; Yilmaz, M. Chiral Schiff base derivatives of calix[4]arene: Synthesis and complexation studies with chiral and achiral amines. Tetrahedron Asymmetry 2006, 17, 2322–2327. [Google Scholar] [CrossRef]
- Naemura, K.; Nishikawa, Y.; Fuji, J.; Hirose, K.; Tobe, Y. Preparation of homochiral phenolic crown ethers containing para-substituted phenol moiety and chiral subunits derived from (S)-1-phenylethane-1,2-diol: Their chiral recognition behaviour in complexation with neutral amines. Tetrahedron Asymmetry 1997, 8, 873–882. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Swinarew, A.; Morejko, B.; Grobelny, Z.; Stolarzewicz, A.; Gražulevičius, J.V.; Getautis, V. Synthesis and spectral analysis of hyperbranched poly(ethers) containing carbazole or hydrazone groups. In Proceedings of the Photonics Applications in Astronomy, Communications, Industry, and High-Energy Physics Experiments 2006; Romaniuk, R.S., Ed.; International Society for Optics and Photonics: Washington, WA, USA, 2006. [Google Scholar]
- Mecea, V.M. Is quartz crystal microbalance really a mass sensor? Sensors Actuators A Phys. 2006, 128, 270–277. [Google Scholar] [CrossRef]
- Kochman, A.; Krupka, A.; Grissbach, J.; Kutner, W.; Gniewińska, B.; Nafalski, L. Design and Performance of a New Thin-Layer Radial-Flow Holder for a Quartz Crystal Resonator of an Electrochemical Quartz Crystal Microbalance. Electroanalysis 2006, 18, 2168–2173. [Google Scholar] [CrossRef]
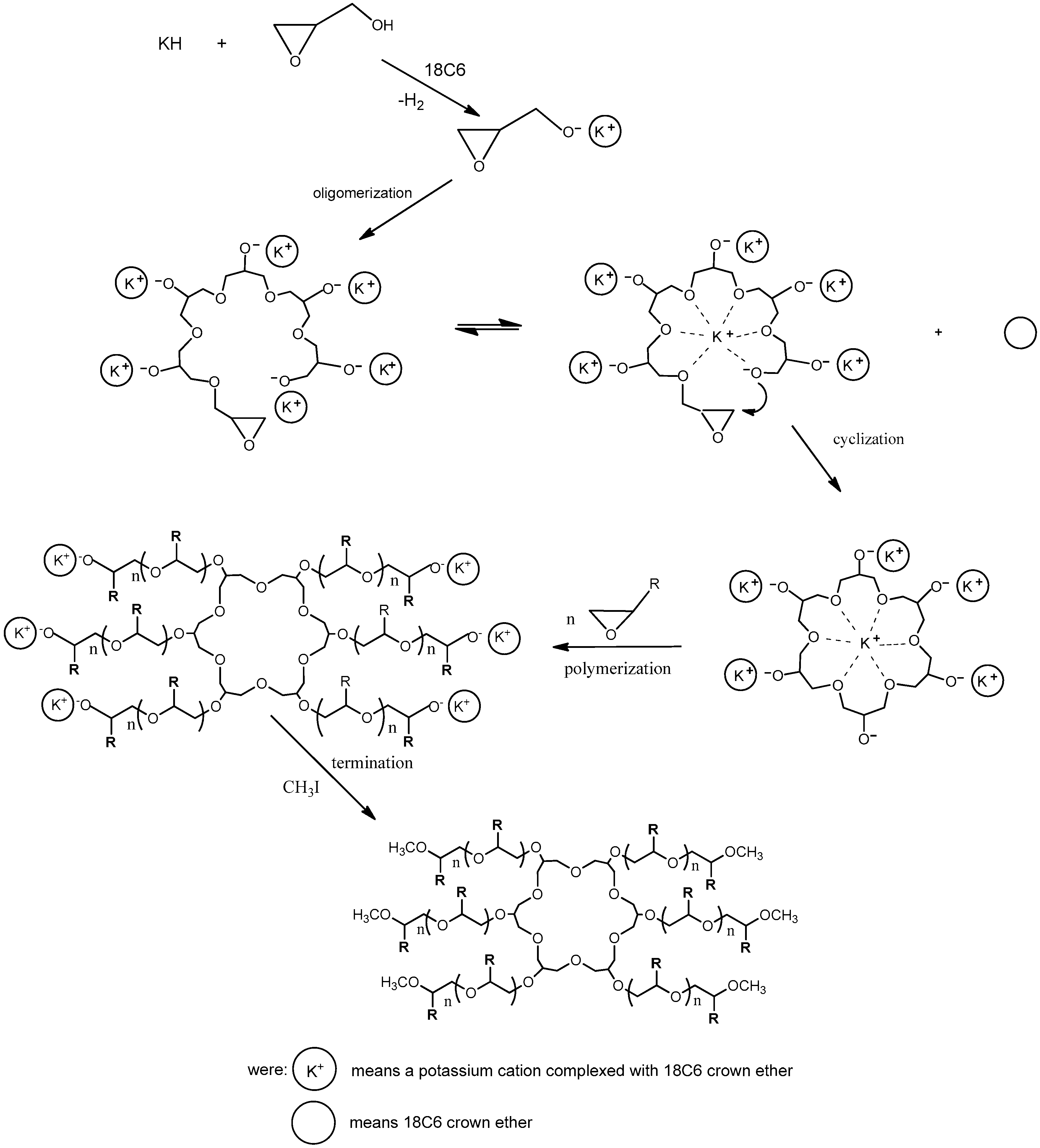

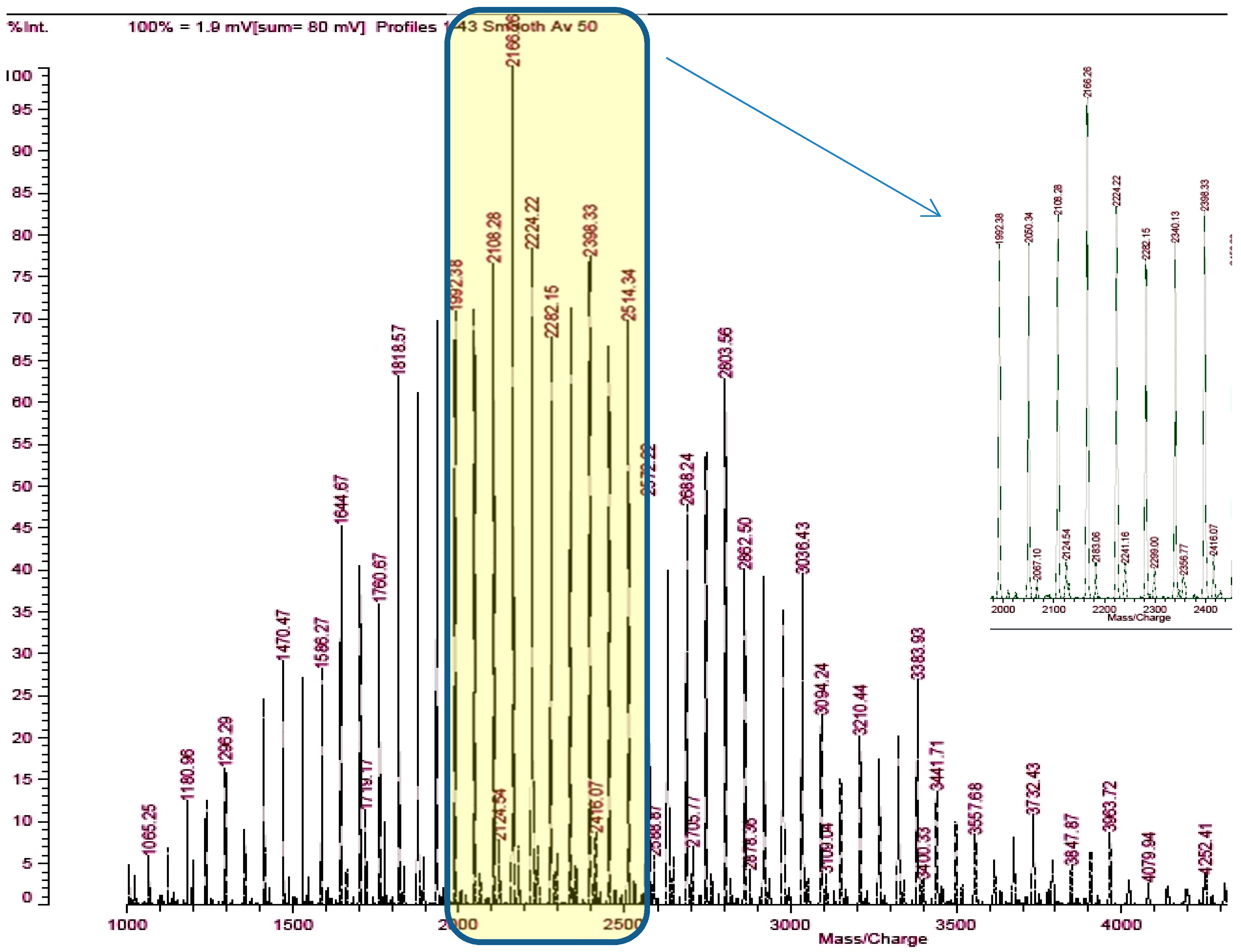
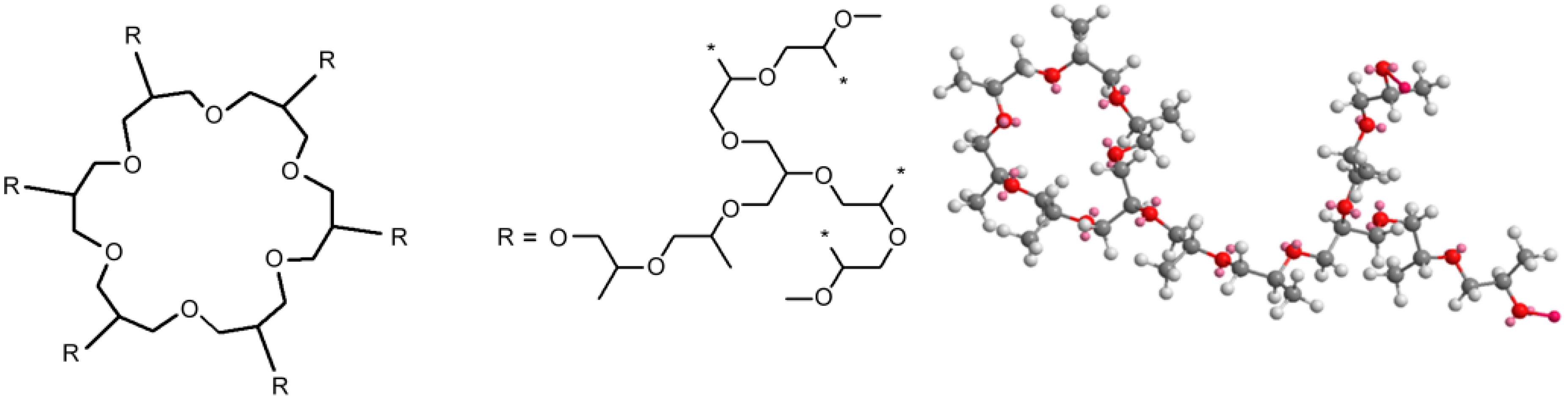


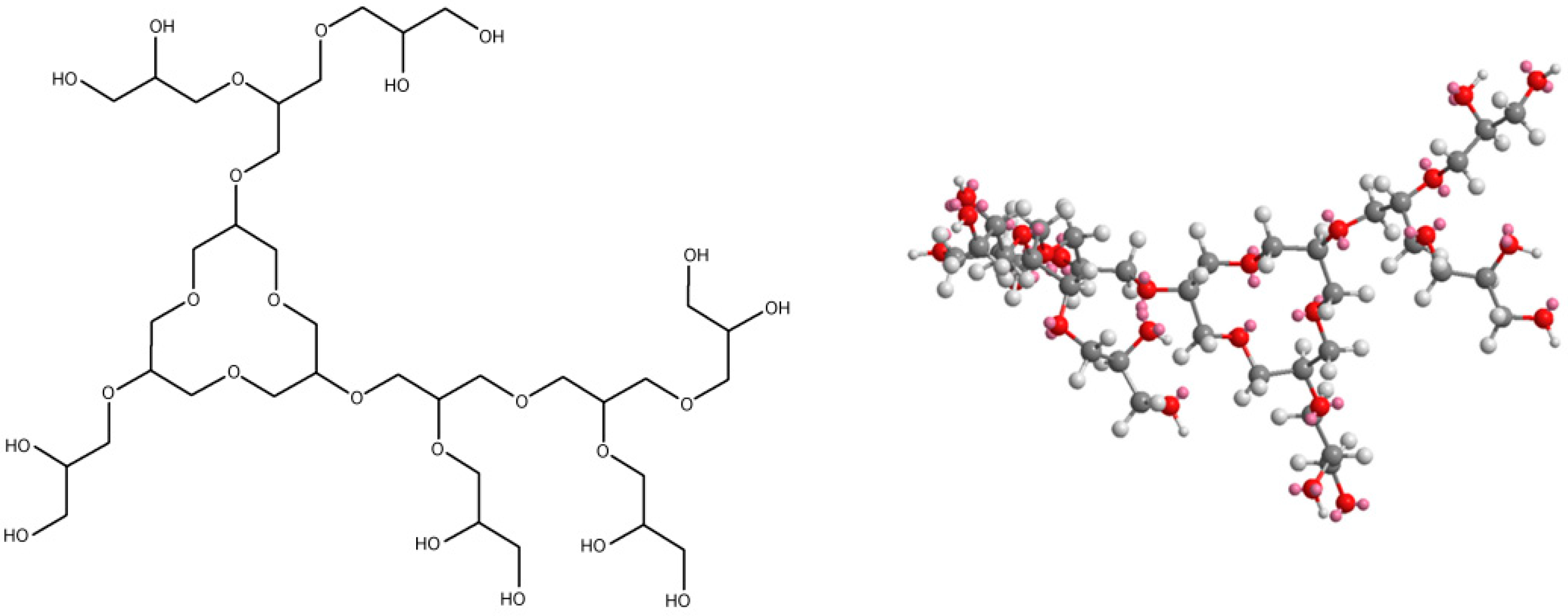

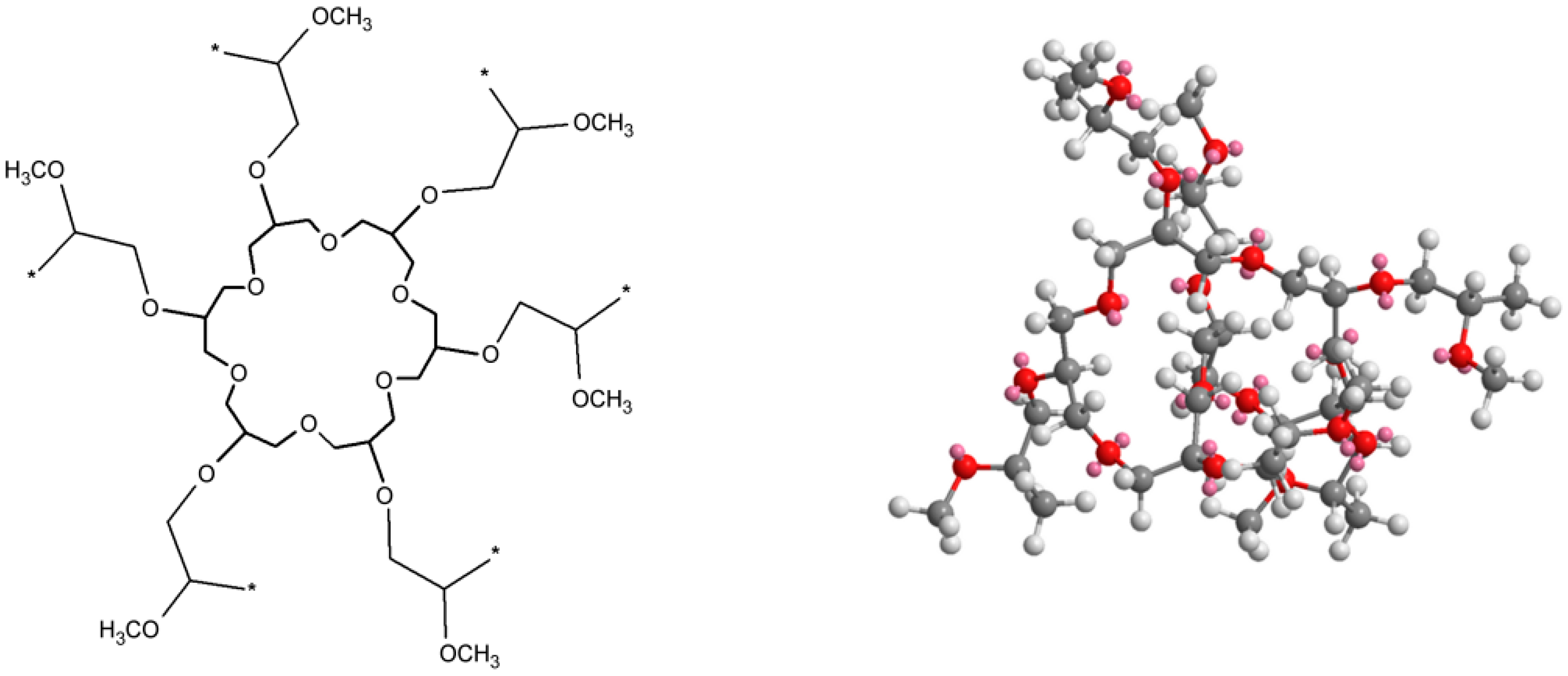
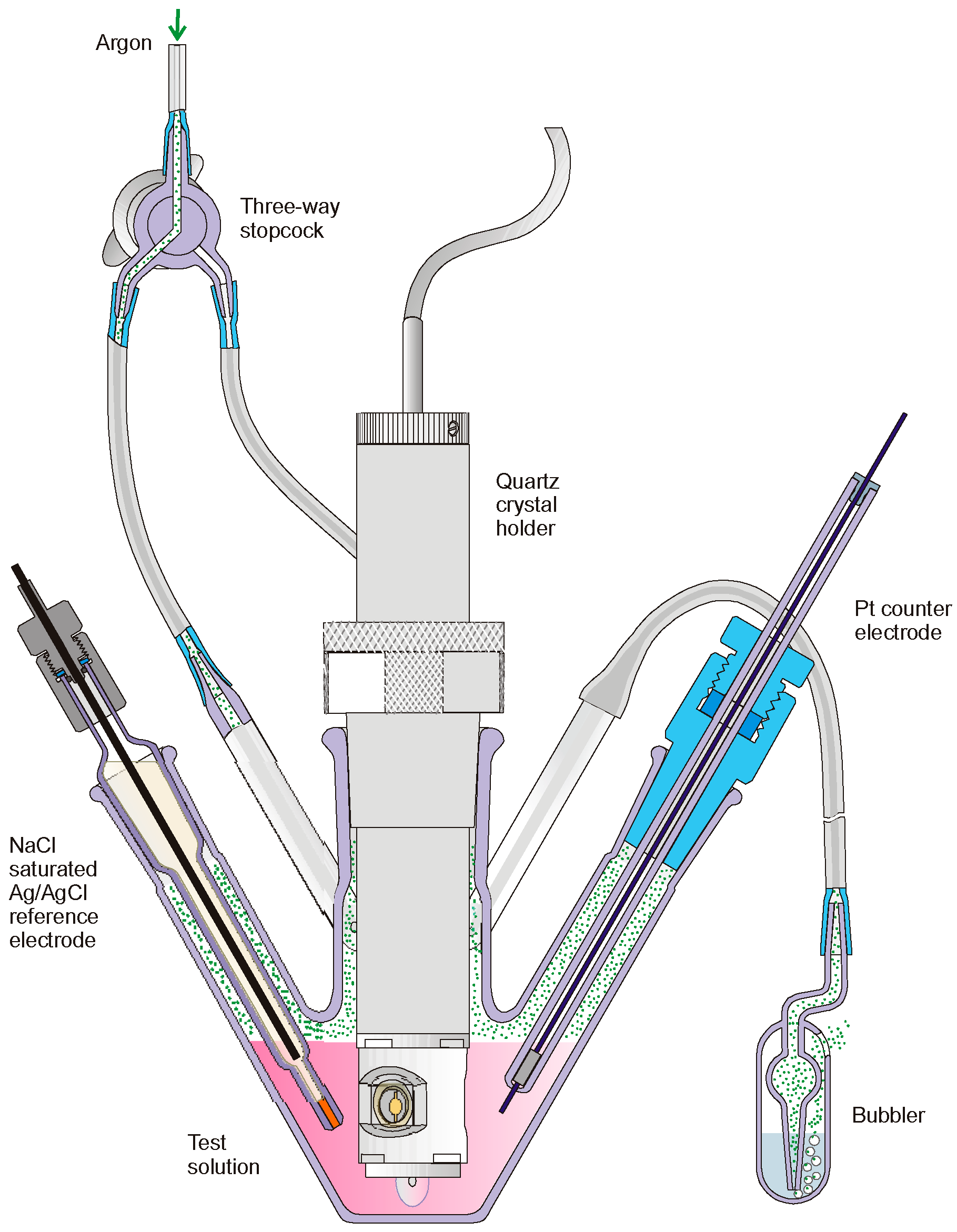
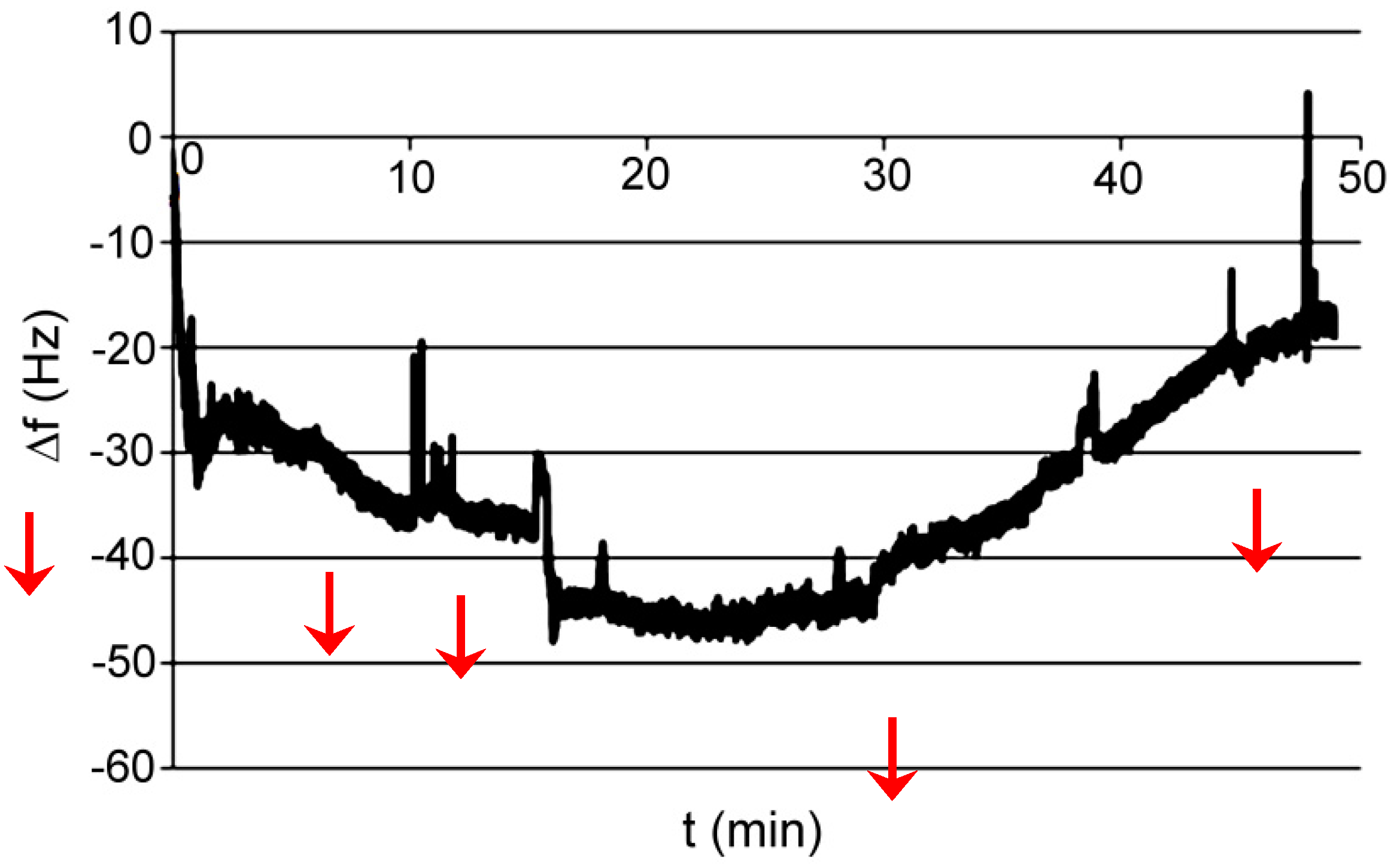
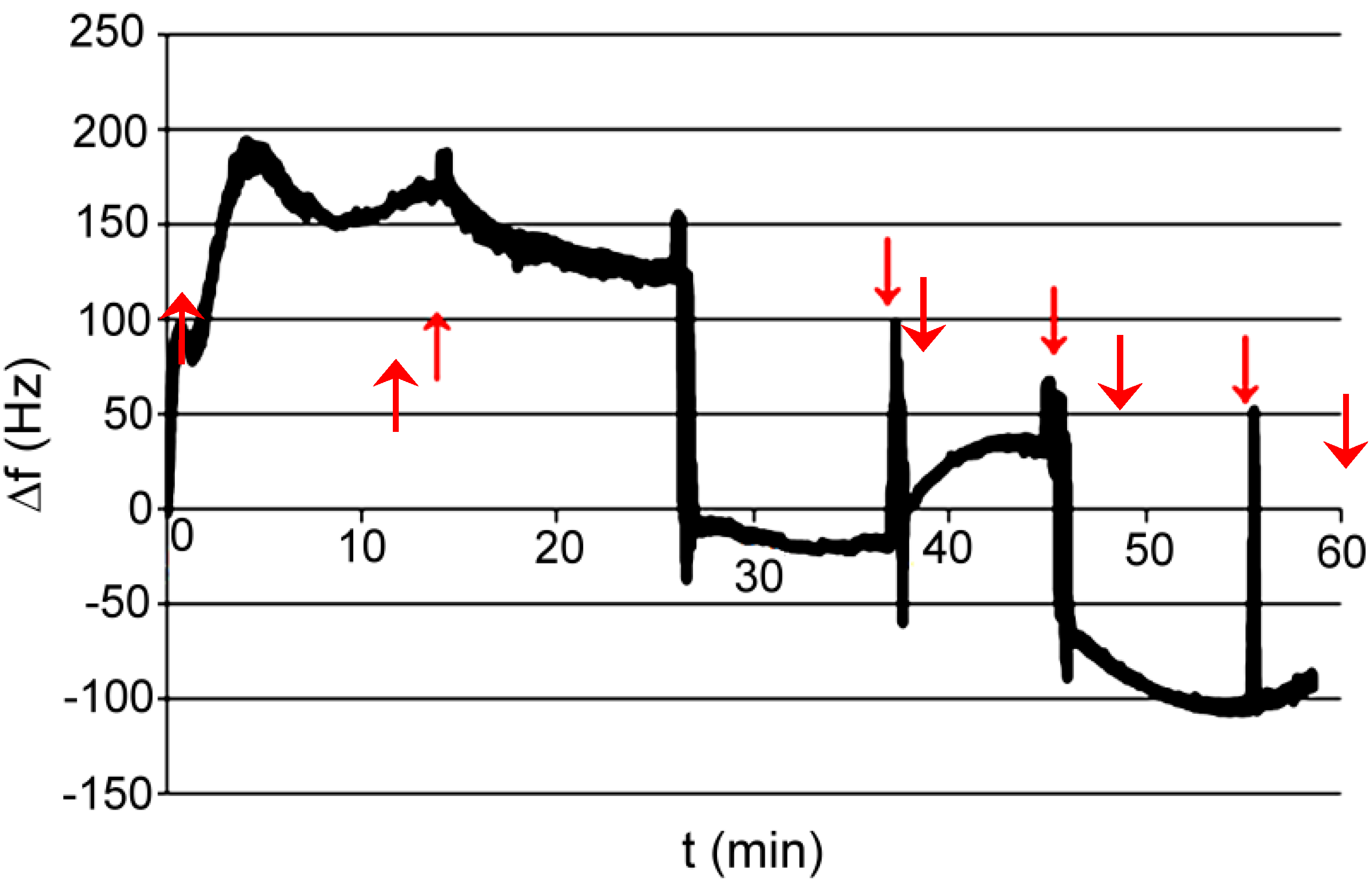


| Series | Potassium Adduct * | Number of Units | m/z Experimental | m/z Theoretical |
|---|---|---|---|---|
| A | Gx(PO)yHxK+ | x = 6, y = 27 | 2050.34 | 2050.42 |
| x = 6, y = 28 | 2108.28 | 2108.46 | ||
| x = 6, y = 29 | 2166.26 | 2166.5 | ||
| x = 6, y = 30 | 2224.22 | 2224.54 | ||
| x = 6, y = 31 | 2282.15 | 2282.58 | ||
| B | Gx(PO)yHxK+ | x = 7, y = 26 | 2067.1 | 2066.42 |
| x = 7, y = 27 | 2124.54 | 2124.46 | ||
| x = 7, y = 28 | 2183.06 | 2182.5 | ||
| x = 7, y = 29 | 2241.16 | 2240.54 | ||
| x = 7, y = 30 | 2299 | 2298.58 |
| Resonant Frequency, MHz | 5 | 10 |
| Surface Shape | plano-convex | plano-plano |
| Quartz Crystal Diameter, mm | 14.0 | |
| Electrode Diameter, mm | 5.0 | |
| Resonant Frequency Range, MHz | 4.90 to 5.05 | 9.90 to 10.05 |
| Sensitivity, ng/Hz cm2 | 17.7 | 4.2 |
| Mass Resolution, ng | 0.35 | 0.08 |
| Detectability (at Signal/Noise = 3), ng | 3.3 | 1.1 |
| Maximum Mass Load, mg | 140 | 50 |
| Electrode Material | Au, Ag, Pt, Pd or Ti; customized electrodes are also available (vacuum deposited or cathodically sputtered) | |
| Quartz Crystal Holder | Dip type; wetted parts are made of Kel-F® and PTFE with the Au plated electrode contacts; diameter 25 mm; length 165 mm; different holders are necessary for 5 MHz and 10 MHz quartz resonators | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swinarew, A.S.; Swinarew, B.; Gabor, J.; Popczyk, M.; Kubik, K.; Stanula, A.; Waśkiewicz, Z.; Rosemann, T.; Knechtle, B. New Kind of Polymer Materials Based on Selected Complexing Star-Shaped Polyethers. Polymers 2019, 11, 1554. https://doi.org/10.3390/polym11101554
Swinarew AS, Swinarew B, Gabor J, Popczyk M, Kubik K, Stanula A, Waśkiewicz Z, Rosemann T, Knechtle B. New Kind of Polymer Materials Based on Selected Complexing Star-Shaped Polyethers. Polymers. 2019; 11(10):1554. https://doi.org/10.3390/polym11101554
Chicago/Turabian StyleSwinarew, Andrzej Szymon, Beata Swinarew, Jadwiga Gabor, Magdalena Popczyk, Klaudia Kubik, Arkadiusz Stanula, Zbigniew Waśkiewicz, Thomas Rosemann, and Beat Knechtle. 2019. "New Kind of Polymer Materials Based on Selected Complexing Star-Shaped Polyethers" Polymers 11, no. 10: 1554. https://doi.org/10.3390/polym11101554
APA StyleSwinarew, A. S., Swinarew, B., Gabor, J., Popczyk, M., Kubik, K., Stanula, A., Waśkiewicz, Z., Rosemann, T., & Knechtle, B. (2019). New Kind of Polymer Materials Based on Selected Complexing Star-Shaped Polyethers. Polymers, 11(10), 1554. https://doi.org/10.3390/polym11101554










