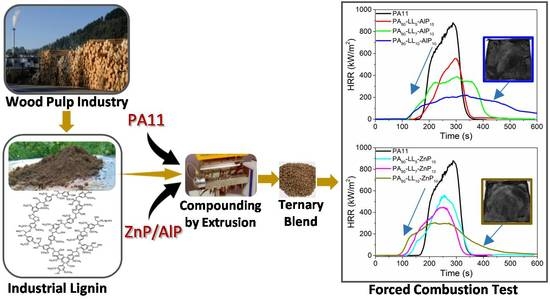
Valorization of Industrial Lignin as Biobased Carbon Source in Fire Retardant System for Polyamide 11 Blends
Abstract
1. Introduction
2. Experimental
2.1. Materials and Processing
2.2. Blends Preparation
2.3. Morphology
2.4. Thermal Decomposition
2.5. Fire Behavior
3. Results and Discussion
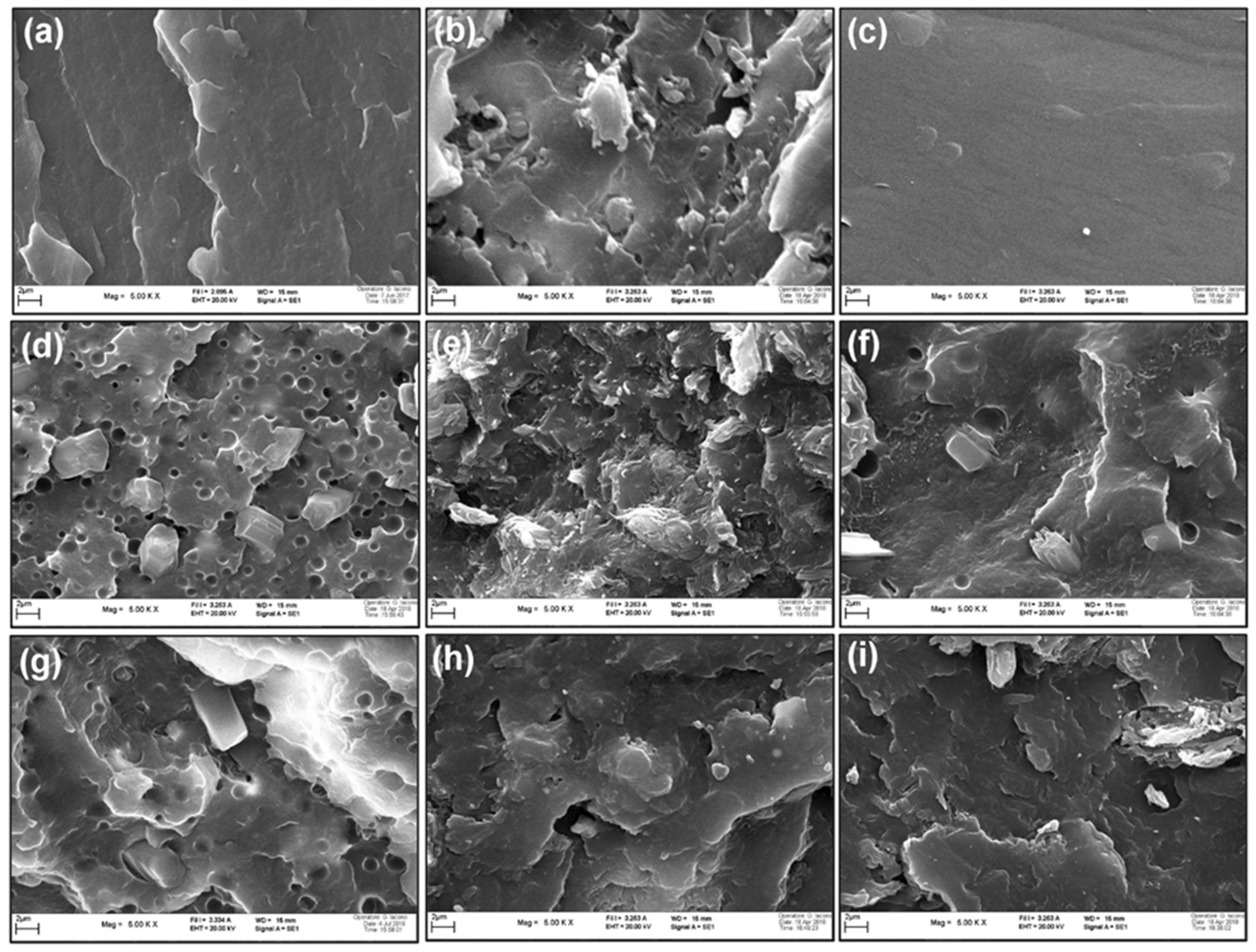
3.1. Morphology of Blends
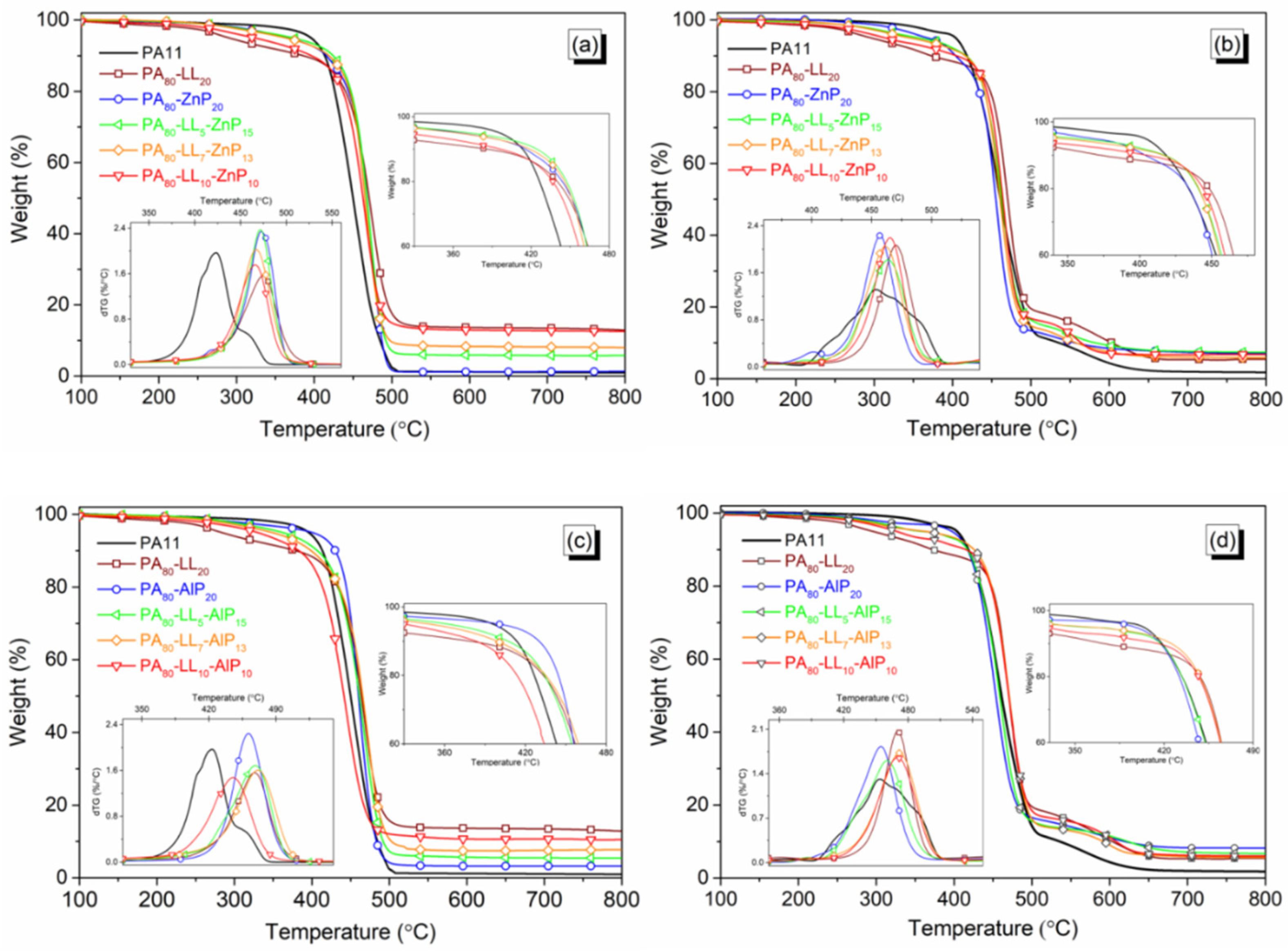
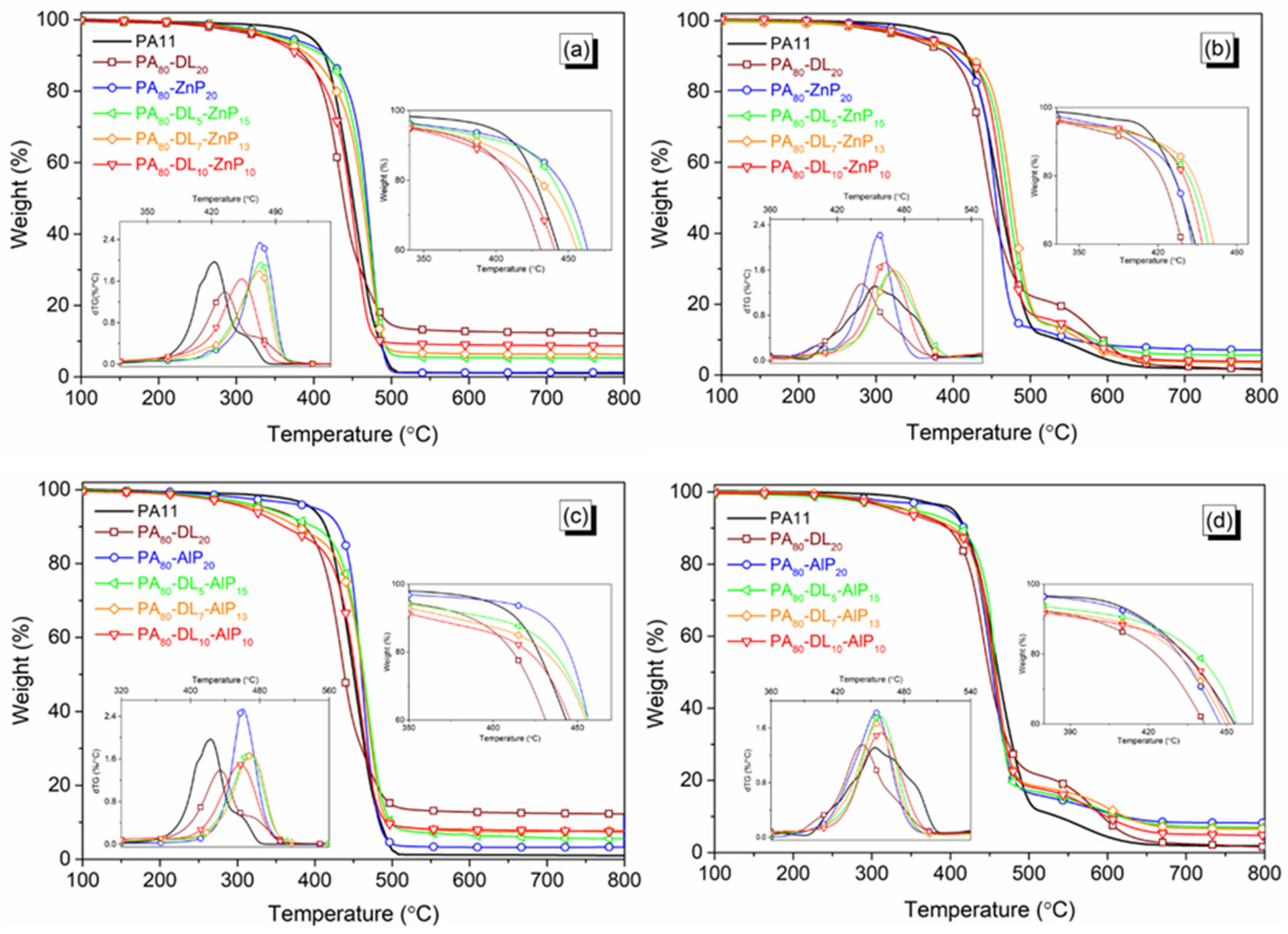
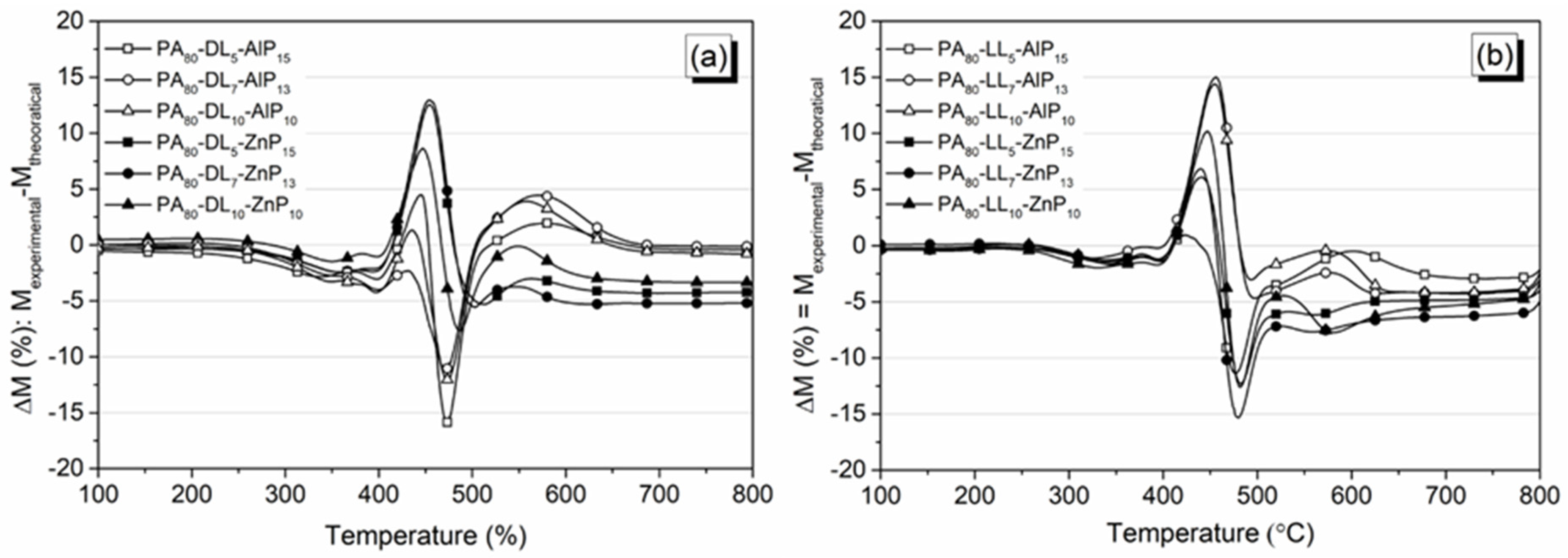
3.2. Decomposition Behavior
3.3. Flammability Behavior
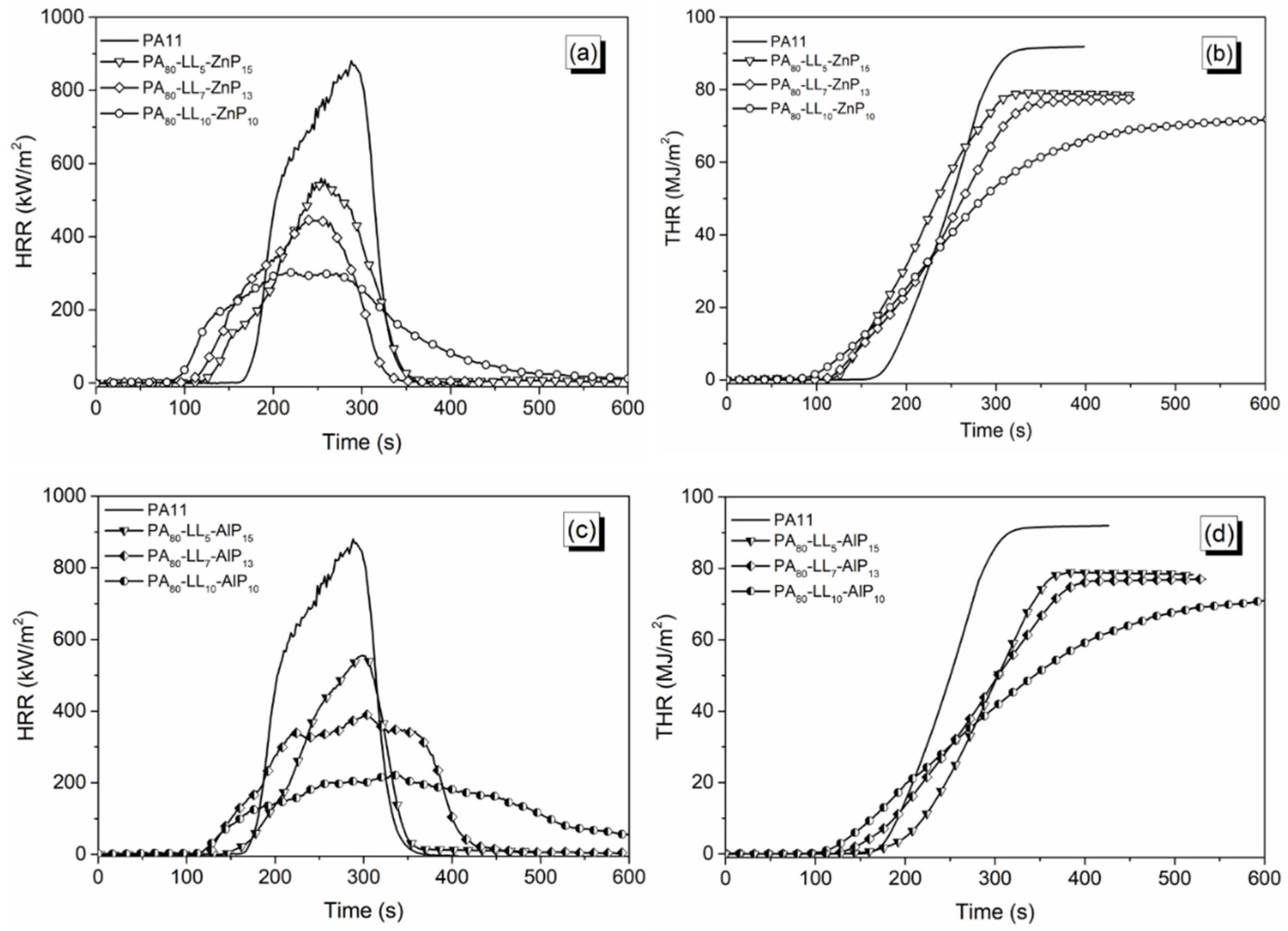
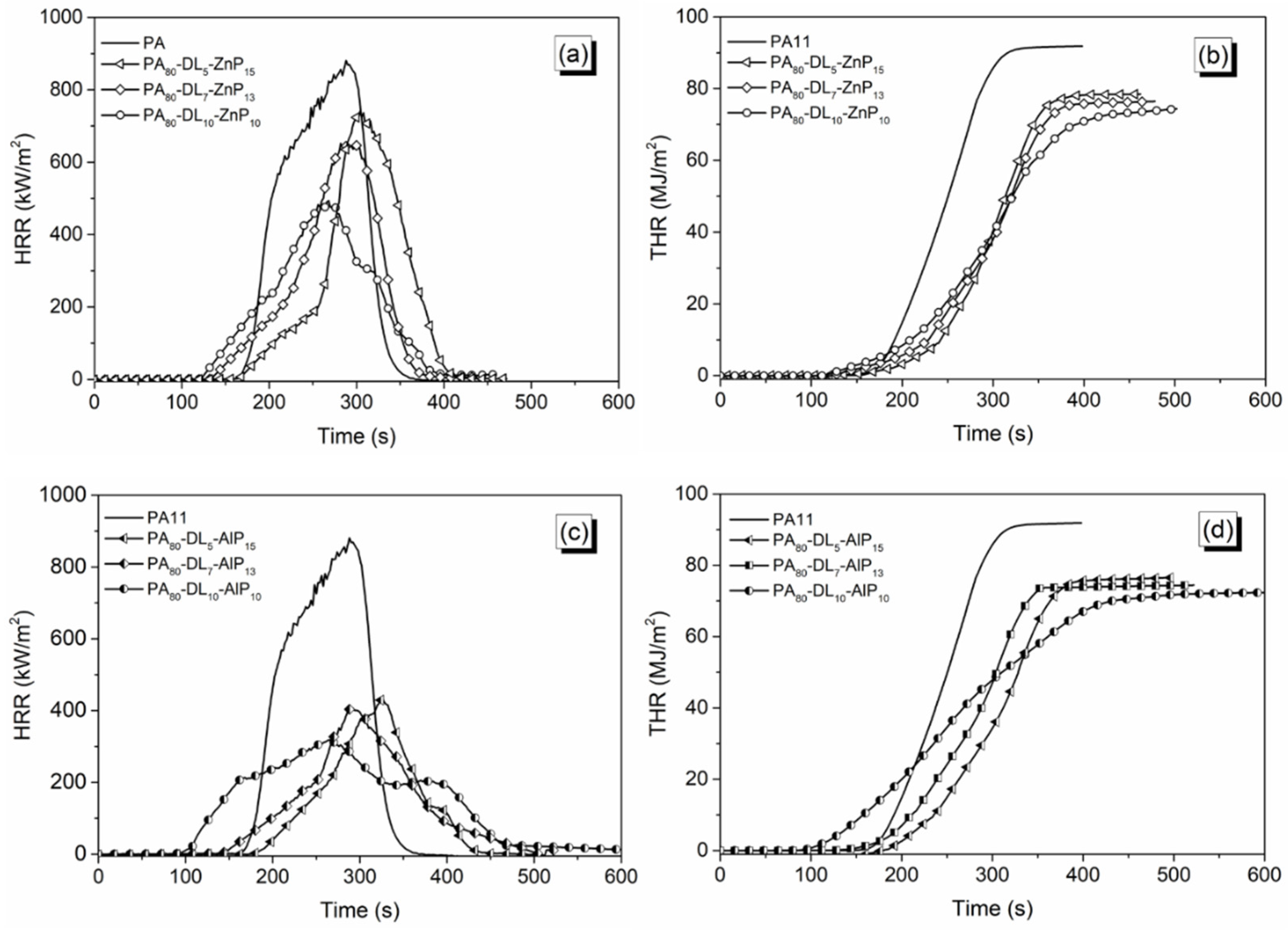
3.4. Forced-Combustion Behavior
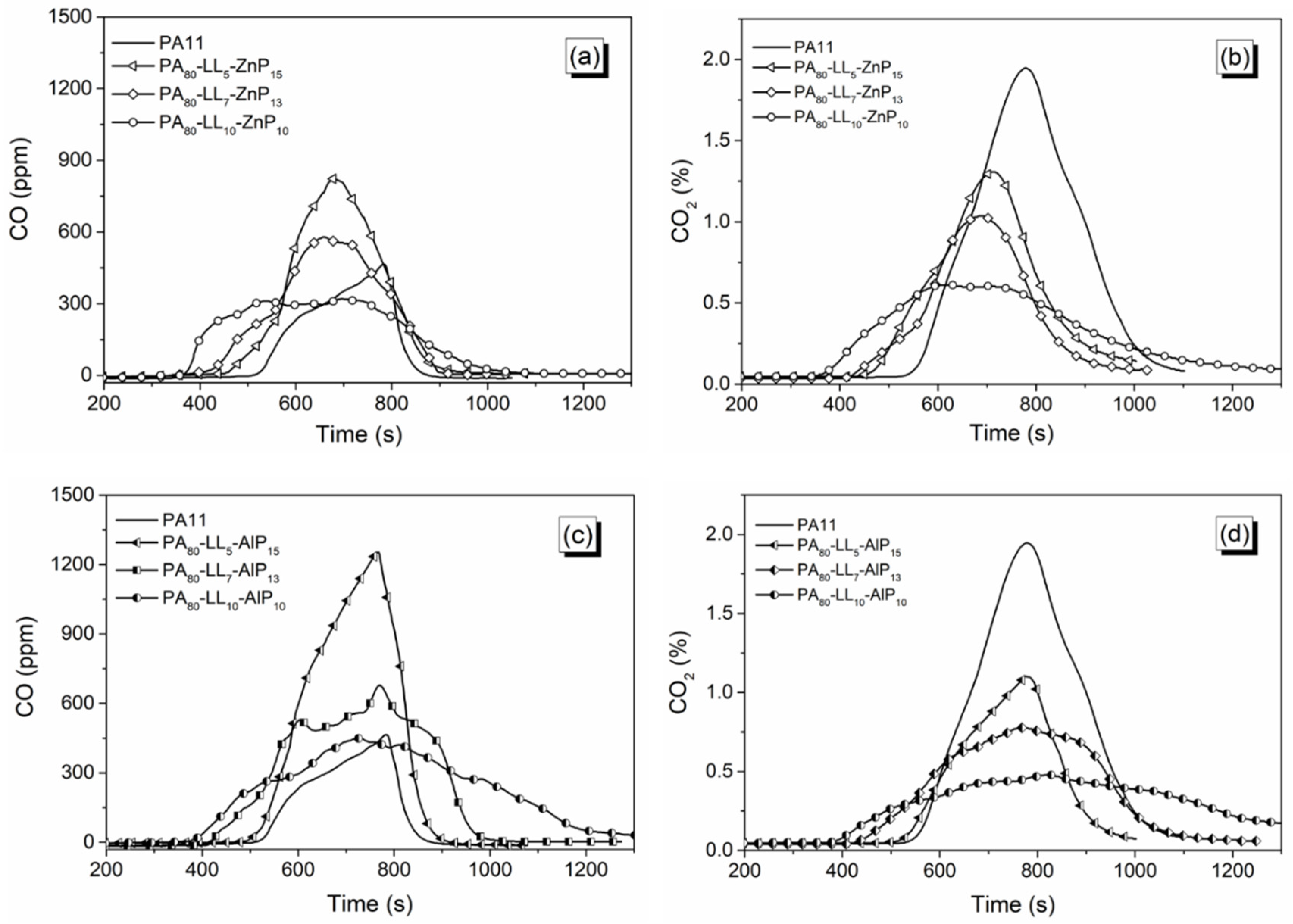
3.5. Smoke and CO Release
3.6. Morphology of Char Residue
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morgan, A.B.; Wilkie, C.A. Non-Halogenated Flame Retardant Handbook; Scrivener Publishing: Beverly, MA, USA, 2014. [Google Scholar]
- Rilsan® PA11: Created from a Renewable Source; Arkema Inc.: Colombes, France, 2012.
- Kuciel, S.; Kuzniar, P.; Liber-Kneć, A. Polyamides from renewable sources as matrices of short fiber reinforced biocomposites. Polimery 2012, 57, 627–634. [Google Scholar] [CrossRef]
- Holbery, J.; Houston, D. Natural-Fiber-Reinforced Polymer Composites in Automotive Applications. JOM 2006, 58, 80–86. [Google Scholar] [CrossRef]
- da Costa, A.P.; Botelho, E.C.; Costa, M.L.; Narita, N.E.; Tarpani, J.R. A review of welding technologies for thermoplastic composites in aerospace applications. J. Aerosp. Technol. Manag. 2012, 4, 255–265. [Google Scholar] [CrossRef]
- Lignin Products Global Market Size, Sales Data 2017–2022; Reuters: London, UK, 2017; Available online: https://www.reuters.com/brandfeatures/venture-capital/article?id=4789 (accessed on 2 August 2018).
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Polat, Y.; Stojanovska, E.; Negawo, T.A. Lignin as an Additive for Advanced Composites. Green Biocompos. 2017, 71–89. [Google Scholar] [CrossRef]
- Cicala, G.; Saccullo, G.; Blanco, I.; Samal, S.; Battiato, S.; Dattilo, S.; Saake, B. Polylactide/lignin blends: Effects of processing conditions on structure and thermo-mechanical properties. J. Therm. Anal. Calorim. 2017, 130, 515–524. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Hechenleitner, A.A.W.; Job, A.E.; Radovanocic, E.; Pineda, E.A.G. Thermal and photochemical stability of poly(vinyl alcohol)/modified lignin blends. Polym. Degrad. Stab. 2006, 91, 1192–1201. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W.O.S.; George, G. Thermal stability and miscibility of poly(hydroxybutyrate) and soda lignin blends. Ind. Crops Prod. 2010, 32, 656–661. [Google Scholar] [CrossRef]
- Blanco, I.; Cicala, G.; Latteri, A.; Saccullo, G.; El-Sabbagh, A.M.M.; Ziegmann, G. Thermal characterization of a series of lignin-based polypropylene blends. J. Therm. Anal. Calorim. 2017, 127, 147–153. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Lu, W.; Li, Q.; Zhang, Y.; Yu, H.; Hirose, S.; Hatakeyama, H.; Matsumoto, Y. Lignosulfonate/APP IFR and its flame retardancy in lignosulfonate-based rigid polyurethane foams. J. Wood Sci. 2018, 64, 287–293. [Google Scholar] [CrossRef]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Malucelli, G.; Guan, J. Thermal Stability and Fire Retardant Properties of Polyamide 11 Microcomposites Containing Different Lignins. Ind. Eng. Chem. Res. 2017, 56, 13704–13714. [Google Scholar] [CrossRef]
- Hörold, S. Phosphorus-based and Intumescent Flame Retardants. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 221–254. [Google Scholar]
- Kleiner, H.-J.; Budzinsky, W.; Kirsch, G. Low-Flammability Polyamide Molding Materials. US5773556A, 30 June 1998. Available online: https://patents.google.com/patent/US5773556A (accessed on 2 August 2018).
- Braun, U.; Bahr, H.; Schartel, B. Fire retardancy effect of aluminium phosphinate and melamine polyphosphate in glass fibre reinforced polyamide 6. E-Polymers 2010, 10. [Google Scholar] [CrossRef]
- Jenewein, E.; Kleiner, H.-J. Synergistic Flame Protection Agent Combination for Thermoplastic Polymers. EP0892829B1, 02 April 1997. Available online: https://patents.google.com/patents/EP0892829B1 (accessed on 12 August 2018).
- Braun, U.; Schartel, B.; Fichera, M.A.; Jäger, C. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6,6. Polym. Degrad. Stab. 2007, 92, 1528–1545. [Google Scholar] [CrossRef]
- Didane, N.; Giraud, S.; Devaux, E.; Lemort, G. Development of fire resistant PET fibrous structures based on phosphinate-POSS blends. Polym. Degrad. Stab. 2012, 97, 879–885. [Google Scholar] [CrossRef]
- Mandlekar, N.; Malucelli, G.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Guan, J. Fire retardant action of zinc phosphinate and polyamide 11 blend containing lignin as a carbon source. Polym. Degrad. Stab. 2018, 153, 63–74. [Google Scholar] [CrossRef]
- Troitzsch, J. Plastics Flammability Handbook: Principles, Regulations, Testing, and Approval; Hanser: Munich, Germany, 2004. [Google Scholar]
- ISO 5660. Fire Test, Reaction to Fire, Rate of Heat Release (Cone Calorimeter Method); International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Song, P.; Cao, Z.; Fu, S.; Fang, Z.; Wu, Q.; Ye, J. Thermal degradation and flame retardancy properties of ABS/lignin: Effects of lignin content and reactive compatibilization. Thermochim. Acta 2011, 518, 59–65. [Google Scholar] [CrossRef]
- Sicken, M.; Schlosser, E.; Wanzke, W.; Burghardt, D. Fusible Zinc Phosphinate. US20040176506A1, 9 September 2004. [Google Scholar]
- Battegazzore, D.; Alongi, J.; Fontaine, G.; Frache, A.; Bourbigot, S.; Malucelli, G. Bulk vs. surface flame retardancy of fully bio-based polyamide 10,10. RSC Adv. 2015, 5, 39424–39432. [Google Scholar] [CrossRef]
- Vannier, A.; Duquesne, S.; Bourbigot, S.; Alongi, J.; Camino, G.; Delobel, R. Investigation of the thermal degradation of PET, zinc phosphinate, OMPOSS and their blends—Identification of the formed species. Thermochim. Acta 2009, 495, 155–166. [Google Scholar] [CrossRef]
- Sut, A.; Greiser, S.; Jäger, C.; Schartel, B. Aluminium diethylphosphinate versus ammonium polyphosphate: A comprehensive comparison of the chemical interactions during pyrolysis in flame-retarded polyolefine/poly(phenylene oxide). Thermochim. Acta 2016, 640, 74–84. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Role of methoxyl group in char formation from lignin-related compounds. J. Anal. Appl. Pyrolysis 2009, 84, 79–83. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Sturm, H.; Schartel, B. Flame retardancy mechanisms of metal phosphinates in combination with melamine cyanurate in glass-fiber reinforced poly (1,4-butylene terephthalate): The influence of metal cation. Polym. Adv. Technol. 2008, 19, 680–692. [Google Scholar] [CrossRef]
- Jakab, E.; Till, F.; Székely, T.; Faix, O. Thermogravimetry/mass spectrometry of various lignosulfonates as well as of a kraft and acetosolv lignin. Holzforschung 1991, 45, 355–360. [Google Scholar] [CrossRef]
- Didane, N.; Giraud, S.; Devaux, E. Fire performances comparison of back coating and melt spinning approaches for PET covering textiles. Polym. Degrad. Stab. 2012, 97, 1083–1089. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Flame retardant mechanisms of red phosphorus and magnesium hydroxide in high impact polystyrene. Macromol. Chem. Phys. 2004, 205, 2185–2196. [Google Scholar] [CrossRef]
- Lewin, M.; Weil, E. Mechanisms and Modes of Action in Flame Retardancy of Polymers; Woodhead Publishing Ltd.: Cambridge, UK, 2001; pp. 31–68. [Google Scholar]
- Mouritz, A.P.; Mathys, Z.; Gibson, A.G. Heat release of polymer composites in fire. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1040–1054. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J.M. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.; Zhao, J. Synthesis, application and flame retardancy mechanism of a novel flame retardant containing silicon and caged bicyclic phosphate for polyamide 6. Polym. Degrad. Stab. 2011, 96, 1508–1515. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Effect of red phosphorus and melamine polyphosphate on the fire behavior of HIPS. J. Fire Sci. 2005, 23, 5–30. [Google Scholar] [CrossRef]
- Huang, W.; He, W.; Long, L.; Yan, W.; He, M.; Qin, S.; Yu, J. Highly efficient flame-retardant glass-fiber-reinforced polyamide 6T system based on a novel DOPO-based derivative: Flame retardancy, thermal decomposition, and pyrolysis behaviour. Polym. Degrad. Stab. 2018, 148, 26–41. [Google Scholar] [CrossRef]
- Zhan, Z.; Xu, M.; Li, B. Synergistic effects of sepiolite on the fl ame retardant properties and thermal degradation behaviors of polyamide 66/aluminum diethylphosphinate composites. Polym. Degrad. Stab. 2015, 117, 66–74. [Google Scholar] [CrossRef]




| Sample | Polyamide 11 (wt %) | Lignin (L 1 in wt %) | Phosphinate (P 1 in wt %) |
|---|---|---|---|
| PA11 | 100 | - | - |
| PA80-L20 | 80 | 20 | - |
| PA80-P20 | 80 | - | 20 |
| PA80-L5-P15 | 80 | 5 | 15 |
| PA80-L7-P13 | 80 | 7 | 13 |
| PA80-L10-P10 | 80 | 10 | 10 |
| Samples | T5% (°C) | Tmax (°C) | MMLR (%/min) | RExp 1 700 °C (%) | RCal 1 (%) | Tmax1 (°C) | Tmax2 (°C) | MMLR (%/min) | RExp 1 700 °C (%) | RCal 1 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Atmosphere: Nitrogen | Atmosphere: Air | |||||||||
| PA11 | 396 | 423 | 2 | 1 | - | 454 | 574 | 1.3 | 2 | - |
| PA80-LL5-ZnP15 | 373 | 472 | 2.4 | 5.8 | 6.7 | 464 | 553 | 1.8 | 7.6 | 12.5 |
| PA80-LL7-ZnP13 | 364 | 467 | 2 | 8.2 | 7.5 | 462 | 543 | 2 | 6 | 12.3 |
| PA80-LL10-ZnP10 | 326 | 465 | 1.8 | 12.7 | 8.6 | 465 | 559 | 2.2 | 6.8 | 12.1 |
| PA80-LL5-AlP15 | 363 | 468 | 1.7 | 5.5 | 6.2 | 460 | 611 | 1.6 | 7.1 | 10.0 |
| PA80-LL7-AlP13 | 349 | 470 | 1.6 | 7.6 | 7.1 | 472 | 610 | 1.8 | 5.9 | 10.2 |
| PA80-LL10-AlP10 | 330 | 445 | 1.5 | 10.7 | 8.3 | 470 | 612 | 1.7 | 6.2 | 10.4 |
| PA80-DL5-ZnP15 | 359 | 473 | 2 | 5.4 | 5.8 | 468 | 573 | 1.7 | 5.8 | 10.1 |
| PA80-DL7-ZnP13 | 347 | 472 | 1.8 | 6.4 | 6.3 | 474 | 577 | 1.6 | 3.8 | 9.0 |
| PA80-DL10-ZnP10 | 339 | 453 | 1.6 | 8.9 | 6.9 | 462 | 564 | 1.7 | 4.1 | 7.4 |
| PA80-DL5-AlP15 | 334 | 469 | 1.7 | 5.7 | 5.4 | 458 | 601 | 2.2 | 7.3 | 7.6 |
| PA80-DL7-AlP13 | 325 | 470 | 1.6 | 7.4 | 5.9 | 455 | 604 | 1.7 | 6.8 | 6.9 |
| PA80-DL10-AlP10 | 313 | 457 | 1.5 | 7.8 | 6.6 | 455 | 583 | 1.6 | 5.1 | 5.7 |
| Samples | 1st Flame t1 (s) | 2nd Flame t2 (s) | Combustion time (t1 + t2) | Cotton Ignition | Dripping | Rating |
|---|---|---|---|---|---|---|
| PA11 | 11 ± 1 | 7 ± 1 | 18 ± 1 | Yes | Yes | V2 |
| PA80-LL5-ZnP15 | 11 ± 1 | 3 ± 1 | 14 ± 1 | Yes | Yes | V2 |
| PA80-LL7-ZnP13 | 4 ± 1 | 3 ± 1 | 7 ± 1 | No | Yes | V1 |
| PA80-LL10-ZnP10 | 6 ± 1 | 3 ± 1 | 9 ± 1 | No | Yes | V1 |
| PA80-LL5-AlP15 | 16 ± 1 | 6 ± 2 | 22 ± 1 | Yes | Yes | V2 |
| PA80-LL7-AlP13 | 24 ± 2 | 4 ± 1 | 28 ± 2 | Yes | Yes | V2 |
| PA80-LL10-AlP10 | 8 ± 1 | 4 ± 1 | 12 ± 2 | Yes | Yes | V2 |
| PA80-DL5-ZnP15 | 10 ± 1 | 4 ± 1 | 14 ± 2 | Yes | Yes | V2 |
| PA80-DL7-ZnP13 | 11 ± 1 | 4 ± 1 | 15 ± 2 | Yes | Yes | V2 |
| PA80-DL10-ZnP10 | 7 ± 1 | 3 ± 1 | 10 ± 1 | Yes | Yes | V2 |
| PA80-DL5-AlP15 | 22 ± 2 | 5 ± 1 | 27 ± 2 | Yes | Yes | V2 |
| PA80-DL7-AlP13 | 19 ± 1 | 4 ± 1 | 23 ± 2 | Yes | Yes | V2 |
| PA80-DL10-AlP10 | 18 ± 1 | 3 ± 1 | 20 ± 1 | Yes | Yes | V2 |
| Samples | TTI (s) | PHRR (kW/m2) | Reduction (%) | THR (MJ/m2) | EHC (kJ/g) | TSR (m2/m2) | CO Yield (g/kg) | CO2 Yield (kg/kg) | CO2/CO | Residue (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PA11 | 154 ± 3 | 884 ± 4 | - | 92 ± 4 | 33.8 ± 0.6 | 1033 ± 1 | 33 ± 1 | 2.6 ± 0.1 | 79 | 0.6 ± 0.1 |
| PA80-LL5-ZnP15 | 112 ± 12 | 560 ± 40 | 37 | 79 ± 2 | 29.3 ± 0.6 | 1799 ± 48 | 88 ± 1 | 1.9 ± 0.1 | 22 | 5.9 ± 0.2 |
| PA80-LL7-ZnP13 | 92 ± 9 | 443 ± 21 | 50 | 77 ± 4 | 28.8 ± 0.7 | 1652 ± 151 | 92 ± 1 | 2.1 ± 0.1 | 23 | 6.2 ± 0.2 |
| PA80-LL10-ZnP10 | 86 ± 9 | 315 ± 11 | 64 | 73 ± 2 | 28.4 ± 0.4 | 1691 ± 26 | 72 ± 2 | 2.0 ± 0.1 | 28 | 8.5 ± 0.3 |
| PA80-LL5-AlP15 | 142 ± 11 | 554 ± 33 | 37 | 78 ± 6 | 30.3 ± 1 | 1959 ± 28 | 98 ± 3 | 1.9 ± 0.1 | 19 | 5.8 ± 0.3 |
| PA80-LL7-AlP13 | 124 ± 10 | 420 ± 27 | 52 | 77 ± 3 | 29.6 ± 0.3 | 2034 ± 37 | 85 ± 3 | 2.0 ± 0.1 | 24 | 7.1 ± 0.3 |
| PA80-LL10-AlP10 | 108 ± 12 | 230 ± 14 | 74 | 72 ± 4 | 29.4 ± 0.4 | 1995 ± 18 | 71 ± 6 | 2.1 ± 4 | 30 | 11.5 ± 0.3 |
| PA80-DL5-ZnP15 | 150 ± 18 | 740 ± 23 | 16 | 79 ± 2 | 29.6 ± 1.3 | 1720 ± 36 | 71 ± 3 | 2.2 ± 0.1 | 31 | 3.8 ± 0.2 |
| PA80-DL7-ZnP13 | 128 ± 14 | 678 ± 36 | 23 | 77 ± 3 | 30.2 ± 0.4 | 1800 ± 88 | 73 ± 4 | 2.1 ± 0.1 | 29 | 5.2 ± 1.5 |
| PA80-DL10-ZnP10 | 116 ± 13 | 500 ± 48 | 43 | 75 ± 7 | 30.3 ± 1.2 | 1745 ± 62 | 61 ± 6 | 2.1 ± 0.1 | 34 | 8.2 ± 0.5 |
| PA80-DL5-AlP15 | 174 ± 14 | 424 ± 39 | 42 | 76 ± 4 | 28 ± 1 | 1819 ± 40 | 99 ± 2 | 2.0 ± 0.1 | 20 | 7.7 ± 0.5 |
| PA80-DL7-AlP13 | 140 ± 12 | 406 ± 34 | 54 | 74 ± 5 | 31 ± 1 | 1929 ± 45 | 90 ± 3 | 2.0 ± 0.1 | 22 | 8.6 ± 0.5 |
| PA80-DL10-AlP10 | 95 ± 7 | 320 ± 10 | 64 | 72 ± 1 | 29 ± 1.3 | 1967 ± 68 | 86 ± 5 | 2.1 ± 0.1 | 24 | 10.4 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Guan, J. Valorization of Industrial Lignin as Biobased Carbon Source in Fire Retardant System for Polyamide 11 Blends. Polymers 2019, 11, 180. https://doi.org/10.3390/polym11010180
Mandlekar N, Cayla A, Rault F, Giraud S, Salaün F, Guan J. Valorization of Industrial Lignin as Biobased Carbon Source in Fire Retardant System for Polyamide 11 Blends. Polymers. 2019; 11(1):180. https://doi.org/10.3390/polym11010180
Chicago/Turabian StyleMandlekar, Neeraj, Aurélie Cayla, François Rault, Stéphane Giraud, Fabien Salaün, and Jinping Guan. 2019. "Valorization of Industrial Lignin as Biobased Carbon Source in Fire Retardant System for Polyamide 11 Blends" Polymers 11, no. 1: 180. https://doi.org/10.3390/polym11010180
APA StyleMandlekar, N., Cayla, A., Rault, F., Giraud, S., Salaün, F., & Guan, J. (2019). Valorization of Industrial Lignin as Biobased Carbon Source in Fire Retardant System for Polyamide 11 Blends. Polymers, 11(1), 180. https://doi.org/10.3390/polym11010180