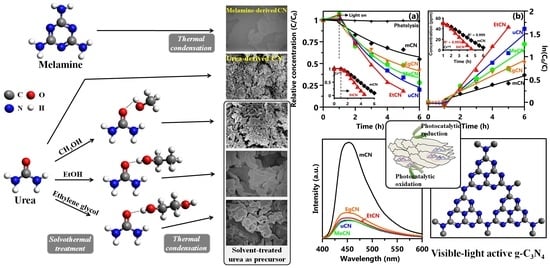
Photocatalytic Dye and Cr(VI) Degradation Using a Metal-Free Polymeric g-C3N4 Synthesized from Solvent-Treated Urea
Abstract
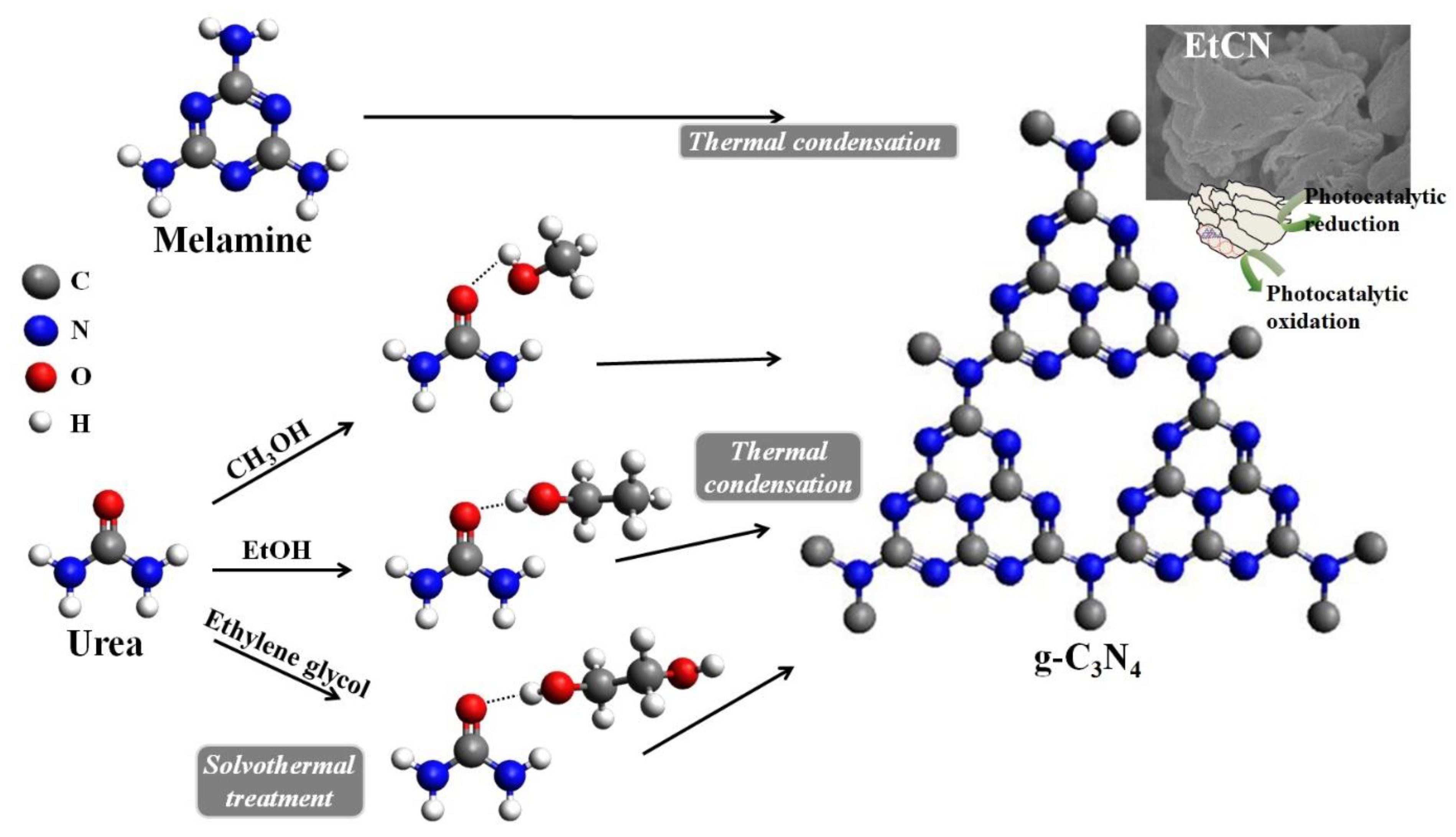
:1. Introduction
2. Experimental Procedures
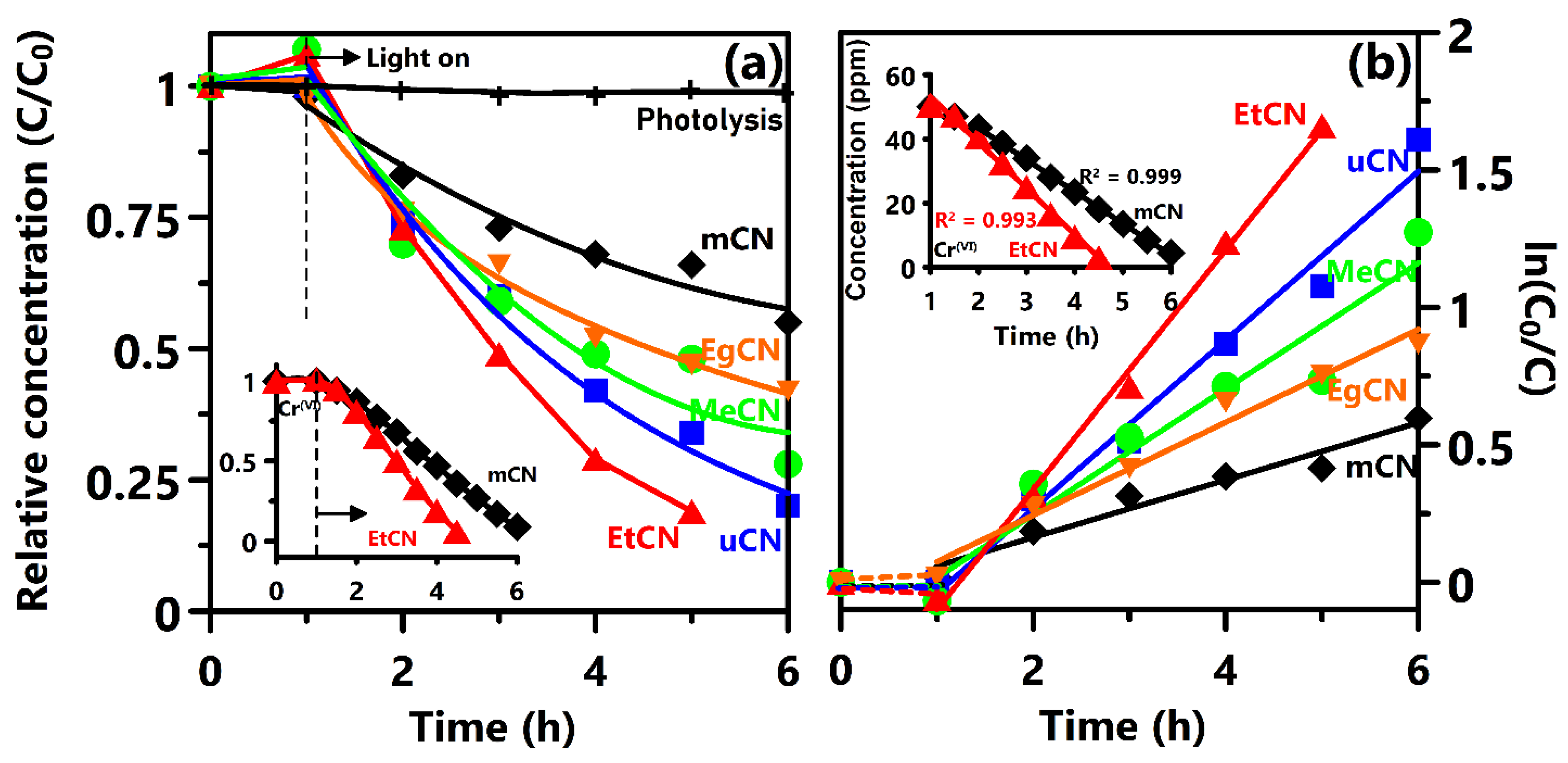
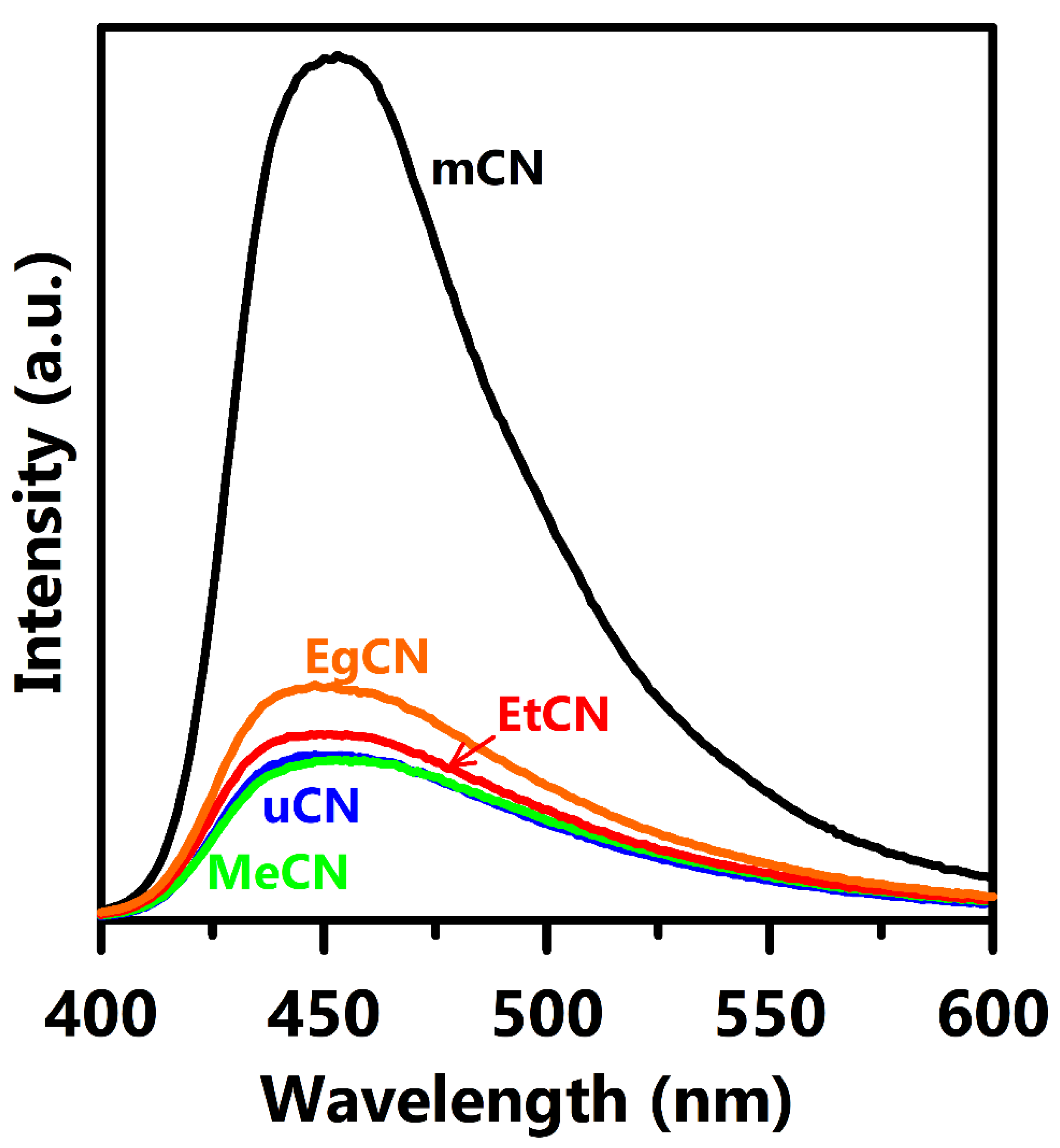
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| C0 | dye concentration in time t0 (M). |
| C | dye concentration in time t (M). |
| Ds | crystallite size obtained from Scherrer method (nm). |
| DW-H | crystallite size obtained from Williamson-Hall method (nm). |
| k | first-order reaction rate constant (h−1). |
| k’ | zero-order reaction rate constant (ppm h−1). |
| K | shape factor (/) |
| t0 | initial irradiation time (h) |
| t | irradiation time (h) |
| βhkl | full width at half maximum at (hkl) plane. |
| ε | lattice strain obtained from the Williamson-Hall method. |
| λ | wavelength of incident X-ray (1.5406 Å) |
| θ | XRD diffraction angle (degree). |
References
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; O’Donnell, K.; Ang, H.M.; Tade, M.O.; Wang, S. Metal-free melem/g-C3N4 hybrid photocatalysts for water treatment. J. Colloid Interf. Sci. 2016, 464, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, G.; Cuomo, F.; Ambrosone, L.; Colella, M.; Ceglie, A.; Venditti, F.; Lopez, F. Photocatalytic degradation of a model textile dye using carbon-doped titanium dioxide and visible light. J. Water Process Eng. 2017, 20, 71–77. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Wu, Z.; Jiang, L.; Li, H. Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr(VI) reduction. J. Hazard. Mater. 2015, 286, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bian, Y.; Qin, H.; Zhang, Y.; Bian, Z. Photocatalytic reduction behavior of hexavalent chromium on hydroxyl modified titanium dioxide. Appl. Catal. B Environ. 2017, 206, 293–299. [Google Scholar] [CrossRef]
- Venditti, F.; Ceglie, A.; Palazzo, G.; Colafemmina, G.; Lopez, F. Removal of chromate from water by a new CTAB–silica gelatin composite. J. Colloid Interf. Sci. 2007, 310, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Hu, C.C.; Lin, Y.R.; Yang, H.C. Recent development of g-C3N4-based hydrogels as photocatalysts: A minireview. ChemSusChem 2018. [Google Scholar] [CrossRef]
- Hu, C.C.; Huang, H.X.; Lin, Y.F.; Yoshida, M.; Chen, T.H. Decoration of SrTiO3 nanofibers by BiOI for photocatalytic methyl orange degradation under visible light irradiation. J. Taiwan Inst. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Chen, S.; Lu, W.; Han, J.; Zhong, H.; Xu, T.; Wang, G.; Chen, W. Robust three-dimensional g-C3N4@cellulose aerogel enhanced by cross-linked polyester fibers for simultaneous removal of hexavalent chromium and antibiotics. Chem. Eng. J. 2019, 359, 119–129. [Google Scholar] [CrossRef]
- Hu, C.C.; Shen, J.J.; Chang, A.L.; Wei, T.C. Microwave plasma torch synthesis of ZnAl oxides as adsorbent and photocatalyst for organic compounds removal. Powder Technol. 2019, 344, 454–462. [Google Scholar] [CrossRef]
- Hu, C.C.; Chen, T.S.; Huang, H.X. Heterojunction of n-type Sr2TiO4 with p-type Bi5O7I with enhanced photocatalytic activity under irradiation of simulated sunlight. Appl. Surf. Sci. 2017, 426, 536–544. [Google Scholar] [CrossRef]
- Hu, C.C.; Wang, M.S. Photoluminescence and photocatalysis of gallium oxynitride synthesized from nitridation of Ga2O3. ECS J. Solid State Sci. Technol. 2017, 6, Q3001–Q3006. [Google Scholar] [CrossRef]
- Hu, C.C.; Huang, H.H.; Huang, Y.C. N-doped NaTaO3 synthesized from a hydrothermal method for photocatalytic water splitting under visible light irradiation. J. Energy Chem. 2017, 26, 515–521. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Nikokavoura, C. Graphene and g-C3N4 based photocatalysts for NOx removal: A review. Appl. Surf. Sci. 2018, 430, 18–52. [Google Scholar] [CrossRef]
- Hu, C.; Hung, W.Z.; Wang, M.S.; Lu, P.J. Phosphorus and sulfur codoped g-C3N4 as an efficient metal-free photocatalyst. Carbon 2018, 127, 374–383. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.S.; Hung, W.Z. Influence of solvothermal synthesis on the photocatalytic degradation activity of carbon nitride under visible light irradiation. Chem. Eng. Sci. 2017, 167, 1–9. [Google Scholar] [CrossRef]
- Hu, C.; Chu, Y.C.; Wang, M.S.; Wu, X.H. Rapid synthesis of g-C3N4 spheres using microwave-assisted solvothermal method for enhanced photocatalytic activity. J. Photochem. Photobiol. A-Chem. 2017, 348, 8–17. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.C.; Kang, X.; Wang, L.; Liu, G.; Cheng, H.M. Selective breaking of hydrogen bonds of layered carbon nitride for visible light photocatalysis. Adv. Mater. 2016, 28, 6471–6477. [Google Scholar] [CrossRef]
- Yea, S.; Wang, R.; Wu, M.Z.; Yuan, Y.P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Redemann, C.E.; Lucas, H.J. Some derivatives of cyameluric acid and probable structures of melam, melem and melon. J. Am. Chem. Soc. 1940, 62, 842–846. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, F.; Goettmann, M.; Antonietti, J.O.; Müller, R.; Schlögl, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Fang, H.B.; Luo, Y.; Zeng, Y.Z.; Ma, W.; Tao, X. Facile large-scale synthesis of urea-derived porous graphitic carbon nitride with extraordinary visible-light spectrum photodegradation. Ind. Eng. Chem. Res. 2016, 55, 4506–4514. [Google Scholar] [CrossRef]
- Dai, K.; Lu, L.H.; Liu, Q.; Zhu, G.P.; Wei, X.Q.; Bai, J.; Xuan, L.L.; Wang, H. Sonication assisted preparation of graphene oxide/graphitic-C3N4 nanosheet hybrid with reinforced photocurrent for photocatalyst applications. Dalton. T. 2014, 43, 6295–6299. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.B.; Gong, Y.J.; Zhang, J.S.; Zhan, L.; Ma, L.L.; Fang, Z.Y.; Vajtai, R.; Wang, X.C.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Li, Y.H.; Wang, Z.Y.; Ho, W.K. Enhanced visible light photocatalytic activity and oxidation ability of porous graphene-like g-C3N4 nanosheets via thermal exfoliation. Appl. Surf. Sci. 2015, 358, 393–403. [Google Scholar] [CrossRef]
- Yang, Y.F.; Chen, J.J.; Mao, Z.Y.; An, N.; Wang, D.J.; Fahlman, B.D. Ultrathin g-C3N4 nanosheets with an extended visible-light-responsive range for significant enhancement of photocatalysis. RSC Adv. 2017, 7, 2333–2341. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Zak, K.; Majid, W.H.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Guo, Q.; Xie, Y.; Wang, X.; Zhang, S.; Huo, T.; Lv, S. Synthesis of carbon nitride nanotubes with the C3N4 stoichiometry via a benzene-thermal process at low temperatures. Chem. Commun. 2004, 0, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, R.; Lin, J.; Zhu, Y. Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Environ. Sci. 2011, 4, 2922–2929. [Google Scholar] [CrossRef]
- Cao, S.W.; Low, J.X.; Yu, J.G.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Yang, D.; Shi, J.; Nan, Y.; Sun, Y.; Jiang, Z. Three-dimensional porous aerogel constructed by g-C3N4 and graphene oxide nanosheets with excellent visible-light photocatalytic performance. ACS Appl. Mater. Inter. 2015, 7, 25693–25701. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. Visible light Cr(VI) reduction and organic chemical oxidation by TiO2 photocatalysis. Environ. Sci. Technol. 2005, 39, 6251–6259. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Qiao, L.L.; Zhu, A.Q.; Liu, Y.; Pan, J. Constructing 2D BiOCl/C3N4 layered composite with large contact surface for visible-light-driven photocatalytic degradation. Appl. Surf. Sci. 2017, 426, 897–905. [Google Scholar] [CrossRef]
- Yan, G.; Feng, X.J.; Xiao, L.G.; Xi, W.G.; Tan, H.Q.; Shi, H.F.; Wang, Y.H.; Li, Y.G. Tuning of the photocatalytic performance of g-C3N4 by polyoxometalates under visible light. Dalton. T. 2017, 46, 16019–16024. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.R.; Huang, G.F.; Li, D.F.; Tian, Q.N.; Yang, K.; Si, Y.; Chang, S.; Zhang, X.A.; Huang, W.Q. Novel β-C3N4/CuO nanoflakes: Facile synthesis and unique photocatalytic performance. J. Phys. D Appl. Phys. 2017, 50, 1–9. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Gong, T.T.; Xian, Q.M.; Xie, J.M. Graphite-like carbon nitride coupled with tiny Bi2S3 nanoparticles as 2D/0D heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 444, 75–86. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zhang, L.L.; Yin, M.H.; Xie, D.Y.; Chen, J.Q.; Yin, J.Z.; Fu, Y.S.; Zhao, P.S.; Zhong, H.; Zhao, Y.J.; et al. Ultrathin g-C3N4 films supported on Attapulgite nanofibers with enhanced photocatalytic performance. Appl. Surf. Sci. 2018, 440, 170–176. [Google Scholar] [CrossRef]
- Yan, M.; Ma, Y.; Zhang, H.; Ye, B.; Dong, X. Enhanced photocatalytic activity of graphitic carbon nitride/cadmium sulfide heterojunctions by protonating treatment. J. Phys. Chem. Solids. 2018, 116, 50–57. [Google Scholar] [CrossRef]
- Gao, Y.; Lin, J.; Zhang, Q.; Yu, H.; Ding, F.; Xu, B.; Sun, Y.; Xu, Z. Facile synthesis of heterostructured YVO4/g-C3N4/Ag photocatalysts with enhanced visible-light photocatalytic performance. Appl. Catal. B Environ. 2017, 224, 586–593. [Google Scholar] [CrossRef]
- Li, H.; Li, N.; Wang, M.; Zhao, B.; Long, F. Synthesis of novel and stable g-C3N4-Bi2WO6 hybrid nanocomposites and their enhanced photocatalytic activity under visible light irradiation. Royal Soc. Open Sci. 2018, 5, 171419. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liang, W.; Guo, J.; Tang, H.; Liu, S. MoS2 quantum dots decorated g-C3N4/Ag heterostructures for enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2018, 430, 234–242. [Google Scholar] [CrossRef]
- Nithya, J.; Do, J.; Kang, M. Fabrication of flower-like copper cobaltite/graphitic-carbon nitride (CuCo2O4/g-C3N4 ) composite with superior photocatalytic activity. J. Ind. Eng. Chem. 2018, 57, 405–415. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Shi, Q.; Cai, Z.; Yang, Z. Acid-treated g-C3N4 with improved photocatalytic performance in the reduction of aqueous Cr(VI) under visible-light. Sep. Purif. Technol. 2015, 142, 251–257. [Google Scholar] [CrossRef]
- Xiao, D.; Dai, K.; Qu, Y.; Yin, Y.; Chen, H. Hydrothermal synthesis of α-Fe2O3/g-C3N4 composite and its efficient photocatalytic reduction of Cr(VI) under visible light. Appl. Surf. Sci. 2015, 358, 181–187. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Zhang, Y.; Yang, Z.; Zhu, A.; Dionysiou, D. Enhancement of the Cr(VI) adsorption and photocatalytic reduction activity of g-C3N4 by hydrothermal treatment in HNO3 aqueous solution. Appl. Catal. A Gen. 2016, 521, 9–18. [Google Scholar] [CrossRef]
- Wei, H.; Hou, C.; Zhang, Y.; Nan, Z. Scalable low temperature in air solid phase synthesis of porous flower-like hierarchical nanostructure SnS2 with superior performance in the adsorption and photocatalytic reduction of aqueous Cr(VI). Sep. Purif. Technol. 2017, 189, 153–161. [Google Scholar] [CrossRef]
- Huang, W.; Liu, N.; Zhang, X.; Wu, M.; Tang, L. Metal organic framework g-C3N4/MIL-53(Fe) heterojunctions with enhanced photocatalytic activity for Cr(VI) reduction under visible light. Appl. Surf. Sci. 2017, 425, 107–116. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Z.; Lin, F.; Guo, F.; Alamry, K.A.; Taib, L.A.; Asiri, A.M.; Wang, X. Sulfur-doped carbon nitride polymers for photocatalytic degradation of organic pollutant and reduction of Cr(VI). Molecules 2017, 22, 572. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.A.; Leng, D.; Lang, S.B.; Wang, W.B. Fabrication of g-C3N4/SnS2/SnO2 nanocomposites for promoting photocatalytic reduction of aqueous Cr(VI) under visible light. Chem. Eng. J. 2017, 335, 491–500. [Google Scholar] [CrossRef]
- Lamkhao, S.; Rujijanagul, G.; Randorn, C. Fabrication of g-C3N4 and a promising charcoal property towards enhanced chromium(VI) reduction and wastewater treatment under visible light. Chemosphere 2017, 193, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tang, L.; Zeng, G.; Zhu, Z.; Yan, M.; Zhou, Y.Y.; Wang, J.; Liu, Y.; Wang, J. Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism. Appl. Catal. B Environ. 2017, 203, 343–354. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, Z.; Shen, X.; Zhu, B.; Macharia, D.K.; Chen, Z.; Zhang, L. Synthesis of Au nanoparticle-decorated carbon nitride nanorods with plasmon-enhanced photoabsorption and photocatalytic activity for removing various pollutants from water. J. Hazard. Mater. 2018, 344, 1188–1197. [Google Scholar] [CrossRef] [PubMed]

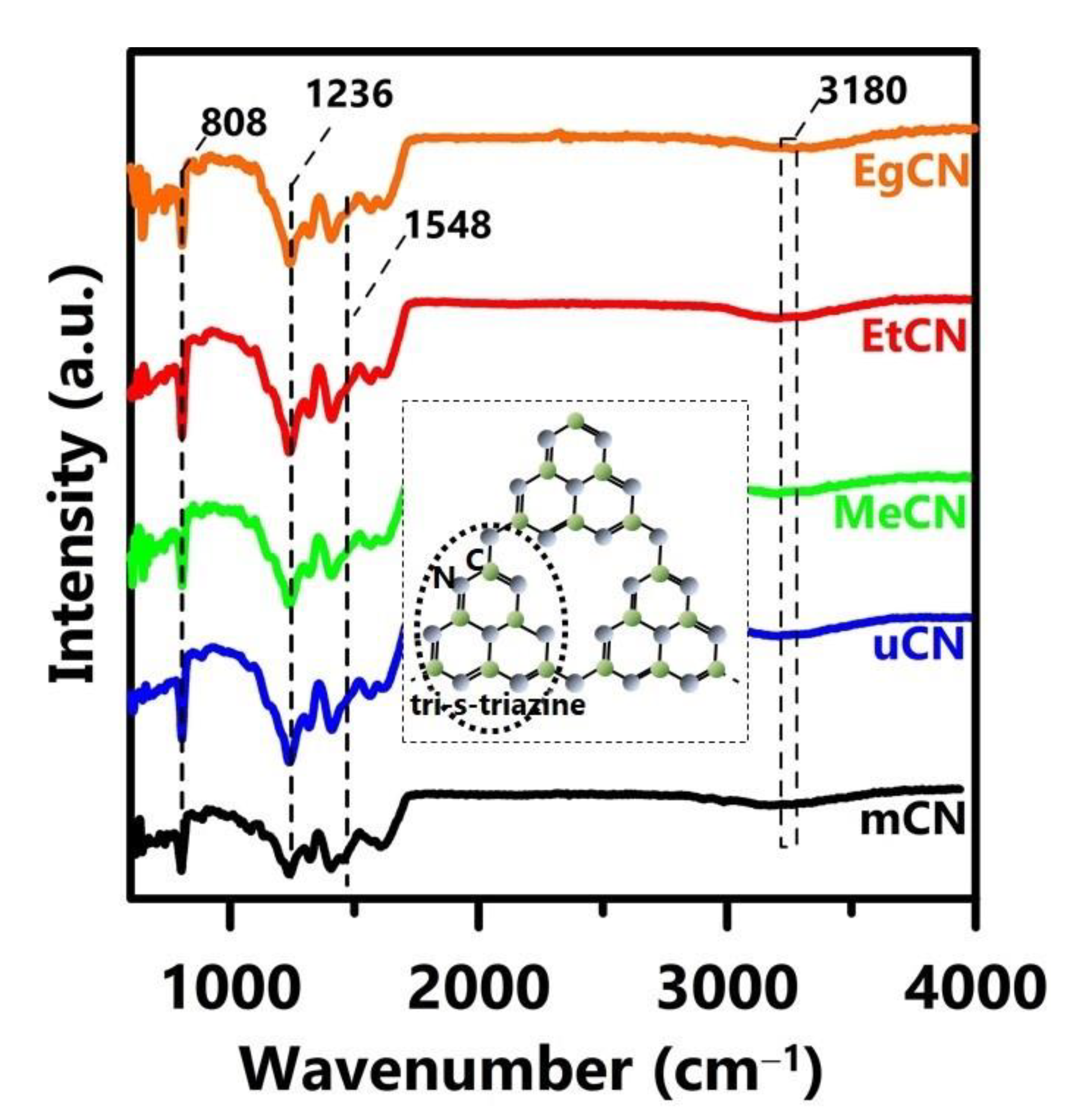
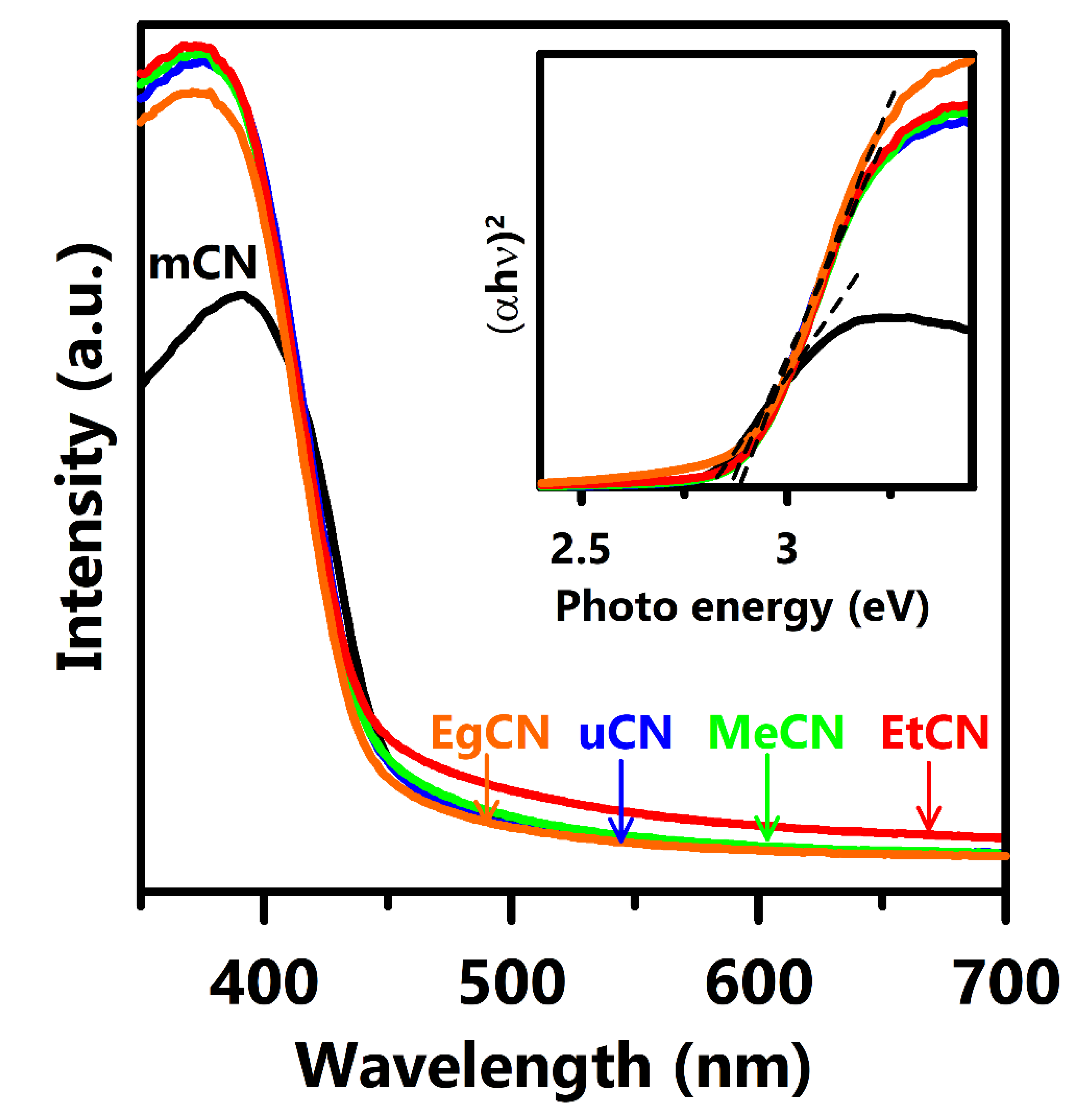
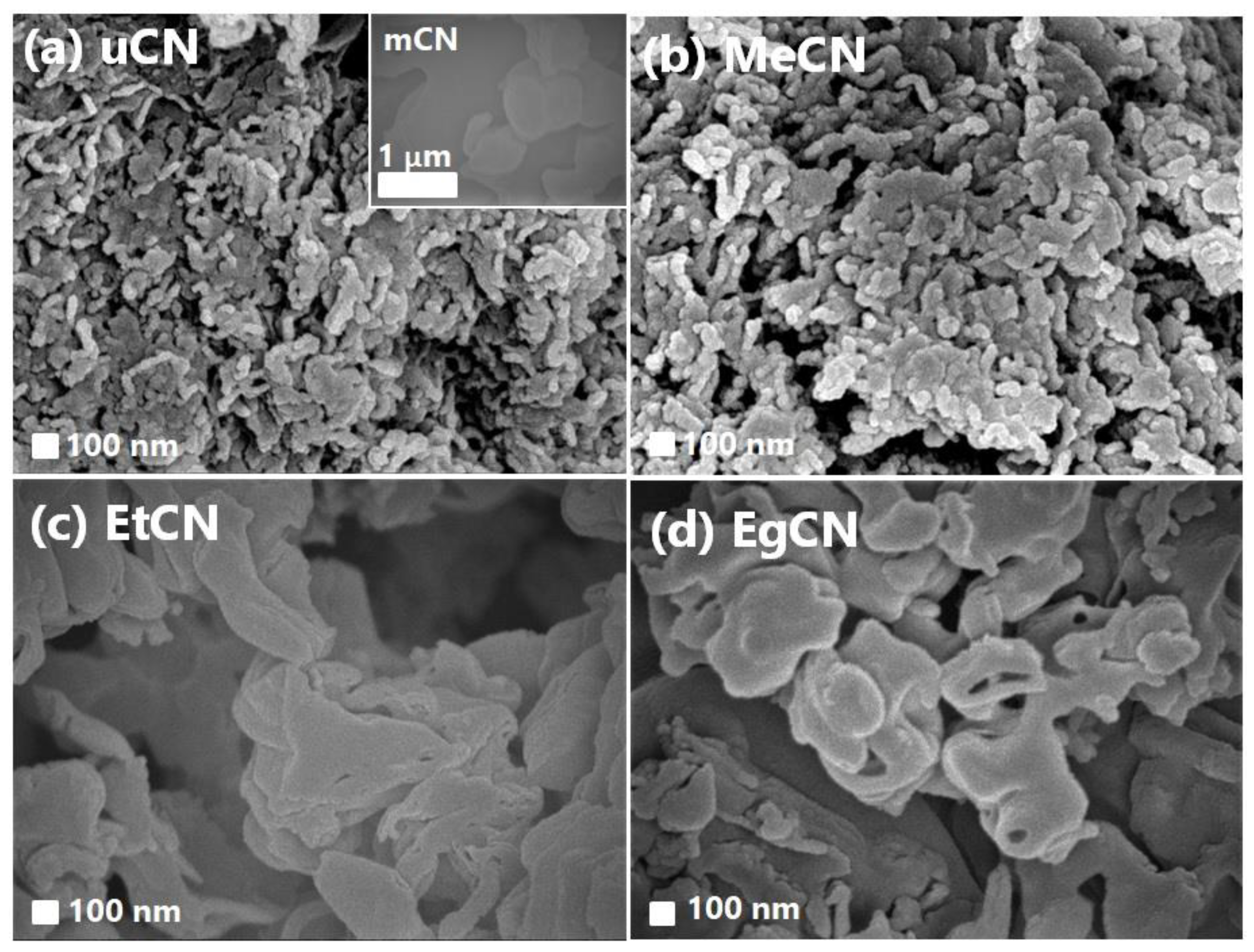
| Samples | Williamson–Hall Method | Scherrer Method | ||
|---|---|---|---|---|
| ε | DW–H (nm) | Ds(100) (nm) | Ds(002) (nm) | |
| uCN | 0.0405 | 2.494 | 1.52 | 3.49 |
| MeCN | 0.0414 | 2.478 | 1.71 | 3.82 |
| EtCN | 0.0403 | 2.453 | 2.12 | 3.74 |
| EgCN | 0.0413 | 2.476 | 1.72 | 3.84 |
| Samples | Light Source | Rate Constant, k (h−1) | Amounts of Catalyst (g) | MO Concentration (ppm) | Year | Ref. |
| g-C3N4/BiOCl | 300 W Xe lamp with an optical filter (>420 nm) | 0.0858 | 0.01 | 10 | 2017 | [36] |
| g-C3N4/POM (Polyoxometalates) | 300 W Xe lamp with an optical filter (>420 nm) | 2.28 | 0.05 | 20 | 2017 | [37] |
| β-C3N4/CuO | 300 W UV lamp | 0.0311 | 0.005 | 10 | 2017 | [38] |
| g-C3N4/Bi2S3 | 350 W Xe lamp | 0.2874 | 0.05 | 10 | 2018 | [39] |
| g-C3N4 | 500 W Xe lamp with an optical filter (>420 nm) | 0.1266 | 0.25 | 10 | 2018 | [40] |
| g-C3N4/CdS | 300 W Xe lamp with an optical filter (>420 nm) | 0.306 | 0.1 | 10 | 2018 | [41] |
| g-C3N4/YVO4 | 300 W Xe lamp | 0.6786 | 0.02 | 20 | 2018 | [42] |
| g-C3N4/Bi2WO6 | 500 W Xe lamp with an optical filter (>420 nm) | 0.5994 | 0.05 | 5 | 2018 | [43] |
| g-C3N4/Ag/MoS2 | 350 W Xe lamp with an optical filter (>420 nm) | 0.78 | 0.05 | 10 | 2018 | [44] |
| g-C3N4/CuCo2O4 | 150 W Xe lamp | 0.138 | 0.03 | 10 | 2018 | [45] |
| Samples | Light source | Rate Constant, k (min−1) | Amounts of Catalyst (g) | Concentration of K2Cr2O7 (ppm) | Year | Ref. |
| acid-treated g-C3N4 | Visible light with an optical filter (>420 nm) | / | 0.3 | 50 | 2015 | [46] |
| g-C3N4/α-Fe2O3 | 300 W Xe lamp | / | 0.1 | 10 | 2015 | [47] |
| g-C3N4 | Visible light with an optical filter (>420 nm) | 0.0025 | 0.3 | 50 | 2016 | [48] |
| g-C3N4/SnS2 | Visible light with an optical filter (>420 nm) | 0.0109 | 0.3 | 50 | 2017 | [49] |
| g-C3N4/MIL53(Fe) | 500 W Xe lamp with an optical filter (760 > λ > 420 nm) | 0.004 | 0.02 | 10 | 2017 | [50] |
| S-doped g-C3N4 | 300 W Xe lamp with an optical filter (>400 nm) | 0.0036 | 0.01 | 5 | 2017 | [51] |
| g-C3N4/SnS2/SnO2 | 300 W Xe lamp | 0.001 | 0.05 | 20 | 2017 | [52] |
| g-C3N4 | 30 W white LED | / | 0.05 | 2.94 | 2017 | [53] |
| g-C3N4 nanosheet | 300 W Xe lamp with an optical filter (>400 nm) | / | 0.05 | 20 | 2017 | [54] |
| g-C3N4/Au | 300 W Xe lamp with an optical filter (>400 nm) | / | 0.02 | 20 | 2018 | [55] |
| g-C3N4 obtained from ethanol-treated urea | 350 W Xe lamp (>400 nm) | MO: 0.436 h−1 Cr(VI): 14 ppm h−1 | 0.25 | MO: 2.5 × 10−5 M (8.2 ppm) K2Cr2O7 solution: 50 ppm | This work (2018) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Chu, Y.-C.; Lin, Y.-R.; Yang, H.-C.; Wang, K.-H. Photocatalytic Dye and Cr(VI) Degradation Using a Metal-Free Polymeric g-C3N4 Synthesized from Solvent-Treated Urea. Polymers 2019, 11, 182. https://doi.org/10.3390/polym11010182
Hu C, Chu Y-C, Lin Y-R, Yang H-C, Wang K-H. Photocatalytic Dye and Cr(VI) Degradation Using a Metal-Free Polymeric g-C3N4 Synthesized from Solvent-Treated Urea. Polymers. 2019; 11(1):182. https://doi.org/10.3390/polym11010182
Chicago/Turabian StyleHu, Chechia, Yi-Ching Chu, Yan-Ru Lin, Hung-Chun Yang, and Ke-Hsuan Wang. 2019. "Photocatalytic Dye and Cr(VI) Degradation Using a Metal-Free Polymeric g-C3N4 Synthesized from Solvent-Treated Urea" Polymers 11, no. 1: 182. https://doi.org/10.3390/polym11010182
APA StyleHu, C., Chu, Y.-C., Lin, Y.-R., Yang, H.-C., & Wang, K.-H. (2019). Photocatalytic Dye and Cr(VI) Degradation Using a Metal-Free Polymeric g-C3N4 Synthesized from Solvent-Treated Urea. Polymers, 11(1), 182. https://doi.org/10.3390/polym11010182