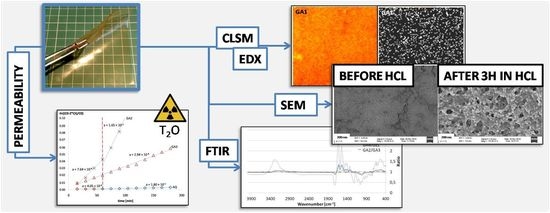
Soft Gelatin Films Modified with Cellulose Acetate Phthalate Pseudolatex Dispersion—Structure and Permeability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films
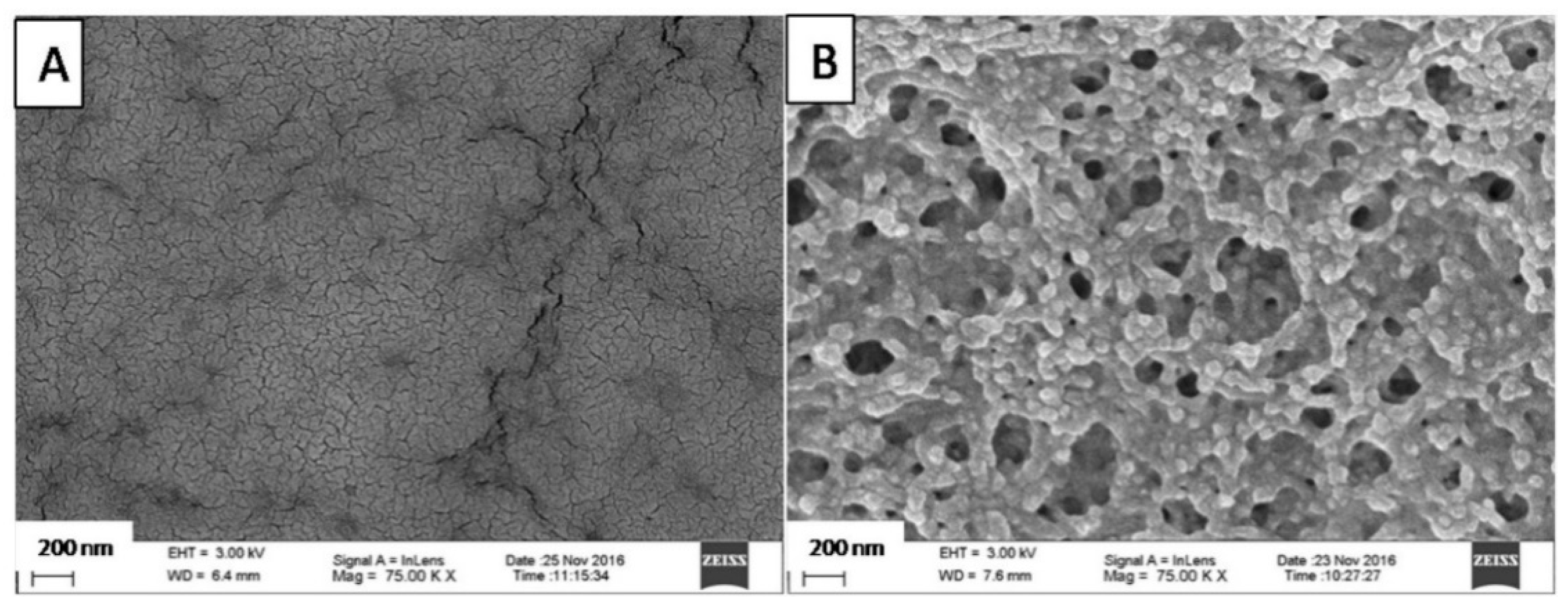
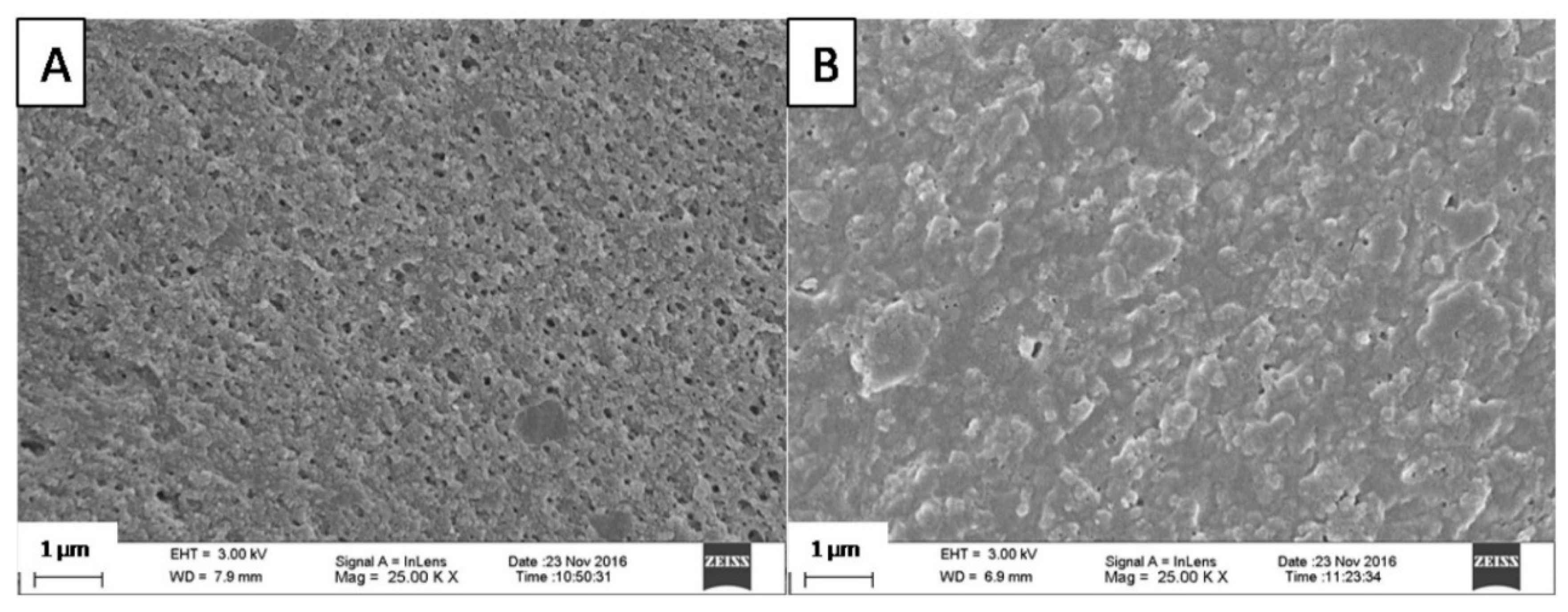
2.3. Scanning Electron Microscopy (SEM)
2.4. Energy-Dispersive X-ray Spectroscopy (EDX)
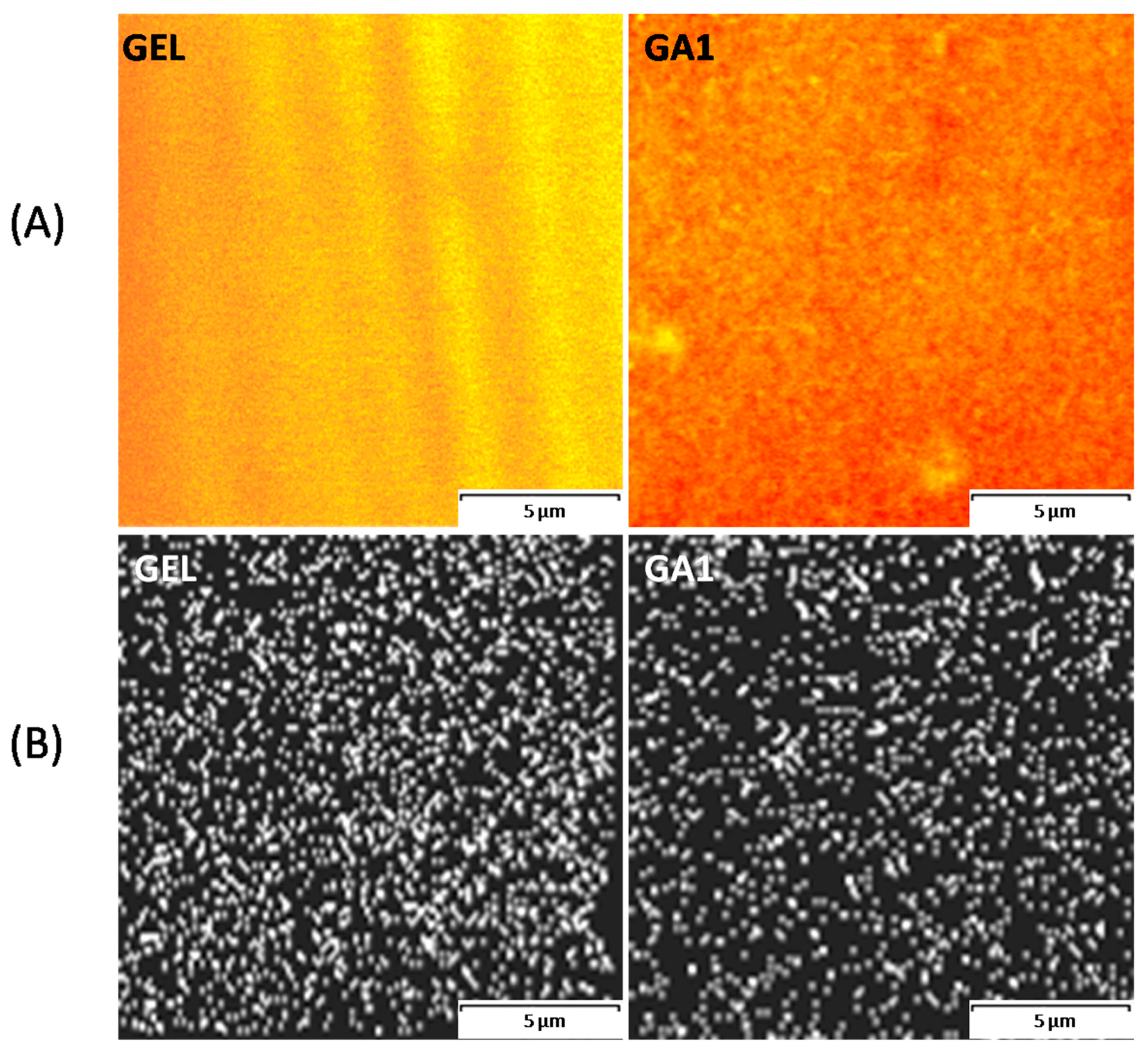
2.5. Confocal Laser Scanning Microscopy (CLSM)
2.6. Attenuated Total Reflection-Fourier Transform Infra-Red (ATR-FTIR) Spectroscopy
2.7. Swelling and Fraction Soluble in HCl
2.8. Permeability Study
3. Results and Discussion
3.1. Microscopic Structure
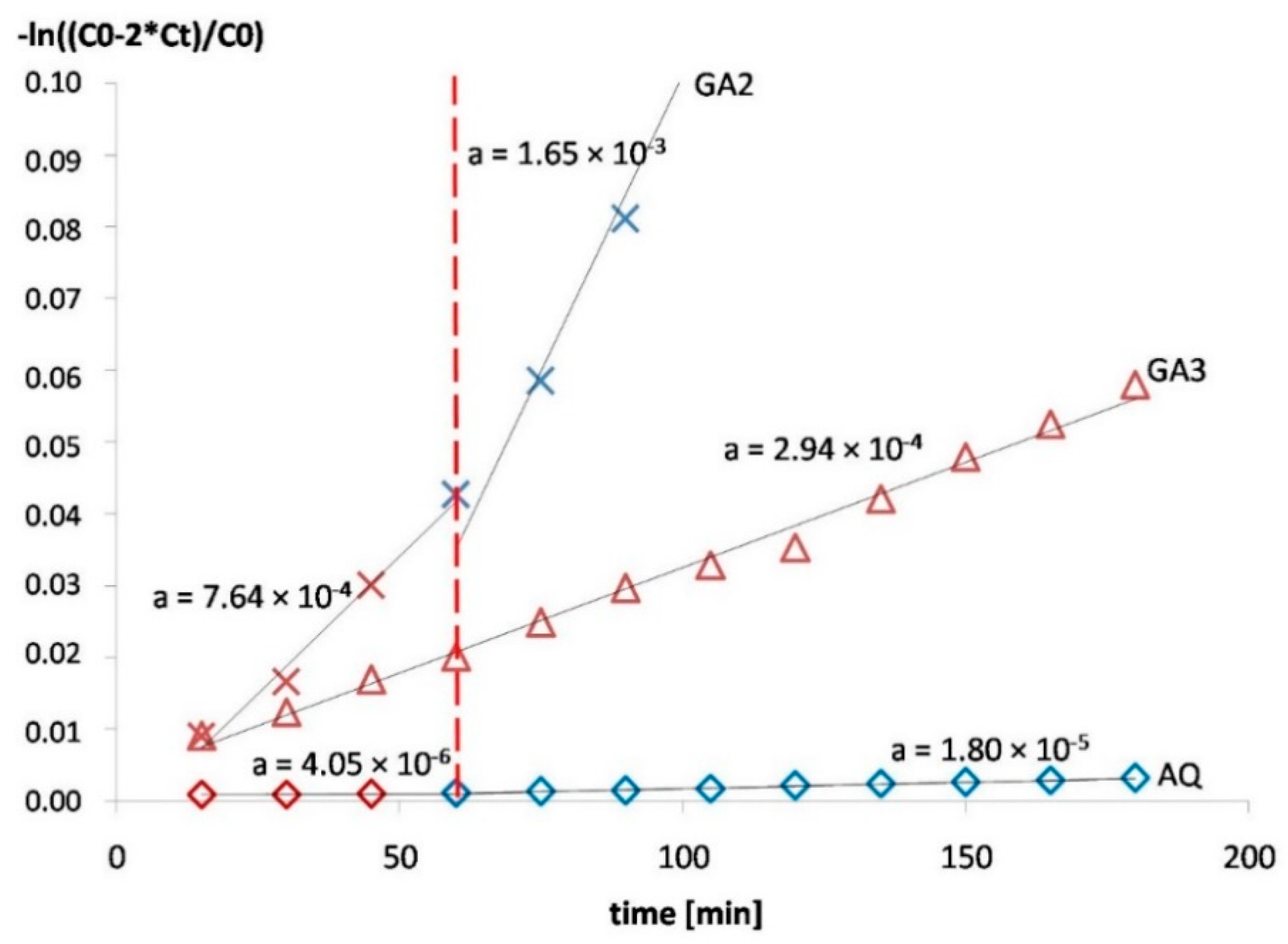
3.2. Permeability
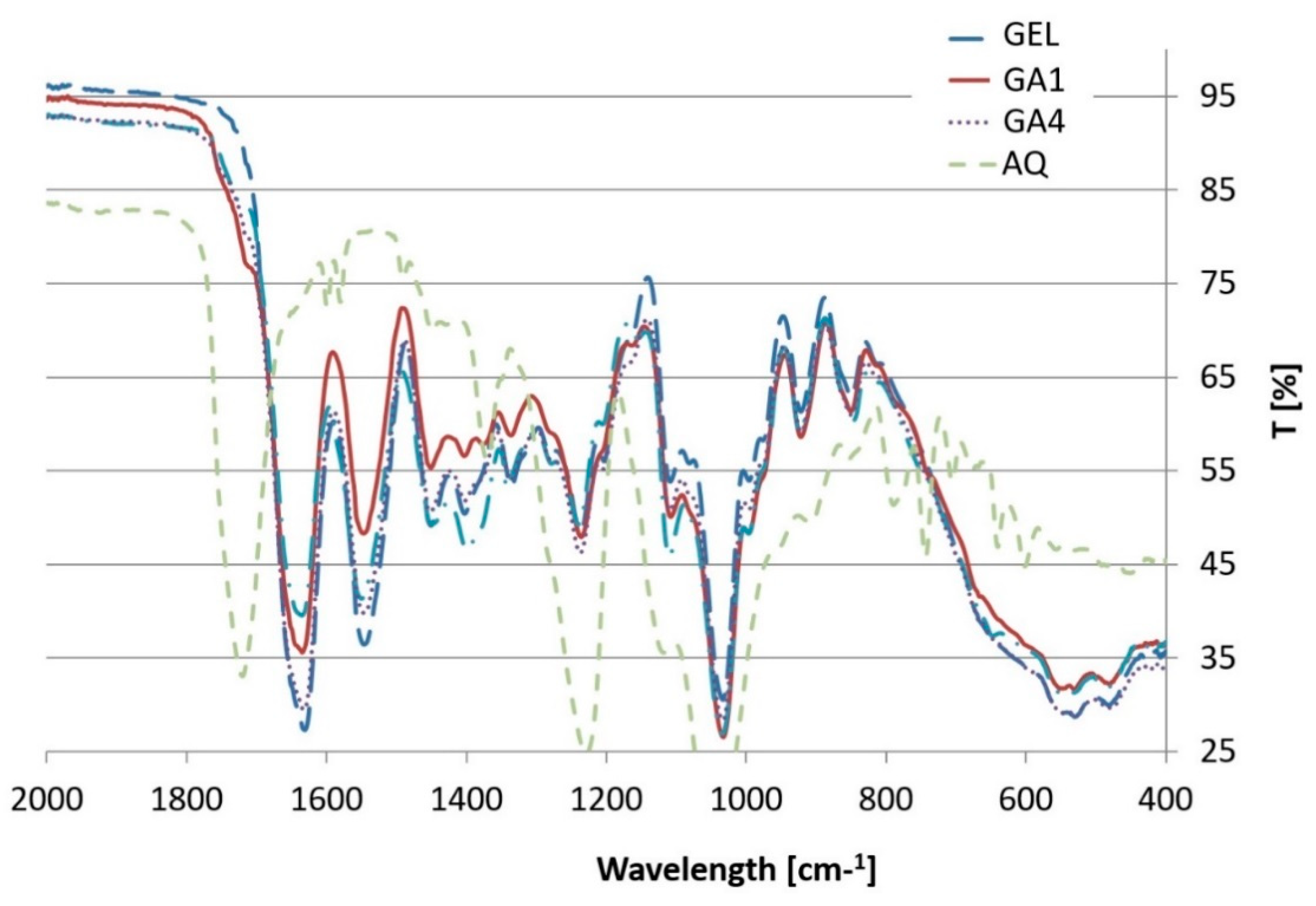
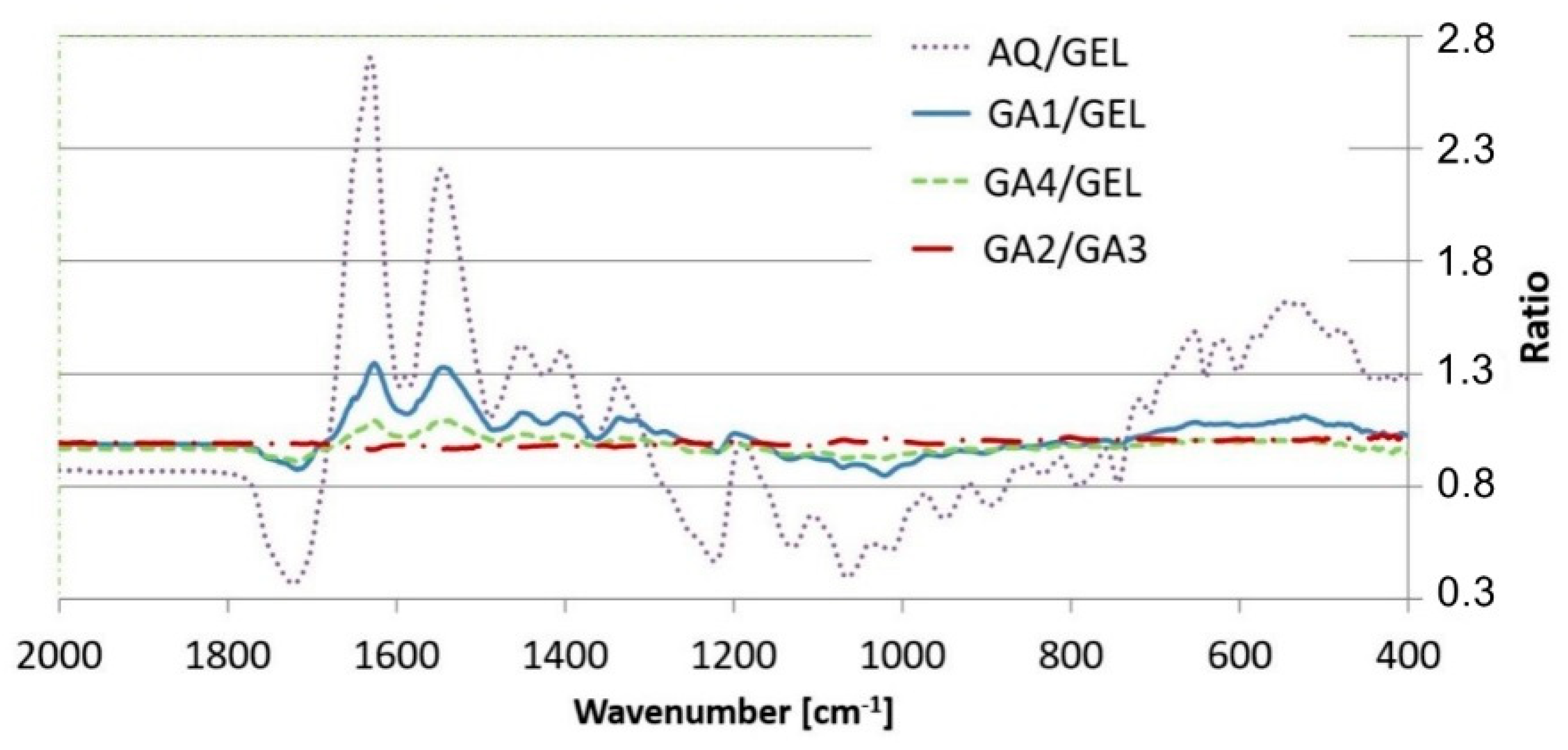
3.3. ATR-FTIR
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esposito, E.; Cortesi, R.; Nastruzzi, C. Gelatin microspheres: Influence of preparation parameters and thermal treatment on chemico-physical and biopharmaceutical properties. Biomaterials 1996, 17, 2009–2020. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Flaker, C.H.C.; Lourenço, R.V.; Bittante, A.M.Q.B.; Sobral, P.J.A. Gelatin-based nanocomposite films: A study on montmorillonite dispersion methods and concentration. J. Food Eng. 2015, 167, 65–70. [Google Scholar] [CrossRef]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Devi, N.; Kakati, D.K. Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. J. Food Eng. 2013, 117, 193–204. [Google Scholar] [CrossRef]
- Djabourov, M.; Papon, P. Influence of thermal treatments on the structure and stability of gelatin gels. Polymer 1983, 24, 537–542. [Google Scholar] [CrossRef]
- Nur Hanani, Z.; Roos, Y.H.; Kerry, J.P. Use of beef, pork and fish gelatin sources in the manufacture of films and assessment of their composition and mechanical properties. Food Hydrocoll. 2012, 29, 144–151. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Karim, A.; Bhat, R. Gelatin alternatives for the food industry: Recent developments, challenges and prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Rabadiya, B.; Rabadiya, P. Review: Capsule shell material from gelatin to non animal origin material. Int. J. Pharm. Res. BioSci. 2013, 2, 42–71. [Google Scholar]
- Cerea, M.; Foppoli, A.; Maroni, A.; Palugan, L.; Zema, L.; Sangalli, M.E. Dry coating of soft gelatin capsules with HPMCAS. Drug Dev. Ind. Pharm. 2008, 34, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A.; Haase, M.M.; Shah, N.H.; Zhang, G.; Infeld, M.H.; Malick, A.W.; Mcginity, J.W. Physical and enteric properties of soft gelatin capsules coated with Eutragit L30 D-55. Int. J. Pharm. 1995, 113, 17–24. [Google Scholar] [CrossRef]
- Fox, S.H.; Paterson, O.L. Enteric gelatin Capsule Shell or Envelope. U.S. Patent 2390088A, 1945. [Google Scholar]
- Bogin, H.H. Enteric Capsule. U.S. Patent 2491475, 1949. [Google Scholar]
- Hassan, E.M.; Fatmi, A.A.; Chidambaram, N. Enteric Composition for the Manufacture of Soft Capsule Wall. U.S. Patent 8685445, 2014. [Google Scholar]
- Teles, H.; Van Duijnhoven, H.; Bayarri, M. Enteric Soft Capsule Compositions. WO Application WO 2015195989A1, 2015. [Google Scholar]
- Maciejewski, B.; Weitschies, W.; Schneider, F.; Sznitowska, M. Gastroresistant gelatin films prepared by addition of cellulose acetate phthalate. Pharmazie 2017, 72, 324–328. [Google Scholar] [PubMed]
- Peh, K.K.; Wong, C.F. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J. Pharm. Pharm. Sci. 1999, 2, 53–61. [Google Scholar] [PubMed]
- Hjaertstam, J.; Hjertberg, T. Studies of the water permeability and mechanical properties of a film made of an ethyl cellulose-ethanol-water ternary mixture. J. Appl. Polym. Sci. 1999, 74, 2056–2062. [Google Scholar] [CrossRef]
- Andersson, H.; Hjärtstam, J.; Stading, M.; von Corswant, C.; Larsson, A. Effects of molecular weight on permeability and microstructure of mixed ethyl-hydroxypropyl-cellulose films. Eur. J. Pharm. Sci. 2013, 48, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Van den Mooter, G.; Samyn, C.; Kinget, R. Characterization of colon-specific azo polymers: A study of the swelling propertoes and the permeability of isolated polymer films. Int. J. Pharm. 1994, 111, 127–136. [Google Scholar] [CrossRef]
- Tromp, R.H.; Van de Velde, F.; Van Riel, J.; Paques, M. Confocal scanning light microscopy (CSLM) on mixtures of gelatine and polysaccharides. Food Res. Int. 2001, 34, 931–938. [Google Scholar] [CrossRef]
- Felder, C.B.; Blanco-Prieto, M.J.; Heizmann, J.; Merkle, H.P.; Gander, B. Ultrasonic atomization and subsequent polymer desolvation for peptide and protein microencapsulation into biodegradable polyesters. J. Microencapsul. 2003, 20, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Weiss, G.; Knoch, A.; Laicher, A.; Stanislaus, F.; Daniels, R. Simple coacervation of hydroxypropyl methylcellulose phthalate (HPMCP)I. Temperature and pH dependency of coacervate formation. Int. J. Pharm. 1995, 124, 87–96. [Google Scholar]
- Williams, R.O.; Liu, J. Influence of processing and curing conditions on beads coated with an aqueous dispersion of cellulose acetate phthalate. Eur. J. Pharm. Biopharm. 2000, 49, 243–252. [Google Scholar] [CrossRef]
- Pranoto, Y.; Lee, C.M.; Park, H.J. Characterizations of fish gelatin films added with gellan and kappa-carrageenan. LWTFood Sci. Technol. 2007, 40, 766–774. [Google Scholar]
- Michon, C.; Cuvelier, G.; Launay, B.; Parker, A. Viscoelastic properties of iota-carrageenan/gelatin mixtures. Carbohydr. Polym. 1996, 31, 161–169. [Google Scholar] [CrossRef]
- Hashim, D.M.; Man, Y.B.C.; Norakasha, R.; Shuhaimi, M.; Salmah, Y.; Syahariza, Z.A. Potential use of Fourier transform infrared spectroscopy for differentiation of bovine and porcine gelatins. Food Chem. 2010, 118, 856–860. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Effect of heat treatment of film-forming solution on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. J. Food Eng. 2010, 96, 66–73. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Ilyin, S.O.; Maklakova, A.A.; Kulichikhin, V.G.; Malkin, A.Y. The rheology of gelatin hydrogels modified by κ-carrageenan. LWT Food Sci. Technol. 2015, 63, 1–8. [Google Scholar] [CrossRef]

| Symbol | % Aquacoat CPD * | Composition of Dry Film (%) | |||
|---|---|---|---|---|---|
| Gelatin | Aquacoat CPD | Glycerol | ί-Carrageenan | ||
| GA1 | 30% | 48.1 | 20.6 | 31.3 | - |
| GA2 | 25% | 51.5 | 17.2 | 31.3 | - |
| GA3 | 25% | 50.0 | 17.2 | 31.3 | 1.5 |
| GA4 | 10% | 61.8 | 6.9 | 31.3 | - |
| GEL | 0% | 68.7 | - | 31.3 | - |
| AQ | 100% | - | 100.0 | - | - |
| Sample | Content (% m/m) in the Investigated Films | Residue (% of the Initial Mass) * | ||
|---|---|---|---|---|
| Aquacoat CPD Content | Iota-Carrageenan | |||
| Total | CAP | |||
| GA1 | 20.6% | 15.8% | - | 16.4 ± 2.1% |
| GA2 | 17.2% | 13.2% | - | 15.5 ± 1.2% |
| GA3 | 17.2% | 13.2% | 1.5% | 38.3 ± 5.1% |
| GA4 | 6.9% | 5.3% | - | 12.4 ± 2.0% |
| Films | Permeation of Water * | ||||
|---|---|---|---|---|---|
| Sample | Aquacoat % (Based on Total Polymer Content) | Thickness (µm) | Cumulative Amount after 3 h (% of the Total Amount) | Average Permeability (cm2/s) | |
| 15–60 min | 60–180 min | ||||
| GA1 | 30% | 540 ± 17 | 7.6% | 2.65 × 10−6 | 4.02 × 10−6 |
| GA2 | 25% | 509 ± 27 | 10.9% | 3.53 × 10−6 | 5.95 × 10−6 |
| GA3 | 25% (+1.5% carrageenan) | 622 ± 35 | 2.3% | 1.1 × 10−6 | |
| GA4 | 10% | 446 ± 17 | n/a | 8.19 × 10−6 | Disintegration after 100–150 min |
| AQ | 100% | 600 ± 17 | 0.15% | 1.67 × 10−8 | 7.58 × 10−8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejewski, B.; Ström, A.; Larsson, A.; Sznitowska, M. Soft Gelatin Films Modified with Cellulose Acetate Phthalate Pseudolatex Dispersion—Structure and Permeability. Polymers 2018, 10, 981. https://doi.org/10.3390/polym10090981
Maciejewski B, Ström A, Larsson A, Sznitowska M. Soft Gelatin Films Modified with Cellulose Acetate Phthalate Pseudolatex Dispersion—Structure and Permeability. Polymers. 2018; 10(9):981. https://doi.org/10.3390/polym10090981
Chicago/Turabian StyleMaciejewski, Bartosz, Anna Ström, Anette Larsson, and Małgorzata Sznitowska. 2018. "Soft Gelatin Films Modified with Cellulose Acetate Phthalate Pseudolatex Dispersion—Structure and Permeability" Polymers 10, no. 9: 981. https://doi.org/10.3390/polym10090981
APA StyleMaciejewski, B., Ström, A., Larsson, A., & Sznitowska, M. (2018). Soft Gelatin Films Modified with Cellulose Acetate Phthalate Pseudolatex Dispersion—Structure and Permeability. Polymers, 10(9), 981. https://doi.org/10.3390/polym10090981