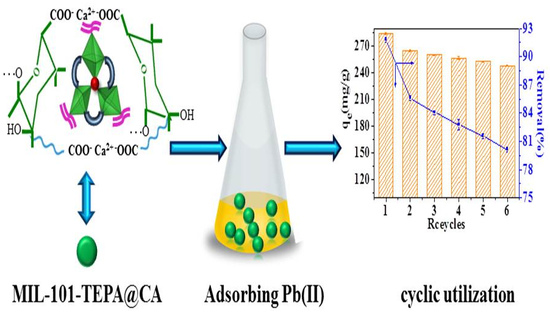
Fabrication of Composite Beads Based on Calcium Alginate and Tetraethylenepentamine-Functionalized MIL-101 for Adsorption of Pb(II) from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MIL-101-TEPA@CA Beads
2.2.1. Preparation of MIL-101
2.2.2. Preparation of MIL-101-TEPA
2.2.3. Preparation of MIL-101-TEPA@CA Beads
2.3. Characterization
2.4. Adsorption Experiment
2.5. Reutilization Test
3. Results and Discussion
3.1. Synthesis of MIL-101-TEPA@CA
3.2. Characterization
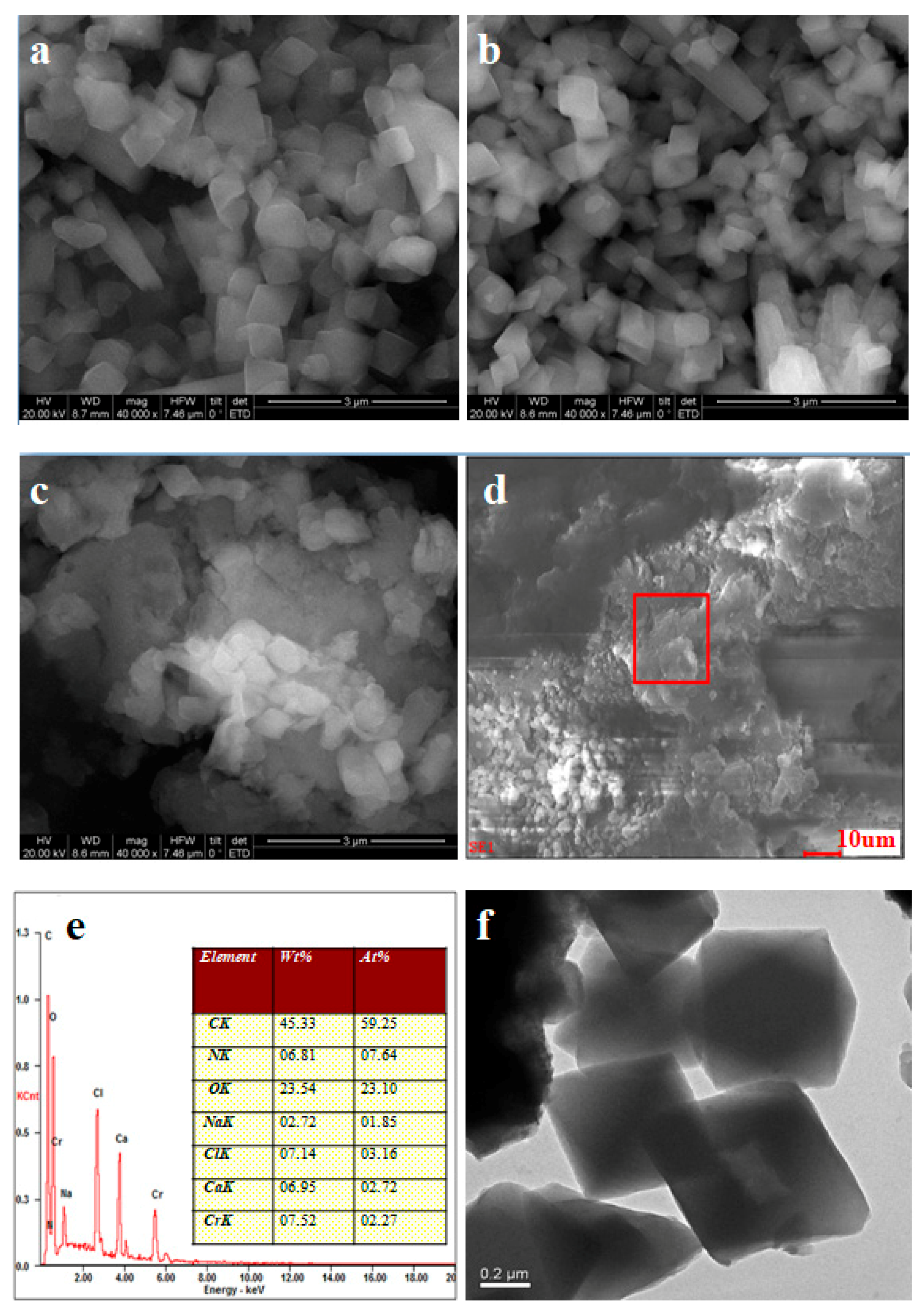
3.2.1. SEM Observation
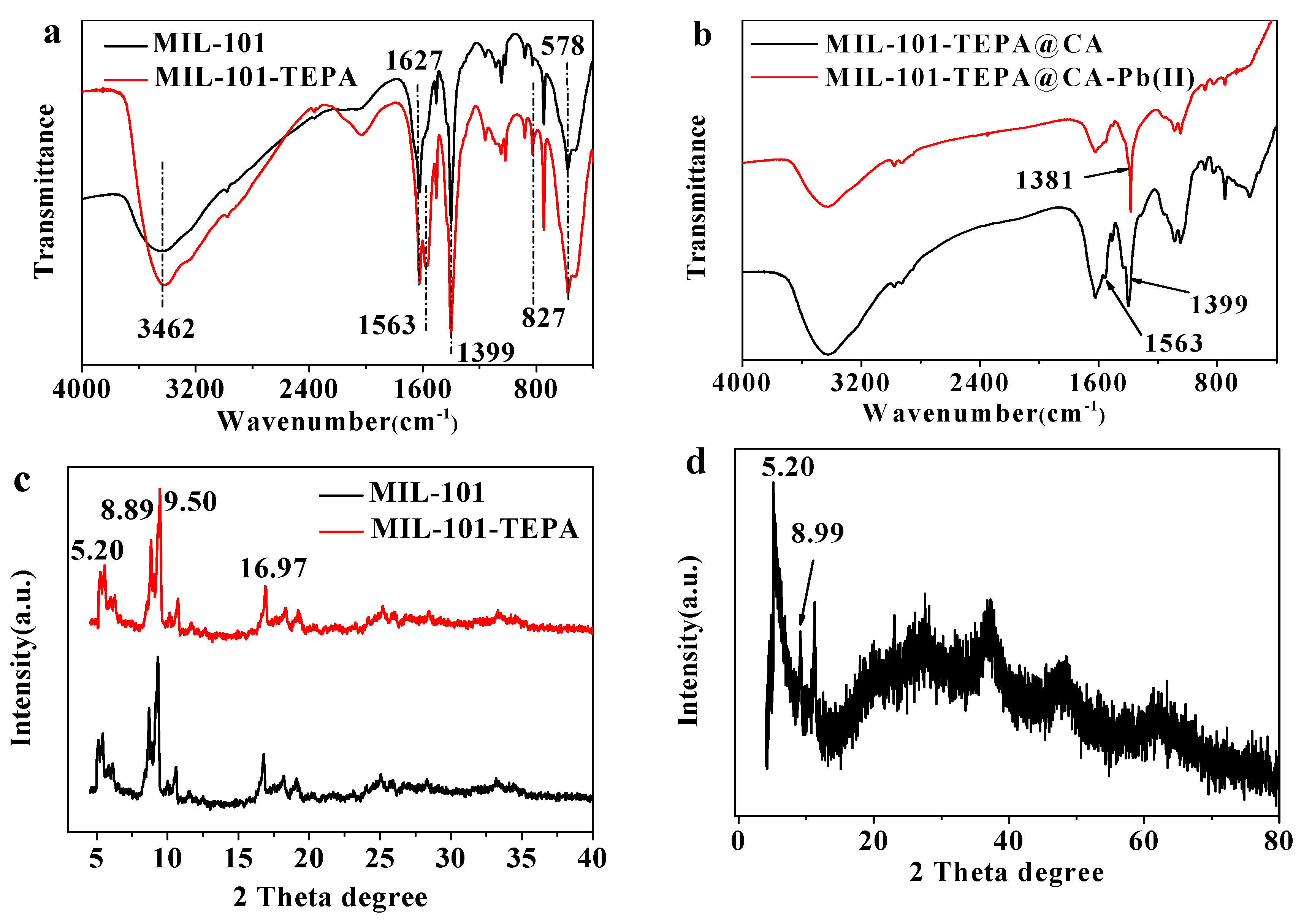
3.2.2. FTIR Analysis
3.2.3. XRD Analysis
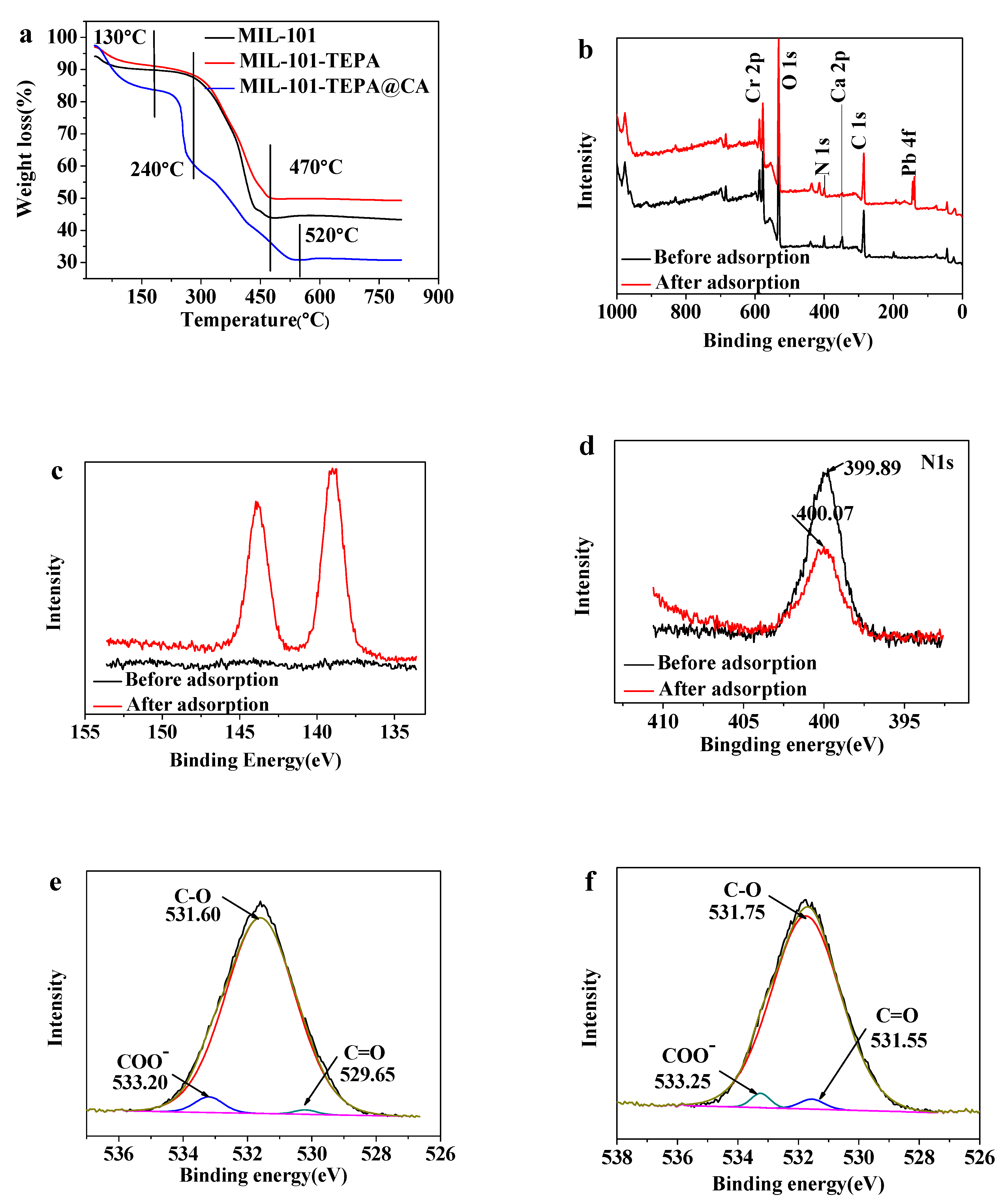
3.2.4. TGA Analysis
3.2.5. XPS Analysis
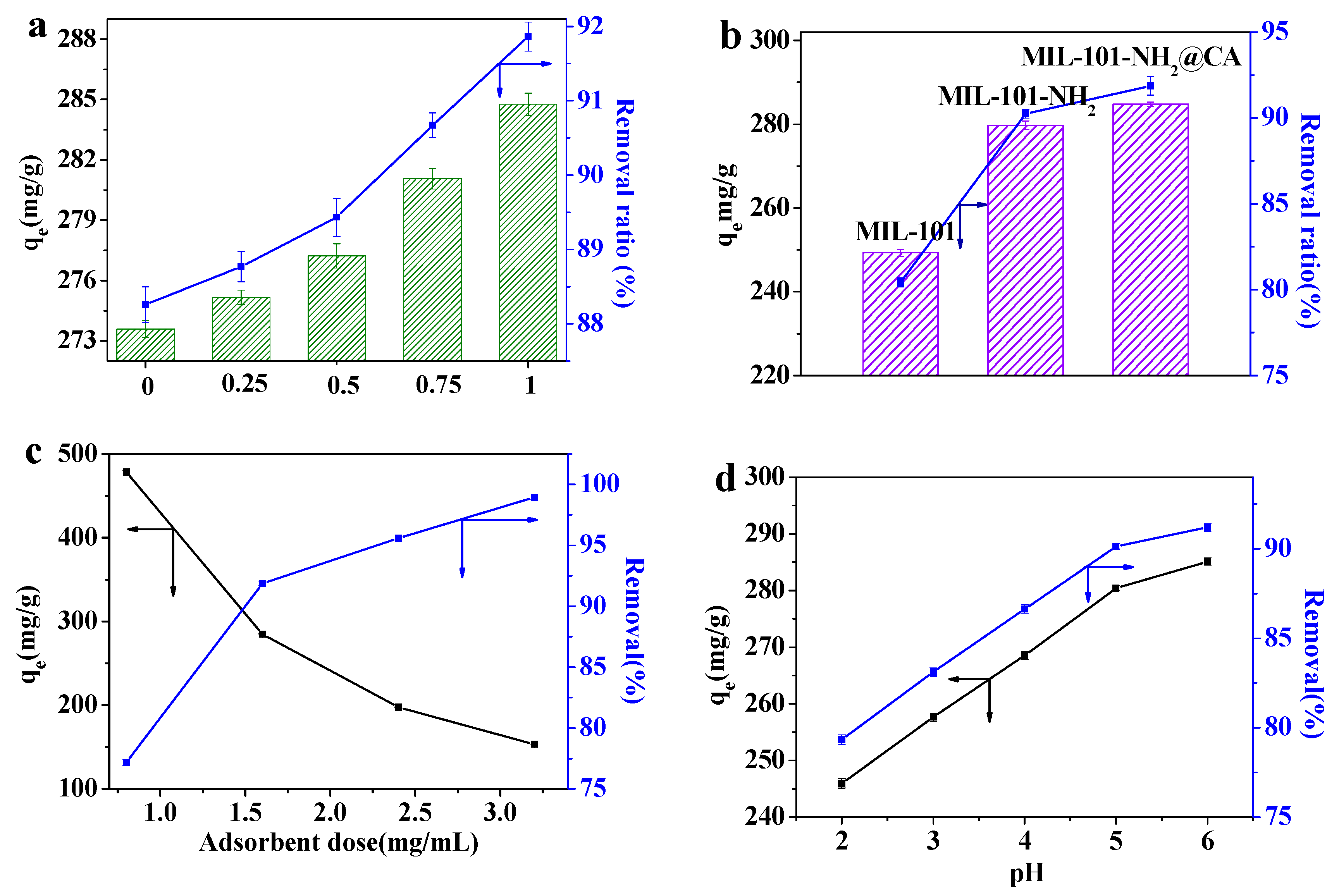
3.3. Optimization of the Adsorption Conditions
3.3.1. Comparison of Adsorption Capacity
3.3.2. Effect of the Adsorbent Dose
3.3.3. Effect of the pH of Pb(II) Solution
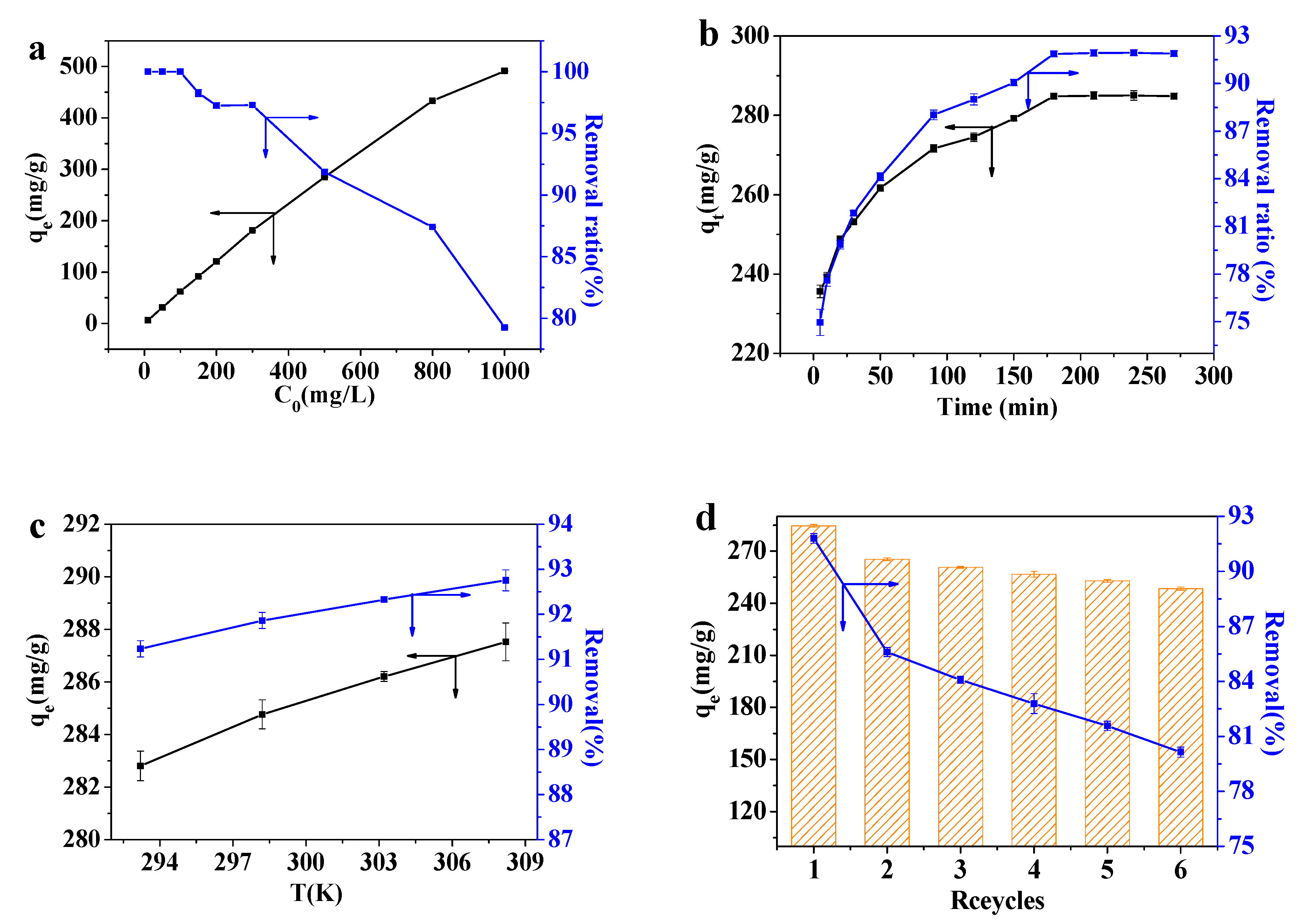
3.3.4. Effect of the Initial Concentration of Pb(II)
3.3.5. Effect of the Adsorption Time
3.3.6. Effect of the Adsorption Temperature
3.3.7. Effect of the Recycling Process
3.4. Adsorption Mechanism
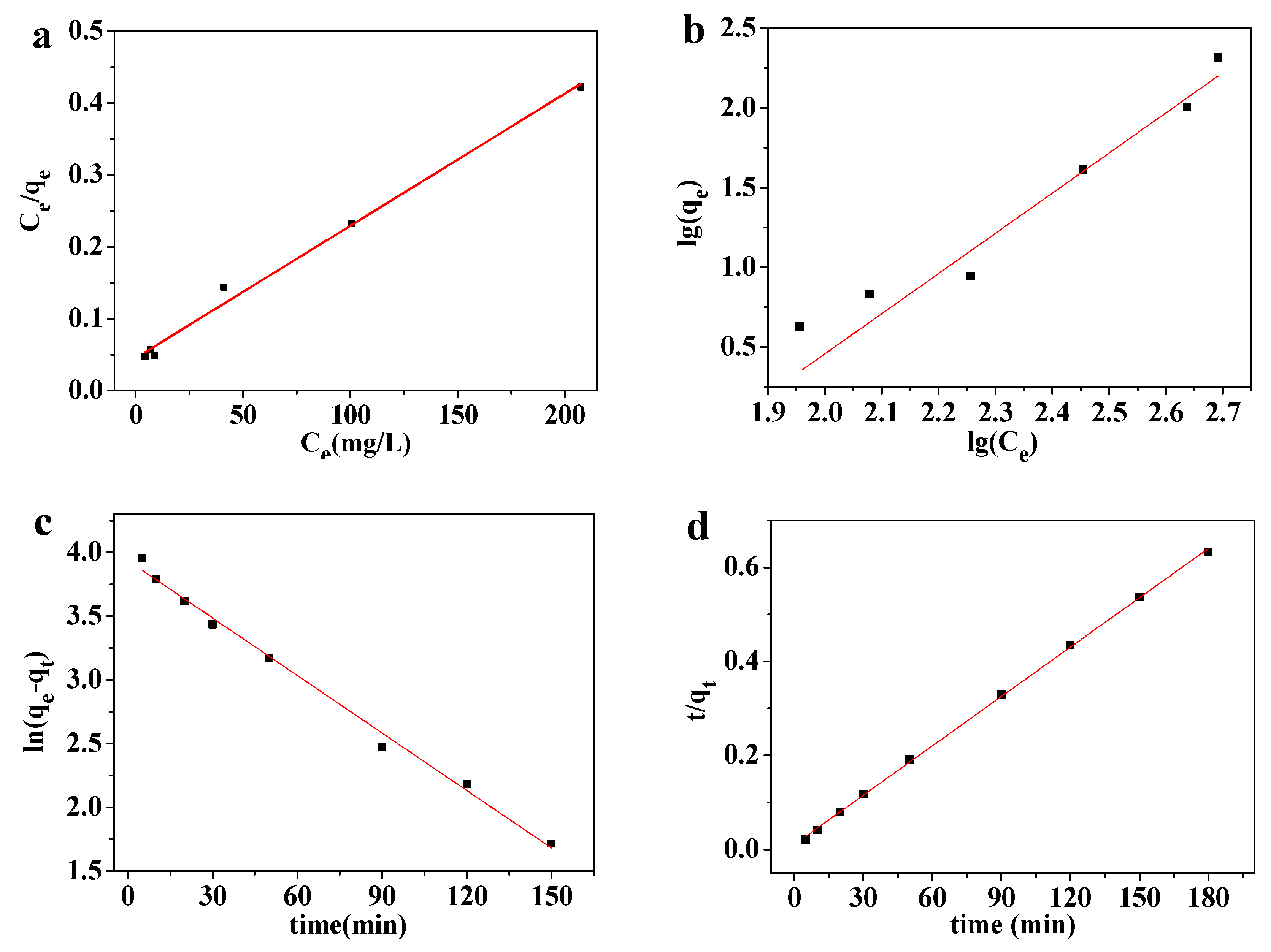
3.4.1. Adsorption Isotherm
3.4.2. Adsorption Kinetics Study
3.4.3. Adsorption Thermodynamics Study
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Beqa, L.; Singh, A.K.; Khan, S.A.; Senapati, D.; Arumugam, S.R.; Ray, P.C. Gold nanoparticle-based simple colorimetric and ultrasensitive dynamic light scattering assay for the selective detection of Pb(II) from paints, plastics, and water samples. ACS Appl. Mater. Interfaces 2017, 3, 668–673. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Thi, H.P.N.; Nguyen, T.A.; Bhosale, S. Effective removal of Pb(II) using graphene@ternary oxides composite as adsorbent in aqueous media. New J. Chem. 2017, 41, 14627–14634. [Google Scholar] [CrossRef]
- Senger, M.R.; Rico, E.P.; De, B.A.M.; Frazzon, A.P.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Exposure to Hg2+ and Pb2+ changes NTPDase and ecto-5′-nucleotidase activities in central nervous system of zebrafish (Danio rerio). Toxicology 2006, 226, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.L.; Zhang, Y.L.; Jiang, Y.; Zeng, G.M.; Cui, Z.H.; Liu, K.; Deng, C.H.; Niu, Q.Y.; Deng, J.H.; Huan, S.Y. Continuous adsorption of Pb(II) and methylene blue by engineered graphite oxide coated sand in fixed-bed column. Appl. Surf. Sci. 2015, 330, 148–157. [Google Scholar] [CrossRef]
- Chakravarty, S.; Mohanty, A.; Sudha, T.N.; Upadhyay, A.K.; Konar, J.; Sircar, J.K.; Madhukar, A.; Gupta, K.K. Removal of Pb(II) ions from aqueous solution by adsorption using bael leaves (Aegle marmelos). J. Hazard. Mater. 2010, 173, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kamala-Kannan, S.; Lee, K.J.; Park, Y.J.; Shea, P.J.; Lee, W.H.; Kim, H.M.; Oh, B.T. Removal of Pb(II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem. Eng. J. 2013, 217, 54–60. [Google Scholar] [CrossRef]
- Parlayıcı, Ş.; Pehlivan, E. Removal of metals by Fe3O4 loaded activated carbon prepared from plum stone (Prunus nigra): Kinetics and modelling study. Powder Technol. 2017, 317, 23–30. [Google Scholar] [CrossRef]
- Chen, Y.; He, M.; Wang, C.; Wei, Y. A novel polyvinyltetrazole-grafted resin with high capacity for adsorption of Pb(II), Cu(II) and Cr(III) ions from aqueous solutions. J. Mater. Chem A 2014, 2, 10444–10453. [Google Scholar] [CrossRef]
- Wang, N.; Jin, R.N.; Omer, A.M.; Ouyang, X. Adsorption of Pb(II) from fish sauce using carboxylated cellulose nanocrystal: Isotherm, kinetics, and thermodynamic studies. Int. J. Biol. Macromol. 2017, 102, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Lü, L.; Li, H.; Fang, L. The adsorption properties of Pb(II) and Cd(II) on functionalized graphene prepared by electrolysis method. J. Hazard. Mater. 2010, 183, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, S.; Sheng, G.; Hu, J.; Tan, X.; Wang, X. Effect of surfactants on Pb(II) adsorption from aqueous solutions using oxidized multiwall carbon nanotubes. Chem. Eng. J. 2011, 166, 551–558. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Hassanpour, S. Selective adsorption of Cr(VI) ions from aqueous solutions using a Cr(VI)-imprinted polymer supported by magnetic multiwall carbon nanotubes. Polymer 2017, 132, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Wang, Y.; Xu, M.; Wang, B. A novel reusable nanocomposite adsorbent, xanthated Fe3O4-chitosan grafted onto graphene oxide, for removing Cu(II) from aqueous solutions. Appl. Surf. Sci. 2016, 367, 327–334. [Google Scholar] [CrossRef]
- Yang, X.; Zou, L.; Zhou, H.-C. Anchor installation on porous polymer networks (PPNs) for high CO2 uptake. Polymer 2017, 126, 303–307. [Google Scholar] [CrossRef]
- Wang, N.; Ouyang, X.K.; Yang, L.Y. Fabrication of a magnetic cellulose nanocrystal/metal-organic framework composite for removal of Pb(II) from water. ACS Sustain. Chem. Eng. 2017, 5, 10447–10458. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Lv, M.; Xu, Y.; Yang, H.; Liu, X.; Wei, F. Selective adsorption of cationic dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Bradshaw, D.; Garai, A.; Huo, J. Metal–organic framework growth at functional interfaces: Thin films and composites for diverse applications. Chem. Soc. Rev. 2012, 43, 2344–2381. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Khutia, A.; Rammelberg, H.U.; Schmidt, T.; Henninger, S.; Janiak, C. Water sorption cycle measurements on functionalized MIL-101Cr for heat transformation application. Chem. Mater. 2013, 25, 790–798. [Google Scholar] [CrossRef]
- Huo, S.H.; Yan, X.P. Facile magnetization of metal-organic framework MIL-101 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples. Analyst 2012, 137, 3445–3451. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ding, L.; Luo, J. Adsorptive removal of pb(II) ions from aqueous samples with amino-functionalization of metal-organic frameworks mil-101(Cr). J. Chem. Eng. Data 2015, 60, 1732–1743. [Google Scholar] [CrossRef]
- Lu, J.; Yang, H.; Hao, J.; Wu, C.; Liu, L.; Xu, N.; Linhardt, R.J.; Zhang, Z. Impact of hydrolysis conditions on the detection of mannuronic to guluronic acid ratio in alginate and its derivatives. Carbohydr. Polym. 2015, 122, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Mørch, Y.A.; Donati, I.; Strand, B.L.; Skjåk-Braek, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb(II) removal using sodium alginate-carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Choi, E.J.; Jhung, S.H. Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, X.; Wang, X.; Wang, Q.; Ji, Z.; Wang, X.; Wu, T.; Gao, C. Highly and stably water permeable thin film nanocomposite membranes doped with MIL-101(Cr) nanoparticles for reverse osmosis application. Materials 2016, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Pan, F.; Yang, S.; Ding, H.; Jiang, Z.; Wang, B.; Li, Z.; Cao, X. Enhanced pervaporation performance of MIL-101(Cr) filled polysiloxane hybrid membranes in desulfurization of model gasoline. Chem. Eng. Sci. 2015, 135, 479–488. [Google Scholar] [CrossRef]
- Shafiei, M.; Alivand, M.S.; Rashidi, A.; Samimi, A.; Mohebbi-Kalhori, D. Synthesis and adsorption performance of a modified micro-mesoporous MIL-101(Cr) for VOCs removal at ambient conditions. Chem. Eng. J. 2018, 341, 164–174. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Q.; Kong, T. Tetraethylenepentamine-modified MCM-41/silica gel with hierarchical mesoporous structure for CO2 capture. Chem. Eng. J. 2015, 273, 472–480. [Google Scholar] [CrossRef]
- Li, X.; Mao, Y.; Leng, K.; Ye, G.; Sun, Y.; Xu, W. Synthesis of amino-functionalized MIL-101(Cr) with large surface area. Mater. Lett. 2017, 197, 192–195. [Google Scholar] [CrossRef]
- Yu, Z.; Deschamps, J.; Hamon, L.; Prabhakaran, P.K.; Pré, P. Hydrogen adsorption and kinetics in MIL-101(Cr) and hybrid activated carbon- MIL-101(Cr) materials. Int. J. Hydrogen Energy 2017, 42, 8021–8031. [Google Scholar] [CrossRef]
- Hernández-Morales, V.; Nava, R.; Acosta-Silva, Y.J.; Macías-Sánchez, S.A.; Pérez-Bueno, J.J.; Pawelec, B. Adsorption of lead (II) on SBA-15 mesoporous molecular sieve functionalized with –NH2 groups. Micropor. Mesopor. Mater. 2012, 160, 133–142. [Google Scholar] [CrossRef]
- Deng, S.; Ting, Y.P. Fungal biomass with grafted poly(acrylic acid) for enhancement of Cu(II) and Cd(II) biosorption. Langmuir 2005, 21, 5940–5948. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Mostofi, M.; Alimohammadi, M.; Mckay, G.; Yetilmezsoy, K.; Albadarin, A.B.; Heibati, B.; Alghouti, M.; Mubarak, N.M.; Sahu, J.N. High-performance removal of toxic phenol by single-walled and multi-walled carbon nanotubes: Kinetics, adsorption, mechanism and optimization studies. J. Ind. Eng. Chem. 2016, 35, 63–74. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Jańczuk, B.; Zdziennicka, A.; Jańczuk, B.; Zdziennicka, A.; Jańczuk, B. Thermodynamic parameters of some biosurfactants and surfactants adsorption at water-air interface. J. Mol. Liq. 2017, 243, 236–244. [Google Scholar] [CrossRef]
- Stromer, B.S.; Woodbury, B.; Williams, C.F. Tylosin sorption to diatomaceous earth described by langmuir isotherm and freundlich isotherm models. Chemosphere 2018, 193, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gu, J.; Yin, N. Efficient removal of Pb(II) and Cd(II) using NH2-functionalized zr-mofs via rapid microwave-promoted synthesis. Ind. Eng. Chem. Res. 2017, 56, 1880–1887. [Google Scholar] [CrossRef]
- He, Y.; Liu, Q.; Hu, J.; Zhao, C.; Peng, C.; Yang, Q.; Wang, H.; Liu, H. Efficient removal of Pb(II) by amine functionalized porous organic polymer through post-synthetic modification. Sep. Purif. Technol. 2017, 180, 142–148. [Google Scholar] [CrossRef]
- Li, J.; Zuo, K.; Wu, W.; Xu, Z.; Yi, Y.; Jing, Y.; Dai, H.; Fang, G. Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II). Carbohydr. Polym. 2018, 196, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, L.V.A.; Gil, L.F. Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by succinylated mercerized cellulose modified with triethylenetetramine. Carbohydr. Polym. 2009, 77, 142–149. [Google Scholar] [CrossRef]
- Zhao, Q.; Ren, L.; Zhou, H.; Cao, T.; Chen, P. Enhanced adsorption of Pb(II) by Al(OH)3/(PAA-CO-PAM) sub-microspheres with three-dimensional interpenetrating network structure. Chem. Eng. J. 2014, 250, 6–13. [Google Scholar] [CrossRef]
- Jayabrata, M.; Samit, K.R. Chitosan based nano composite adsorbent—Synthesis, characterization and application for adsorption of binary mixtures of Pb(II) and Cd(II) from water. Carbohydr. Polym. 2018, 182, 159–171. [Google Scholar]
- Katal, R.; Hasani, E.; Farnam, M.; Baei, M.S.; Ghayyem, M.A. Charcoal ash as an adsorbent for Ni(II) adsorption and its application for wastewater treatment. J. Chem. Eng. Data 2012, 57, 374–383. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Li, M.; Zhou, S.; Xue, A.; Xing, W. Adsorption of Hg2+ from aqueous solution onto polyacrylamide/attapulgite. J. Hazard. Mater. 2009, 171, 640–646. [Google Scholar] [CrossRef] [PubMed]

| T (K) | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qm(mg/g) | b (L/mg) | R2 | KF | n | R2 | |
| 298.2 | 543.48 | 0.040 | 0.9910 | 1.27×10−4 | 0.38 | 0.9473 |
| C0 (mg/L) | 10 | 50 | 100 | 200 | 300 | 500 | 800 | 1000 |
| RL | 0.67 | 0.29 | 0.17 | 0.091 | 0.063 | 0.038 | 0.024 | 0.020 |
| Adsorbents | qmax(mg/g) | Adsorption Mechanism | References |
|---|---|---|---|
| NH2-MIL-101(Cr) | 81.09 | electrostatic interaction | [39] |
| POP-NH2 | 523.6 | coordinated complexation | [40] |
| NFC/PEI | 357.14 | chemical adsorption | [41] |
| Cellulose 4 | 192.3 | coordinated complexation, electrostatic interaction | [42] |
| Al(OH)3/(PAA-CO-PAM) | 106.2 | chemical adsorption | [43] |
| Cs-PMA/HNT | 357.38 | coordinated complexation, electrostatic interaction | [44] |
| MIL-101-TEPA@CA | 543.48 | coordinated complexation, electrostatic interaction | this work |
| qe, exp | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| qe, cal | k1 | R2 | qe, cal | k2 | R2 | |
| 284.76 | 10.70 | 0.02390 | 0.9930 | 285.71 | 0.0011 | 0.9994 |
| (kJ/mol) T (K) | (kJ/mol) | (J/mol∙K) | |||
|---|---|---|---|---|---|
| 293.2 | 298.2 | 303.2 | 308.2 | ||
| −4.54 | −4.82 | −5.07 | −5.31 | 10.30 | −0.051 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Yang, L.-Y.; Wang, Y.-g.; Ouyang, X.-k. Fabrication of Composite Beads Based on Calcium Alginate and Tetraethylenepentamine-Functionalized MIL-101 for Adsorption of Pb(II) from Aqueous Solutions. Polymers 2018, 10, 750. https://doi.org/10.3390/polym10070750
Wang N, Yang L-Y, Wang Y-g, Ouyang X-k. Fabrication of Composite Beads Based on Calcium Alginate and Tetraethylenepentamine-Functionalized MIL-101 for Adsorption of Pb(II) from Aqueous Solutions. Polymers. 2018; 10(7):750. https://doi.org/10.3390/polym10070750
Chicago/Turabian StyleWang, Nan, Li-Ye Yang, Yang-guang Wang, and Xiao-kun Ouyang. 2018. "Fabrication of Composite Beads Based on Calcium Alginate and Tetraethylenepentamine-Functionalized MIL-101 for Adsorption of Pb(II) from Aqueous Solutions" Polymers 10, no. 7: 750. https://doi.org/10.3390/polym10070750
APA StyleWang, N., Yang, L.-Y., Wang, Y.-g., & Ouyang, X.-k. (2018). Fabrication of Composite Beads Based on Calcium Alginate and Tetraethylenepentamine-Functionalized MIL-101 for Adsorption of Pb(II) from Aqueous Solutions. Polymers, 10(7), 750. https://doi.org/10.3390/polym10070750