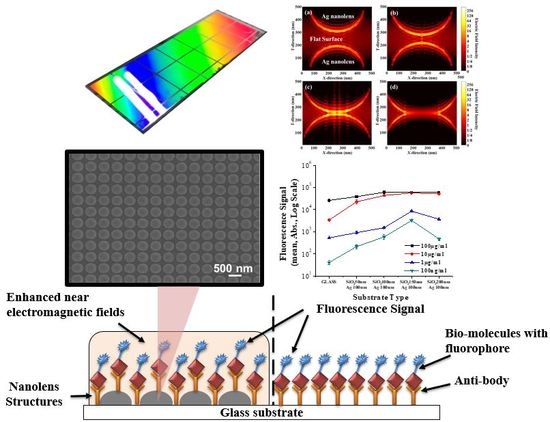
Fabrication of Highly Packed Plasmonic Nanolens Array Using Polymer Nanoimprinted Nanodots for an Enhanced Fluorescence Substrate
Abstract
:1. Introduction
2. Experimental Methods
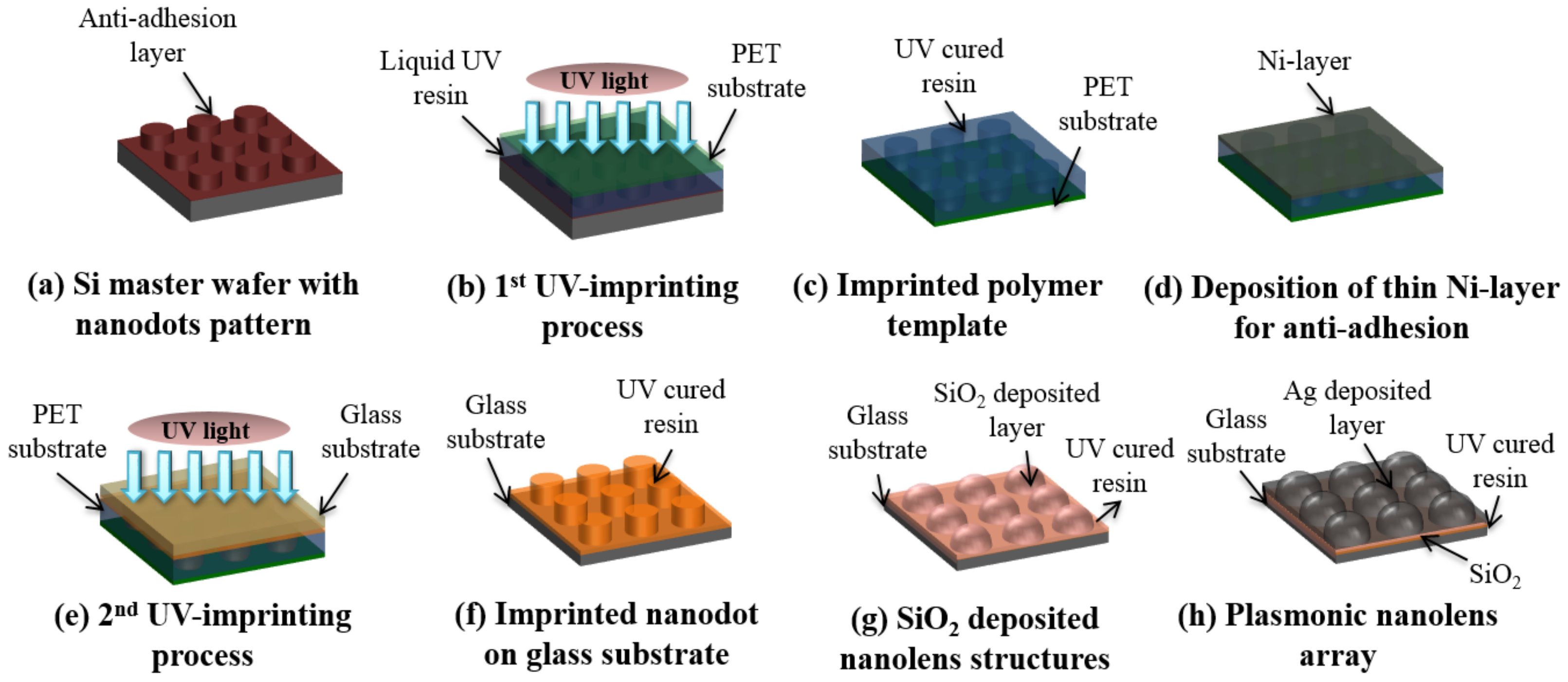
2.1. Fabrication of Plasmonic Nanolens Array
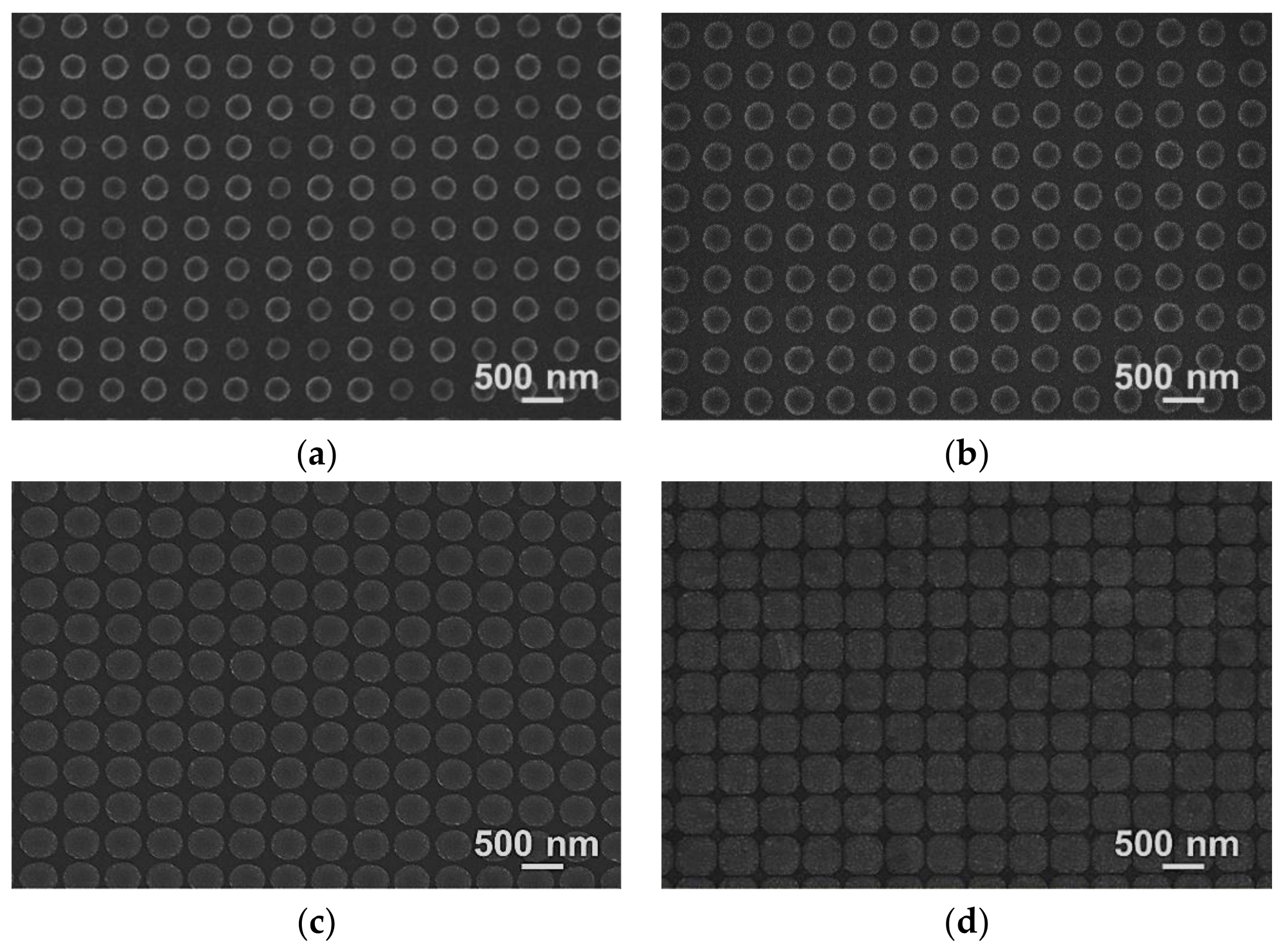
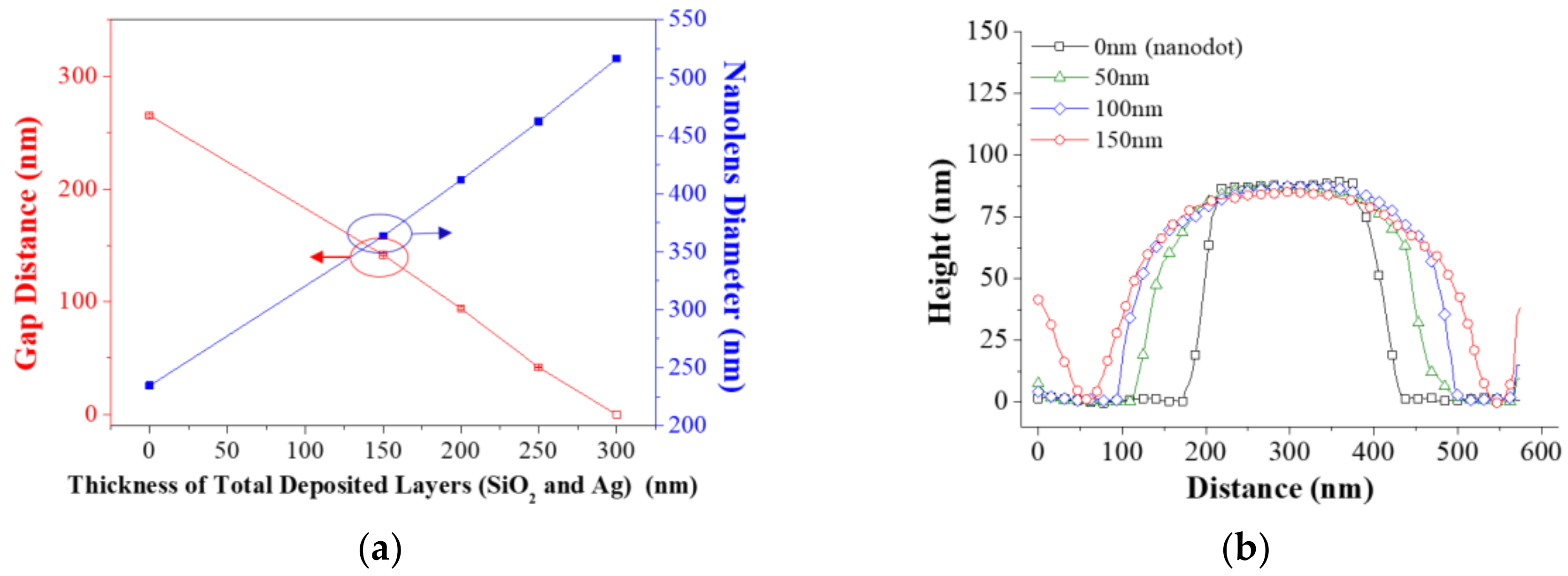
2.2. Evaluation of Geometrical Characteristics
2.3. Fluorescence Signal Measurements
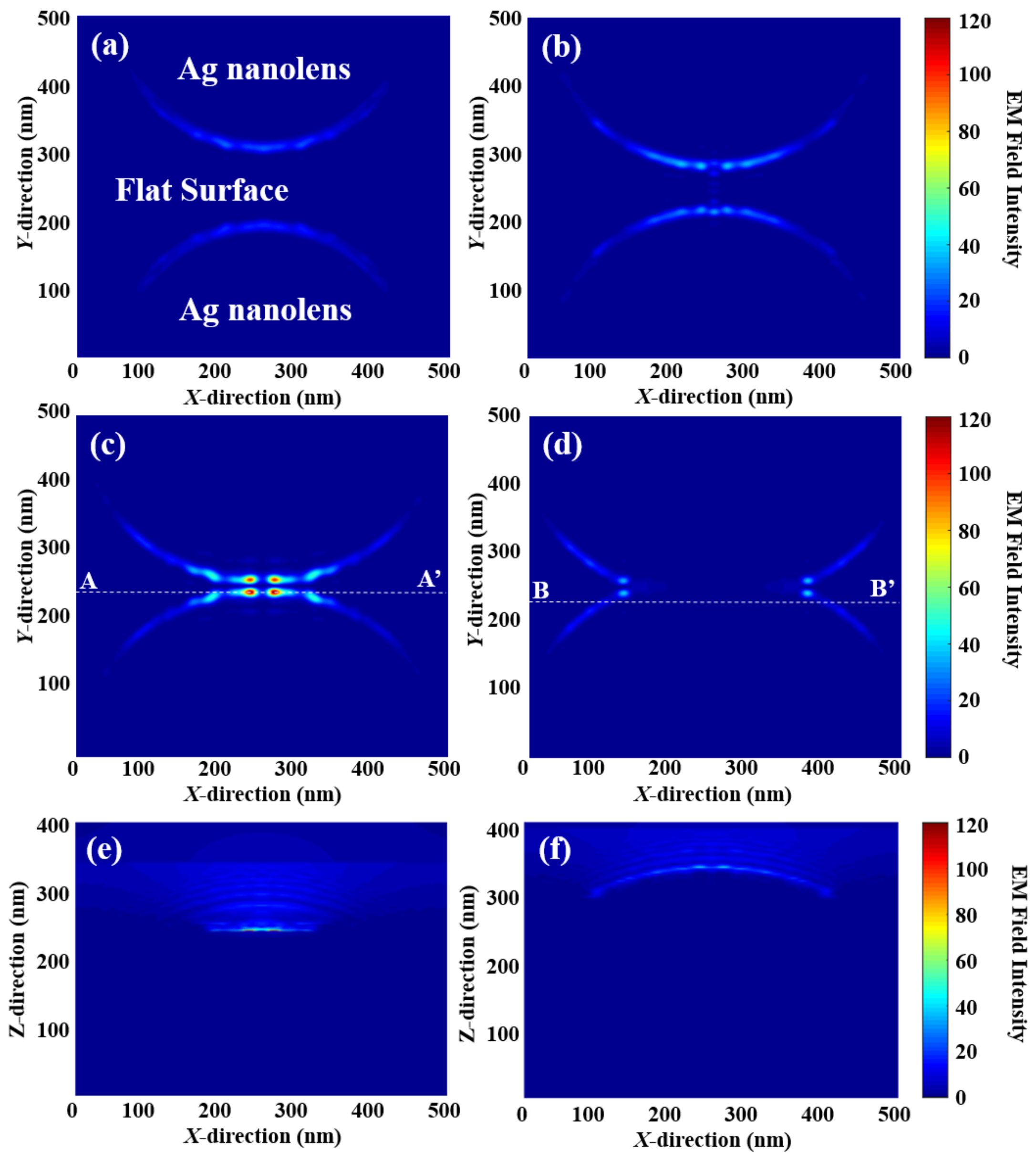
2.4. Electromagnetic Analysis
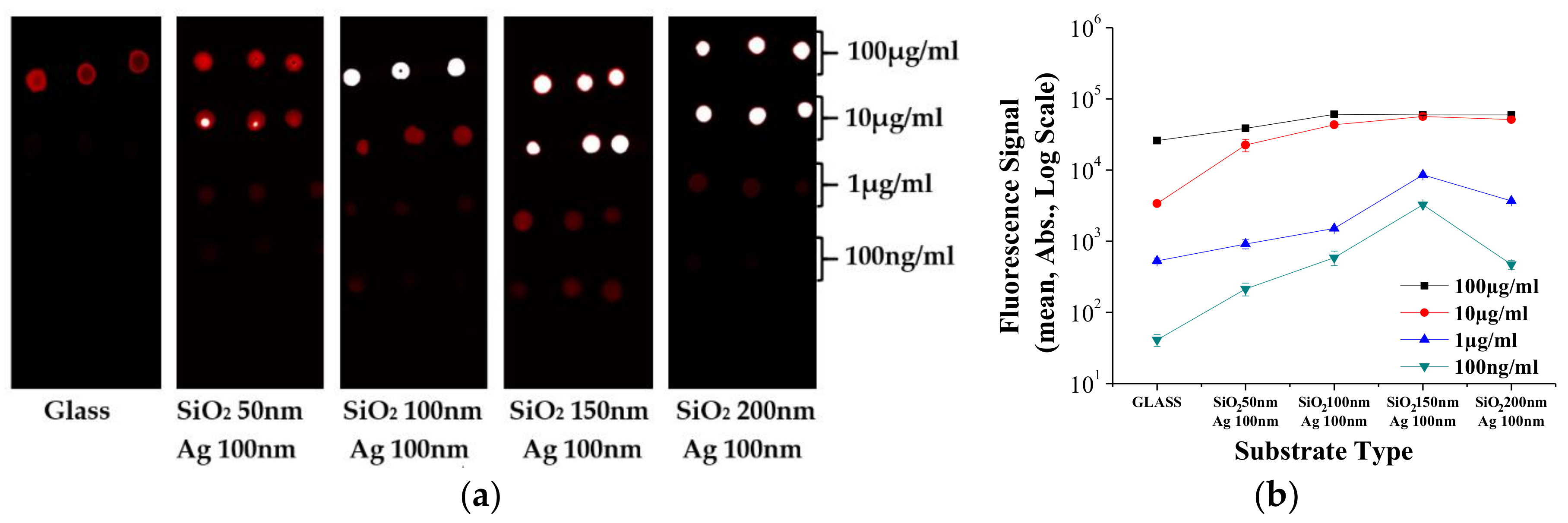
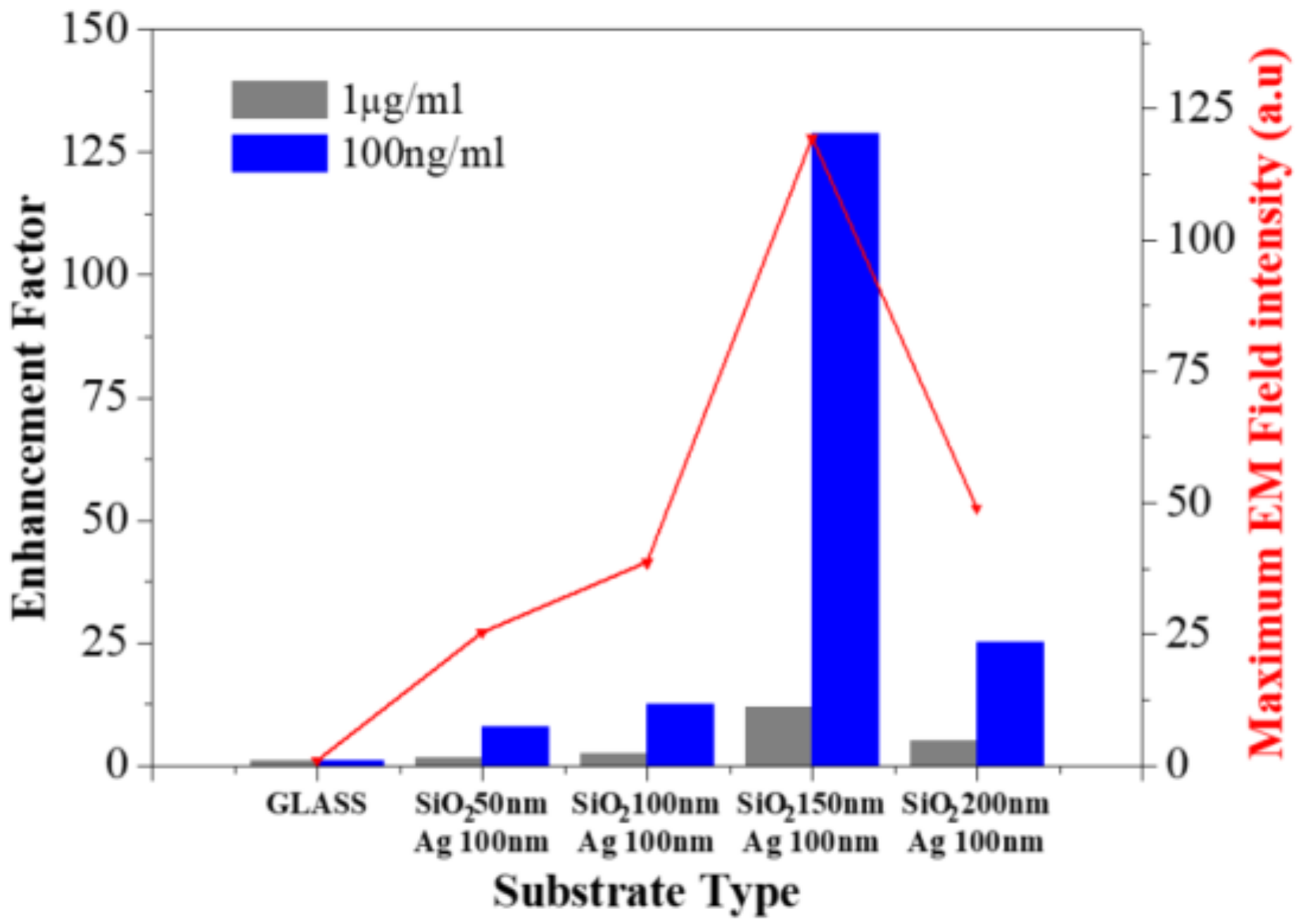
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Schena, M.; Shalon, D.; Davis, R.W.; Brown, P.O. Quantitative monitoring of gene-expression patterns with a complementary-DNA microarray. Science 1995, 270, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Emili, A.Q.; Cagney, G. Large-scale functional analysis using peptide or protein arrays. Nat. Biotechnol. 2000, 18, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Wark, A.W.; Corn, R.M. Microarray methods for protein biomarker detection. Analyst 2008, 133, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Canales, R.D.; Luo, Y.; Willey, J.C.; Austermiller, B.; Barbacioru, C.C.; Boysen, C.; Hunkapiller, K.; Jensen, R.V.; Knight, C.R.; Lee, K.Y.; et al. Evaluation of DNA microarray results with quantitative gene expression platforms. Nat. Biotechnol. 2006, 24, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Sreekanth, K.V.; Shang, J.; Yu, T.; Chen, C.K.; Yin, F.; Baillargeat, D.; Coquet, P.; Ho, H.P.; Kabashin, A.V.; Yong, K.T. Graphene–Gold Metasurface Architectures for Ultrasensitive Plasmonic Biosensing. Adv. Mater. 2015, 27, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hartmann, G.; Wu, Z.; Scarabelli, L.; Rajeeva, B.B.; Jarrett, J.W.; Perillo, E.P.; Dunn, A.K.; Liz-Marzán, L.M.; Hwang, G.S.; Zheng, Y. Controlling Plasmon-Enhanced Fluorescence via Intersystem Crossing in Photoswitchable Molecules. Small 2017, 13, 1701763. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Flynn, J.D.; Isaacoff, B.P.; Rowland, D.J.; Biteen, J.S. Super-Resolving the Distance-Dependent Plasmon-Enhanced Fluorescence of Single Dye and Fluorescent Protein Molecules. J. Phys. Chem. C 2015, 119, 19350–19358. [Google Scholar] [CrossRef]
- Geddes, C.D.; Lakowicz, J.R. Metal-enhanced fluorescence. J. Fluoresc. 2002, 12, 121–129. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Ju, J.; Byeon, E.; Han, Y.A.; Kim, S.M. Fabrication of a substrate for ag-nanorod metal-enhanced fluorescence using the oblique angle deposition process. Micro Nano Lett. 2013, 8, 370–373. [Google Scholar] [CrossRef]
- Zhang, J.; Matveeva, E.; Gryczynski, I.; Leonenko, Z.; Lakowicz, J.R. Metal-enhanced fluoroimmunoassay on a silver film by vapor deposition. J. Phys. Chem. B 2005, 109, 7969–7975. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.I.; Strohsahl, C.M.; Leach, K.E.; Krauss, T.D.; Miller, B.L. Label-free DNA detection on nanostructured ag surfaces. Acs Nano 2009, 3, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Pompa, P.P.; Martiradonna, L.; Della Torre, A.; Della Sala, F.; Manna, L.; De Vittorio, M.; Calabi, F.; Cingolani, R.; Rinaldi, R. Metal-enhanced fluorescence of colloidal nanocrystals with nanoscale control. Nat. Nanotechnol. 2006, 1, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, T.D.; Guo, S.H.; Szmacinski, H.; Phaneuf, R.J. Systematic study of the size and spacing dependence of ag nanoparticle enhanced fluorescence using electron-beam lithography. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Pompa, P.P.; Martiradonna, L.; Torre, A.D.; Carbone, L.; del Mercato, L.L.; Manna, L.; De Vittorio, M.; Calabi, F.; Cingolani, R.; Rinaldi, R. Fluorescence enhancement in colloidal semiconductor nanocrystals by metallic nanopatterns. Sensors Actuators B: Chem. 2007, 126, 187–192. [Google Scholar] [CrossRef]
- Corrigan, T.D.; Guo, S.; Phaneuf, R.J.; Szmacinski, H. Enhanced fluorescence from periodic arrays of silver nanoparticles. J. Fluoresc. 2005, 15, 777. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.A.; Byeon, E.; Kim, J.; Kim, S.M. A simulation model considering sidewall deposition for the precise prediction of the performance of label-free photonic-crystal biosensors. J. Nanosci. Nanotechnol. 2012, 12, 5429–5434. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Smith, E.A.; Natan, M.J.; Keating, C.D. The distance-dependence of colloidal au-amplified surface plasmon resonance. J. Phys. Chem. B 2004, 108, 10973–10980. [Google Scholar] [CrossRef]
- Haynes, C.L.; Van Duyne, R.P. Nanosphere lithography: A versatile nanofabrication tool for studies of size-dependent nanoparticle optics. J. Phys. Chem. B 2001, 105, 5599–5611. [Google Scholar] [CrossRef]
- Michieli, N.; Pilot, R.; Russo, V.; Scian, C.; Todescato, F.; Signorini, R.; Agnoli, S.; Cesca, T.; Boziob, R.; Mattei, G. Oxidation effects on the SERS response of silver nanoprism arrays. RSC Adv. 2017, 7, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Badshah, M.A.; Jang, H.; Kim, Y.K.; Kim, T.-H.; Kim, S.-M. Development of a rapid cure polydimethylsiloxane replication process with near-zero shrinkage. J. Micro/Nanolithogr. MEMS MOEMS 2014, 13, 033006. [Google Scholar] [CrossRef]
- Hilczer, M.; Traytak, S.; Tachiya, M. Electric field effects on fluorescence quenching due to electron transfer. J. Chem. Phys. 2001, 115, 11249–11253. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badshah, M.A.; Kim, J.; Jang, H.; Kim, S.-m. Fabrication of Highly Packed Plasmonic Nanolens Array Using Polymer Nanoimprinted Nanodots for an Enhanced Fluorescence Substrate. Polymers 2018, 10, 649. https://doi.org/10.3390/polym10060649
Badshah MA, Kim J, Jang H, Kim S-m. Fabrication of Highly Packed Plasmonic Nanolens Array Using Polymer Nanoimprinted Nanodots for an Enhanced Fluorescence Substrate. Polymers. 2018; 10(6):649. https://doi.org/10.3390/polym10060649
Chicago/Turabian StyleBadshah, Mohsin Ali, Jun Kim, Hoyoung Jang, and Seok-min Kim. 2018. "Fabrication of Highly Packed Plasmonic Nanolens Array Using Polymer Nanoimprinted Nanodots for an Enhanced Fluorescence Substrate" Polymers 10, no. 6: 649. https://doi.org/10.3390/polym10060649
APA StyleBadshah, M. A., Kim, J., Jang, H., & Kim, S.-m. (2018). Fabrication of Highly Packed Plasmonic Nanolens Array Using Polymer Nanoimprinted Nanodots for an Enhanced Fluorescence Substrate. Polymers, 10(6), 649. https://doi.org/10.3390/polym10060649