Novel PSMA-Coated On-Off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Measurements
2.2. Synthesis of DSA
2.3. Preparation of Aggregation-Induced Emission Dots
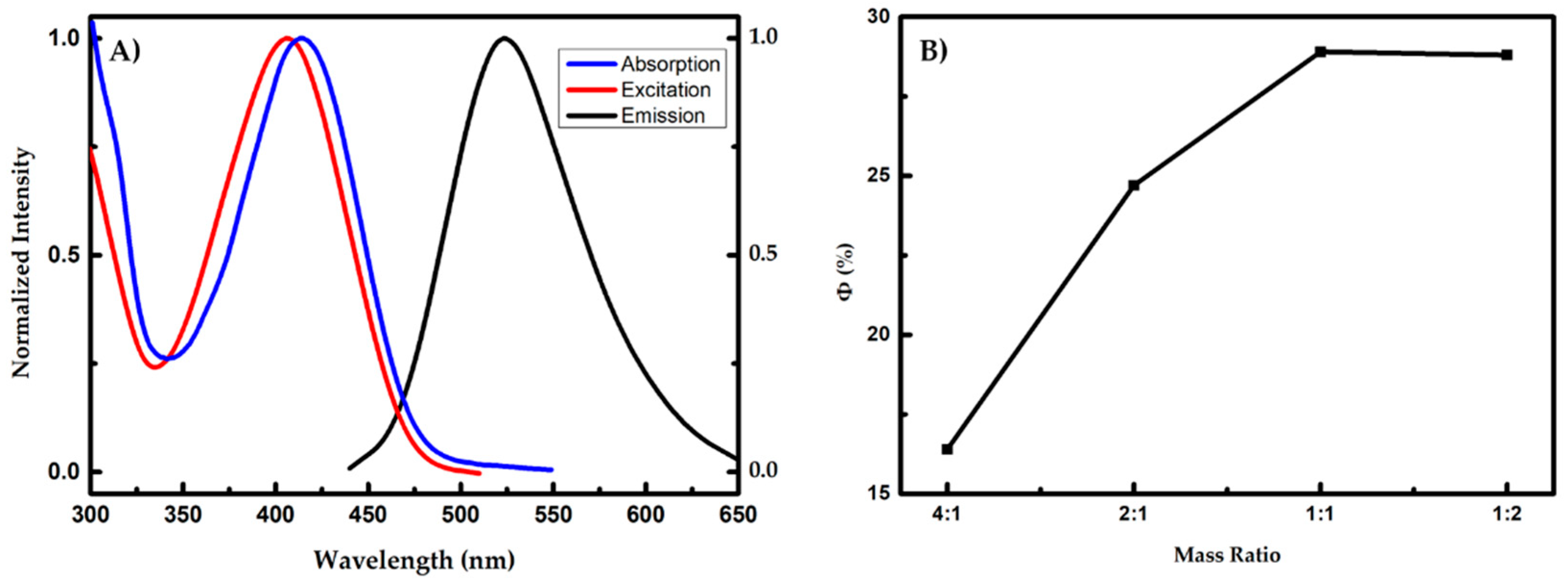
2.4. Determination of Quantum Yields
2.5. Detection of Cu2+, Fe3+ and Cysteine
3. Results and Discussion
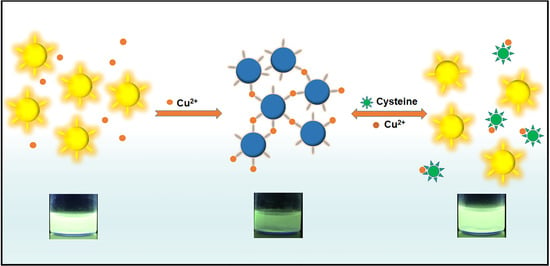
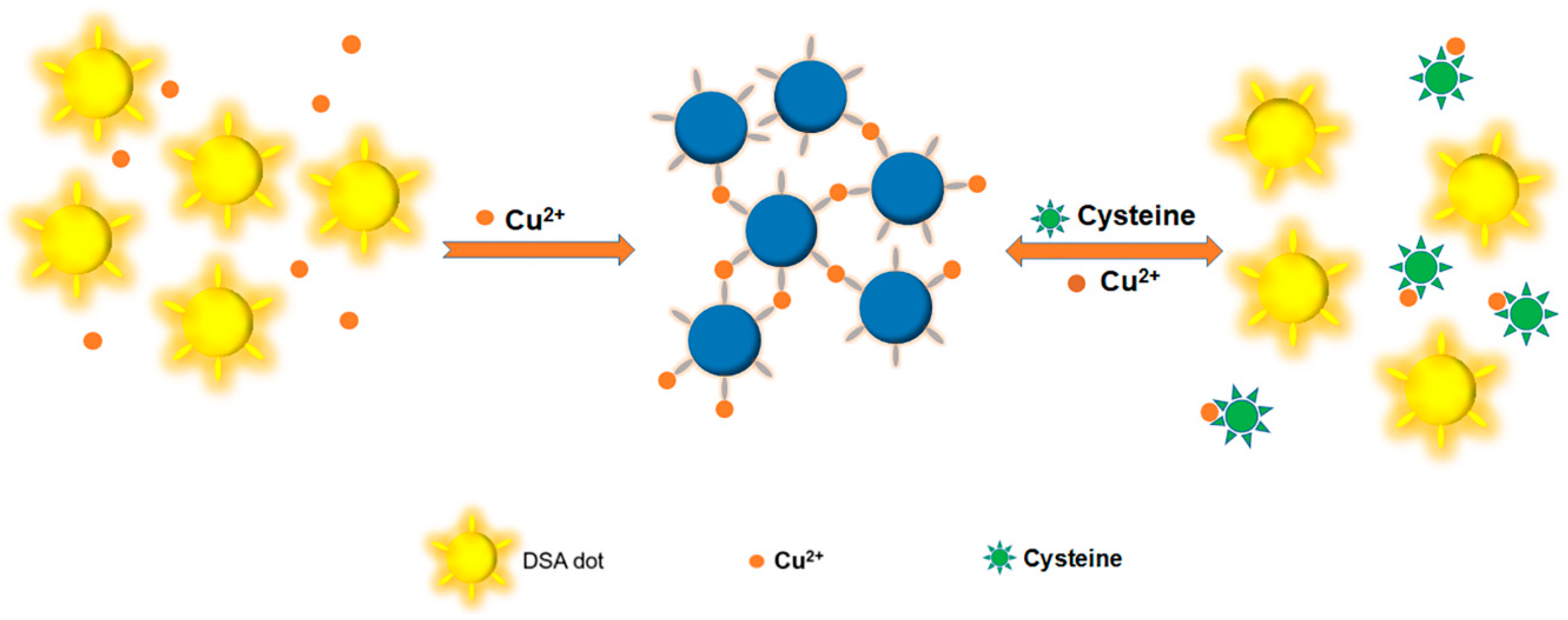
3.1. Mechanism for the Detection
3.2. Preparation and Characterization of Aggregation-Induced Emission Dots Based Probe
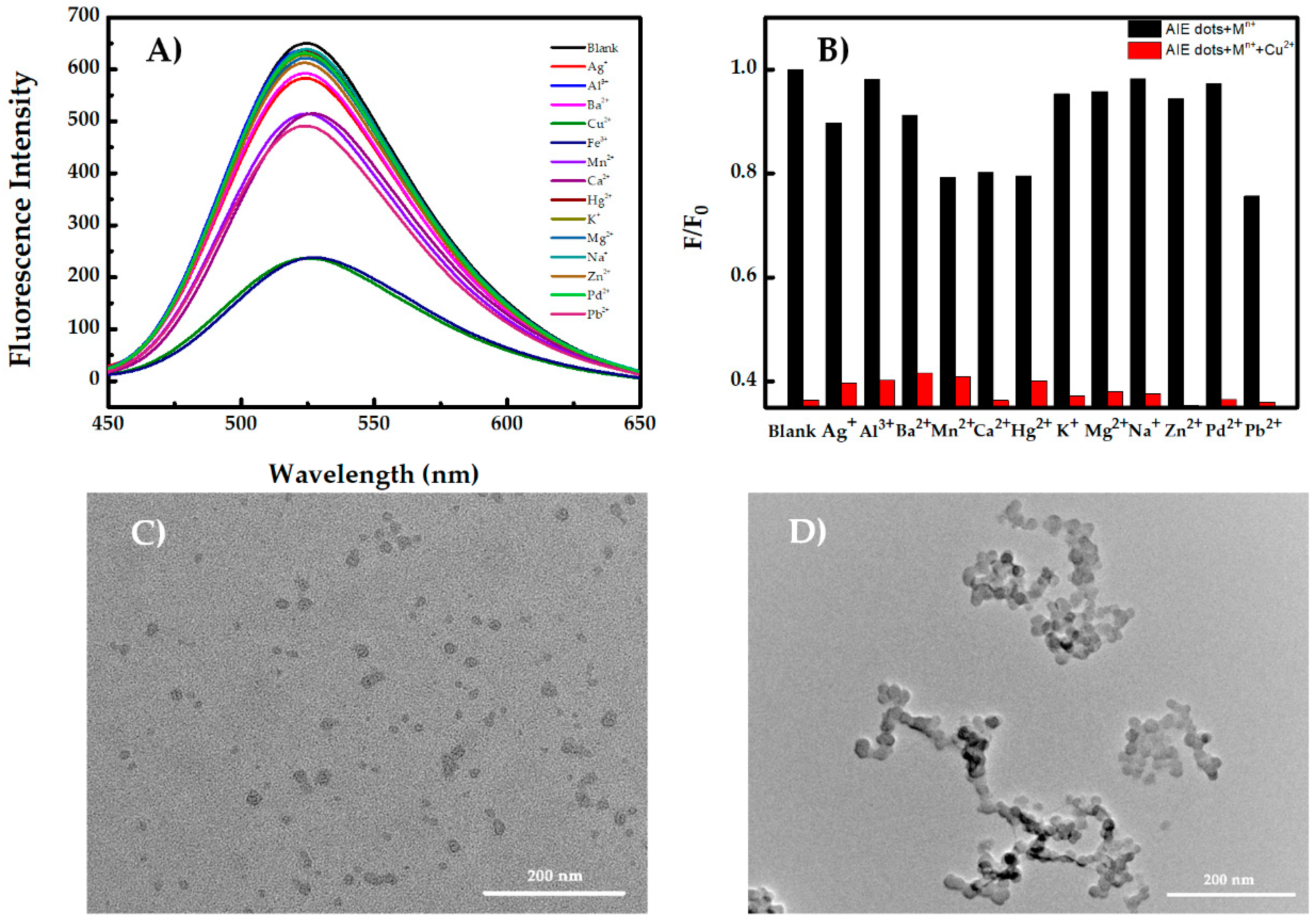
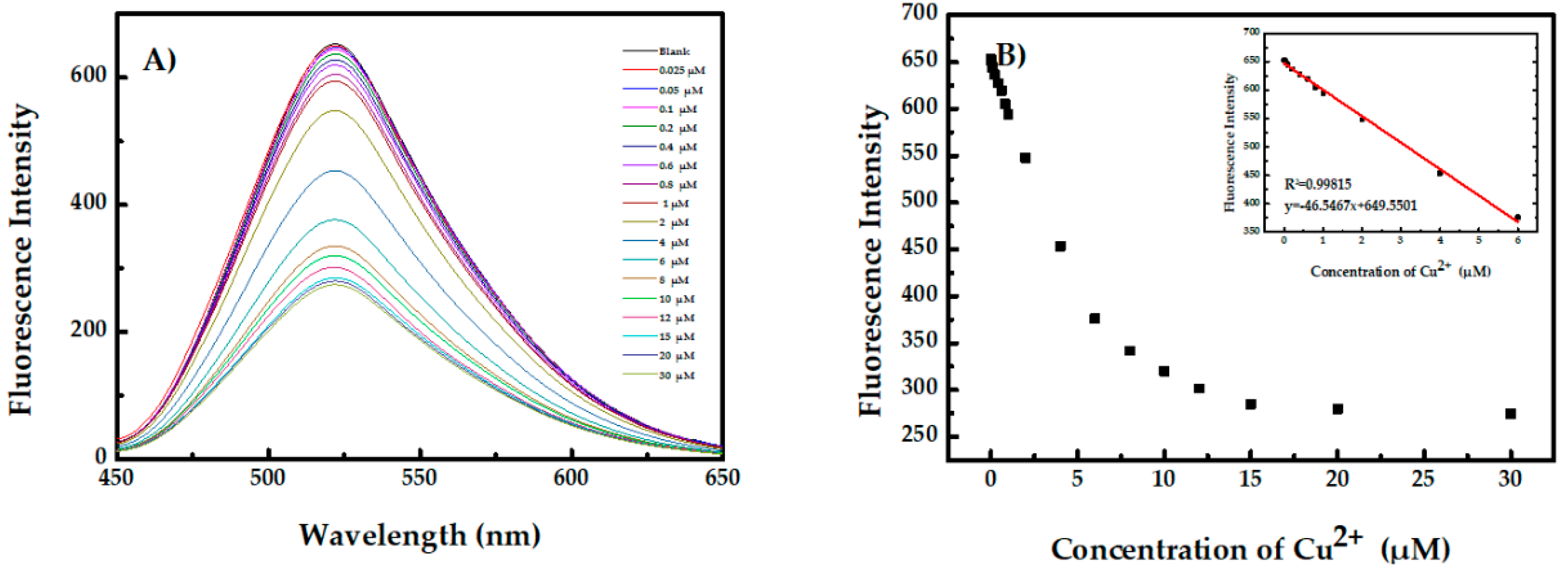
3.3. Aggregation-Induced Emission Dots for Recognition of Cu2+ and Fe3+
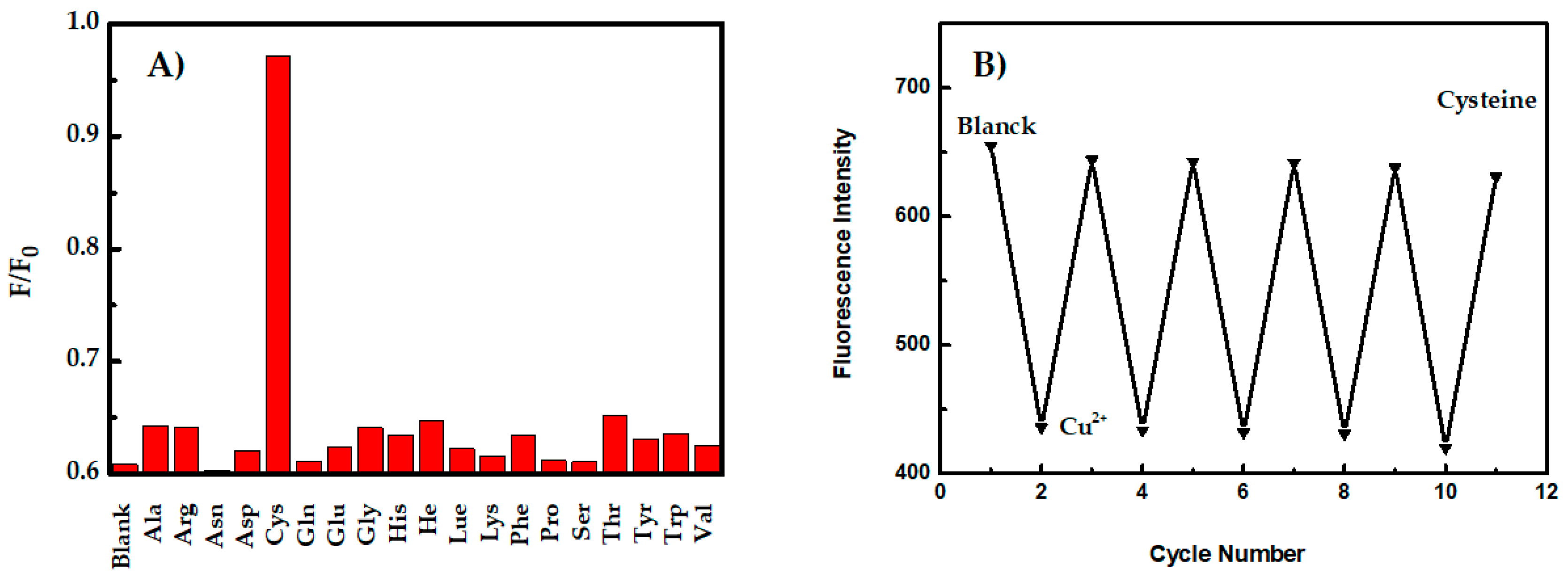
3.4. Detection of Cysteine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brewer, G.J. Risks of Copper and Iron Toxicity during Aging in Humans. Chem. Res. Toxicol. 2010, 23, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Lee, K.H. Optical sensor: A promising strategy for environmental and biomedical monitoring of ionic species. RSC Adv. 2015, 5, 72150–72287. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef] [PubMed]
- Angelémartínez, C.; Goodman, C. Metal-mediated DNA damage and cell death: Mechanisms, detection methods, and cellular consequences. Metallomics 2014, 6, 1358–1381. [Google Scholar] [CrossRef] [PubMed]
- Zidar, J.; Pirc, E.T. Copper(II) ion binding to cellular prion protein. J. Chem. Inf. Model. 2008, 48, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Robertson, J.D. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Maynard, C.J.; Cappai, R. Overexpression of Alzheimer’s disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 2002, 277, 44670–44676. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, D.A.; Pinchuk, I. The Mechanism of Copper-Induced Peroxidation. Biophys. J. 2017, 112, 377a. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, B. Fluorescence “turn on” detection of mercuric ion based on bis(dithiocarbamato)copper(II) complex functionalized carbon nanodots. Anal. Chem. 2014, 86, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Fahrni, C.J. A fluorogenic probe for the copper(I)-catalyzed azide-alkyne ligation reaction: Modulation of the fluorescence emission via 3(n, π*)-1(π, π*) inversion. J. Am. Chem. Soc. 2004, 126, 8862–8863. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y. Cyclam-functionalized carbon dots sensor for sensitive and selective detection of copper(II) ion and sulfide anion in aqueous media and its imaging in live cells. Sens. Actuators B-Chem. 2016, 224, 298–306. [Google Scholar] [CrossRef]
- Wen, T.; Qu, F. A facile, sensitive, and rapid spectrophotometric method for copper(II) ion detection in aqueous media using polyethyleneimine. Arab. J. Chem. 2013, 79, 1680–1685. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z. A reversible fluorescent chemosensor for selective and sequential detection of copper ion and sulfide. Dyes Pigment. 2016, 125, 292–298. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H. Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper(II) ions. Bionsens. Bioelectron. 2017, 91, 306–312. [Google Scholar] [CrossRef] [PubMed]
- El-Khairy, L.; Ueland, P.M. Plasma total cysteine as a risk factor for vascular disease: The European Concerted Action Project. Circulation 2001, 103, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A.; Riese, R.J.; Shi, G.P. Emerging roles for cysteine proteases in human biology. Annu. Rev. Physiol. 2003, 59, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.Y.; Guan, Y.S. Bodipy-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 2012, 134, 18928–18931. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shi, L. Silver-Ion-Mediated DNAzyme Switch for the Ultrasensitive and Selective Colorimetric Detection of Aqueous Ag+ and Cysteine. Chem. Eur. J. 2010, 15, 3347–3350. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, N. Highly selective red- and green-emitting two-photon fluorescent probes for cysteine detection and their bio-imaging in living cells. Chem. Commun. 2012, 48, 3442–3444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Tang, S. A novel colorimetric assay for rapid detection of cysteine and Hg2+ based on gold clusters. Talanta 2016, 146, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. A ratiometric fluorescent chemosensor for the detection of cysteine in aqueous solution at neutral pH. Luminescence 2017, 32, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Nam, S.W. A highly selective ratiometric near-infrared fluorescent cyanine sensor for cysteine with remarkable shift and its application in bioimaging. Chem. Sci. 2012, 3, 2760–2765. [Google Scholar] [CrossRef]
- Liu, X.; Xi, N. Highly selective phosphorescent nanoprobes for sensing and bioimaging of homocysteine and cysteine. J. Mater. Chem. 2012, 22, 7894–7901. [Google Scholar] [CrossRef]
- Shen, J.S.; Li, D.H. Metal-metal-interaction-facilitated coordination polymer as a sensing ensemble: A case study for cysteine sensing. Langmuir 2011, 27, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Jiang, X. Facile synthesis of N-acetyl-l-cysteine capped ZnS quantum dots as an eco-friendly fluorescence sensor for Hg2+. Talanta 2011, 85, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Guang, S. An effective real-time colorimeteric sensor for sensitive and selective detection of cysteine under physiological conditions. Analyst 2011, 136, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Galal, A. A novel sensor of cysteine self-assembled monolayers over gold nanoparticles for the selective determination of epinephrine in presence of sodium dodecyl sulfate. Analyst 2012, 137, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tian, W. Graphene quantum dots/l-cysteine coreactant electrochemiluminescence system and its application in sensing lead(II) ions. ACS Appl. Mater. Interfaces 2014, 6, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Garcíasantamarina, S.; Boronat, S. Reversible Cysteine Oxidation in Hydrogen Peroxide Sensing and Signal Transduction. Biochemistry 2015, 53, 2560–2580. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Gu, X. Two-photon AIE bio-probe with large Stokes shift for specific imaging of lipid droplets. Chem. Sci. 2017, 5440–5446. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, R. A reusable and naked-eye molecular probe with aggregation-induced emission (AIE) characteristics for hydrazine detection. J. Mater. Chem. B 2017, 5, 3565–3571. [Google Scholar] [CrossRef]
- Li, X.; Olivares, D. Differential sensing of oils by conjugates of serum albumins and 9,10-distyrylanthracene probes: a cautionary tale. Supramol. Chem. 2017, 29, 308–314. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, J. “AIE + ESIPT” ratiometric fluorescent probe for monitoring sulfur dioxide with distinct ratiometric fluorescence signals in mammalian cells, mouse embryonic fibroblast and zebrafish. J. Mater. Chem. B 2018, 1973–1983. [Google Scholar] [CrossRef]
- Li, Q.Y.; Ma, Z. AIE-active tetraphenylethene functionalized metal-organic framework for selective detection of nitroaromatic explosives and organic photocatalysis. Chem. Commun. 2016, 52, 11284–11287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhu, W. Selective fluorescent probes for spermine and 1-adamantanamine based on the supramolecular structure formed between AIE-active molecule and cucurbit[n]urils. Sens. Actuators B-Chem. 2018, 261, 602–607. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, X.; Peng, Z. A “Turn-On” Fluorescent Chemosensor with the Aggregation-Induced Emission Characteristic for High-Sensitive Detection of Ce Ions. Sens. Actuators B-Chem. 2018, 267, 351–356. [Google Scholar] [CrossRef]
- He, J.; Xu, B. Aggregation-Induced Emission in the Crystals of 9,10-Distyrylanthracene Derivatives: The Essential Role of Restricted Intramolecular Torsion. J. Phys. Chem. C 2009, 113, 9892–9899. [Google Scholar] [CrossRef]
- Li, Q.; Wu, X. Tailoring the Fluorescence of AIE-Active Metal–Organic Frameworks for Aqueous Sensing of Metal Ions. ACS Appl. Mater. Interfaces 2018, 10, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Jin, Y. Highly Fluorescent, Photostable, Conjugated Polymer Dots with Amorphous, Glassy-State, Coarsened Structure for Bioimaging. Adv. Opt. Mater. 2015, 3, 78–86. [Google Scholar] [CrossRef]
- Feng, G.; Mao, D. Polymeric nanorods with aggregation-induced emission characteristics for enhanced cancer targeting and imaging. Nanoscale 2018, 10, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, H. Cation-driven luminescent self-assembled dots of copper nanoclusters with aggregation-induced emission Highly Fluorescent, Photostable, Conjugated Polymer Dots with Amorphous, Glassy-State, Coarsened Structure for Bioimaging on for β-galactosidase activity monitoring. J. Mater. Chem. B 2017, 5, 78–86. [Google Scholar] [CrossRef]
- Li, K.; Jiang, G. Impact of cyclic topology: Odd–even glass transition temperatures and fluorescence quantum yields in molecularly-defined macrocycles. Polym. Chem. 2017, 8. [Google Scholar] [CrossRef]
- Gupta, A.S.; Paul, K. A fluorescent probe with “AIE + ESIPT” characteristics for Cu2+, and F−, ions estimation. Sens. Actuators B-Chem. 2017, 246, 653–661. [Google Scholar] [CrossRef]
- Feng, H.T.; Song, S. Self-assembled tetraphenylethylene macrocycle nanofibrous materials for the visual detection of copper(II) in wate. J. Phys. Chem. C 2014, 2, 2353–2359. [Google Scholar] [CrossRef]
- Zhao, M.; Qian, Z. Fabrication of Stable and Luminescent Copper Nanocluster-Based AIE Particles and Their Application in β-Galactosidase Activity Assay. ACS Appl. Mater. Interfaces 2017, 9, 32887–32895. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.; Liu, N.; Li, F.; Fu, W.; Zhou, Y.; Zhang, Y. Novel PSMA-Coated On-Off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine. Polymers 2018, 10, 786. https://doi.org/10.3390/polym10070786
Jiang R, Liu N, Li F, Fu W, Zhou Y, Zhang Y. Novel PSMA-Coated On-Off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine. Polymers. 2018; 10(7):786. https://doi.org/10.3390/polym10070786
Chicago/Turabian StyleJiang, Rui, Na Liu, Fan Li, Wensheng Fu, Yun Zhou, and Yan Zhang. 2018. "Novel PSMA-Coated On-Off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine" Polymers 10, no. 7: 786. https://doi.org/10.3390/polym10070786
APA StyleJiang, R., Liu, N., Li, F., Fu, W., Zhou, Y., & Zhang, Y. (2018). Novel PSMA-Coated On-Off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine. Polymers, 10(7), 786. https://doi.org/10.3390/polym10070786