Effects of Modified Graphene Oxide on Thermal and Crystallization Properties of PET
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
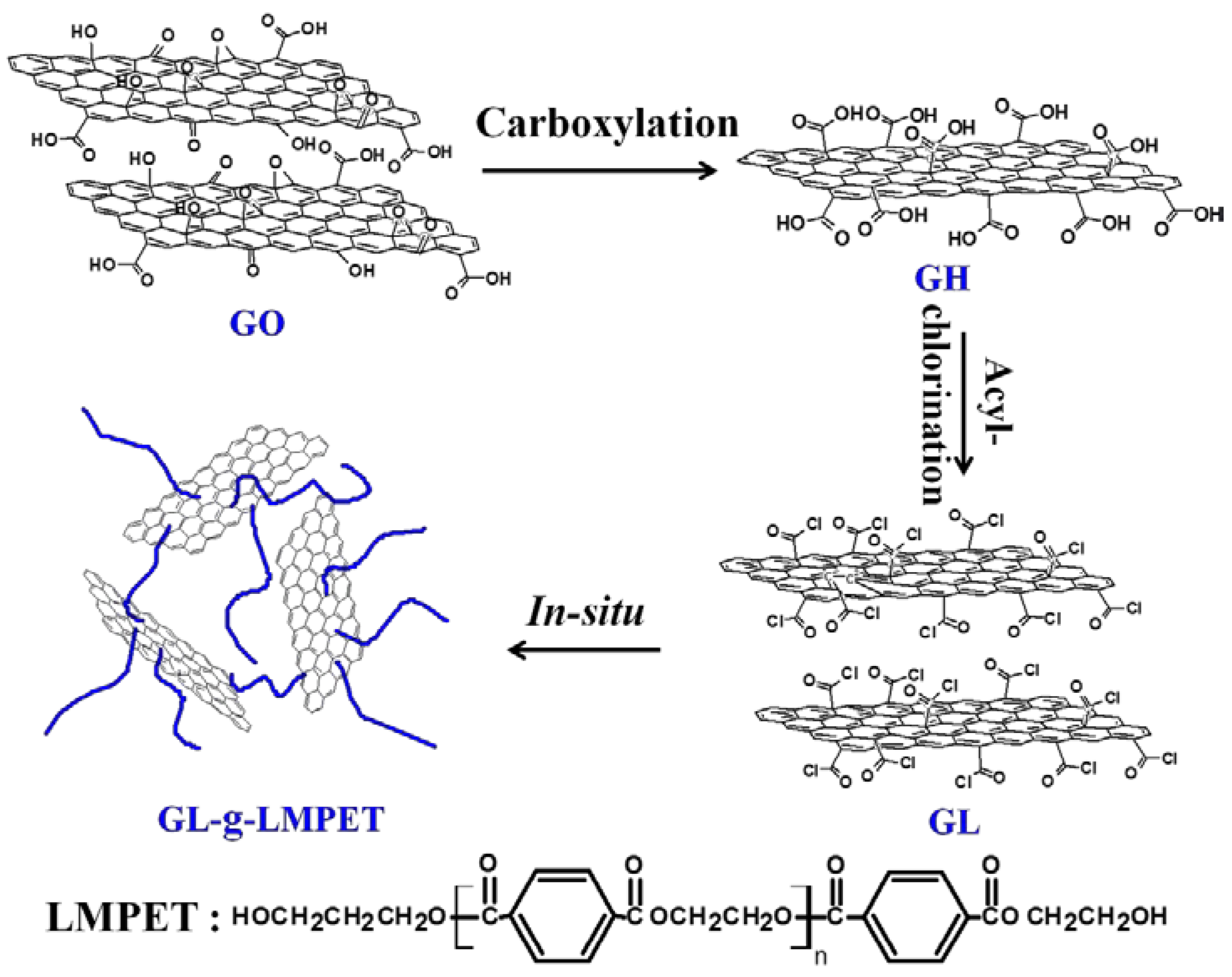
2.2. Surface Modification of GO Nanosheets
2.3. Melt-Blending of PET and GL-g-LMPET
2.4. Characterization
3. Results and Discussion
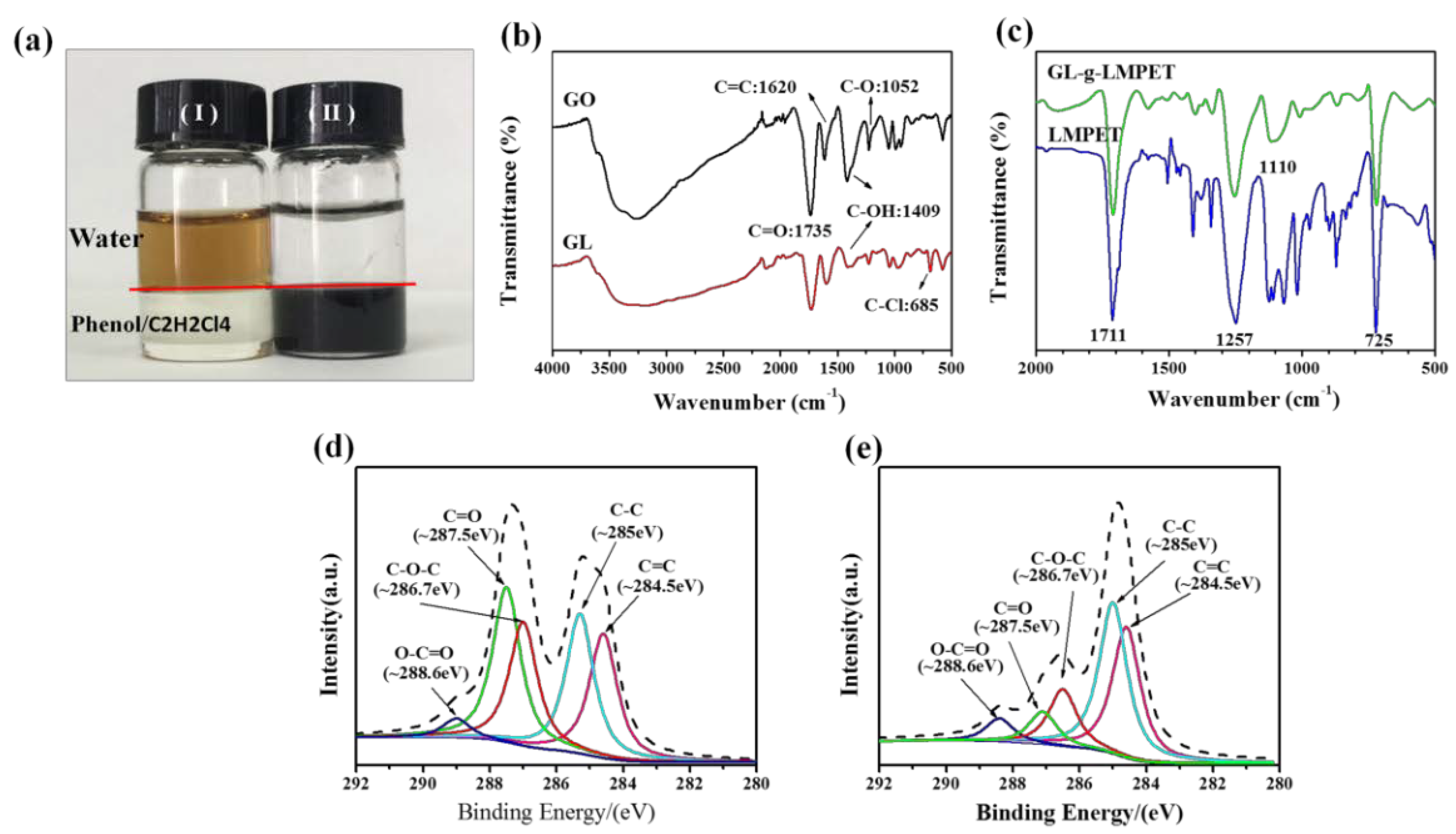
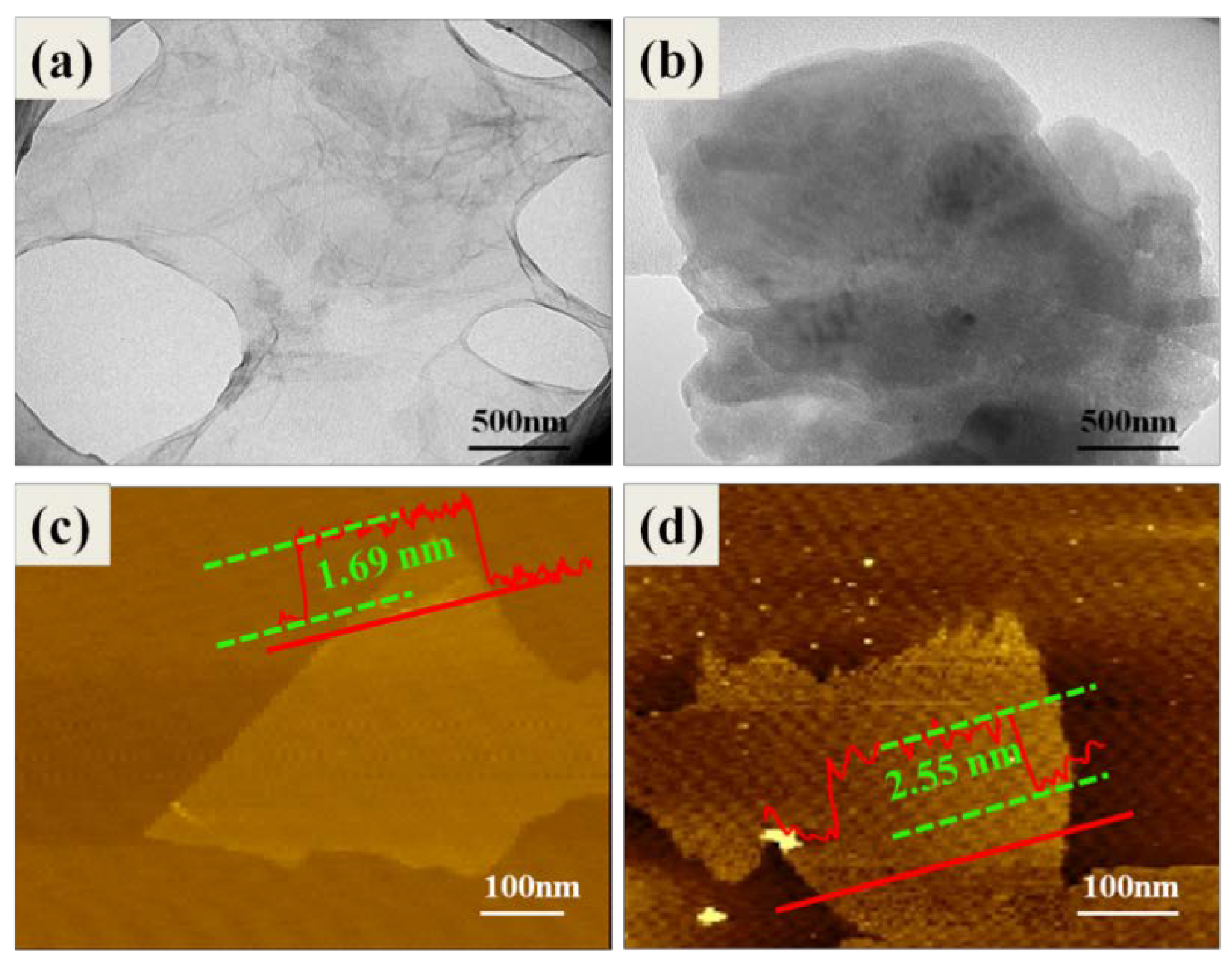
3.1. Structural Characterization of GL-g-LMPET
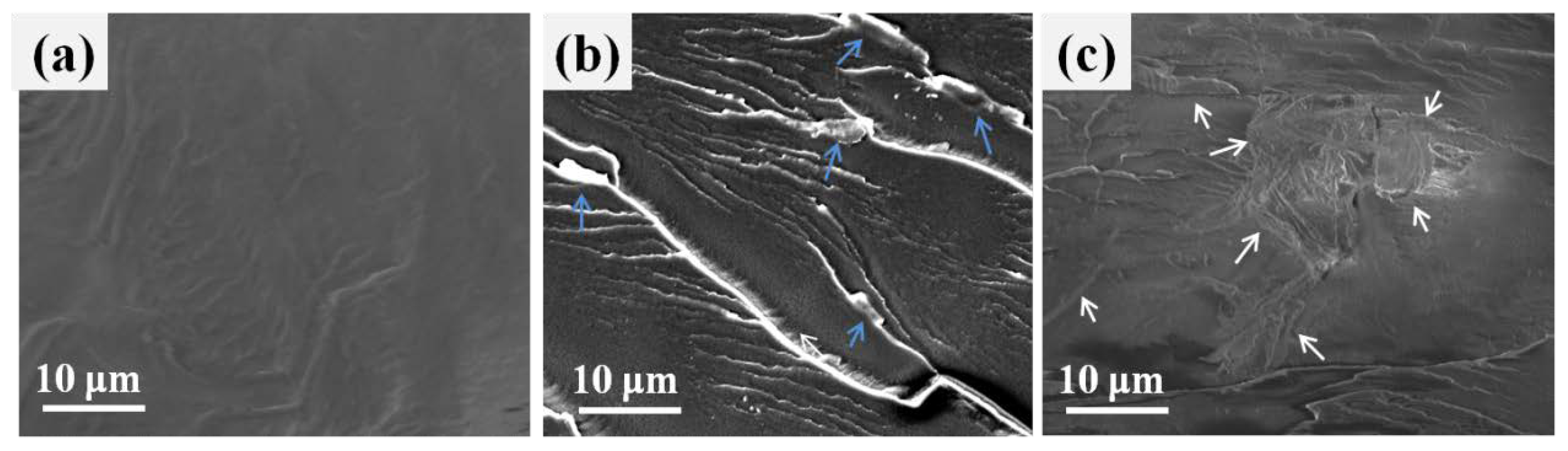
3.2. Dispersion of Various Nano-GO into the Polymer Matrix
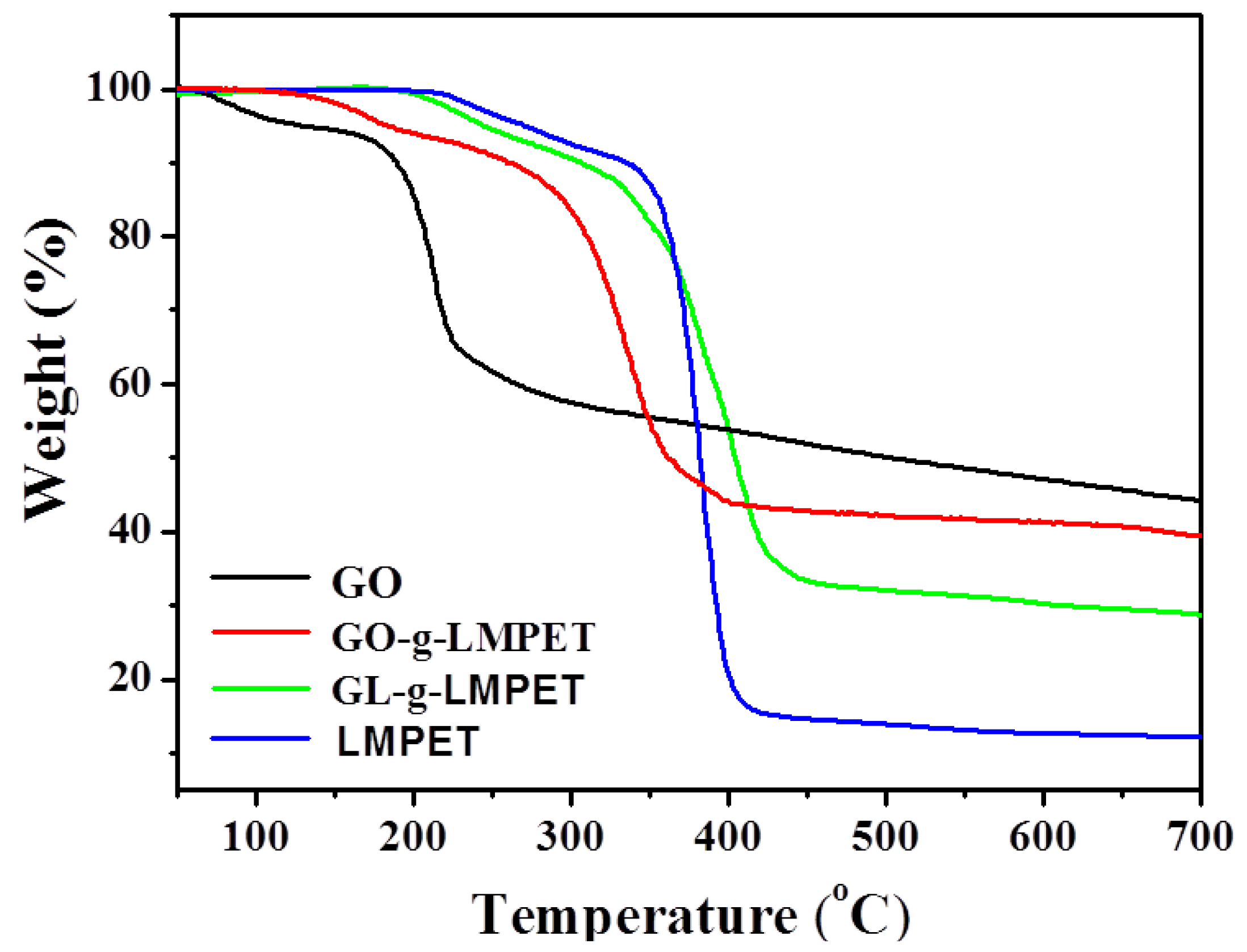
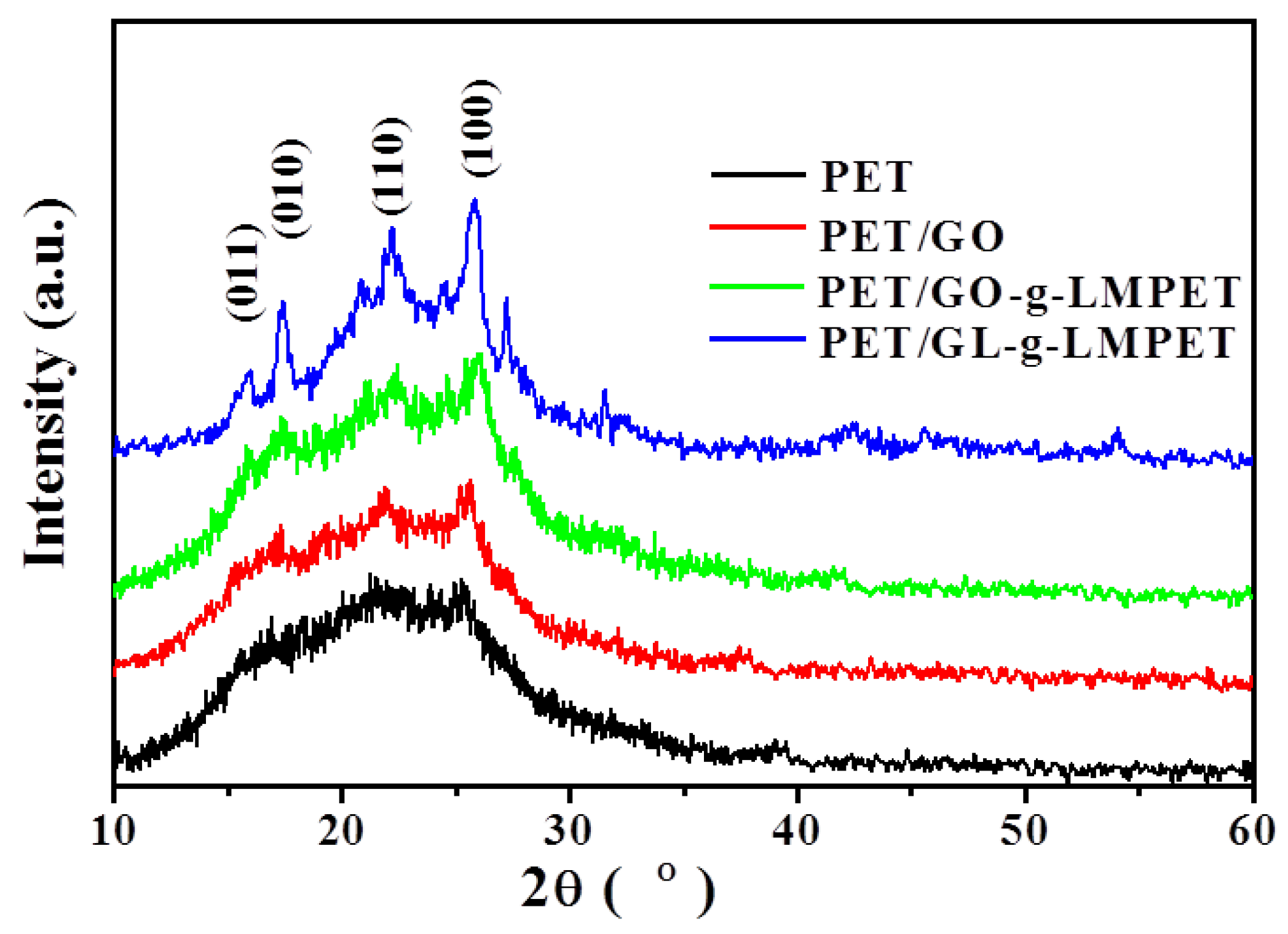
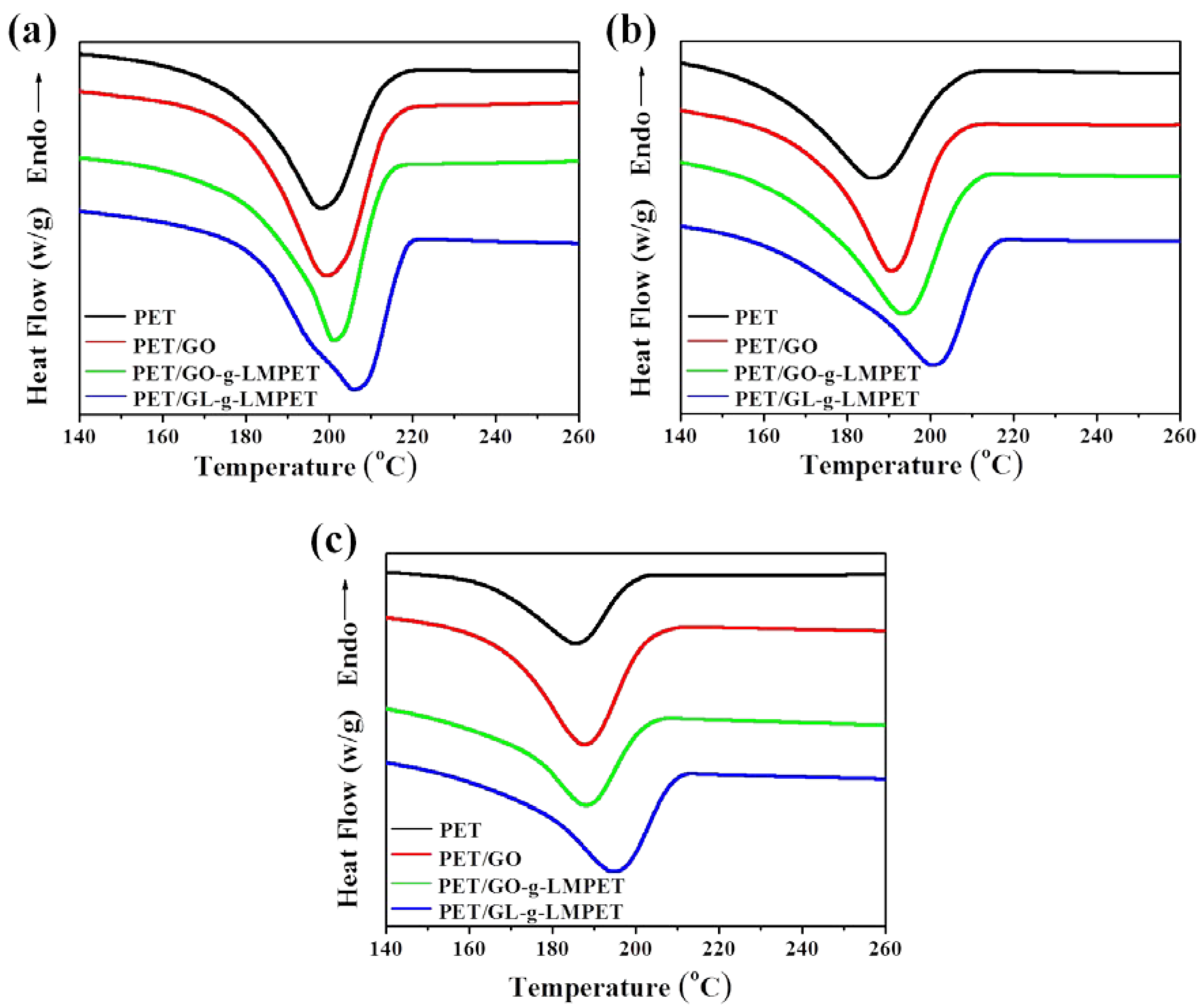
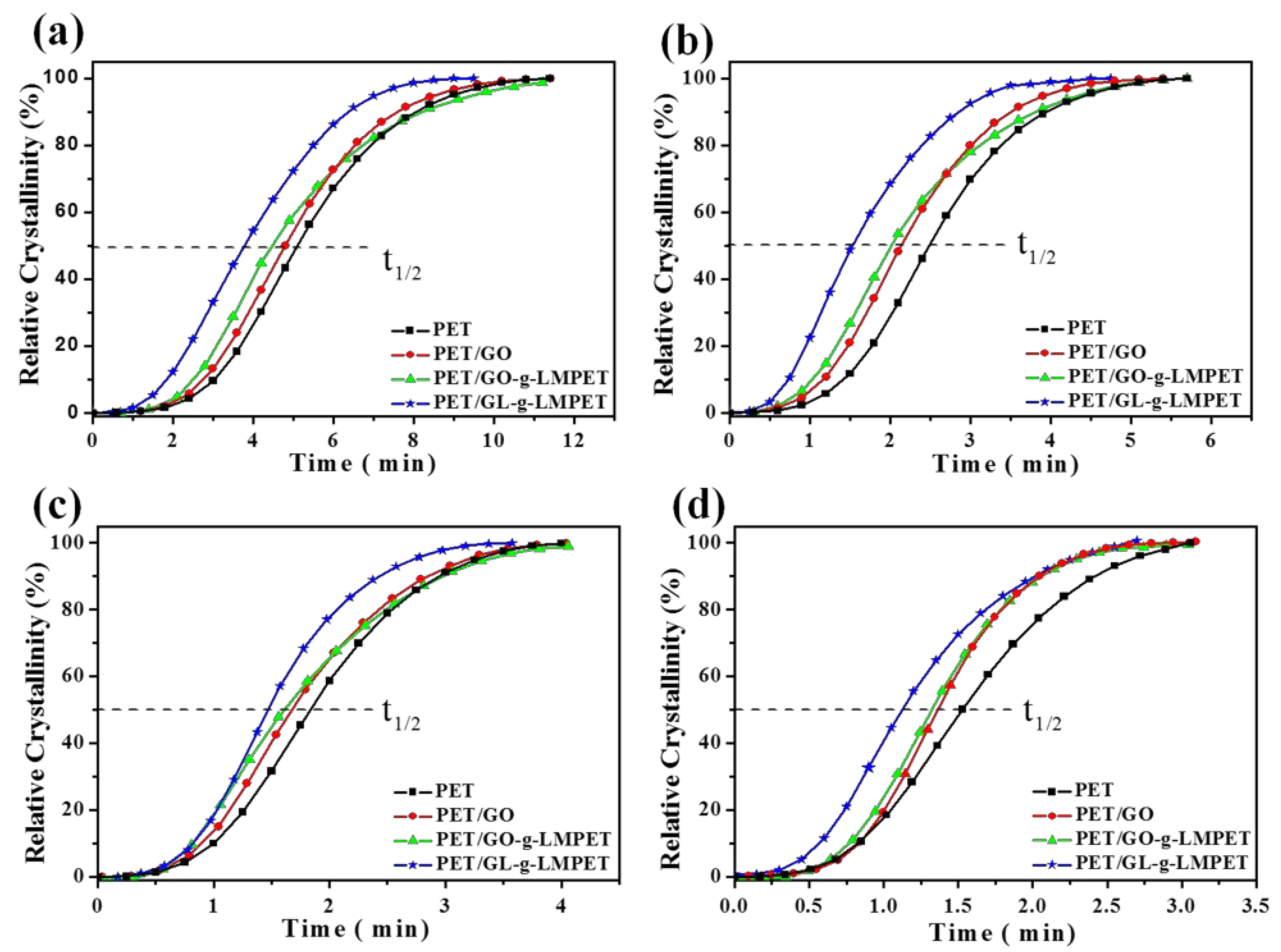
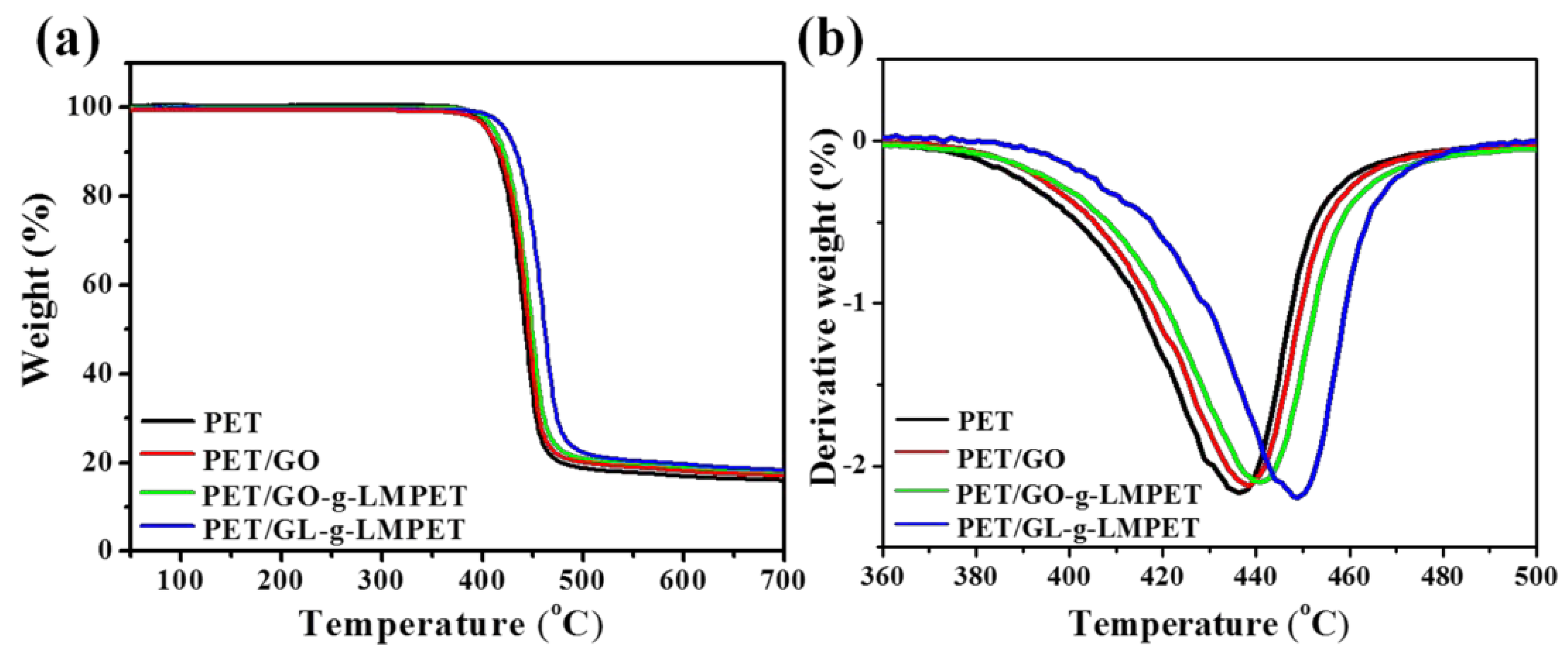
3.3. Influence of Various Nano-GO on Thermal Stability and Crystallization Properties of PET
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bang, H.J.; Kim, H.Y.; Jin, F.L.; Park, S.J. Fibers spun from 1,4-cyclohexanedimethanol-modified polyethylene terephthalate resin. J. Ind. Eng. Chem. 2011, 17, 805–810. [Google Scholar] [CrossRef]
- Miyamae, T.; Yamada, Y.; Uyama, H.; Nozoye, H. Molecular orientation of poly(ethylene terephthalate) and buried interface characterization of tio 2 films on poly(ethylene terephthalate) by using infrared-visible sum-frequency generation. Surf. Sci. 2001, 493, 314–318. [Google Scholar] [CrossRef]
- Tang, X.W.; Guo, W.H.; Gao, Y.J.; Yin, G.R.; Li, B.Y.; Wu, C.F. Preparation of novel super-tough poly(ethylene terephthalate) engineering plastic. Acta Sci. Nat. Univ. Sunyatseni 2005, 44, 39–41. [Google Scholar]
- Malas, A.; Bharati, A.; Verkinderen, O.; Goderis, B.; Moldenaers, P.; Cardinaels, R. Effect of the GO reduction method on the dielectric properties, electrical conductivity and crystalline behavior of PEO/rGO nanocomposites. Polymers 2017, 9, 613. [Google Scholar] [CrossRef]
- Baldenegro-Perez, L.A.; Navarro-Rodriguez, D.; Medellin-Rodriguez, F.J.; Hsiao, B.; Avila-Orta, C.A.; Sics, I. Molecular weight and crystallization temperature effects on poly(ethylene terephthalate) (PET) homopolymers, an isothermal crystallization analysis. Polymers 2014, 6, 583–600. [Google Scholar] [CrossRef]
- Yang, L.; Si, L.P.; Teo, J.K.H.; Toh, C.L.; Lau, S.K.; Ma, J.; Lu, X. A biomimetic approach to enhancing interfacial interactions: Polydopamine-coated clay as reinforcement for epoxy resin. ACS Appl. Mater. Interfaces 2011, 3, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.L.D.; Errico, M.E.; Avella, M. Thermal and morphological characterization of poly(ethylene terephthalate)/calcium carbonate nanocomposites. J. Mater. Sci. 2002, 37, 2351–2358. [Google Scholar] [CrossRef]
- Todorov, L.V.; Martins, C.I.; Viana, J.L.C. Characterization of pet nanocomposites with different nanofillers. Solid State Phenom. 2009, 151, 113–117. [Google Scholar] [CrossRef]
- Anand, K.A.; Agarwal, U.S.; Joseph, R. Carbon nanotubes induced crystallization of poly(ethylene terephthalate). Polymer 2006, 47, 3976–3980. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, H. Preparation and properties of deep dye fibers from poly(ethylene terephthalate)/SiO2 nanocomposites by in situ polymerization. J. Appl. Polym. Sci. 2007, 105, 2363–2369. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.-C.; Gong, L.-X.; Yan, D.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.E.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, L.-Z.; Jiao, L. Graphene highly scattered in porous carbon nanofibers: A binder-free and high-performance anode for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 1698–1705. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Liu, K.; Chen, L.; Chen, Y.; Wu, J.; Zhang, W.; Chen, F.; Fu, Q. Preparation of polyester/reduced graphene oxide composites via in situ melt polycondensation and simultaneous thermo-reduction of graphene oxide. J. Mater. Chem. 2011, 21, 8612–8617. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Z.; Liu, T.; Yin, Z.; Zhou, X.; Chen, S.; Xie, L.; Boey, F.; Zhang, H.; Huang, W. Multilayer stacked low-temperature-reduced graphene oxide films: Preparation, characterization, and application in polymer memory devices. Small 2010, 6, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, K.T.; Lee, J.U.; Jo, W.H. Facile method to functionalize graphene oxide and its application to poly(ethylene terephthalate)/graphene composite. ACS Appl. Mater. Interfaces 2012, 4, 4184–4191. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, P.; Bassoli, E.; Bon, S.B.; Valentini, L. Preparation and characterization of poly(butylene terephthalate)/graphene composites by in-situ polymerization of cyclic butylene terephthalate. Polymer 2012, 53, 897–902. [Google Scholar] [CrossRef]
- Chiu, N.-F.; Fan, S.-Y.; Yang, C.-D.; Huang, T.-Y. Carboxyl-functionalized graphene oxide composites as SPR biosensors with enhanced sensitivity for immunoaffinity detection. Biosens. Bioelectron. 2017, 89, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Chen, Y.; Yuan, M.; Li, H.; Xia, X.; Xiong, C. Functionalization of graphene oxide with low molecular weight poly(lactic acid). Polymers 2018, 10, 177. [Google Scholar] [CrossRef]
- Gu, H.; Zhang, H.; Ma, C.; Lyu, S.; Yao, F.; Liang, C.; Yang, X.; Guo, J.; Guo, Z.; Gu, J. Polyaniline assisted uniform dispersion for magnetic ultrafine barium ferrite nanorods reinforced epoxy metacomposites with tailorable negative permittivity. J. Phys. Chem. C 2017, 121, 13265–13273. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Kan, Y.; Qian, X.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290–13298. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, R.; Wang, Z.; Liu, H. Carboxyl-functionalized graphene oxide-polyaniline composite as a promising supercapacitor material. J. Mater. Chem. 2012, 22, 13619–13624. [Google Scholar] [CrossRef]
- Wu, G.; Xu, X.; He, X.; Yan, Y. Preparation and characterization of graphene oxide-modified sapium sebiferum oil-based polyurethane composites with improved thermal and mechanical properties. Polymers 2018, 10, 133. [Google Scholar] [CrossRef]
- Yuan, B.; Bao, C.; Song, L.; Hong, N.; Liew, K.M.; Hu, Y. Preparation of functionalized graphene oxide/polypropylene nanocomposite with significantly improved thermal stability and studies on the crystallization behavior and mechanical properties. Chem. Eng. J. 2014, 237, 411–420. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.Y.; Tang, J.G.; Yang, B. Preparation and properties of PET-PEG-SiO2 co-polymerized hybrid nanoblocks. J. Mater. Eng. 2008, 36, 101–105. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, J.; Zheng, H.; Sun, W.; Jiao, J.; Wang, Y.; Huang, L.; Liu, J.; Wang, W.; Shen, W. Crystallization of poly(ethylene terephthalate) via silica nanoparticles tethered with short diblock peg-pet copolymers. Sci. Adv. Mater. 2016, 8, 1603–1611. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Z.; Ge, Y.; Wang, Y.; Fan, J.; Yin, J. Solvent exfoliated graphene for reinforcement of PMMA composites prepared by in situ polymerization. Mater. Chem. Phys. 2012, 136, 43–50. [Google Scholar] [CrossRef]
- Charles, J. The Aldrich library of infrared spectra. Aldrich Chem. 1975, 1975, 184–191. [Google Scholar]
- Parvinzadeh, M.; Moradian, S.; Rashidi, A.; Yazdanshenas, M.E. Surface characterization of polyethylene terephthalate/silica nanocomposites. Appl. Surf. Sci. 2010, 256, 2792–2802. [Google Scholar] [CrossRef]
- O’Hare, A.; Kusmartsev, F.V.; Kugel, K.I. A stable “flat” form of two-dimensional crystals: Could graphene, silicene, germanene be minigap semiconductors? Nano Lett. 2012, 12, 1045. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wong, S.C. Electrical conductivity and dielectric properties of PMMA/expanded graphite composites. Compos. Sci. Technol. 2003, 63, 225–235. [Google Scholar] [CrossRef]
- Chen, G.H.; Wu, D.J.; Weng, W.G.; Yan, W.L. Dispersion of graphite nanosheets in a polymer matrix and the conducting property of the nanocomposites. Polym. Eng. Sci. 2001, 41, 2148–2154. [Google Scholar] [CrossRef]
- Kassaee, M.Z.; Motamedi, E.; Majdi, M. Magnetic Fe3O4-graphene oxide/polystyrene: Fabrication and characterization of a promising nanocomposite. Chem. Eng. J. 2011, 172, 540–549. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Hajiraissi, R.; Parvinzadeh, M. Preparation of polybutylene terephthalate/silica nanocomposites by melt compounding: Evaluation of surface properties. Appl. Surf. Sci. 2011, 257, 8443–8450. [Google Scholar] [CrossRef]
- Geoghegan, M.; Krausch, G. Wetting at polymer surfaces and interfaces. Prog. Polym. Sci. 2003, 28, 261–302. [Google Scholar] [CrossRef]
- Aoyama, S.; Park, Y.T.; Ougizawa, T.; Macosko, C.W. Melt crystallization of poly(ethylene terephthalate): Comparing addition of graphene vs. Carbon nanotubes. Polymer 2014, 55, 2077–2085. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Yao, X.; Tian, X.; Xie, D.; Zhang, X.; Zheng, K.; Xu, J.; Zhang, G.; Cui, P. Interface structure of poly(ethylene terephthalate)/silica nanocomposites. Polymer 2009, 50, 1251–1256. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Guo, Y.; Song, L.; Hu, Y. Poly(vinyl alcohol) nanocomposites based on graphene and graphite oxide: A comparative investigation of property and mechanism. J. Mater. Chem. 2011, 21, 13942–13950. [Google Scholar] [CrossRef]
- Li, B.; Zhong, W.H. Review on polymer/graphite nanoplatelet nanocomposites. J. Mater. Sci. 2011, 46, 5595–5614. [Google Scholar] [CrossRef]
- Cheng, H.K.F.; Sahoo, N.G.; Tan, Y.P.; Pan, Y.; Bao, H.; Li, L.; Chan, S.H.; Zhao, J. Poly(vinyl alcohol) nanocomposites filled with poly(vinyl alcohol)-grafted graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
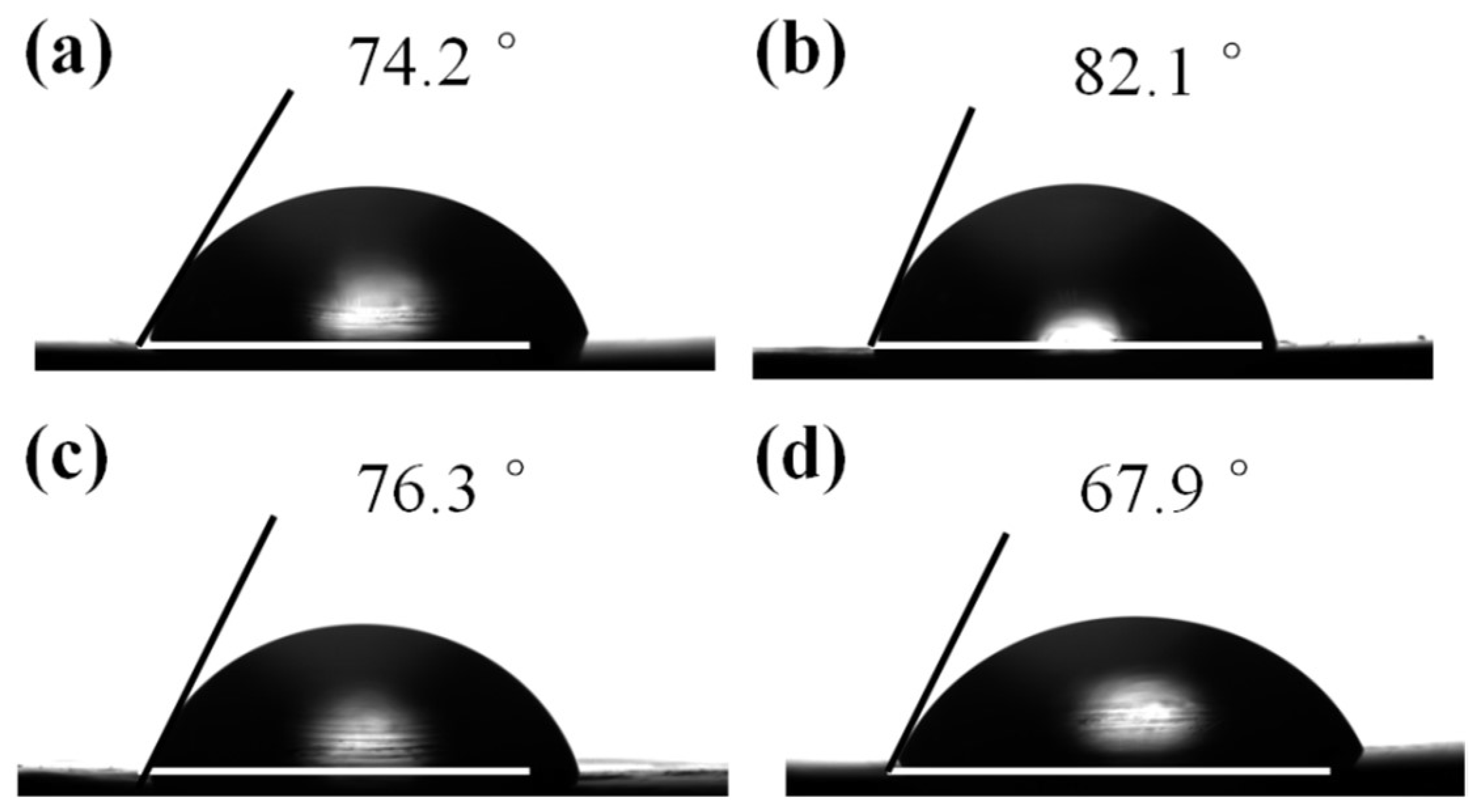
| Sample | Average contact angle (°) | γD |
|---|---|---|
| Pure PET | 74.2 | 39.2 |
| PET/GO | 82.1 | 34.1 |
| PET/GO-g-LMPET | 76.3 | 37.7 |
| PET/GL-g-LMPET | 67.9 | 43.2 |
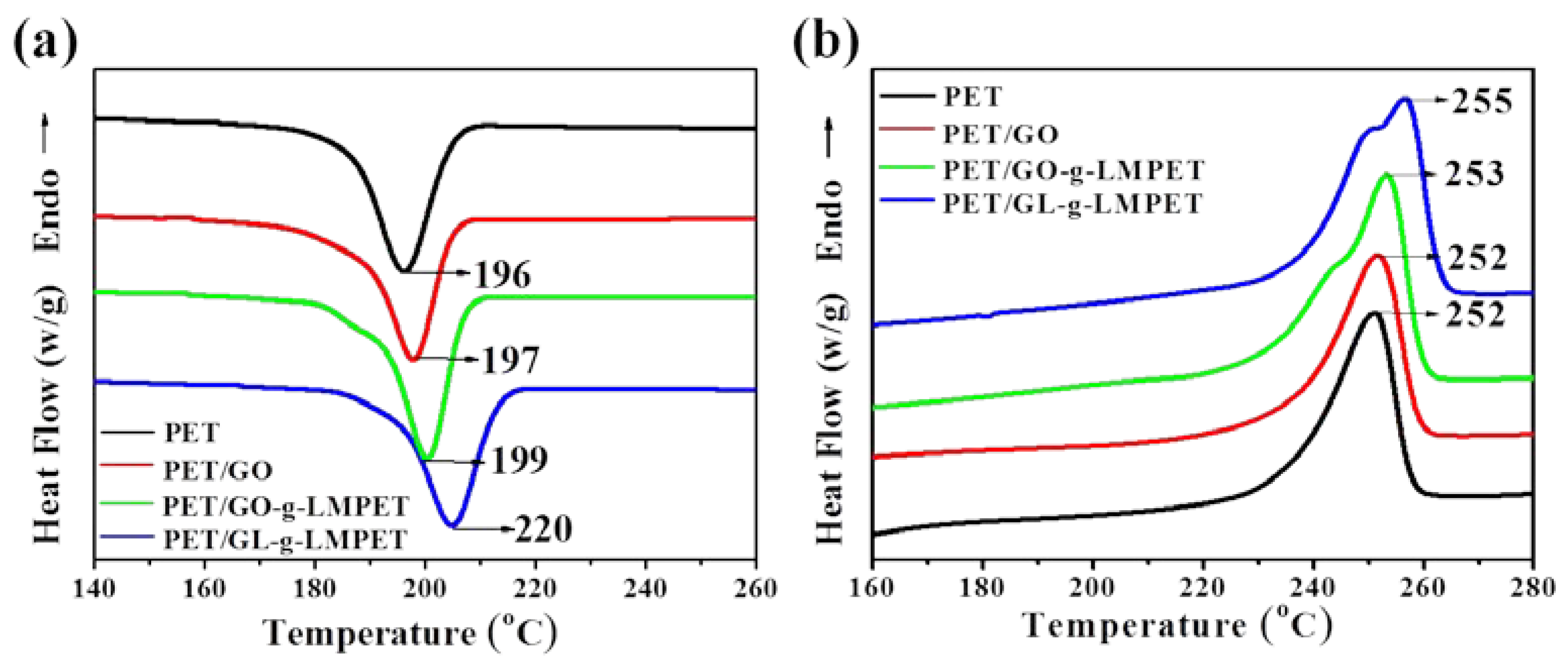
| Sample (0.5 wt %) | Tmc (°C) | Tm (°C) | ΔT (°C) | ΔHm (J/g) | XC (%) |
|---|---|---|---|---|---|
| PET | 196 | 252 | 56 | 33.2 | 23.7 |
| PET/GO | 197 | 252 | 55 | 34.7 | 24.9 |
| PET/GO-g-LMPET | 199 | 253 | 54 | 35.9 | 25.8 |
| PET/GL-g-LMPET | 205 | 255 | 50 | 44.2 | 29.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, L.; Wang, Y.; Wang, S.; Zhang, Y.; Mao, S.; Wang, G.; Liu, J.; Huang, L.; Li, H.; Belfiore, L.A.; et al. Effects of Modified Graphene Oxide on Thermal and Crystallization Properties of PET. Polymers 2018, 10, 613. https://doi.org/10.3390/polym10060613
Xing L, Wang Y, Wang S, Zhang Y, Mao S, Wang G, Liu J, Huang L, Li H, Belfiore LA, et al. Effects of Modified Graphene Oxide on Thermal and Crystallization Properties of PET. Polymers. 2018; 10(6):613. https://doi.org/10.3390/polym10060613
Chicago/Turabian StyleXing, Li, Yao Wang, Shichao Wang, Yu Zhang, Sui Mao, Guanghui Wang, Jixian Liu, Linjun Huang, Hao Li, Laurence A. Belfiore, and et al. 2018. "Effects of Modified Graphene Oxide on Thermal and Crystallization Properties of PET" Polymers 10, no. 6: 613. https://doi.org/10.3390/polym10060613
APA StyleXing, L., Wang, Y., Wang, S., Zhang, Y., Mao, S., Wang, G., Liu, J., Huang, L., Li, H., Belfiore, L. A., & Tang, J. (2018). Effects of Modified Graphene Oxide on Thermal and Crystallization Properties of PET. Polymers, 10(6), 613. https://doi.org/10.3390/polym10060613






