Research on the Methods for the Mass Production of Multi-Scale Organs-On-Chips
Abstract
1. Introduction
2. Materials and Methods
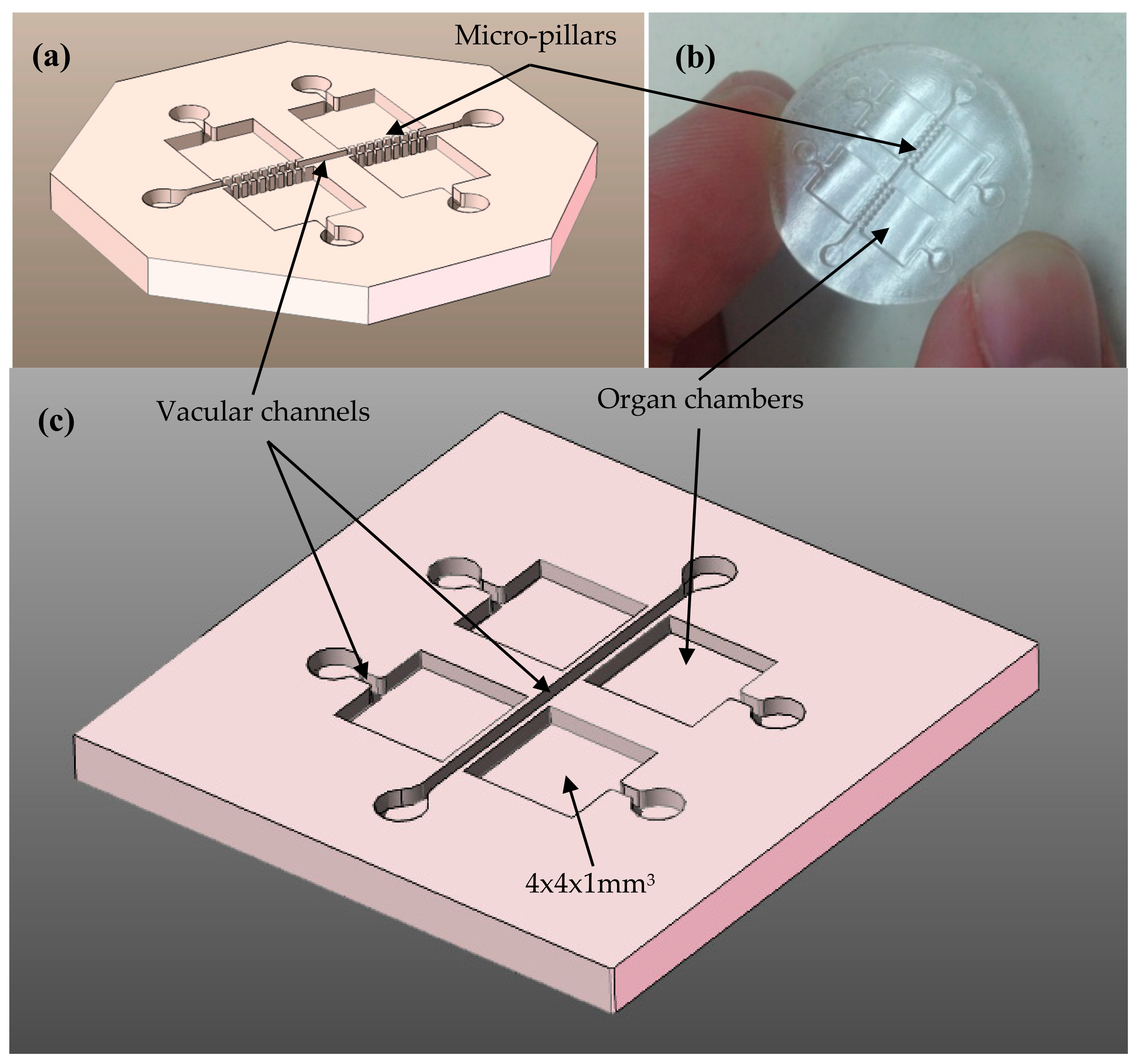
2.1. Design Process
2.2. Manufacturing Process
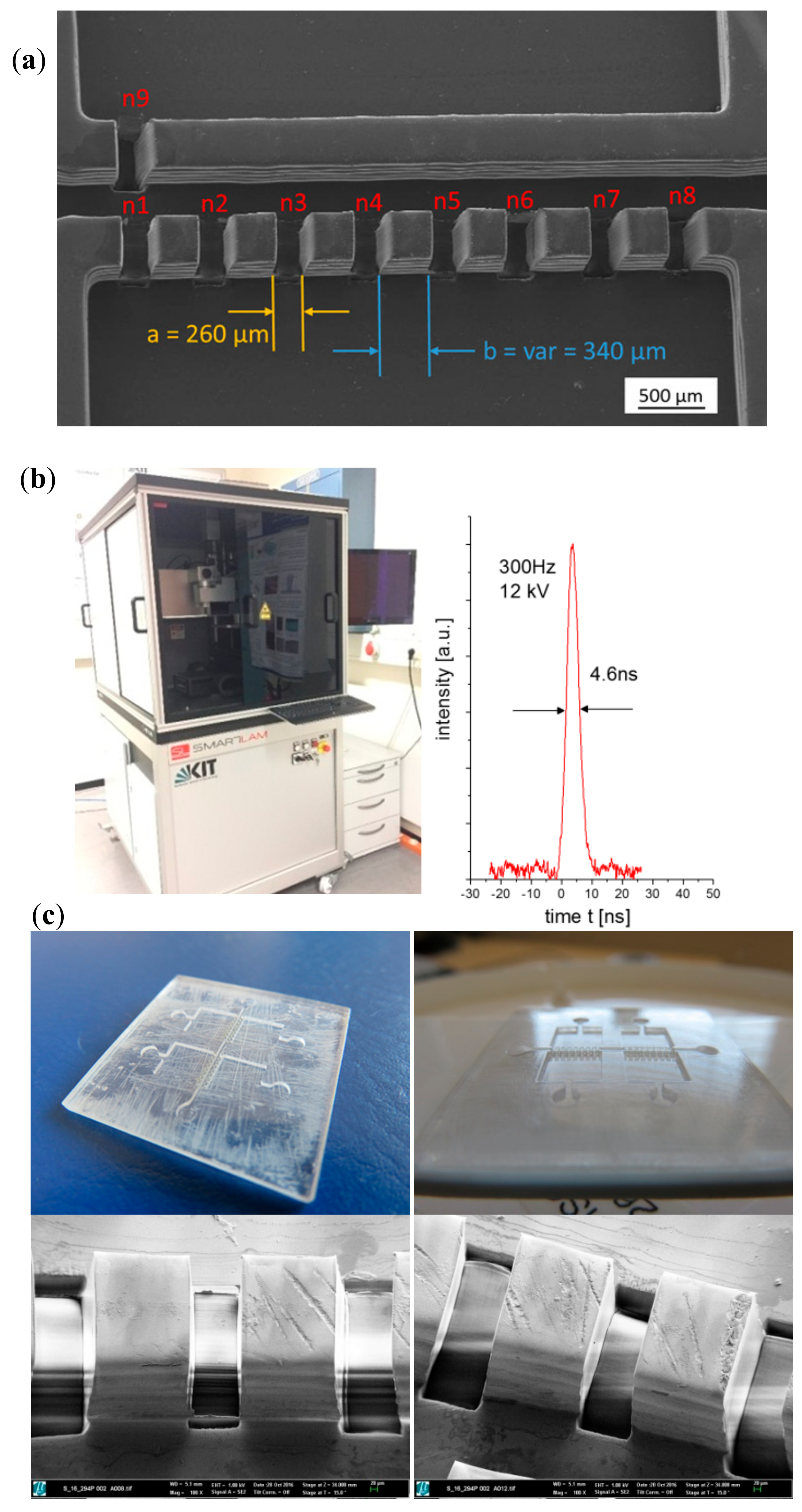
2.2.1. Additive Generation of Master Prototypes or Initial “Green” Parts
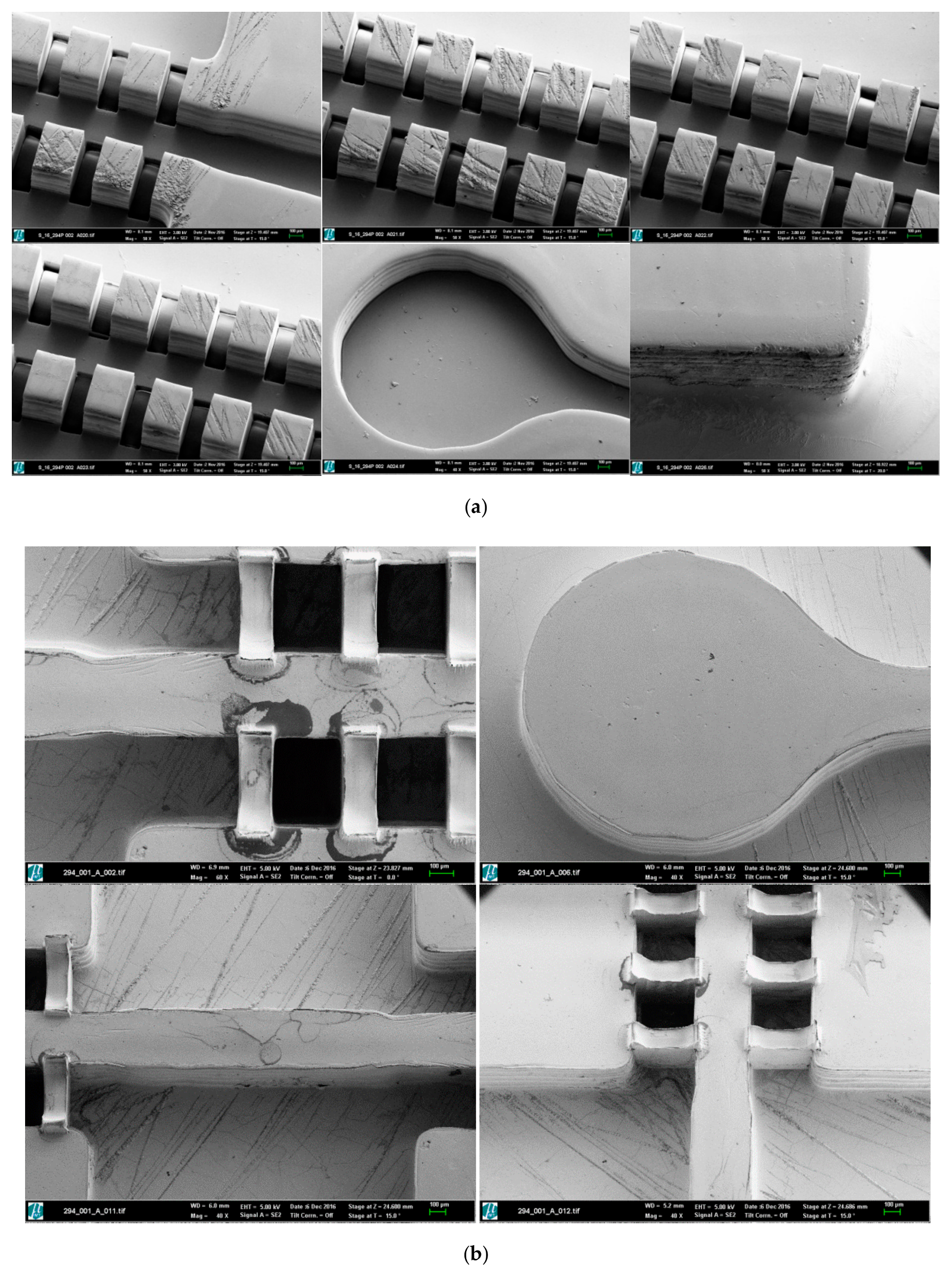
2.2.2. Laser Materials Processing of Master Prototypes
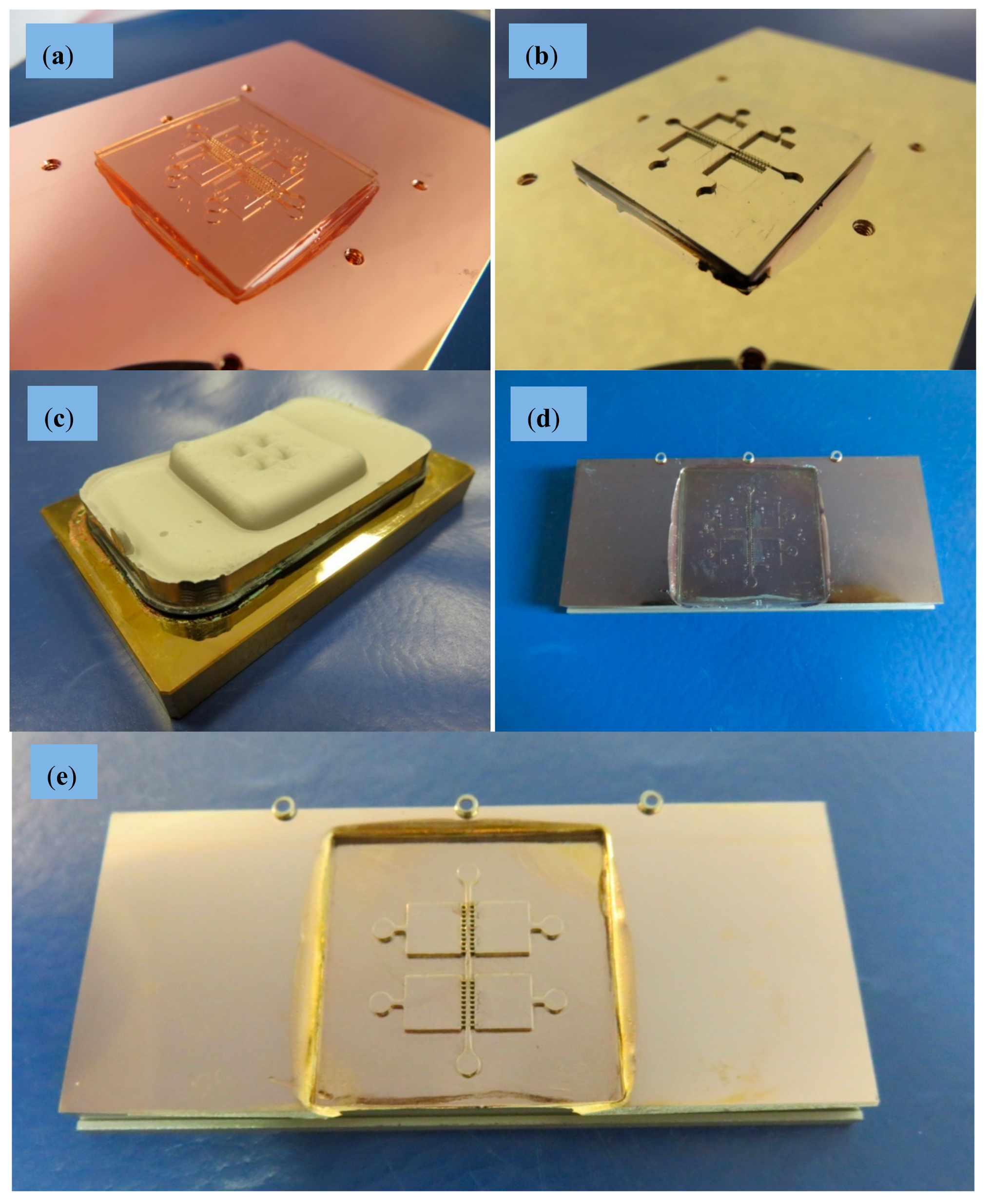
2.2.3. Electroforming and Mechanical Treatment for Mold Insert Fabrication
2.2.4. Micro-Injection Molding of Final Parts
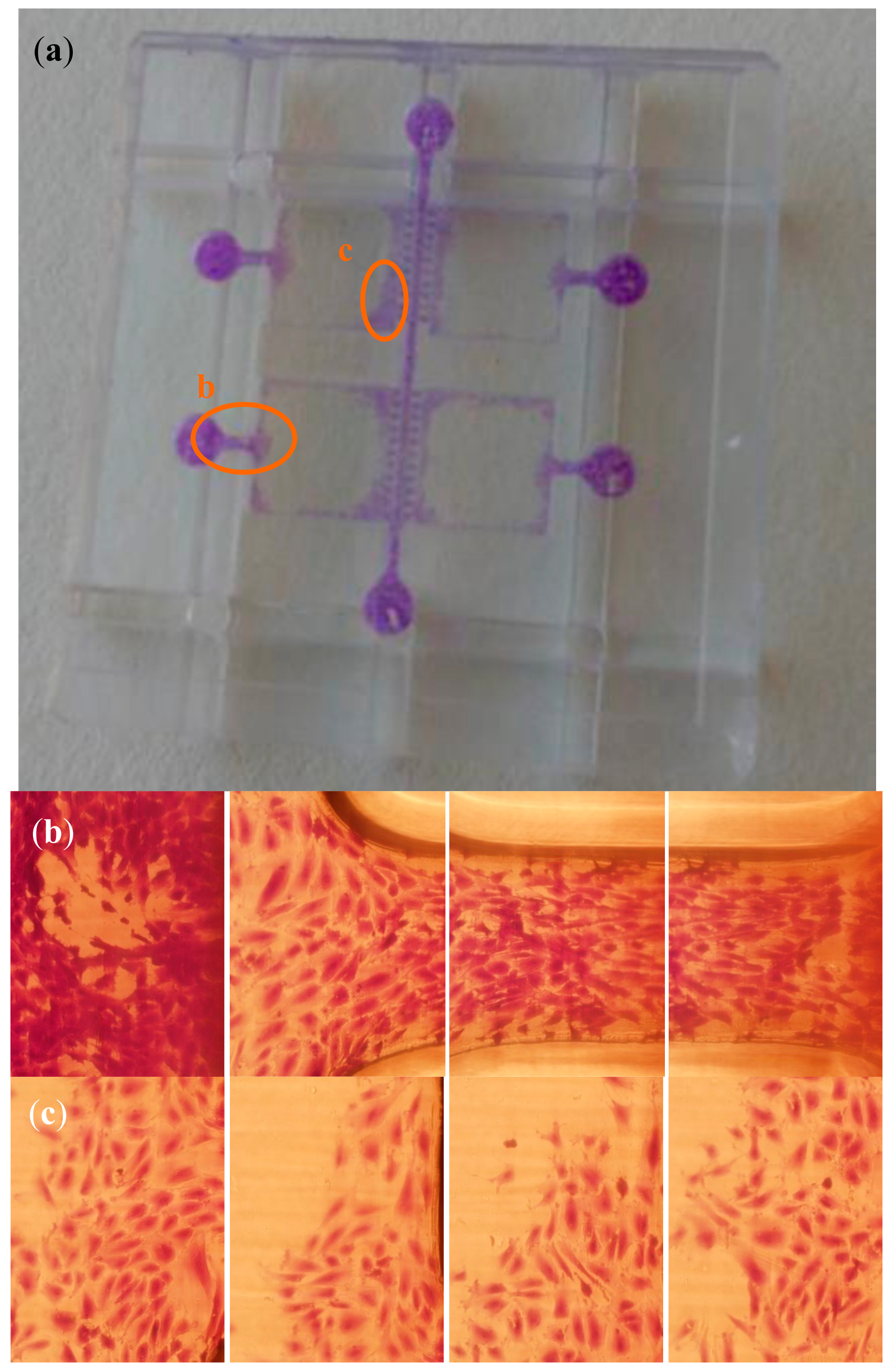
2.3. Cell-Culture Trials for Validation Purposes
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs on chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Kim, H.J.; Fraser, J.P.; Shea, D.E.; Khan, M.; Bahinski, A.; Hamilton, G.A.; Ingber, D.E. Microfabrication of human organs-on-chips. Nat. Protoc. 2013, 8, 2135–2157. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.; Langer, R.; Vacanti, J. Principles of Tissue Engineering, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Jenkins, G.; Mansfield, C.D. Microfluidic Diagnostics: Methods and Protocols; Springer: Berlin, Germany, 2013. [Google Scholar]
- Gad-el-Hak, M. The MEMS Handbook; CRC Press: New York, NY, USA, 2003. [Google Scholar]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft-lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.W.; Phillips, S.T.; Whitesides, G.M. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Ann. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Waldbaur, A.; Rapp, H.; Länge, K.; Rapp, B.E. Let there be chip. Towards rapid prototyping of microfluidic devices: One-step manufacturing processes. Anal. Methods 2011, 3, 2681–2716. [Google Scholar] [CrossRef]
- Schmieder, F.; Ströbel, J.; Rösler, M.; Grünzner, S.; Hohenstein, B.; Klotzbach, U.; Sonntag, F. 3D printing—A key technology for tailored biomedical cell culture labware. Curr. Dir. Biomed. Eng. 2016, 2, 105–108. [Google Scholar]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. LabChip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lantada, A.; Piotter, V.; Plewa, K.; Barié, N.; Guttmann, M.; Wissmann, M. Towards mass production of microtextured microdevices: Linking rapid prototyping with microinjection molding. Int. J. Adv. Manuf. Technol. 2014, 76, 1011–1020. [Google Scholar] [CrossRef]
- Díaz Lantada, A. Handbook of Microsystems for Enhanced Control of Cell Behavior: Fundamentals, Design and Manufacturing Strategies, Applications and Challenges; Springer: Berlin, Germany, 2016. [Google Scholar]
- Mottay, E.; Liu, X.; Zhang, H.; Mazur, E.; Sanatinia, R.; Pfleging, W. Industrial applications of ultrafast laser processing. MRS Bull. 2016, 41, 984–992. [Google Scholar] [CrossRef]
- Pröll, J.; Kohler, R.; Mangang, A.; Ulrich, S.; Ziebert, C.; Pfleging, W. 3D structures in battery materials. JLMNJ. Laser Micro/Nanoeng. 2012, 7, 97–104. [Google Scholar] [CrossRef]
- Wissmann, M.; Guttmann, M.; Hartmann, M.; Hofmann, A.; Hummel, B. Alternative mould insert fabrication technology for micromoulding by galvanic replication. In Proceedings of the 2010 Symposium on Design Test Integration and Packaging of MEMS/MOEMS(DTIP), Seville, Spain, 5–7 May 2010. [Google Scholar]
- Guttmann, M.; Schulz, J.; Saile, V. Lithographic fabrication of mold inserts. In Advanced Micro and Nanosystems, Vol. 3, Microengineering of Metals and Ceramics; Baltes, H., Brand, O., Fedder, G.K., Hierold, C., Korvink, J., Tabata, O., Löhe, D., Hausselt, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 87–219. [Google Scholar]
- Schanz, G.; Bade, K. Microelectroforming of metal, In Advanced Micro and Nanosystems, Vol. 4, Microengineering of Metals and Ceramics; Baltes, H., Brand, O., Fedder, G.K., Hierold, C., Korvink, J., Tabata, O., Löhe, D., Hausselt, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 395–420. [Google Scholar]
- Lennon, D.P.; Haynesworth, S.E.; Bruder, S.P.; Jaiswal, N.; Caplan, A.I. Human and animal mesenchymal progenitor cells from bone marrow: Identification of serum for optimal selection and proliferation. Vitr. Cell. Dev. Boil. 2006, 32, 602. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Ogueta, S.; Muñoz, J.; Obregon, E.; Delgado-Baeza, E.; García-Ruiz, J.P. Prolactin is a component of the human synovial liquid and modulates the growth and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Mol. Cell. Endocrinol. 2002, 190, 51. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells and the NO pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 2695–2696. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, H.; Ynsa, M.D.; Dang, Z.Y.; Torres-Costa, V.; Manso-Silván, M.; Wu, J.F.; Breese, M.B.H.; García-Ruiz, J.P. Conditioned bio-interfaces of silicon / porous silicon micro-patterns lead to the chondrogenesis of hMSCs. RSC Adv. 2015, 5, 92263–92269. [Google Scholar] [CrossRef]
- Díaz Lantada, A.; Alarcón Iniesta, H.; Pareja Sánchez, B.; García-Ruíz, J.P. Free-form rapid-prototyped PDMS scaffolds incorporating growth factors promote chondrogenesis. Adv. Mater. Sci. Eng. 2014, 2014, 612976. [Google Scholar] [CrossRef]
- Díaz Lantada, A.; Alarcón Iniesta, H.; García-Ruíz, J.P. Composite scaffolds for osteochondral repair obtained by combination of additive manufacturing, leaching processes and hMSC-CM functionalization. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 59, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Hengsbach, S.; Díaz Lantada, A. Rapid prototyping of multi-scale biomedical microdevices by combining additive manufacturing technologies. Biomed. Microdevices 2014, 16, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lantada, A.; De Blas Romero, A.; Schwentenwein, M.; Jelinek, C.; Homa, J.; García-Ruíz, J.P. Monolithic 3D labs- and organs-on-chips obtained by lithography-based ceramic manufacture. Int. J. Adv. Manuf. Technol. 2017, 93, 3371–3383. [Google Scholar] [CrossRef]
- Stormonth-Darling, J.M.; Pedersen, R.H.; How, C.; Gadegaard, N. Injection moulding of ultrahigh aspect ratio nanostructures using coated polymer tooling. J. Micromech. Microeng. 2014, 24, 075019. [Google Scholar] [CrossRef]
- Al-Azawi, A.; Smistrup, K.; Kristensen, A. Nanostructuring steel for injection molding tools. J. Micromech. Microeng. 2014, 24, 055023. [Google Scholar] [CrossRef]
- Garcia-Ruiz, J.P.; Matesanz Garcia, A.I.; Perez Souza, A.; Souza Castelo, P. Thiosemicarbazone-Pt(II) complex causes a growth inhibitory effect on human mesenchymal stem cells. Med. Chem. 2015, 11, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, I.; Fazakas, C.; Krizbai, A. In vitro models of the blood-brain barrier. Acta Neurobiol. Experimentalis 2011, 71, 113–128. [Google Scholar]
- Weng, C.; Zhou, M.; Jiang, B.; Lv, H. Improvement on replication quality of electroformed nickel mold inserts with micro/nano-structures. Int. Commun. Heat Mass Transf. 2016, 75, 92–99. [Google Scholar] [CrossRef]
- Zhou, M.; Xiong, X.; Jiang, B.; Weng, C. Fabrication of high aspect ratio nano pillars and micro/nanocombined structures with hydrophobic surface characteristics by injection molding. Appl. Surf. Sci. 2018, 427, 854–860. [Google Scholar] [CrossRef]

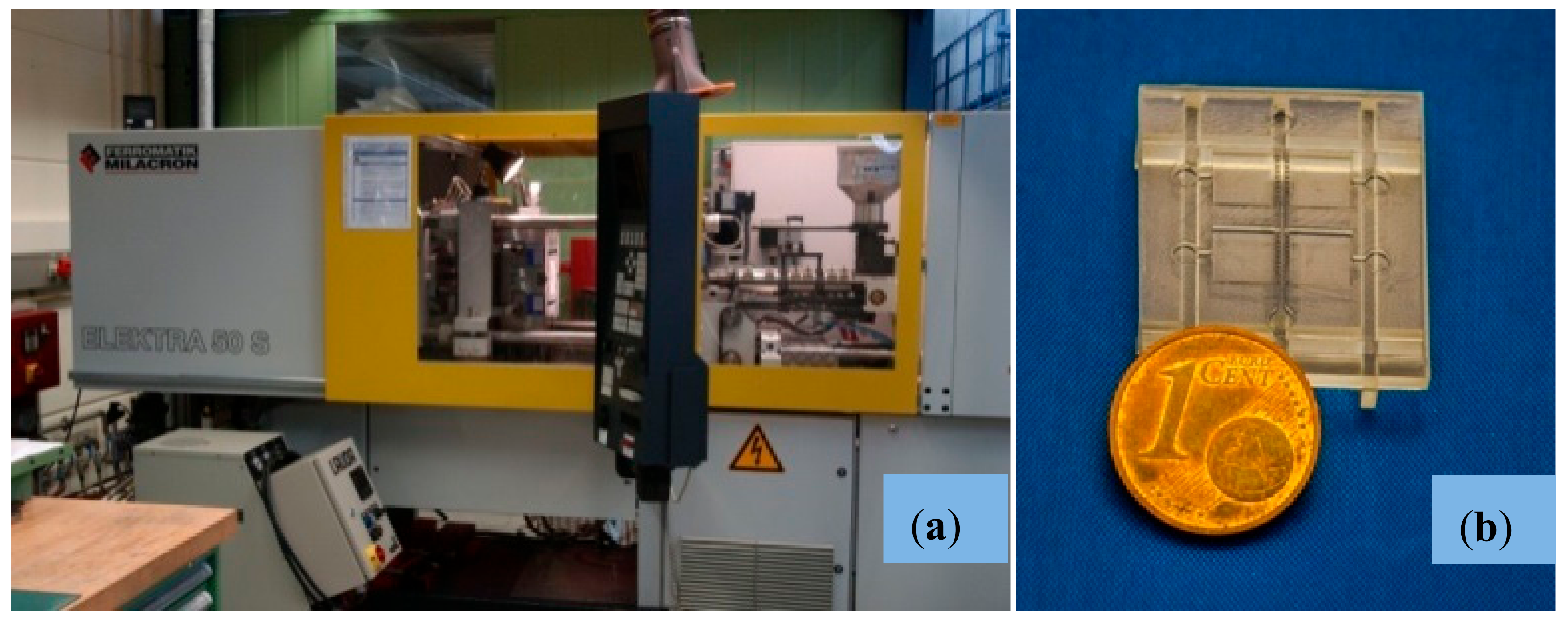
| Feature | Unit | Multi-Organ-Chip Micro-Injected Replica |
|---|---|---|
| Injection pressure | bar | 1300 |
| Injection speed | mm/s | 33 |
| Max. material temperature | °C | 290 |
| Tool temperature at injection | °C | 130 |
| Tool temperature at demolding | °C | 65 |
| Back pressure | bar | 1050 |
| Cycle time | s | ≤300 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz Lantada, A.; Pfleging, W.; Besser, H.; Guttmann, M.; Wissmann, M.; Plewa, K.; Smyrek, P.; Piotter, V.; García-Ruíz, J.P. Research on the Methods for the Mass Production of Multi-Scale Organs-On-Chips. Polymers 2018, 10, 1238. https://doi.org/10.3390/polym10111238
Díaz Lantada A, Pfleging W, Besser H, Guttmann M, Wissmann M, Plewa K, Smyrek P, Piotter V, García-Ruíz JP. Research on the Methods for the Mass Production of Multi-Scale Organs-On-Chips. Polymers. 2018; 10(11):1238. https://doi.org/10.3390/polym10111238
Chicago/Turabian StyleDíaz Lantada, Andrés, Wilhelm Pfleging, Heino Besser, Markus Guttmann, Markus Wissmann, Klaus Plewa, Peter Smyrek, Volker Piotter, and Josefa Predestinación García-Ruíz. 2018. "Research on the Methods for the Mass Production of Multi-Scale Organs-On-Chips" Polymers 10, no. 11: 1238. https://doi.org/10.3390/polym10111238
APA StyleDíaz Lantada, A., Pfleging, W., Besser, H., Guttmann, M., Wissmann, M., Plewa, K., Smyrek, P., Piotter, V., & García-Ruíz, J. P. (2018). Research on the Methods for the Mass Production of Multi-Scale Organs-On-Chips. Polymers, 10(11), 1238. https://doi.org/10.3390/polym10111238







