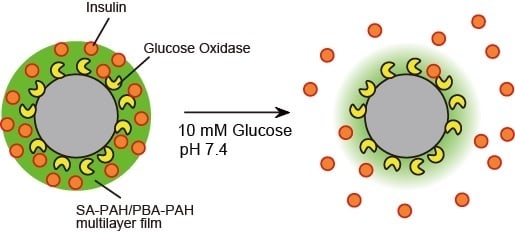
Preparation of Microparticles Capable of Glucose-Induced Insulin Release under Physiological Conditions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Apparatus
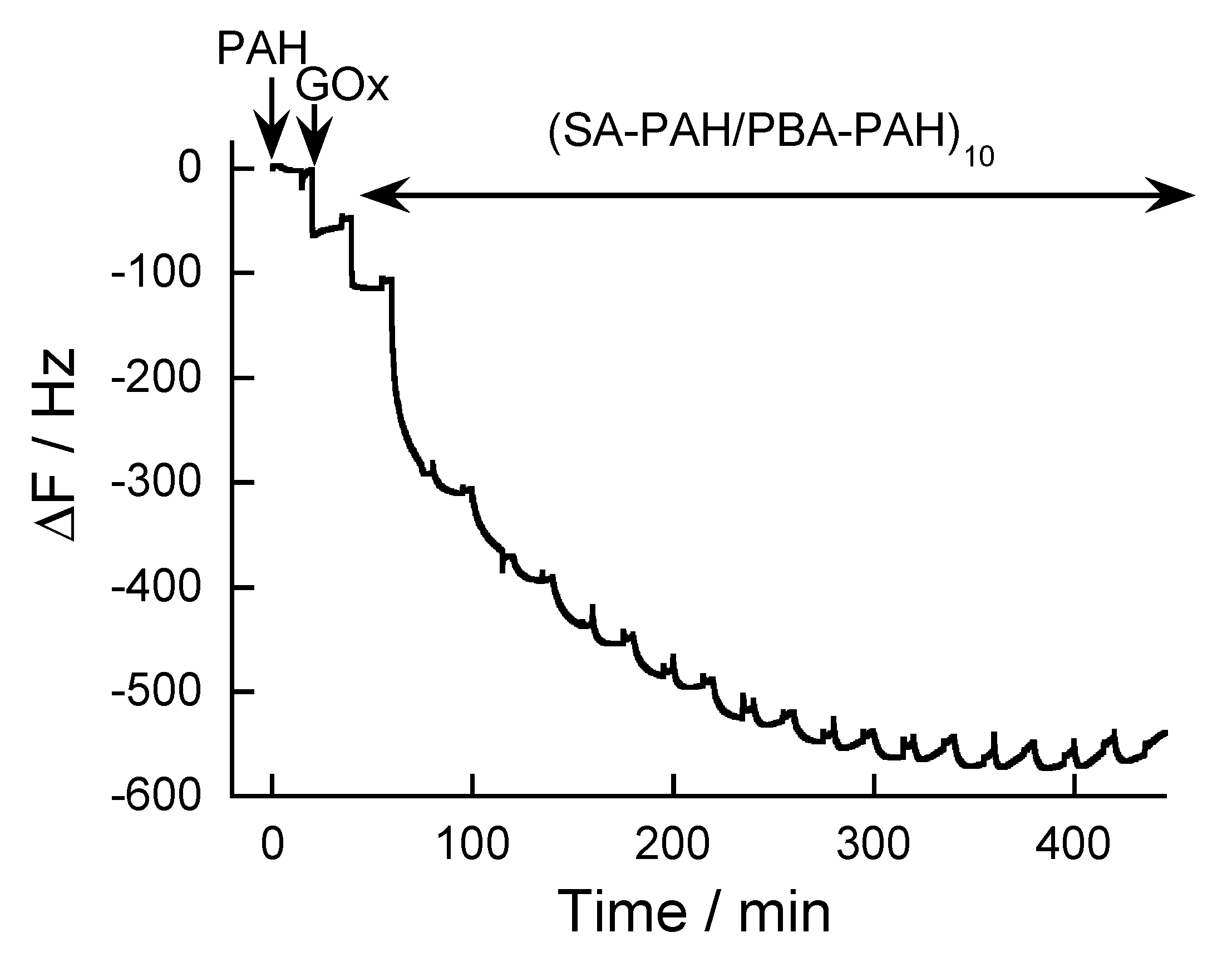
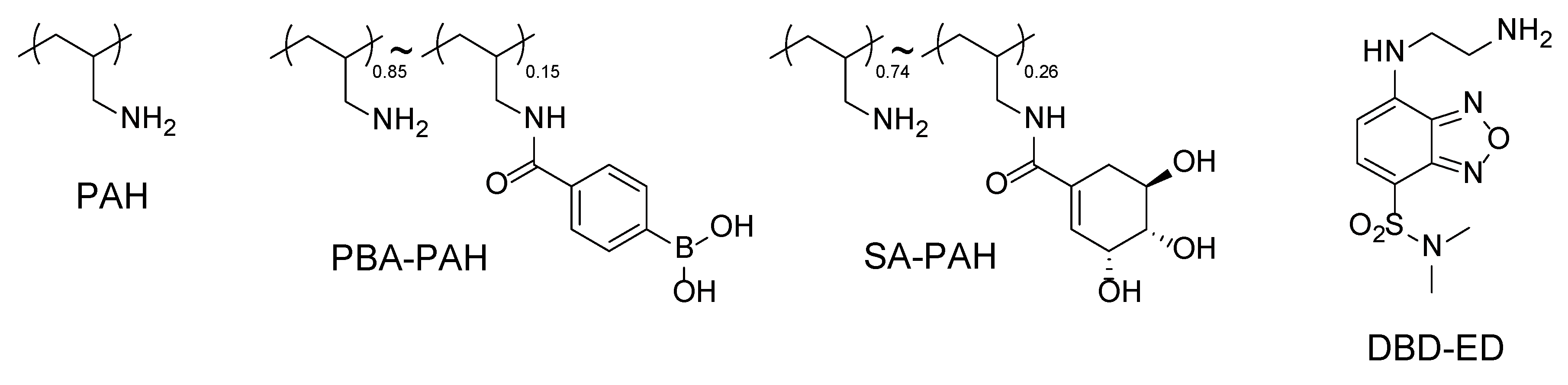
2.3. Preparation of SA-PAH/PBA-PAH Multilayer Films
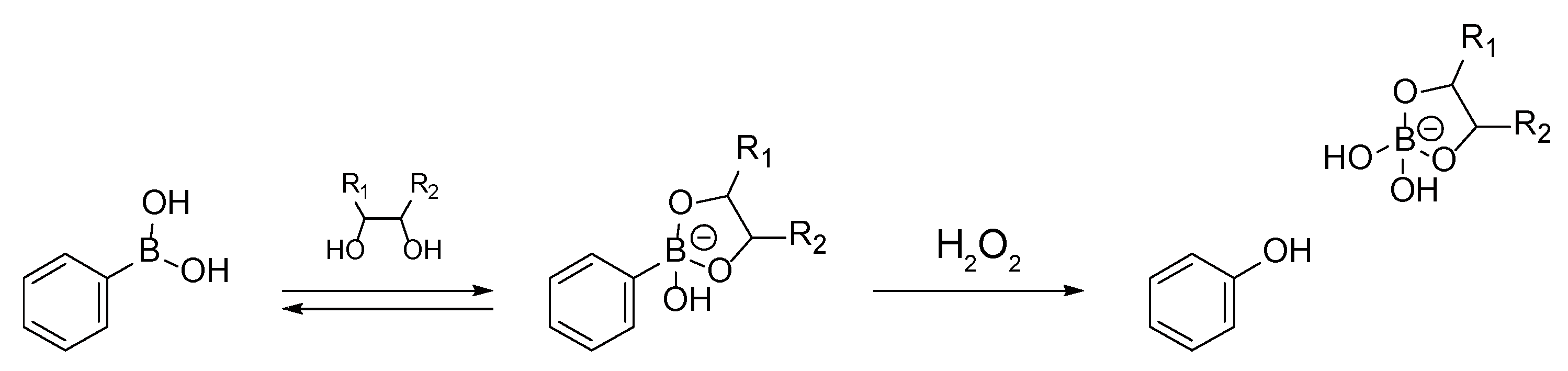
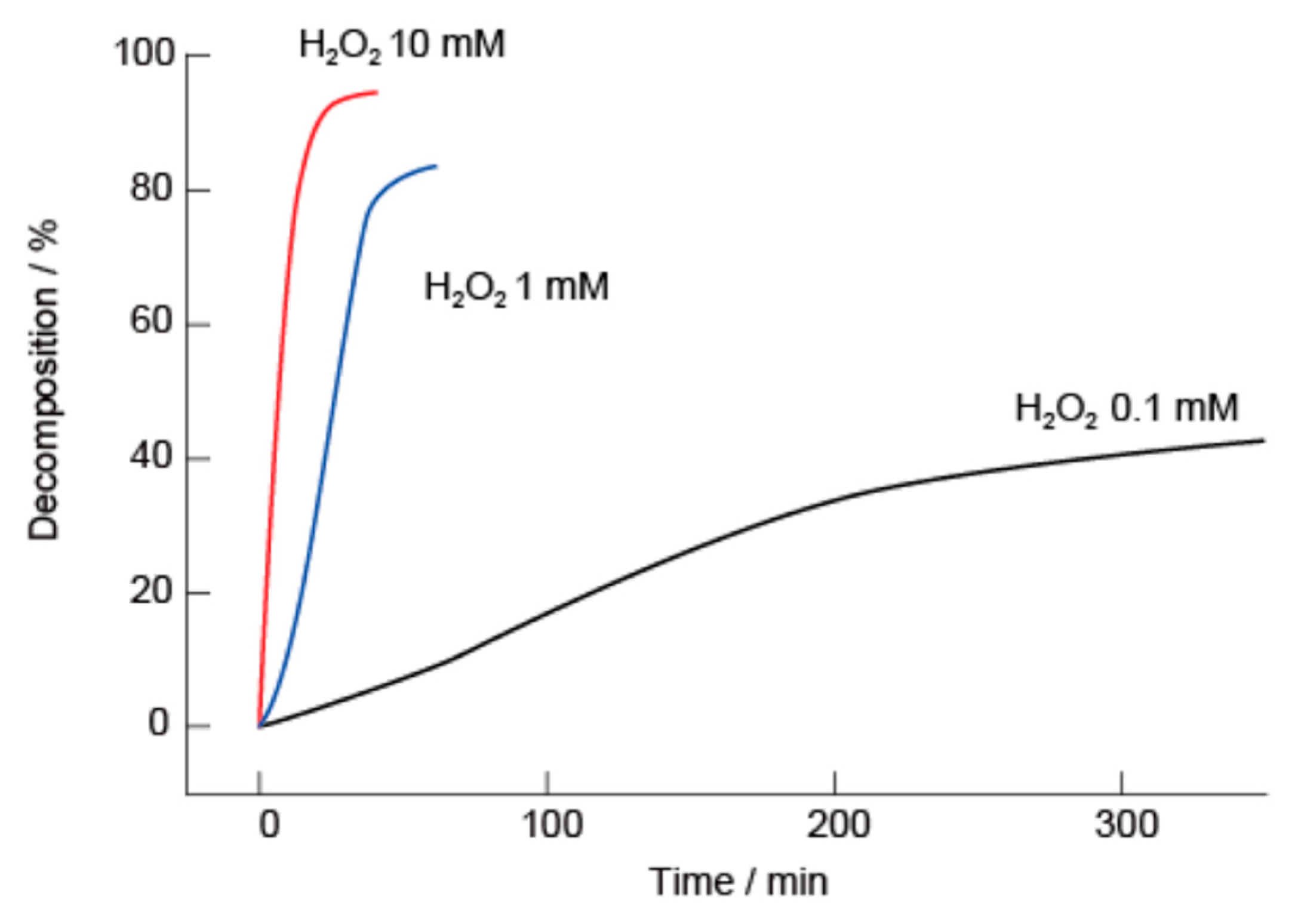
2.4. H2O2- and Glucose-Induced Decomposition of LbL Films
2.5. Preparation of Microparticles Coated with SA-PAH/PBA-PAH Multilayer Films
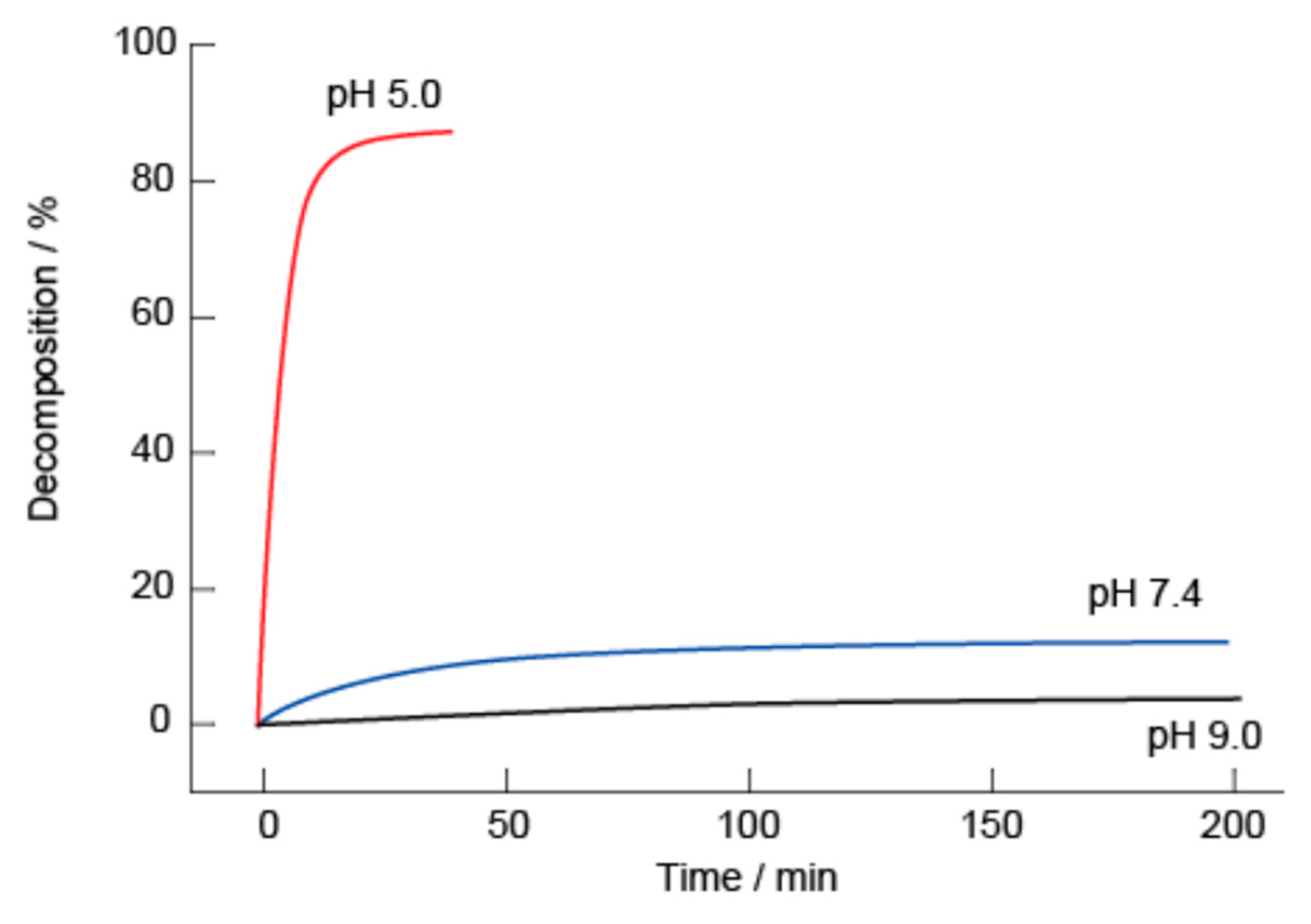
2.6. H2O2- and Glucose-Induced Decomposition of Microparticles Coated with LbL Films
3. Results and Discussion
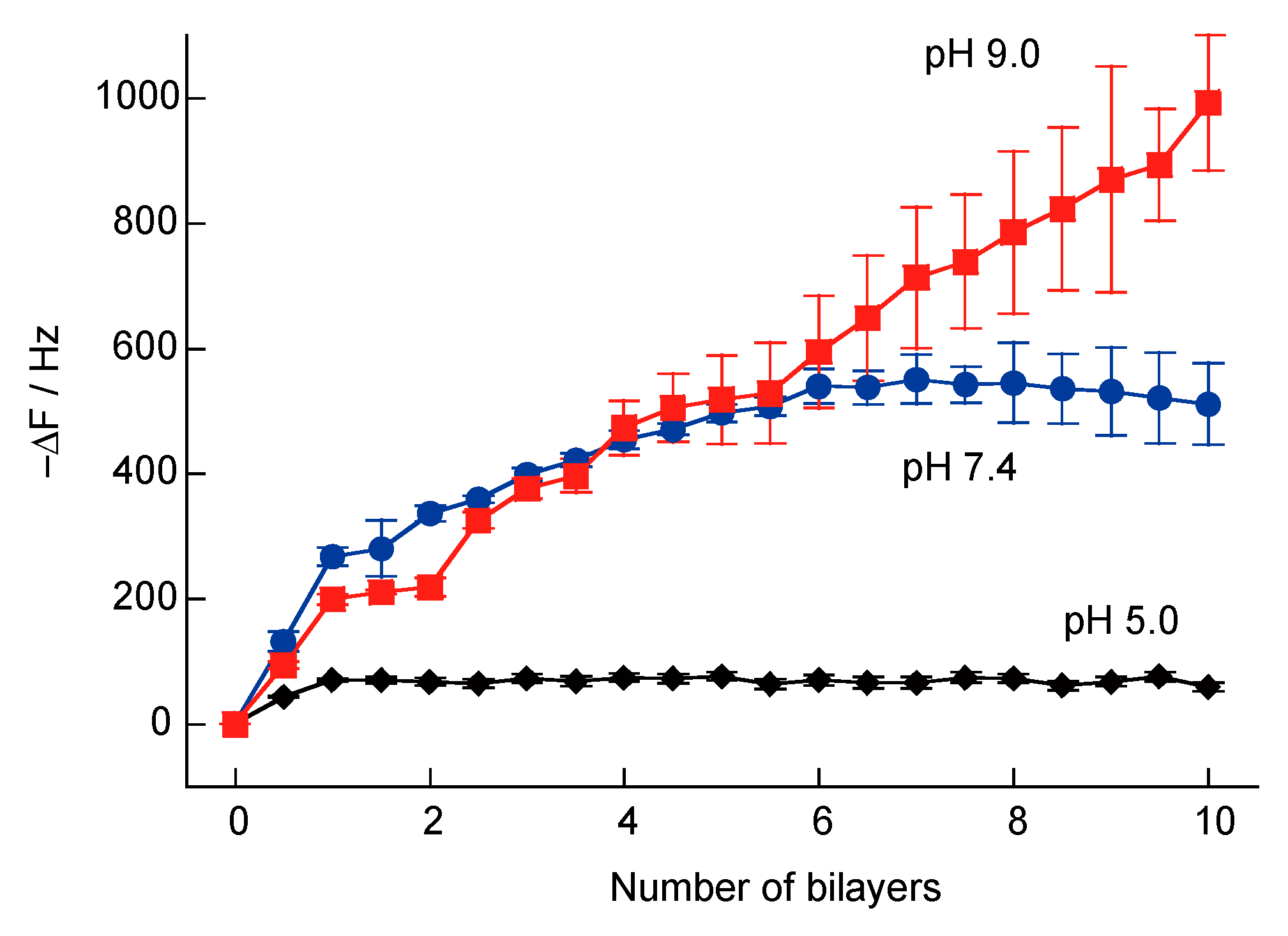
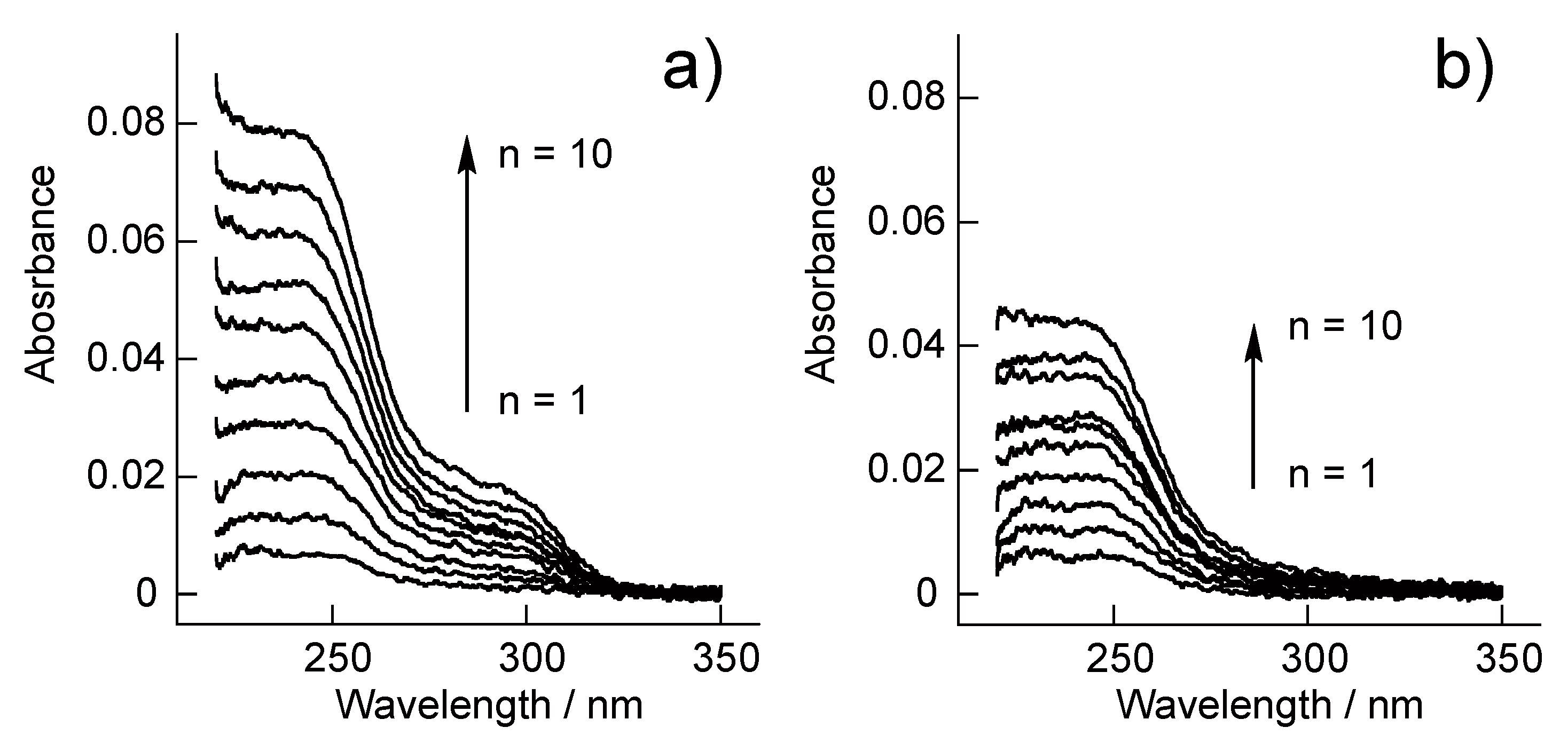
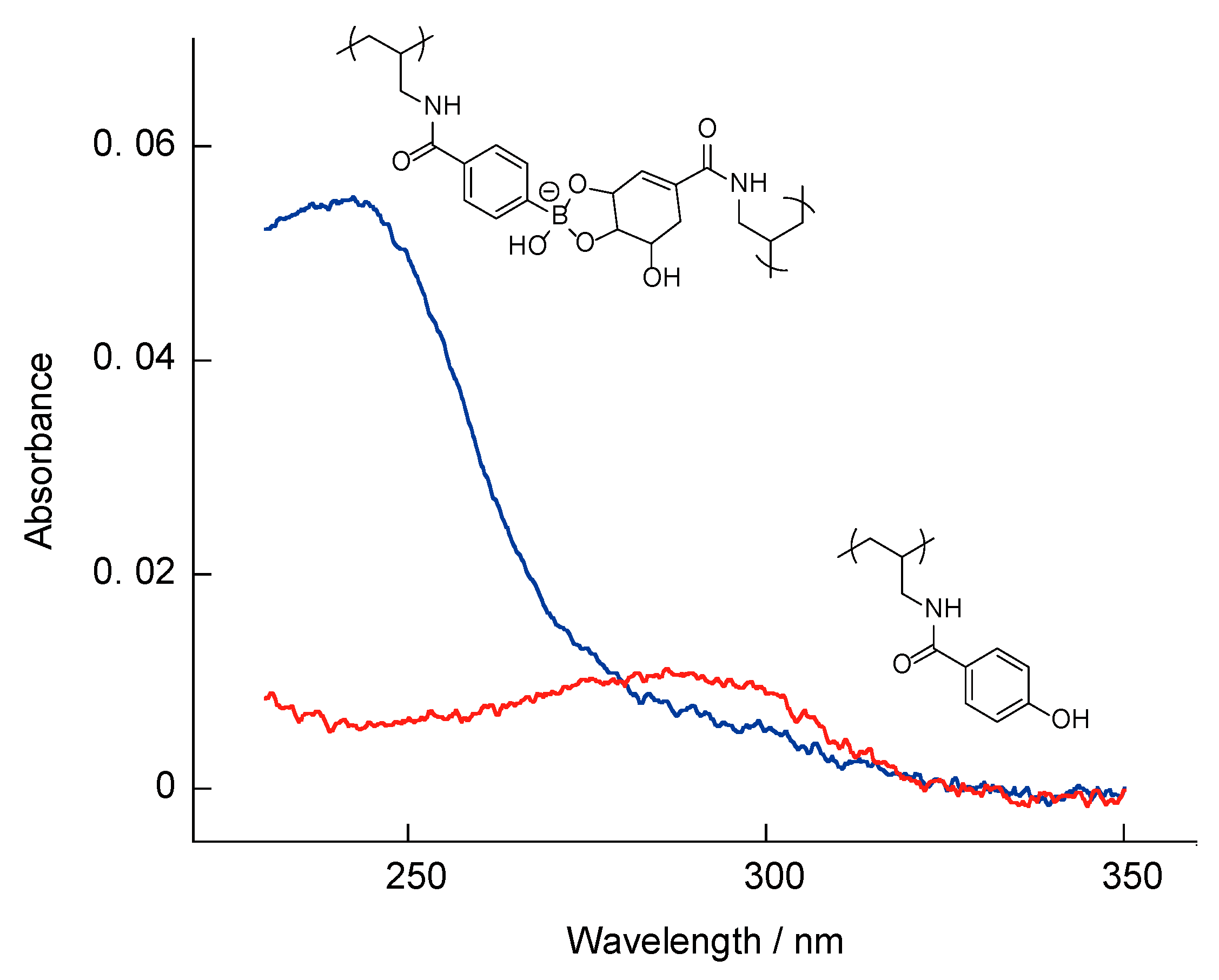
3.1. SA-PAH/PBA-PAH Multilayer Films Characterization
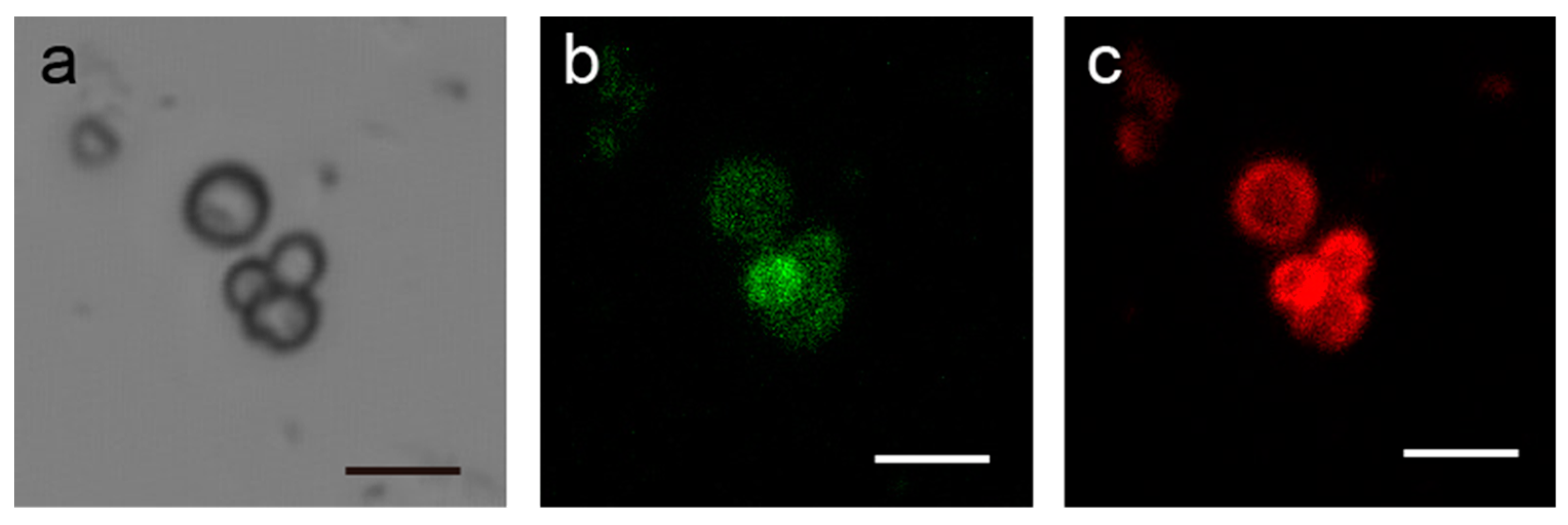
3.2. Preparation of Microparticles Coated with SA-PAH/PBA-PAH Multilayer Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Santini, J.T., Jr.; Cima, M.J.; Langer, R. A controlled-release microchip. Nature 1999, 397, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A.; et al. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano 2008, 27, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.P.; Tey, B.T.; Chan, E.S. Particle designs for the stabilization and controlled-delivery of protein drugs by biopolymers: A case study on insulin. J. Control. Release 2014, 186, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Mȍhwald, H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chem. Int. Ed. Eng. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Sundaramurthy, A.; Sundramoorthy, A.K. Polyelectrolyte capsules preloaded with interconnected alginate matrix: An effective capsule system for encapsulation and release of macromolecules. Int. J. Biol. Macromol. 2018, 107, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Hu, N. pH-Switchable bioelectrocatalysis of hydrogen peroxide on layer-by-layer films assembled by concanavalin A and horseradish peroxidase with electroactive mediator in solution. J. Phys. Chem. B 2010, 114, 9926–9933. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Yan, X.; Fei, J.; Wang, A.; Cui, Y.; Li, J. Triggered release of insulin from glucose-sensitive enzyme multilayer shells. Biomaterials 2009, 30, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, W.; Guo, Z.; Xin, J.; Li, J. Controlled insulin release from glucose-sensitive self-assembled multilayer films based on 21-arm star polymer. Biomaterials 2011, 32, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Shiino, D.; Murata, Y.; Kubo, A.; Kim, Y.J.; Kataoka, K.; Koyama, Y.; Kikuchi, A.; Yokoyama, M.; Sakurai, Y.; Okano, T. Amine containing phenylboronic acid gel for glucose-responsive insulin release under physiological pH. J. Control. Release 1995, 37, 269–276. [Google Scholar] [CrossRef]
- Mandal, D.; Mandal, S.K.; Ghosh, M.; Das, P.K. Phenylboronic acid appended pyrene-based low-molecular-weight injectable hydrogel: Glucose-stimulated insulin release. Chemistry 2015, 21, 12042–12052. [Google Scholar] [CrossRef] [PubMed]
- Takei, C.; Ohno, Y.; Seki, T.; Miki, R.; Seki, T.; Egawa, Y. Sugar-responsive layer-by-layer film composed of phenylboronic acid-appended insulin and poly(vinyl alcohol). Chem. Pharm. Bull. 2018, 66, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Das, B.P.; Tsianou, M. From polyelectrolyte complexes to polyelectrolyte multilayers: Electrostatic assembly, nanostructure, dynamics, and functional properties. Adv. Colloid Interface Sci. 2017, 244, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-based polyelectrolyte multilayers. Curr. Opin. Colloid Interface Sci. 2010, 15, 417–426. [Google Scholar] [CrossRef]
- Yoshida, K.; Sato, K.; Ono, T.; Dairaku, T.; Kashiwagi, Y. Preparation of nafion/polycation layer-by-layer films for adsorption and release of insulin. Polymers 2018, 10, 812. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Guan, Y.; Tan, S.; Xu, J.; Cheng, S.; Zhang, X. Water uptake behavior of hydrogen-bonded PVPON–PAA LBL film. Soft Matter 2006, 2, 699–704. [Google Scholar] [CrossRef]
- Sun, J.; Su, C.; Zhang, X.; Li, J.; Zhang, WB.; Zhao, N.; Xu, J.; Yang, S. Responsive complex capsules prepared with polymerization of dopamine, hydrogen-bonding assembly, and catechol dismutation. J. Colloid Interface Sci. 2018, 513, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Sato, K.; Anzai, J.-I. Disintegration of layer-by-layer assemblies composed of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Biomacromolecules 2005, 6, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Polak, R.; Crouzier, T.; Lim, R.M.; Ribbeck, K.; Beppu, M.M.; Pitombo, R.N.M.; Cohen, R.E.; Rubner, M.F. Sugar-mediated disassembly of mucin/lectin multilayers and their use as pH-Tolerant, on-demand sacrificial layers. Biomacromolecules 2014, 15, 3093–3098. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, J.; Camarada, M.B.; Venkatraman, D.; Ju, H.; Dey, R.S.; Wen, Y. One-step coelectrodeposition-assisted layer-by-layer assembly of gold nanoparticles and reduced graphene oxide and its self-healing three-dimensional nanohybrid for an ultrasensitive DNA sensor. Nanoscale 2018, 3, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Son, K.J.; Koh, W. Metal-enhanced fluorescence using silver nanoparticles-embedded polyelectrolyte multilayer films for microarray-based immunoassays. Colloid Polym. Sci. 2014, 292, 1355–1364. [Google Scholar] [CrossRef]
- Hoffman, K.; Tieke, B. Layer-by-layer assembled membranes containing hexacyclen-hexaacetic acid and polyethyleneimine N-acetic acid and their ion selective permeation behavior. J. Membr. Sci. 2009, 341, 261–267. [Google Scholar] [CrossRef]
- Huang, Z.; Li, M.; Li, N.; Tang, X.; Ouyang, Z. Antibacterial properties enhancement of layer-by-layer self-assembled nanofiltration membranes. J. Nanosci. Nanotechnol. 2018, 18, 4524–4533. [Google Scholar] [CrossRef] [PubMed]
- Ribero, C.; Borges, J.; Costa, A.M.S.; Gaspar, V.M.; Bermudez, V.Z.; Mano, J.F. Preparation of well-dispersed chitosan/alginate hollow multilayered microcapsules for enhanced cellular internalization. Molecules 2018, 23, 625. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ledin, P.A.; Iatridi, Z.; Tsitsilianis, C.; Tsukruk, V.V. Multicompartmental microcapsules with orthogonal programmable two-way sequencing of hydrophobic and hydrophilic cargo release. Angew. Chem. Int. Ed. 2016, 55, 4908–4913. [Google Scholar] [CrossRef] [PubMed]
- Liu, P. Stabilization of layer-by-layer engineered multilayered hollow microspheres. Adv. Colloid Interface Sci. 2014, 207, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ono, T.; Kashiwagi, Y.; Takahashi, S.; Sato, K.; Anzai, J.-I. pH-dependent release of insulin from layer-by-layer-deposited polyelectrolyte microcapsules. Polymers 2015, 7, 1269–1278. [Google Scholar] [CrossRef]
- Koker, S.D.; Hoogenboom, R.; De Geest, B.G. Polymeric multilayer capsules for drug delivery. Chem. Soc. Rev. 2012, 41, 2867–2884. [Google Scholar] [CrossRef] [PubMed]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deilv. Rec. 2011, 63, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Anandhakumar, S.; Gokul, P.; Raichur, A.M. Stimuli-responsive weak polyelectrolyte multilayer films: A thin film platform for self triggered multi-drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Han, J.; Wang, T.; Wei, M.; Evans, D.G.; Duan, X. Temperature-controlled electrochemical switch based on layered double hydroxide/poly(N-isopropylacrylamide) ultrathin films fabricated via layer-by-layer assembly. Langmuir 2012, 28, 9535–9542. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.A.; Gao, H.; Argun, A.A.; Matyjaszewski, K.; Hammond, P.T. All-star polymer multilayers as pH-responsive nanofilms. Macromolecules 2009, 42, 368–375. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Ji, J.; Lin, Q.; Shen, J. Construction and deconstruction of PLL/DNA multilayered films for DNA delivery: Effect of ionic strength. Colloids Surf. B Biointerfaces 2005, 46, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Xing, Y.; Gao, J.; Wang, Z.; Zhang, X. Unconventional layer-by-layer assembly of graphene multilayer films for enzyme-based glucose and maltose biosensing. Langmuir 2010, 26, 15022–15026. [Google Scholar] [CrossRef] [PubMed]
- Mawad, D.; Molino, P.; Gambhir, G.; Loche, J.M.; Officer, D.L. Electrically induced disassembly of electroactive multilayer films fabricated from water soluble polythiophenes. Adv. Funct. Mater. 2012, 22, 5020–5027. [Google Scholar] [CrossRef]
- Seno, M.; Yoshida, K.; Sato, K.; Anzai, J.-I. pH- and sugar-sensitive multilayer films composed of phenylboronic acid (PBA)-modified poly(allylamine hydrochloride) (PBA-PAH) and poly(vinyl alcohol) (PVA): A signigicant effect of PBA content on the film stability. Mater. Sci. Eng. C 2016, 62, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid-diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Egawa, Y.; Miki, R.; Seki, T. Colorimetric sugar sensing using boronic acid-substituted azobenzenes. Materials 2014, 7, 1201–1220. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.D.; Davidson, M.G.; van den Elsen, J.M.H.; Fossey, J.S.; Jenkins, A.T.A.; Jiang, Y.; Kubo, Y.; Marken, F.; Sakurai, K.; Zhao, J.; et al. Exploiting the reversible covalent bonding of boronic acids: Recognition, sensing, and assembly. Acc. Chem. 2013, 46, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Guan, Y.; Zhang, Y.; Zhu, X.X. Layer-by-layer multilayer films linked with reversible boronate ester bonds with glucose-sensitivity under physiological conditions. Soft Matter 2009, 5, 2302–2309. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, S.; Anzai, J.-I. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Salzbrunn, S.; Prakash, G.K.S.; Petasis, N.A.; Olah, G.A. Regioselective conversion of arylboronic acids to phenols and subsequent coupling to symmetrical diaryl ethers. J. Org. Chem. 2001, 66, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takahashi, M.; Ito, M.; Abe, E.; Anzai, J.-I. H2O2-induced decomposition of layer-by-layer films consisting of phenylboronic acid-bearing poly(allylamine) and poly(vinyl alcohol). Langmuir 2014, 30, 9247–9250. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takahashi, M.; Ito, M.; Abe, E.; Anzai, J.-I. Glucose-induced decomposition of layer-by-layer films composed of phenylboronic acid-bearing poly(allylamine) and poly(vinyl alcohol) under physiological conditions. J. Mater. Chem. B 2015, 3, 7796–7802. [Google Scholar] [CrossRef]
- Tong, W.; Dong, W.; Ga, C.; Mȍhwald, H. Multilayer capsules with cell-like topology: Fabrication and spontaneous loading of various substances in aqueous and ethanol solutions. Macromol. Chem. Phys. 2005, 206, 1784–1790. [Google Scholar] [CrossRef]
- Kolasińska, M.; Warszyński, P. The effect of support material and conditioning on wettability of PAH/PSS multilayer films. Bioelectrochemistry 2005, 66, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Suwa, K.; Nagasaka, M.; Niina, S.; Egawa, Y.; Seki, T.; Anzai, J.-I. Sugar response of layer-by-layer films composed of poly(vinyl alcohol) and poly(amidoamine) dendrimer bearing 4-carboxyphenylboronic acid. Colloid Polym. Sci. 2015, 293, 1043–1048. [Google Scholar] [CrossRef]
- Sato, K.; Abe, E.; Takahashi, M.; Anzai, J.-I. Loading and release of fluorescent dye from layer-by-layer film-coated magnetic particles in response to hydrogen peroxide. J. Colloid Interface Sci. 2014, 432, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Shi, K.; Zhang, L.; Tao, A. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: Preparation, in vitro characterization and in vivo evaluation. J. Control. Release 2006, 114, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hashide, R.; Ishii, T.; Takahashi, S.; Sato, K.; Anzai, J.-I. Layer-by-layer films composed of poly(allylamine) and insulin for pH-triggered release of insulin. Colloid Surf. B 2012, 91, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Hashide, R.; Yoshida, K.; Hasebe, Y.; Takahashi, S.; Sato, K.; Anzai, J.-I. Insulin-containing layer-by-layer films deposited on poly(lactic acid) microbeads for pH-controlled release of insulin. Colloid Surf. B 2012, 89, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yoshida, K.; Sato, K.; Anzai, J.-I. Phenylboronic acid-finctionalized layer-by-layer assemblies for biomedical applications. Polymers 2017, 9, 202. [Google Scholar] [CrossRef]



| Glucose | pH 7.4 | pH 7.4 | pH 9.0 |
|---|---|---|---|
| NaCl 150 mM | NaCl 1 M | NaCl 1 M | |
| 1 mM | 16.9 Hz | −2.9 Hz | −18.9 Hz |
| 10 mM | 34.6 Hz | −8.6 Hz | −25.8 Hz |
| 100 mM | 43.8 Hz | 31.7 Hz | −26.8 Hz |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Awaji, K.; Shimizu, S.; Iwasaki, M.; Oide, Y.; Ito, M.; Dairaku, T.; Ono, T.; Kashiwagi, Y.; Sato, K. Preparation of Microparticles Capable of Glucose-Induced Insulin Release under Physiological Conditions. Polymers 2018, 10, 1164. https://doi.org/10.3390/polym10101164
Yoshida K, Awaji K, Shimizu S, Iwasaki M, Oide Y, Ito M, Dairaku T, Ono T, Kashiwagi Y, Sato K. Preparation of Microparticles Capable of Glucose-Induced Insulin Release under Physiological Conditions. Polymers. 2018; 10(10):1164. https://doi.org/10.3390/polym10101164
Chicago/Turabian StyleYoshida, Kentaro, Kazuma Awaji, Seira Shimizu, Miku Iwasaki, Yuki Oide, Megumi Ito, Takenori Dairaku, Tetsuya Ono, Yoshitomo Kashiwagi, and Katsuhiko Sato. 2018. "Preparation of Microparticles Capable of Glucose-Induced Insulin Release under Physiological Conditions" Polymers 10, no. 10: 1164. https://doi.org/10.3390/polym10101164
APA StyleYoshida, K., Awaji, K., Shimizu, S., Iwasaki, M., Oide, Y., Ito, M., Dairaku, T., Ono, T., Kashiwagi, Y., & Sato, K. (2018). Preparation of Microparticles Capable of Glucose-Induced Insulin Release under Physiological Conditions. Polymers, 10(10), 1164. https://doi.org/10.3390/polym10101164