Use of Orange Oil Loaded Pectin Films as Antibacterial Material for Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mango Pectin
2.3. Identification of Major Compounds of Orange Oil
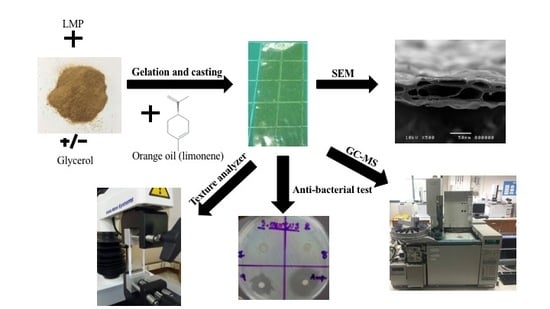
2.4. Preparation of Films Loaded with Orange Oil
2.5. Characterization of Films Loaded with Orange Oil
2.6. Limonene Loading Content
2.7. Film Sterility Test
2.8. Anti-Bacterial Activity of Films Loaded with Orange Oil
2.9. Statistical Analysis
3. Results and Discussion
3.1. Identification of Orange Oil
3.2. Morphology and Mechanical Properties of Film Loaded with Orange Oil
3.3. Limonene Oil Loading Content
3.4. Film Sterility and Anti-Bacterial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vermeiren, L.; Devlieghere, F.; Beest, M.V.; Kruijf, N.D.; Debevere, J. Developments in the active packaging of foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Perez-Perez, C.; Regalado-Gonzalez, C.; Rodriguez-Rodriguez, C.A.; Barbosa-Rodriguez, J.R.; Villasenor-Ortega, F. Incorporation of antimicrobial agents in food packaging films and coatings. In Advances in Agricultural and Food Biotechnology; Research Signpost: Kerala, India, 2006; pp. 193–216. ISBN 8177362690. [Google Scholar]
- Pedroza-Islas, R.; Aguilar-Esperanza, E.; Vernon-Carter, E.J. Obtaining pectins from solids wastes derived from mango (Mangifera indica) processing. AIChE Symp. Ser. 1994, 90, 38–41. [Google Scholar]
- Tandon, D.K.; Garg, N. Mango waste: A potential source of pectin, fiber, and starch. Indian J. Environ. Prot. 1999, 19, 1924–1927. [Google Scholar]
- Müller-Maatsch, J.; Bencivenni, M.; Caligiani, A.; Tedeschi, T.; Bruggeman, G.; Bosch, M.; Petrusan, J.; Van Droogenbroeck, B.; Elst, K.; Sforza, S. Pectin content and composition from different food waste stream. Food Chem. 2016, 15, 37–45. [Google Scholar]
- Magwa, M.L.; Gundidza, M.; Gweru, N.; Humphrey, G. Chemical composition and biological activities of essential oil from the leaves of Sesuvium portulacastrum. J. Ethnopharmacol. 2006, 103, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Matiz, G.; Osorio, M.R.; Camacho, F.; Atencia, M.; Herazo, J. Effectiveness of antimicrobial formulations for acne based on orange (Citrus sinensis) and sweet basil (Ocimum basilicum L) essential oils. Biomedica 2012, 32, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Vimal, M.; Vijaya, P.P.; Mumtaj, P.; Farhath Seema, M.S. Antibacterial activity of selected compounds of essential oils from indigenous plants. J. Chem. Pharm. Res. 2013, 5, 248–253. [Google Scholar]
- Orchard, A.; Vuuren vann, S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid. Based Complement. Alternat. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Chana-Thaworn, J.; Chanthachum, S.; Wittaya, T. Properties and antimicrobial activity of edible films incorporated with kiam wood (Cotyleobium lanceotatum) extract. LWT Food Sci. Technol. 2011, 44, 284–292. [Google Scholar] [CrossRef]
- Assefa, Z.; Admassu, S. Development and characterization of antimicrobial packaging films. J. Food Process. Technol. 2013, 4, 235–241. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food. Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Jantrawut, P.; Chaiwarit, T.; Jantanasakulwong, K.; Brachais, H.C.; Chambin, O. Effect of plasticizer type on tensile property and in vitro indomethacin release of thin films based on low-methoxyl pectin. Polymers 2017, 9, 289–303. [Google Scholar] [CrossRef]
- Jantrawut, P.; Boonsermsukcharoen, K.; Thipnan, K.; Chaiwarit, T.; Hwang, K.M.; Park, E.S. Enhancement of antibacterial activity of orange oil in pectin thin film by microemulsion. Nanomaterials 2018, 8, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, H.S.; Deshmukh, S.R. Development and evaluation of gel-forming ocular films based on xyloglucan. Carbohydr. Polym. 2015, 122, 243–247. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention. Sterlility Test/Microbiological Test. In The United State Pharmacopeia, 40th ed.; United Book Press: Gwynn Oak, MD, USA, 2017; Volume 1, pp. 137–142. [Google Scholar]
- Lee, T.W.; Kim, J.C.; Hwang, S.J. Hydrogel patches containing triclosan for acne treatment. Eur. J. Pharm. Biopharm. 2003, 56, 407–412. [Google Scholar] [CrossRef]
- Högnadóttir, Á.; Rouseff, R.L. Identification of aroma active compounds in orange essence oil using gas chromatography–olfactometry and gas chromatography–mass spectrometry. J. Chromatogr. A 2003, 998, 201–211. [Google Scholar] [CrossRef]
- Qiao, Y.; Xie, B.J.; Zhang, Y.; Zhang, Y.; Fan, G.; Yao, X.L.; Pan, S.Y. Characterization of aroma active compounds in fruit juice and peel oil of Jinchen sweet orange fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O. Molecules 2008, 13, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.G.; Liu, Y.J.; Zhang, J.H.; Zeng, H.Y.; Tang, Y.F.; Zhang, M.L. Chemical composition of essential oil from the peel of Satsuma mandarin. Afr. J. Biotechnol. 2008, 7, 1261–1264. [Google Scholar]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and anti-bacterial properties. Food Hydrocoll. 2014, 36, 9–19. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Nunes, C.; Castro, A.; Ferreira, P.; Coimbra, M.A. Influence of grape pomace extract incorporation on chitosan films properties. Carbohydr. Polym. 2014, 113, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and anti-bacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]

| Compound | GC-MS RT (min) | Peak Area (%) |
|---|---|---|
| 2-Thujene | 3.623 | 0.46 |
| 3-p-Menthene | 3.708 | 0.06 |
| β-Myrcene | 3.874 | 1.06 |
| Octanal | 4.097 | 0.21 |
| 3-Carene | 4.309 | 0.06 |
| Limonene | 4.801 | 84.57 |
| 1,2-Dimethylcyclobutane | 5.597 | 0.13 |
| Linaolool oxide | 5.705 | 0.08 |
| Linalool | 6.375 | 0.77 |
| cis-Limonene oxide | 7.313 | 1.86 |
| trans-Limonene oxide | 7.433 | 0.88 |
| α-Terpineol | 8.990 | 0.25 |
| Decanol | 9.396 | 0.33 |
| Carveol | 9.842 | 0.67 |
| Carvone | 10.592 | 0.76 |
| Farnesol | 13.350 | 0.49 |
| 9,11-Dodecadien-1-ol | 15.719 | 0.10 |
| 1,5-Cyclooctadiene, 1,5-dimethyl | 16.125 | 0.06 |
| Valencene | 18.225 | 0.10 |
| Film Code | Composition | Thickness (μm) | |||
|---|---|---|---|---|---|
| LMP (% w/v of Pectin Solution) | Mango Peel Pectin (% w/v of Pectin Solution) | Glycerol (% w/w Based on Pectin Weight) | Orange Oil (% w/w of Pectin Solution) | ||
| P3M0O | 3 | 0 | - | 3 | 52.94 ± 0.21 |
| P1M2O | 1 | 2 | - | 3 | 58.82 ± 0.23 |
| P1M2GO | 1 | 2 | 40 | 3 | 70.58 ± 0.18 |
| P1M2 | 1 | 2 | - | - | 25.71 ± 0.11 |
| Film Code | Orange Oil Loading (%) | Tensile Strength (MPa) | Elongation (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| P3M0O | 86.17 ± 3.41 a | 6.12 ± 1.18 a | 2.28 ± 0.15 a | 266.04 ± 35.39 a |
| P1M2O | 70.10 ± 1.03 a | 3.12 ± 1.23 b | 2.57 ± 0.10 a | 126.91 ± 42.32 b |
| P1M2GO | 59.25 ± 2.09 b | 4.54 ± 0.18 c | 12.93 ± 0.89 b | 35.24 ± 3.43 c |
| P1M2 | - | 4.98 ± 0.54 c | 6.15 ± 0.88 c | 145.34 ± 31.77 b |
| Sample | Clear Zone Diameter (mm) | Normalized clear Zone (mm) |
|---|---|---|
| P3M0G film | ND | ND |
| P3M0O film | 10.02 ± 0.03 | 10.02 |
| P1M2O film | 8.76 ± 0.01 | 10.77 |
| P1M2GO film | 8.08 ± 0.01 | 11.75 |
| P1M2 film | ND | - |
| Orange oil (100 mg/disc) | 9.64 ± 0.01 | - |
| Tween® 80 | ND | - |
| Ampicillin (6.25 μg/disc) | 9.82 ± 0.01 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiwarit, T.; Ruksiriwanich, W.; Jantanasakulwong, K.; Jantrawut, P. Use of Orange Oil Loaded Pectin Films as Antibacterial Material for Food Packaging. Polymers 2018, 10, 1144. https://doi.org/10.3390/polym10101144
Chaiwarit T, Ruksiriwanich W, Jantanasakulwong K, Jantrawut P. Use of Orange Oil Loaded Pectin Films as Antibacterial Material for Food Packaging. Polymers. 2018; 10(10):1144. https://doi.org/10.3390/polym10101144
Chicago/Turabian StyleChaiwarit, Tanpong, Warintorn Ruksiriwanich, Kittisak Jantanasakulwong, and Pensak Jantrawut. 2018. "Use of Orange Oil Loaded Pectin Films as Antibacterial Material for Food Packaging" Polymers 10, no. 10: 1144. https://doi.org/10.3390/polym10101144
APA StyleChaiwarit, T., Ruksiriwanich, W., Jantanasakulwong, K., & Jantrawut, P. (2018). Use of Orange Oil Loaded Pectin Films as Antibacterial Material for Food Packaging. Polymers, 10(10), 1144. https://doi.org/10.3390/polym10101144