Enhancement of Plasticizing Effect on Bio-Based Polyurethane Acrylate Solid Polymer Electrolyte and Its Properties
Abstract
1. Introduction
2. Experimental
2.1. Material
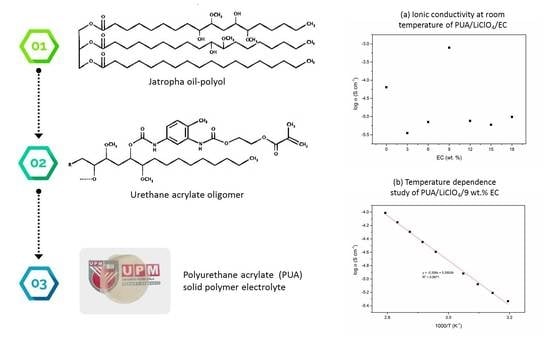
2.2. Preparation of PUA Oligomer
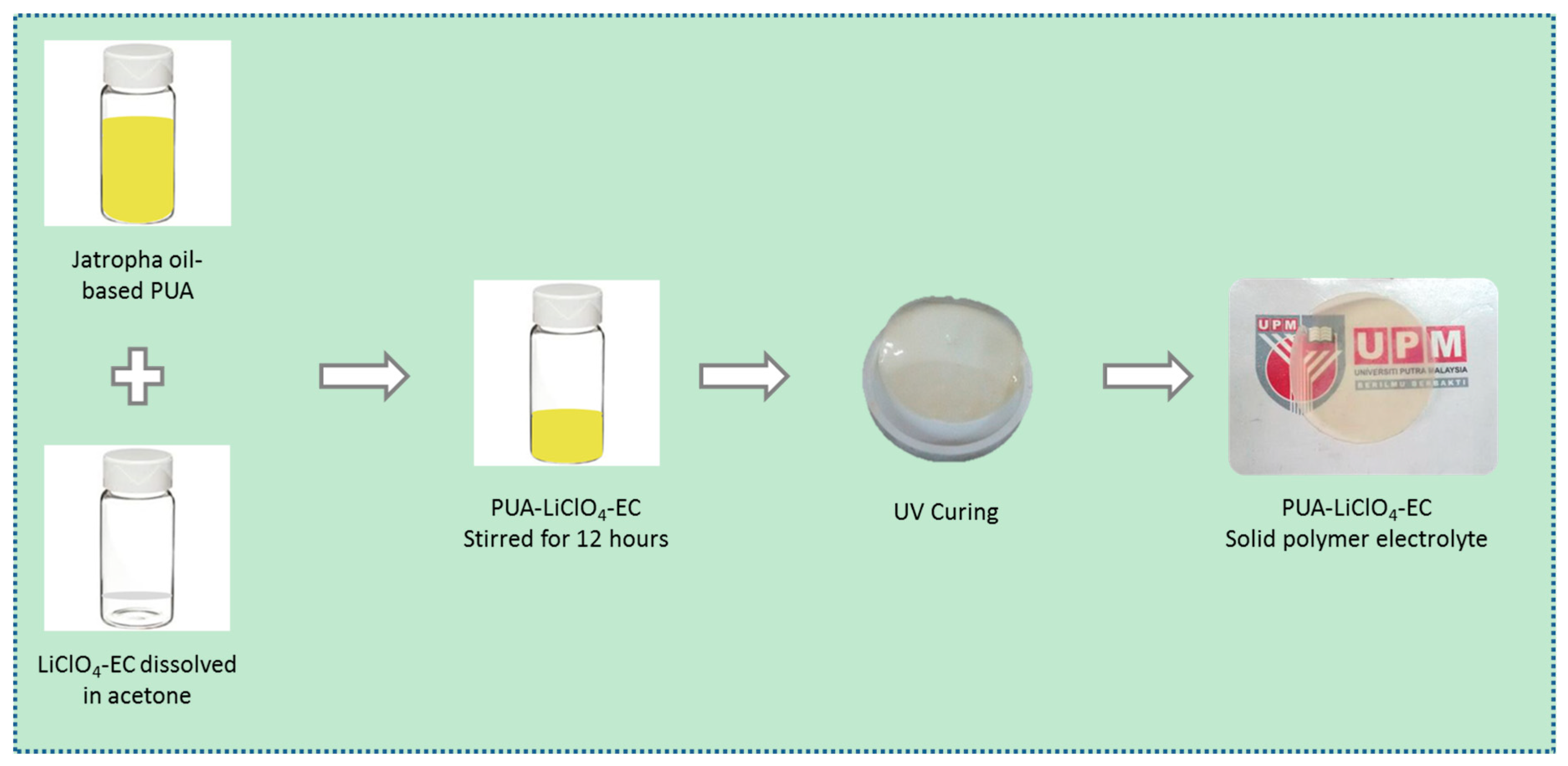
2.3. Preparation of PUA Film Electrolyte
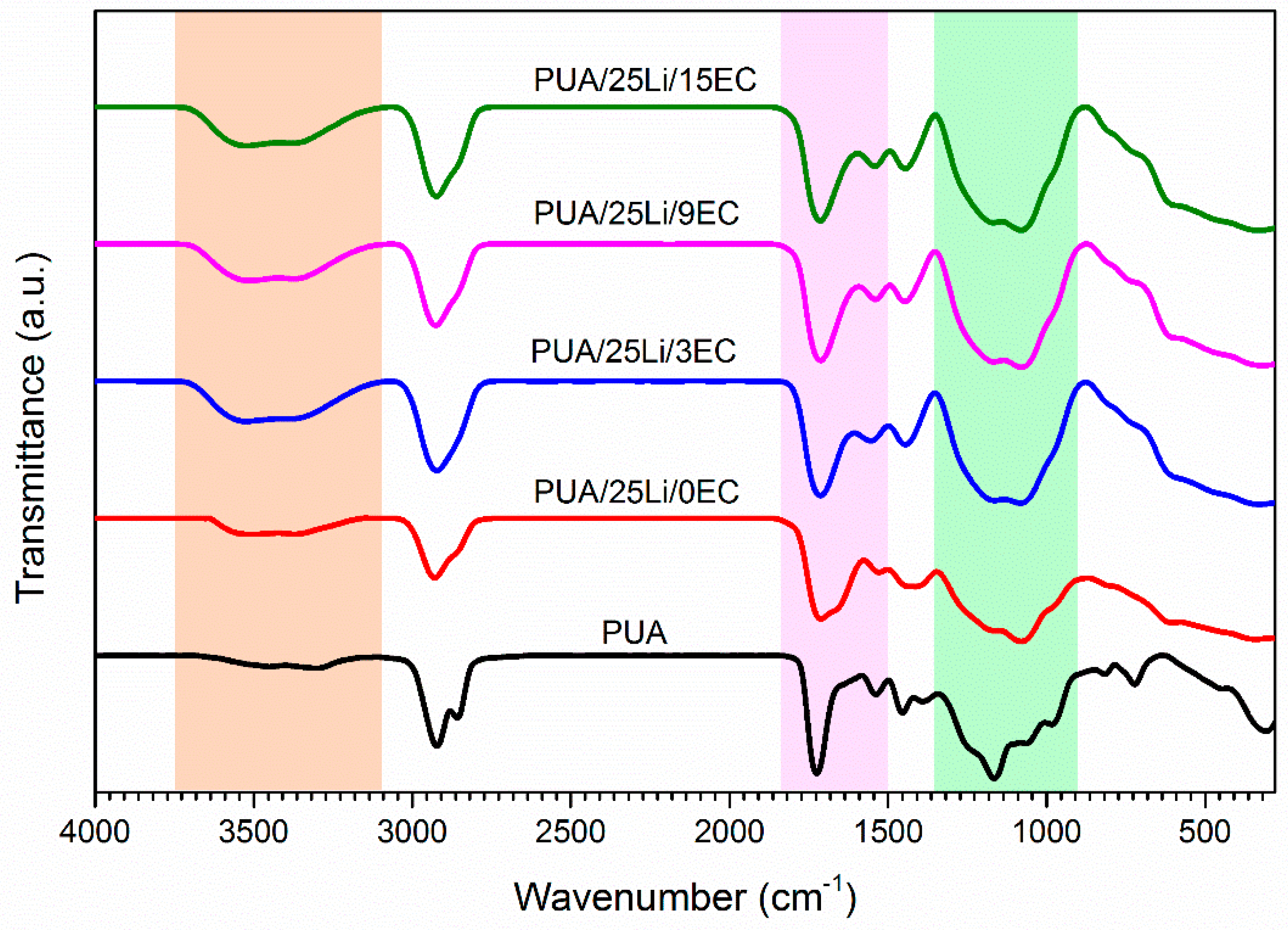
2.4. Characterization of Solid Polymer Electrolyte
Electrochemical Study
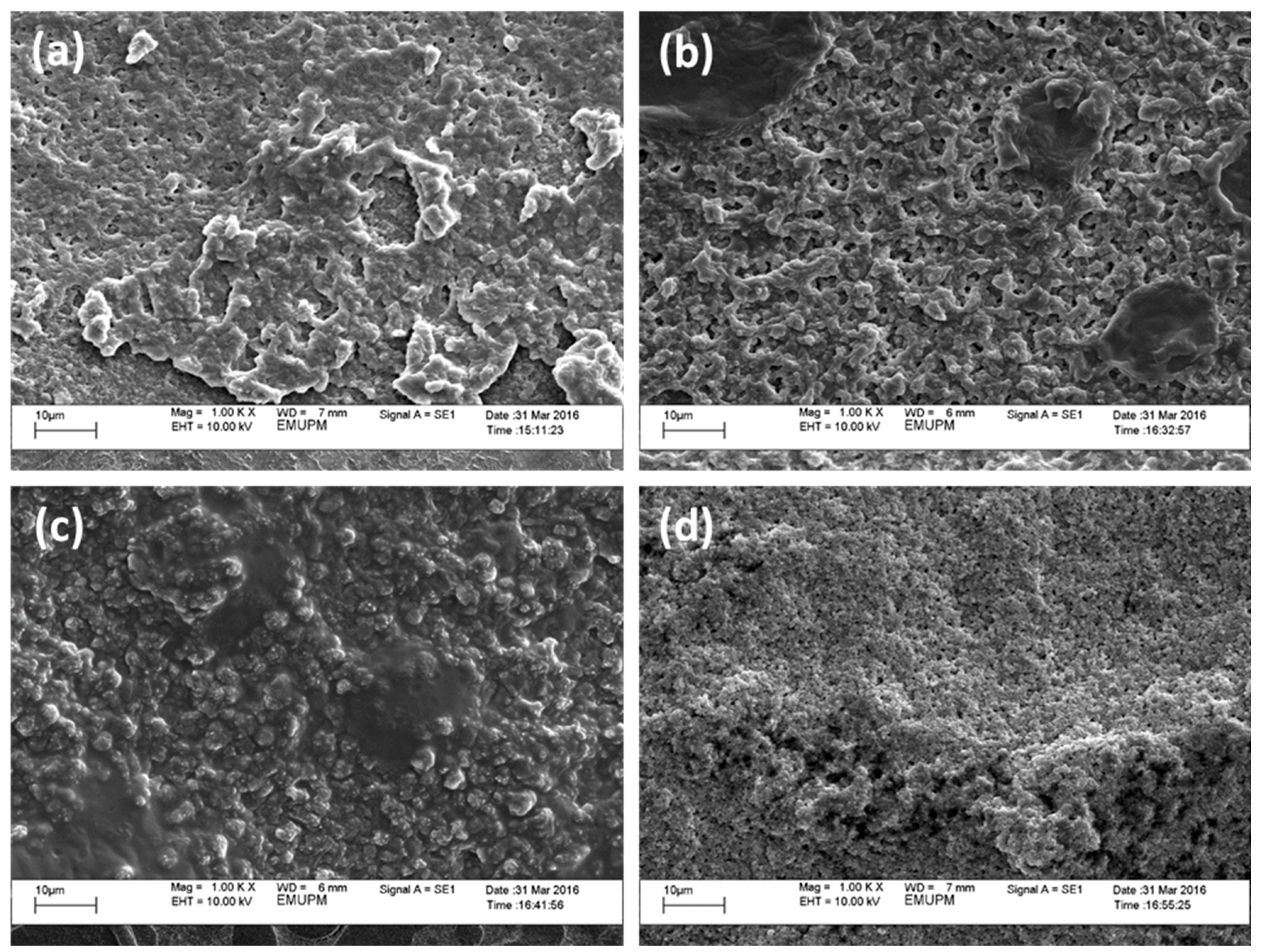
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pradhan, D.K.; Choudhary, R.N.P.; Samantaray, B.K. Studies of dielectric relaxation and AC conductivity behavior of plasticized polymer nanocomposite electrolytes. Int. J. Electrochem. Sci. 2008, 3, 597–608. [Google Scholar]
- Huang, J.; Sun, J.; Zhang, R.; Zou, R.; Yuan, T. Improvement of biodegradability of UV-curable adhesives modified by a novel polyurethane acrylate. Prog. Organ. Coat. 2016, 95, 20–25. [Google Scholar] [CrossRef]
- Zuber, M.; Shah, S.A.A.; Jamil, T.; Asghar, M.I. Performance behaviour of modified cellulosic fabrics using polyurethane acrylate copolymer. Int. J. Biol. Macromol. 2014, 67, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, F.; Xu, B.; Xu, J.; Li, P. Preparation, mechanical properties and surface morphologies of waterborn fluorinated polyurethane acrylate. Prog. Organ. Coat. 2013, 76, 876–883. [Google Scholar] [CrossRef]
- Yaobin, R.; Huiming, P.; Longsi, L.; Jianming, X.; Yongqiang, Y. Synthesis of poylurethane acrylate and application to UV-curable pressure-sensitive adhesive. Des. Monomers Polym. 2005, 8, 383–396. [Google Scholar]
- Hu, Y.; Shang, Q.; Tang, J.; Wang, C.; Zhou, Y. Use of cardanol-based acrylate as reactive diluent in UV –curable castor oil-based polyurethane acrylate resin. Ind. Crops Prod. 2018, 117, 295–302. [Google Scholar] [CrossRef]
- Liu, B.; Nie, J.; He, Y. From rosin to high adhesive polyurethane acrylate: Synthesis and properties. Int. J. Adhes. Adhes. 2016, 66, 99–103. [Google Scholar] [CrossRef]
- Chen, G.; Guan, X.; Xu, R.; Tian, J.; Yang, J. Synthesis and characterization of UV curable castor oil-based polyfunctional polyurethane acrylate via photo-click chemistry and isocyanate polyurethane reaction. Prog. Organ. Coat. 2016, 93, 11–16. [Google Scholar] [CrossRef]
- Li, K.; Shen, Y.; Fei, G.; Wang, H.; Li, J. Preparation and properties of castor oil/ pentaerythritol triacrylate-baed UV curable waterborne poylurethane acrylate. Prog. Organ. Coat. 2015, 78, 146–154. [Google Scholar] [CrossRef]
- Deepak, M.P.; Ganesh, A.P.; Mhaske, S.T. Design and synthesis of bio-based UV curable PU acrylate resin from itaconic acid for coating applications. Des. Monomers Polym. 2017, 20, 269–282. [Google Scholar]
- Barbeau, P.H.; Gerard, J.F.; Magny, B.; Pascault, J.P. Effect of the diisocyanate on the structure and properties of polyurethane acrylate prepolymers. J. Polym. Sci. 2000, 38, 2750–2768. [Google Scholar] [CrossRef]
- Asif, A.; Huang, C.; Shi, W. Structure-property study of waterborne, polyurethane acrylate dispersions based on hyperbranched aliphatic polyester for UV-curable coatings. Colloid Polym. Sci. 2004, 283, 200–208. [Google Scholar] [CrossRef]
- Yap, Y.L.; You, A.H.; Teo, L.L.; Hanapei, H. Inorganic filler sizes effect on ionic conductivity in polyethylene (PEO) composite polymer electrolyte. Int. J. Electrochem. Sci. 2013, 8, 2154–2163. [Google Scholar]
- Wang, W.; Yi, E.; Anthony, J.F.; Richard, M.L.; John, K. Lithium ion conducting poly ethylene oxide based solid electrolyte containing active or passive ceramic nanoparticles. J. Phys. Chem. 2017, 121, 2563–2573. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Low, S.P.; Ahmad, A.; Rahman, M.Y.A. Effect of ethylene carbonate plasticizer and TiO2 nanoparticles on 49% poly(methyl methacrylate) grafted natural rubber-based polymer electrolyte. Ionics 2010, 16, 821–826. [Google Scholar] [CrossRef]
- Pitawala, H.M.J.C.; Dissanayake, M.A.K.L.; Seneviratne, V.A.; Mellander, B.E.; Albinson, I. Effect of plasticizers (EC or PC) on the ionic conductivity and thermal properties of the (PEO)(9)LiTf: Al2O3 nanocomposite polymer electrolyte system. J. Solid State Electrochem. 2008, 12, 783–789. [Google Scholar] [CrossRef]
- Navaratnam, S.; Ramesh, K.; Ramesh, S.; Sanusi, A.; Basirun, W.J.; Arof, A.K. Transport mechanism studies of chitosan electrolyte systems. Electrochim. Acta 2015, 175, 68–73. [Google Scholar] [CrossRef]
- Subban, R.H.Y.; Ahmad, A.H.; Kamarulzaman, N.; Ali, A.M.M. Effects of plasticiser on the lithium ionic conductivity of polymer electrolyte PVC-LiCF3SO3. Ionics 2005, 11, 442–445. [Google Scholar] [CrossRef]
- Imperiyka, M.; Ahmad, A.; Hanifah, S.A.; Rahman, M.Y.A. Preparation and Characterization of Polymer Electrolyte of with Ethylene Carbonate. Int. J. Polym. Sci. 2014, 2014, 638279. [Google Scholar] [CrossRef]
- Tuan Naiwi, T.S.R.; Aung, M.M. Effect of Lithium salt concentration on biopolymer polyurethane acrylate (PUA) of Jatorpha Oil electrolyte. In Proceedings of the International Symposium on advanced polymeric materials (ISAPM 2016), Kuala Lumpur, Malaysia, 16–18 May 2016. [Google Scholar]
- Ibrahim, S.; Ahmad, A.; Mohamed, N.S. Characterization of novel castor oil-based polyurethane polymer electrolytes. Polymers 2015, 7, 747–759. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Rudhziah, S.; Ahmad, A.; Mohamed, N.S. Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymers 2014, 6, 2371–2385. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Lai, H.M.; Isa, M.I.N. Biopolymer materials based carboxymethyl cellulose as a proton conducting biopolymer electrolyte for application in rechargeable proton battery. Electrochim. Acta 2014, 129, 1–13. [Google Scholar] [CrossRef]
- Imperiyka, M.; Ahmad, A.; Hanifah, S.A.; Bella, F. A UV-prepared linear polymer electrolyte membrane for dye-sensitized solar cells. Phys. B 2014, 450, 151–154. [Google Scholar] [CrossRef]
- Liew, C.W.; Ng, H.M.; Numan, A.; Ramesh, S. Poly(acrylic acid)-based hybrid inorganic-organic electrolytes membrane for electrical double layer capacitors application. Polymers 2016, 8, 179. [Google Scholar] [CrossRef]
- Monisha, S.; Mathavan, T.; Selvasekarapandian, S.; Benial, A.M.F.; Latha, M.P. Preparation and characterization of cellulose acetate and lithium nitrate for advanced electrochemical devices. Ionics 2017, 23, 2697–2706. [Google Scholar] [CrossRef]
- Ramesh, S.; Arof, A.K. Ionic conductivity studies of plasticized poly(vinyl chloride) polymer electrolytes. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2001, 85, 11–15. [Google Scholar] [CrossRef]
- Khiar, A.S.A.; Puteh, R.; Arof, A.K. Conductivity studies of a chitosan-based polymer electrolyte. Phys. B Condens. Matter. 2006, 373, 23–27. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Ahmad, A.; Badri, K.H.; Mohamed, N.S.; Rahman, M.Y.A.; Ricardo, C.L.A.; Scardi, P. The potential of polyurethane bio-based solid polymer electrolyte for photoelectrochemical cell application. Int. J. Hydrog. Energy 2014, 39, 3005–3017. [Google Scholar] [CrossRef]
- Digar, M.; Hung, S.L.; Wang, H.L.; Wen, T.C.; Gopalan, A. Study of ionic conductivity and microstructure of a cross-linked polyurethane acrylate electrolyte. Polymer 2002, 43, 681–691. [Google Scholar] [CrossRef]
- Santhosh, P.; Vasudevan, T.; Gopalan, A.; Lee, K. Preparation and properties of new cross-linked polyurethane acrylate electrolytes for lithium batteries. J. Power Sources 2006, 160, 609–620. [Google Scholar] [CrossRef]
- Salih, A.M.; Ahmad, M.B.; Ibrahim, N.A.; Dahlan, K.Z.; Tajau, R.; Mahmood, M.H.; Yunus, W.M.Z.W. Synthesis of radiation curable palm oil-based epoxy acrylate: NMR and FTIR spectroscopic investigations. Molecules 2015, 20, 14191–14211. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.; Kilic, H.; Berkem, M.; Gungor, A. Synthesis by UV-curing and characterization of polyurethane acrylate-lithium salts-based polymer electrolytes in lithium batteries. Chem. Pap. 2014, 68, 1561–1572. [Google Scholar] [CrossRef]
- Ramesh, S.; Ling, O.P. Effect of ethylene carbonate on the ionic conduction in poly(vinylidenefluoride-hexafluoropropylene) based solid polymer electrolytes. Polym. Chem. 2010, 1, 702–707. [Google Scholar] [CrossRef]
- Liao, F.; Zeng, X.; Li, H.; Lai, X.; Zhao, F. Synthesis and properties of UV curable polyurethane acrylates based on two different hydroxyethyl acrylates. J. Cent. South Univ. 2012, 19, 911–917. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Nithya, R.; Rajendran, S. Surface analysis studies on polymer electrolyte membranes using scanning electron microscope and atomic force microscope. Scanning Electron Microsc. 2012, 671–694. [Google Scholar] [CrossRef][Green Version]
- Johan, M.R.; Shy, O.H.; Ibrahim, S.; Mohd Yassin, S.M.; Hui, T.Y. Effects of Al2O3 nanofiller and EC plasticizer on the ionic conductivity enhancement of solid PEO-LiCF3SO3 solid polymer electrolyte. Solid State Ionics 2011, 196, 41–47. [Google Scholar] [CrossRef]

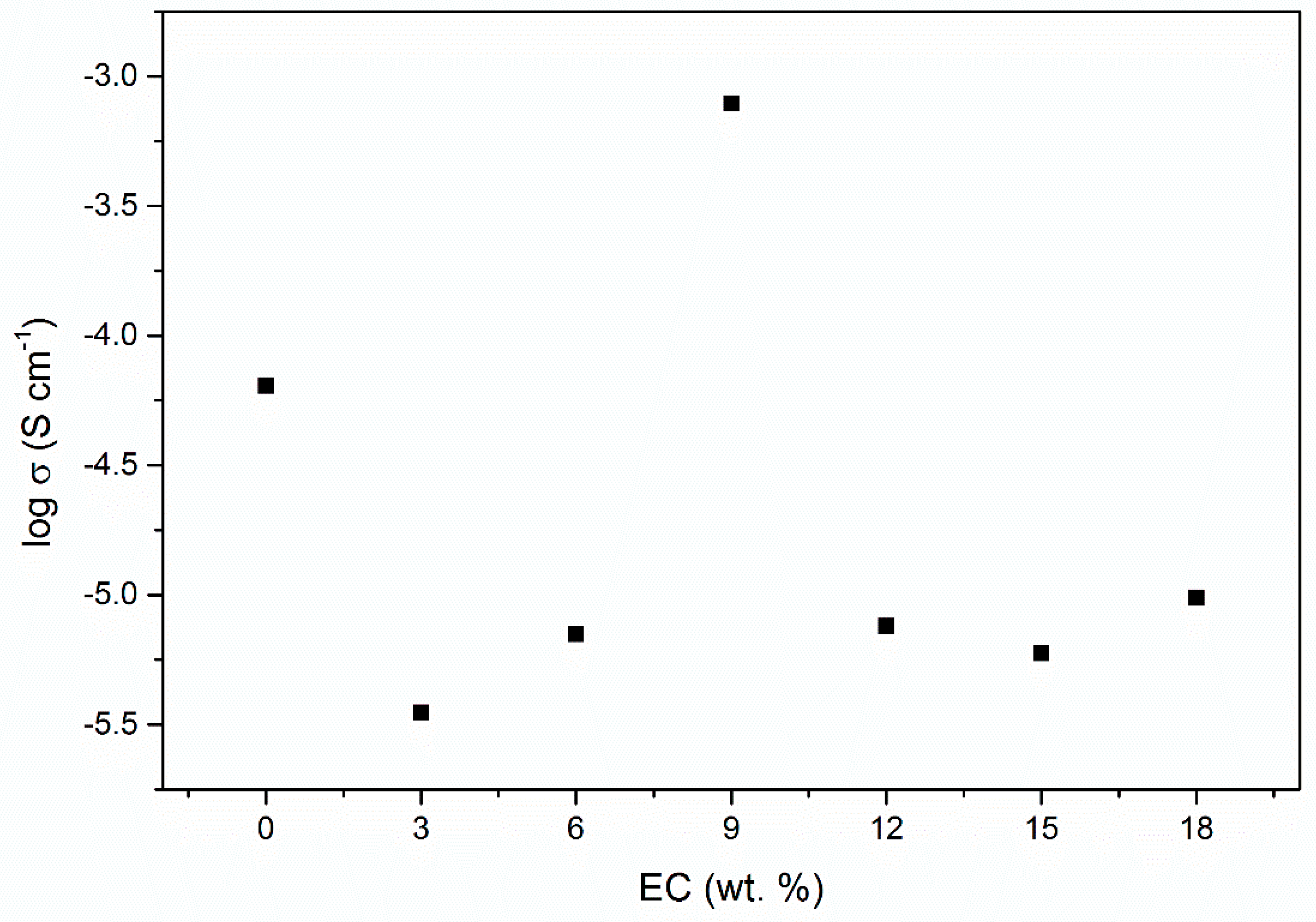
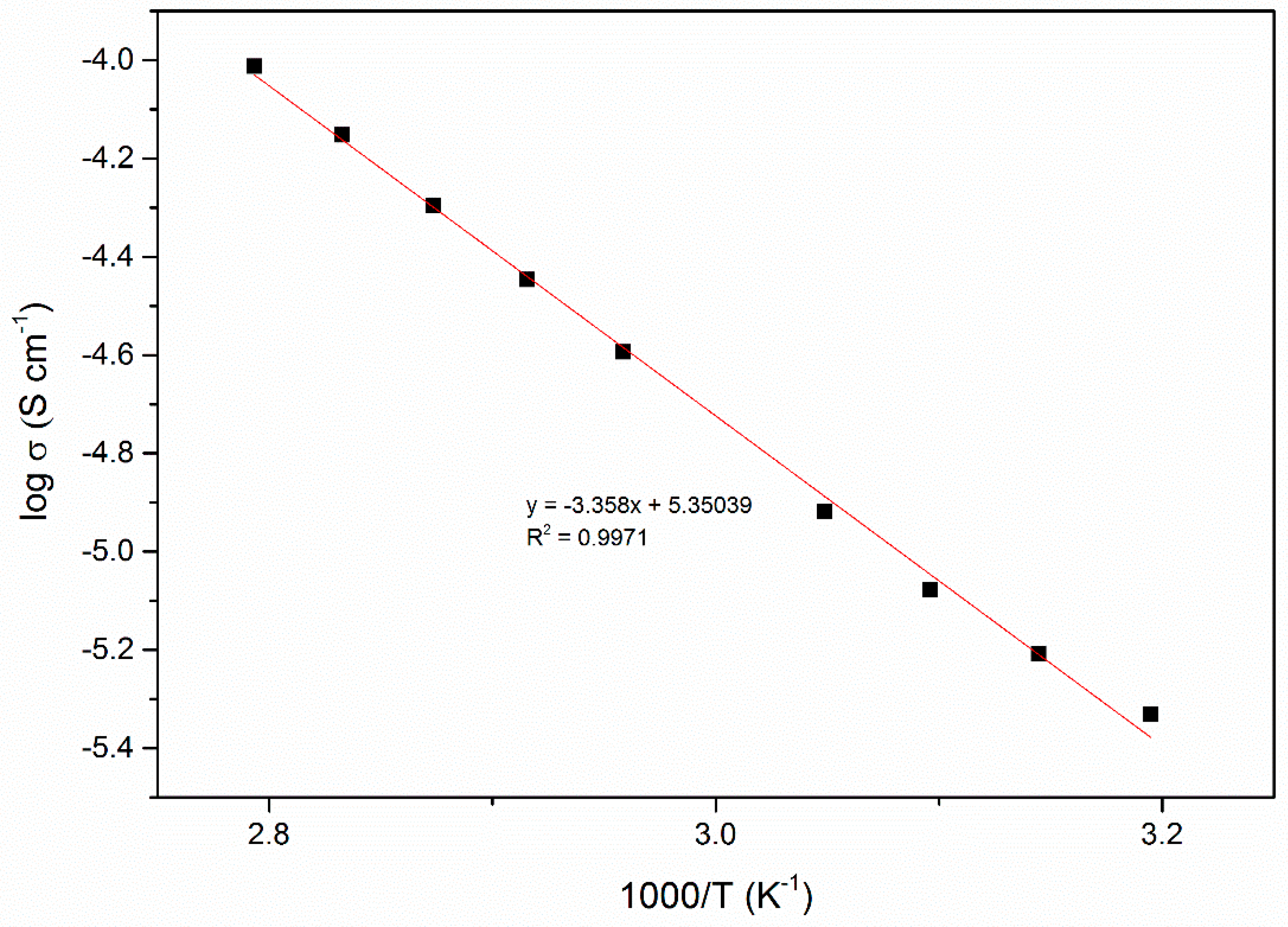

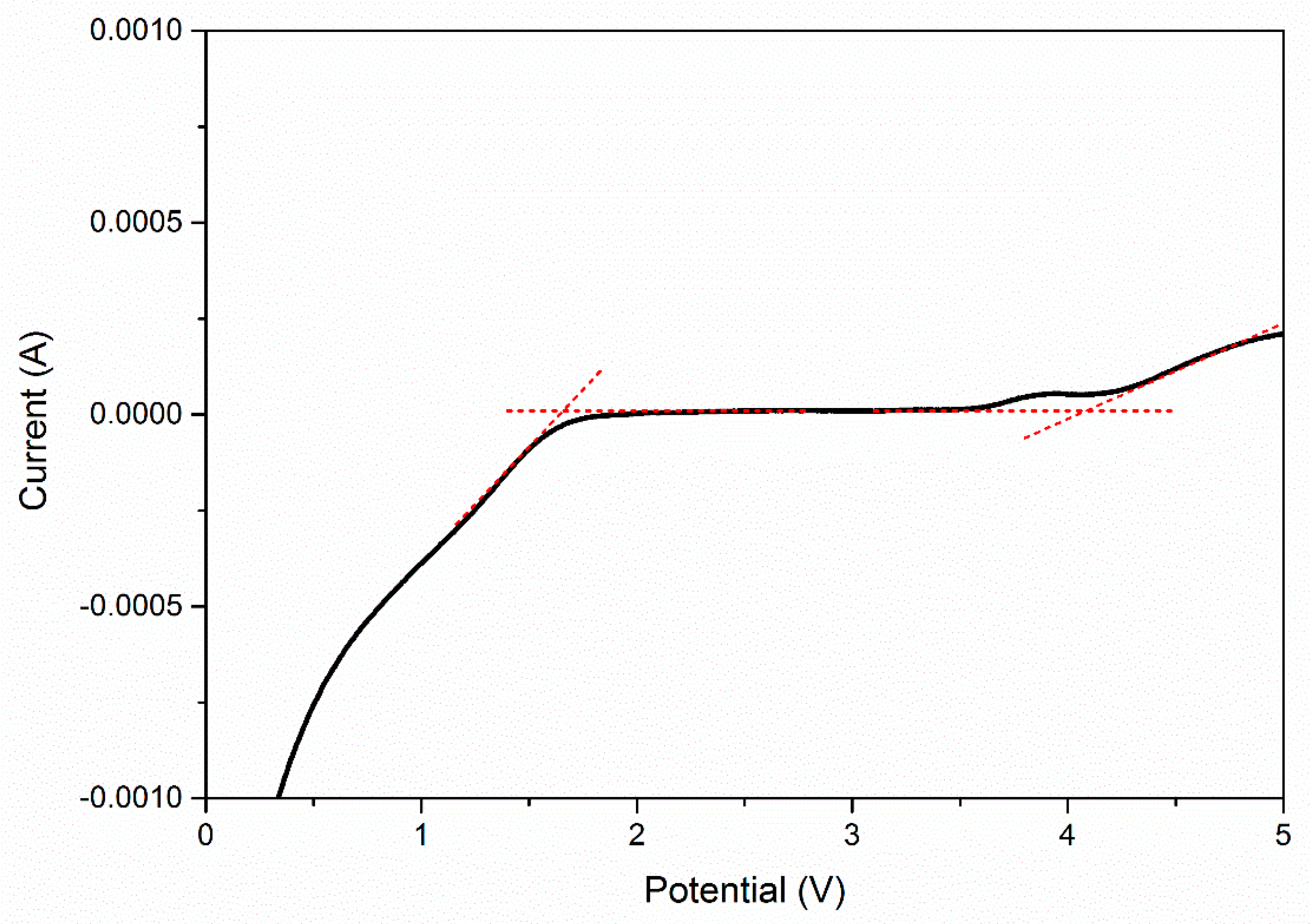
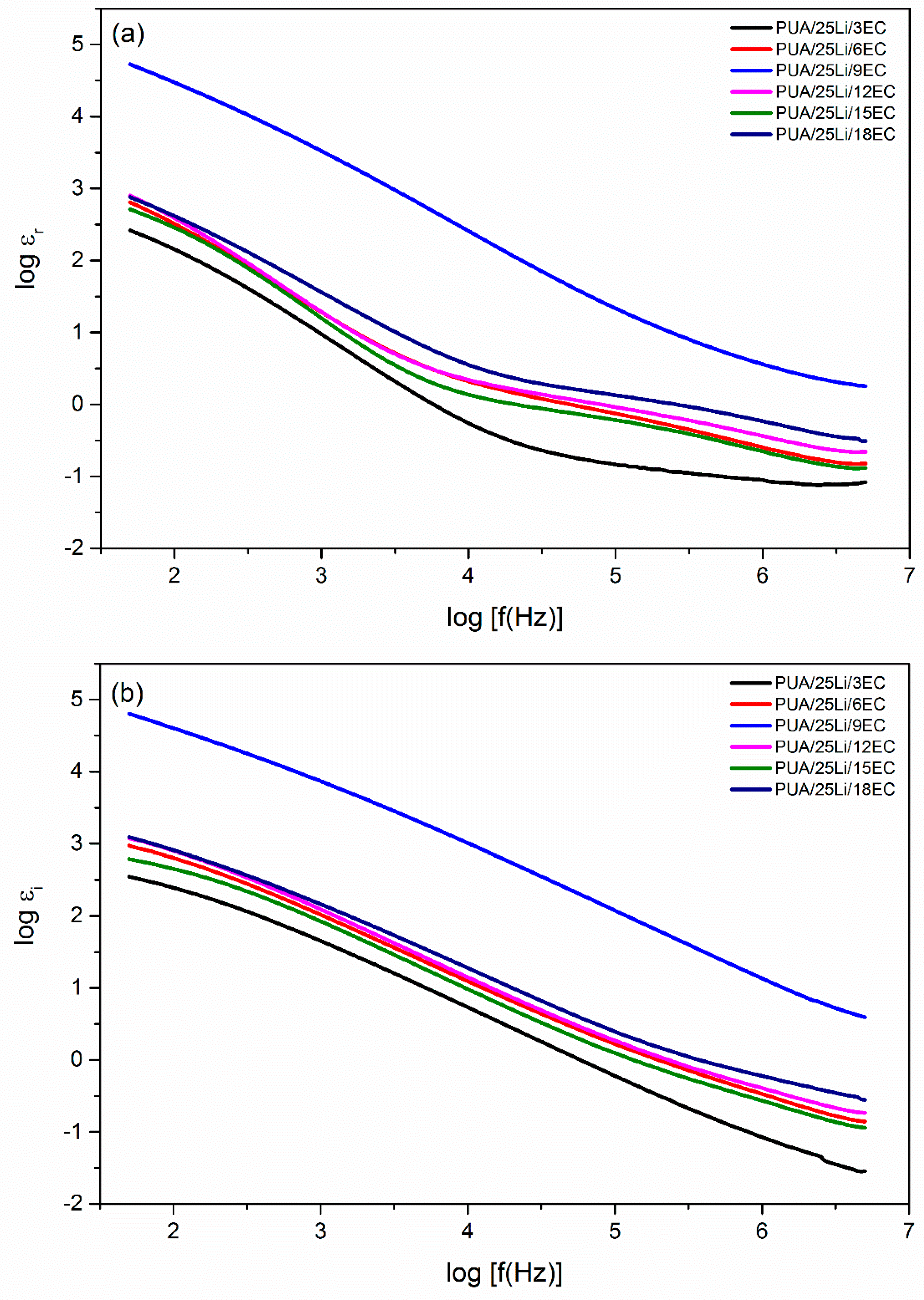
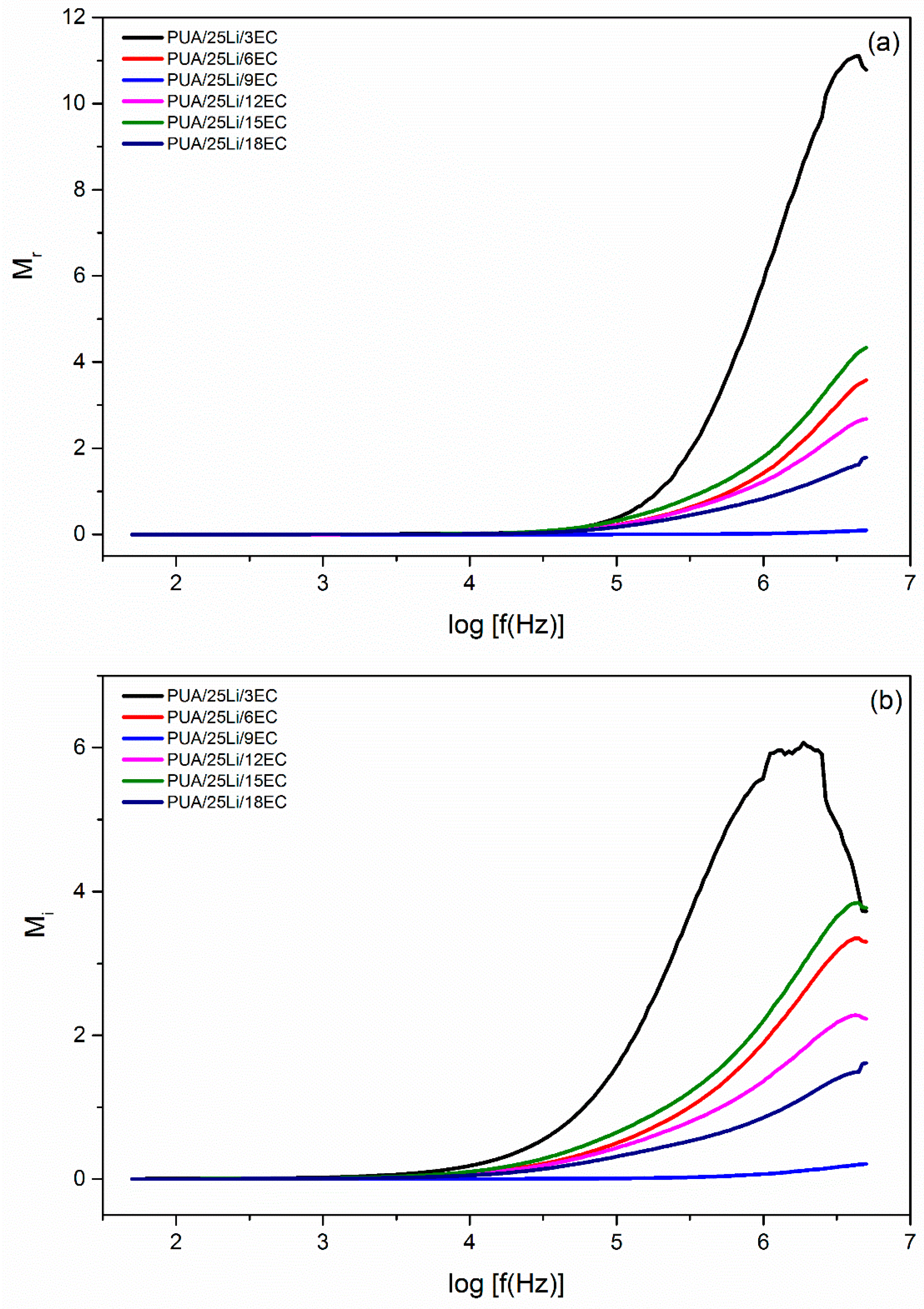

| EC, wt. % | Conductivity, σ (S cm−1) |
|---|---|
| 0% | 6.40 × 10−5 |
| 3% | 3.51 × 10−6 |
| 6% | 7.05 × 10−6 |
| 9% | 7.86 × 10−4 |
| 12% | 7.59 × 10−6 |
| 15% | 5.95 × 10−6 |
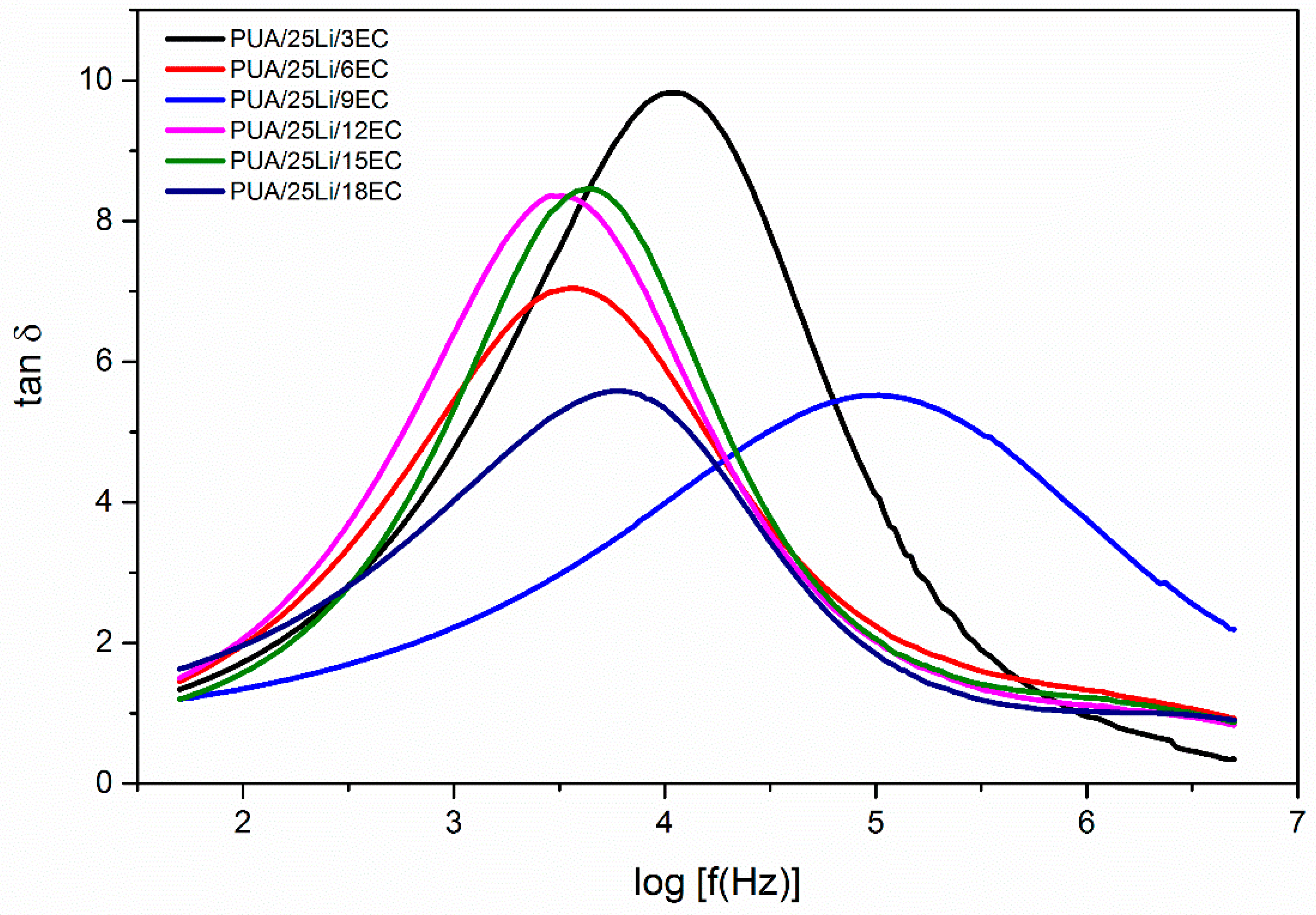
| EC Content (%) | Maximum Peak (Hz) | τ (×10−4 s) |
|---|---|---|
| 3 | 4.04 | 0.912 |
| 6 | 3.56 | 2.75 |
| 9 | 5.02 | 0.0955 |
| 12 | 3.46 | 3.47 |
| 15 | 3.61 | 2.45 |
| 18 | 3.78 | 1.66 |
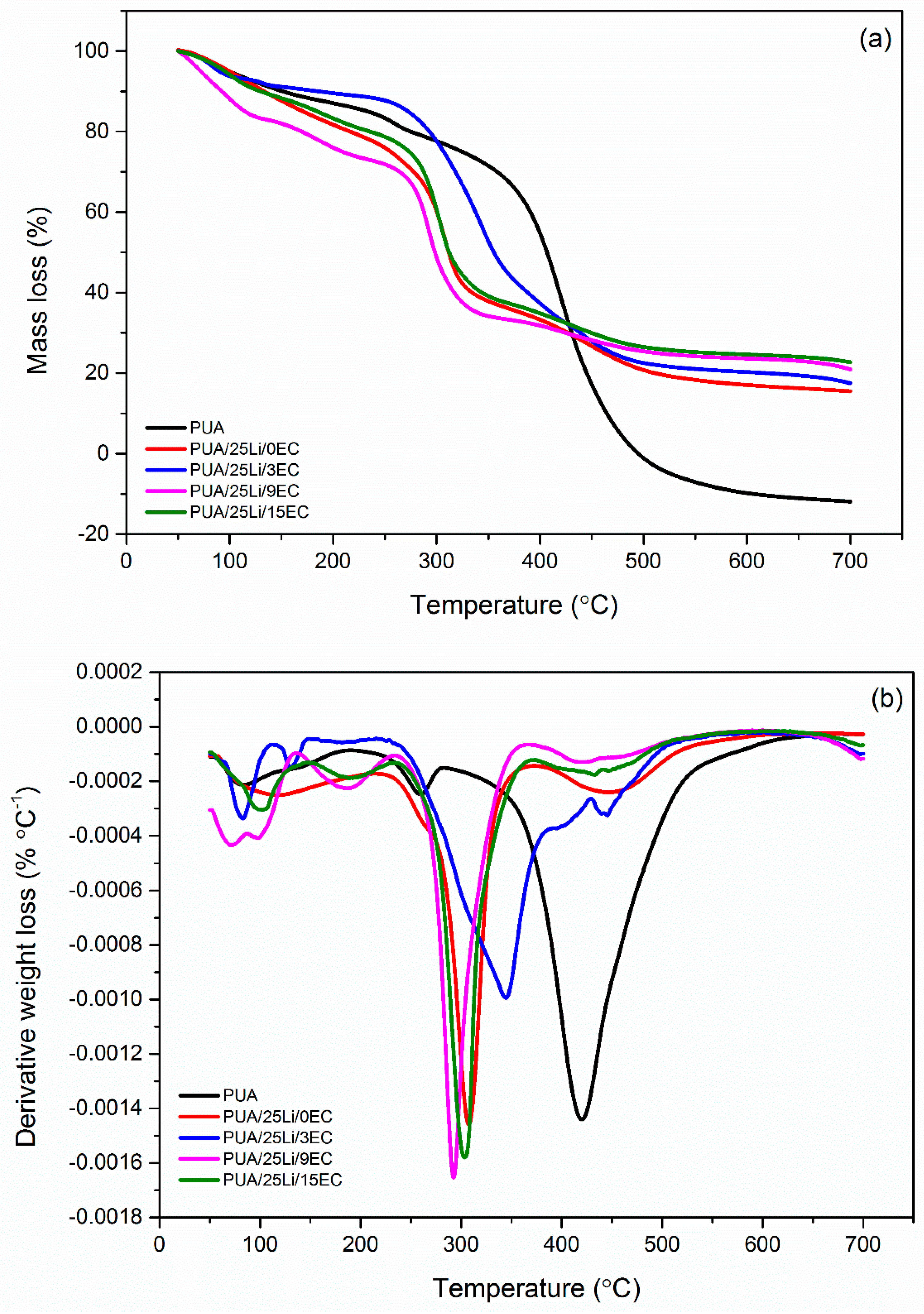
| Composition | Decomposition Stage | Onset | Final | Temp of Max Decomposition (°C) | Weight Loss (%) | Residue (%) |
|---|---|---|---|---|---|---|
| Degradation Temp (°C) | ||||||
| PUA 0 wt. % Li | d1 | 53.38 | 186.75 | 79.43 | 12.26 | 0 |
| d2 | 199.67 | 277.18 | 253.75 | 7.43 | ||
| d3 | 278.02 | 646.83 | 415.77 | 90.45 | ||
| 0 wt. % EC | d1 | 55.98 | 208.62 | 107.07 | 19.62 | 17.34 |
| d2 | 209.87 | 371.83 | 304.55 | 44.59 | ||
| d3 | 372.66 | 579.84 | 447.94 | 18.1 | ||
| 3 wt. % EC | d1 | 53.67 | 108.96 | 82.88 | 6.58 | 21.14 |
| d2 | 112.41 | 146.72 | 129.37 | 1.99 | ||
| d3 | 215.72 | 544.02 | 345.07 | 67.93 | ||
| 9 wt. % EC | d1 | 52.12 | 132.48 | 97.77 | 16.92 | 23.76 |
| d2 | 134.24 | 233.3 | 185.55 | 9.94 | ||
| d3 | 234.36 | 363.81 | 294.33 | 39.45 | ||
| d4 | 368.36 | 585.98 | 424.8 | 9.57 | ||
| 15 wt. % EC | d1 | 54.7 | 141.67 | 97.51 | 11.03 | 18.46 |
| d2 | 146.95 | 230.59 | 187.2 | 8.12 | ||
| d3 | 234.49 | 368.37 | 305.06 | 42.57 | ||
| d4 | 353.33 | 520.54 | 433.31 | 11.09 | ||
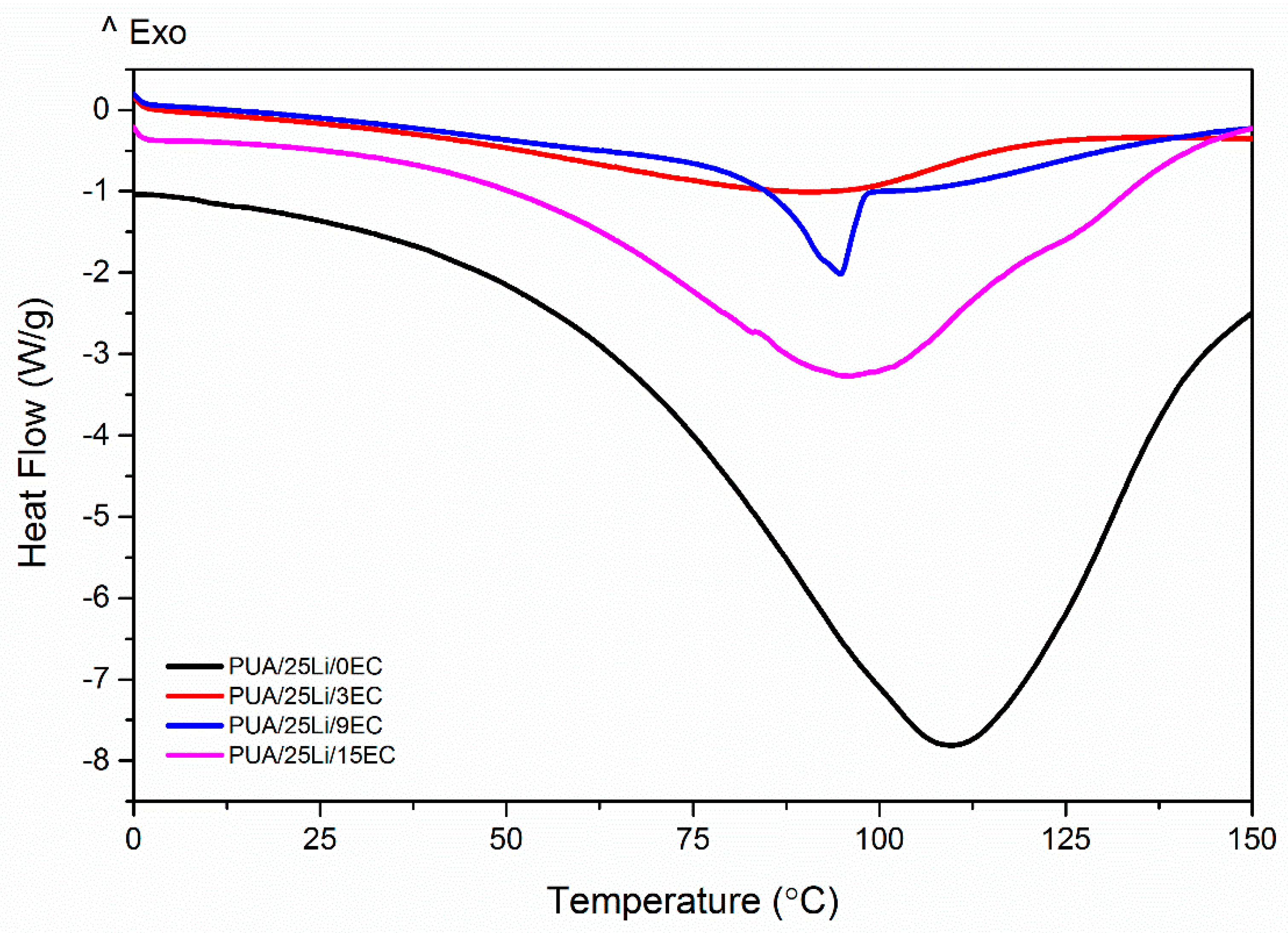
| Sample | Tm1 (°C) | Tm2 (°C) | Tg (°C) |
|---|---|---|---|
| PUA 0 wt. % EC | 108.9 | −15.7 | |
| PUA 3 wt. % EC | 88.94 | ||
| PUA 9 wt. % EC | 94.74 | 156.50 | |
| PUA 15 wt. % EC | 95.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuan. Naiwi, T.S.R.; Aung, M.M.; Ahmad, A.; Rayung, M.; Su’ait, M.S.; Yusof, N.A.; Wynn Lae, K.Z. Enhancement of Plasticizing Effect on Bio-Based Polyurethane Acrylate Solid Polymer Electrolyte and Its Properties. Polymers 2018, 10, 1142. https://doi.org/10.3390/polym10101142
Tuan. Naiwi TSR, Aung MM, Ahmad A, Rayung M, Su’ait MS, Yusof NA, Wynn Lae KZ. Enhancement of Plasticizing Effect on Bio-Based Polyurethane Acrylate Solid Polymer Electrolyte and Its Properties. Polymers. 2018; 10(10):1142. https://doi.org/10.3390/polym10101142
Chicago/Turabian StyleTuan. Naiwi, Tuan Syarifah Rossyidah, Min Min Aung, Azizan Ahmad, Marwah Rayung, Mohd Sukor Su’ait, Nor Azah Yusof, and Khine Zar Wynn Lae. 2018. "Enhancement of Plasticizing Effect on Bio-Based Polyurethane Acrylate Solid Polymer Electrolyte and Its Properties" Polymers 10, no. 10: 1142. https://doi.org/10.3390/polym10101142
APA StyleTuan. Naiwi, T. S. R., Aung, M. M., Ahmad, A., Rayung, M., Su’ait, M. S., Yusof, N. A., & Wynn Lae, K. Z. (2018). Enhancement of Plasticizing Effect on Bio-Based Polyurethane Acrylate Solid Polymer Electrolyte and Its Properties. Polymers, 10(10), 1142. https://doi.org/10.3390/polym10101142