Novel Photocatalytic PVDF/Nano-TiO2 Hollow Fibers for Environmental Remediation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Nano-TiO2 Dispersion
2.3. Nano-TiO2 Particle and Dispersion Characterization
2.4. Hollow Fibers (HFs) Preparation
2.5. Characterization of HF Membranes
2.5.1. HFs Morphology and Elemental Analyses
2.5.2. HFs Porosity, Mechanical Properties and Contact Angle
2.5.3. Bubble Point and Average Pore Size
2.5.4. Stability Tests of Membranes under UV-A Irradiation
2.5.5. Pure Water Permeability (PWP) Measurements
2.5.6. Photocatalytic Activity
3. Results and Discussion
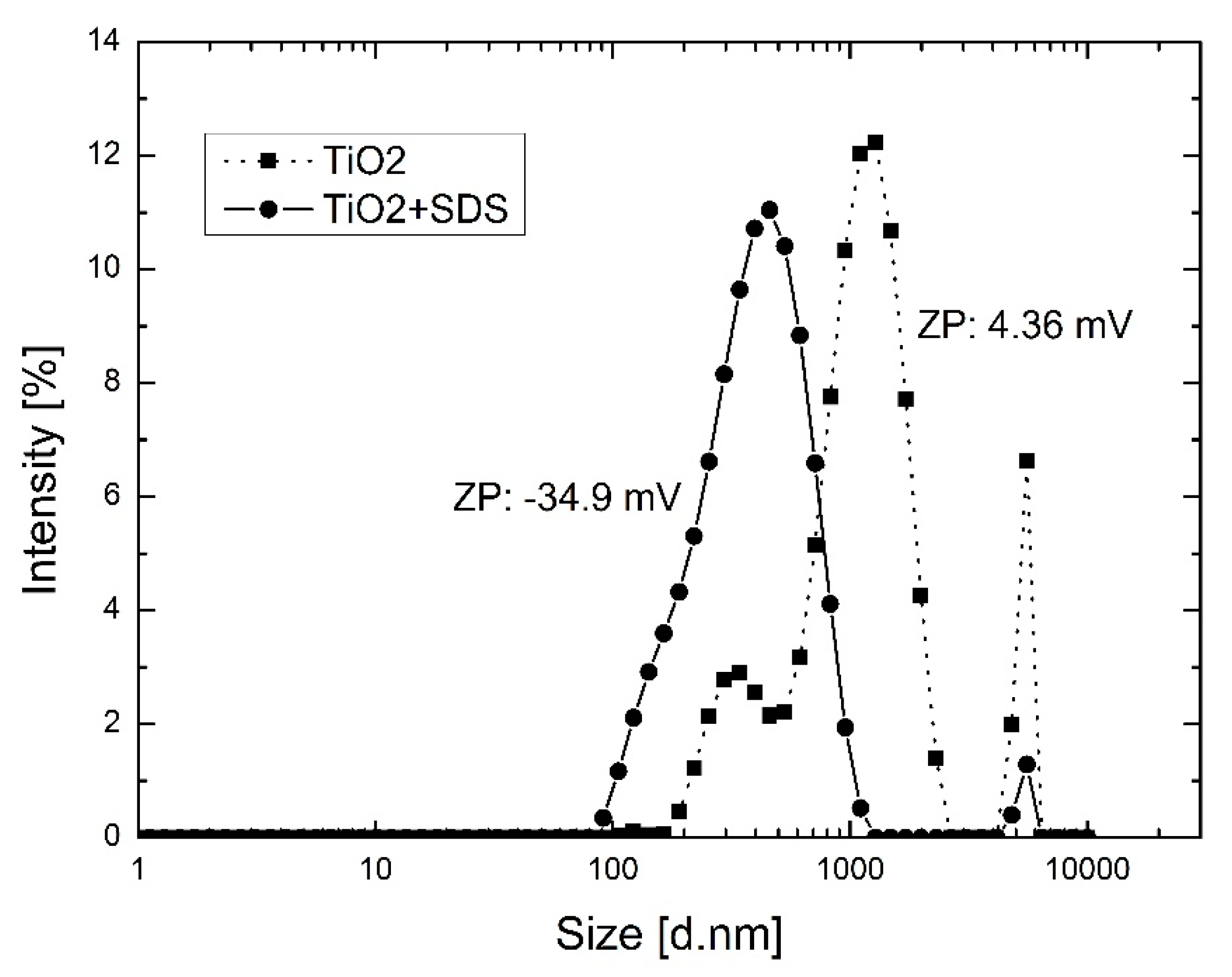
3.1. TiO2 Modification
3.2. HF Characterization
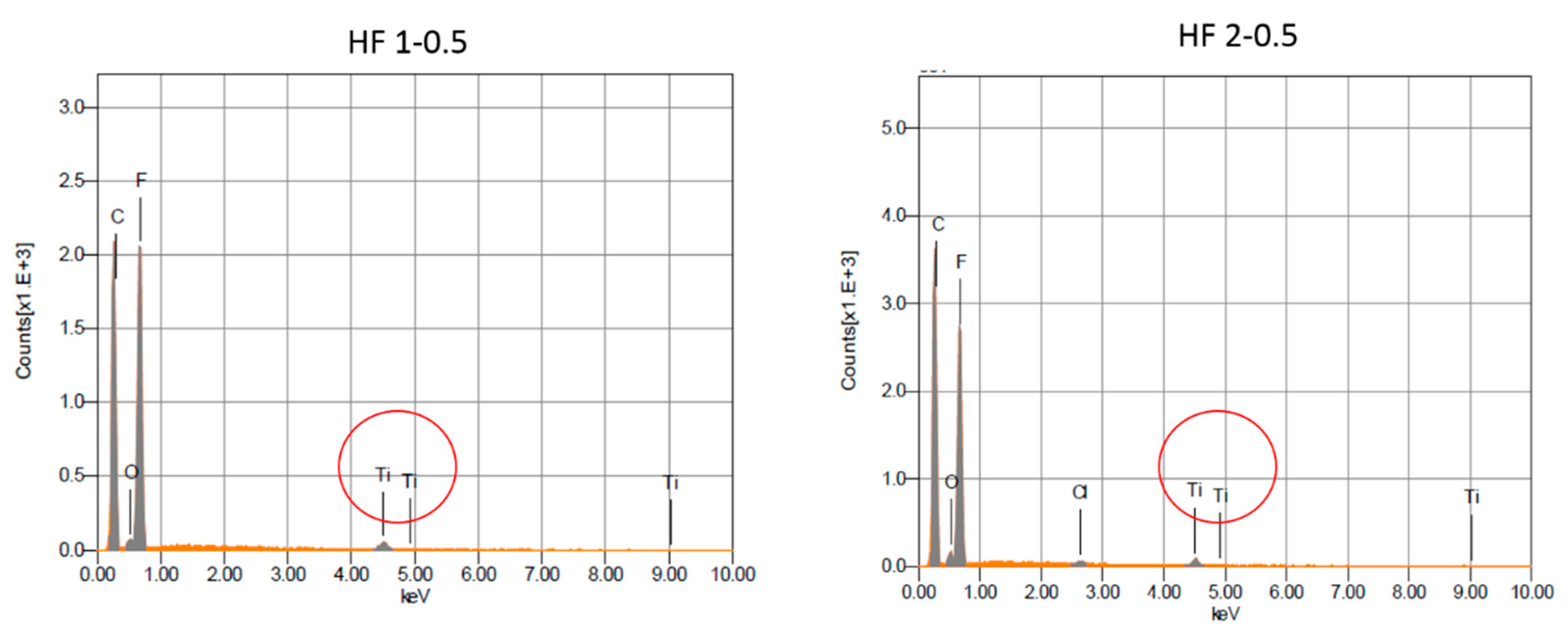
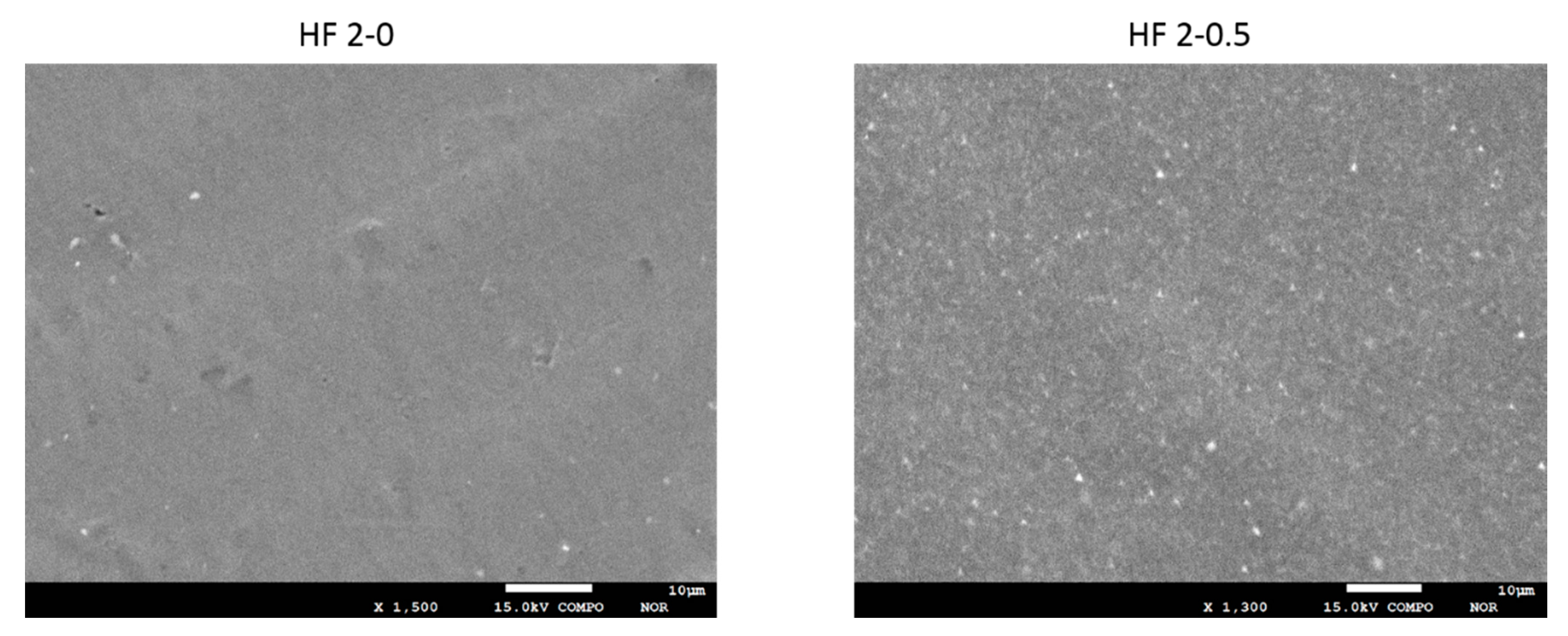
3.2.1. SEM and EDX
3.2.2. Thickness, Porosity and Mechanical Properties
3.2.3. Pore Size
3.2.4. PWP Tests
3.2.5. Permeation Flux Stability under UV Irradiation
3.3. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rogers, P. Facing the freshwater crisis. Sci. Am. 2008, 299, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946. [Google Scholar] [CrossRef] [Green Version]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane engineering in process intensification-An overview. J. Memb. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Drioli, E.; MacEdonio, F. Membrane engineering for water engineering. Ind. Eng. Chem. Res. 2012, 51, 10051–10056. [Google Scholar] [CrossRef]
- Flemming, H.C.; Schaule, G.; Griebe, T.; Schmitt, J. Tamachkiarowa, a Biofouling—The Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Cui, P.; Zhao, X.; Zhou, M.; Wang, L. Photocatalysis-Membrane Separation Coupling Reactor and Its Application. Chin. J. Catal. 2006, 27, 752–754. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Memb. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J. Memb. Sci. 2011, 380, 155–162. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, Z.; Huang, J.; Wang, Y. PVDF membranes with simultaneously enhanced permeability and selectivity by breaking the tradeoff effect via atomic layer deposition of TiO2. J. Memb. Sci. 2013, 442, 57–64. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Memb. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, X.; Chen, S.; Zhao, H.; Zhao, Y. Fabrication of photocatalytic membrane and evaluation its efficiency in removal of organic pollutants from water. Sep. Purif. Technol. 2006, 50, 147–155. [Google Scholar] [CrossRef]
- Loddo, V.; Palmisano, L.; Marino, T.; Molinari, R. 23—Membranes for photocatalysis in water and wastewater treatment. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2011; pp. 746–768. ISBN 9781845699697. [Google Scholar]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Loukidou, M.X.; Zouboulis, A.I. Comparison of two biological treatment processes using attached-growth biomass for sanitary landfill leachate treatment. Environ. Pollut. 2001, 111, 273–281. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ahmad, A.L.; Lim, J.K.; Ooi, B.S. Preparation and characterization of PVDF/TiO2 mixed matrix membrane via in situ colloidal precipitation method. Desalination 2012, 295, 61–69. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; You, Y.; Meng, H.; Zhang, J.; Xu, X. Preparation, performance and adsorption activity of TiO2 nanoparticles entrapped PVDF hybrid membranes. Appl. Surf. Sci. 2012, 263, 660–665. [Google Scholar] [CrossRef]
- You, S.J.; Semblante, G.U.; Lu, S.C.; Damodar, R.A.; Wei, T.C. Evaluation of the antifouling and photocatalytic properties of poly(vinylidene fluoride) plasma-grafted poly(acrylic acid) membrane with self-assembled TiO2. J. Hazard. Mater. 2012, 237–238, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Memb. Sci. 2012, 415–416, 850–863. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Mollahosseini, A.; Rajaeian, B. Structural and performance properties of UV-assisted TiO2 deposited nano-composite PVDF/SPES membranes. Desalination 2012, 285, 31–38. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ooi, B.S.; Ahmad, A.L. Study on PVDF-TiO2 mixed-matrix membrane behaviour towards humic acid adsorption. J. Water Process Eng. 2015, 15, 99–106. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Lu, J.; Zhao, J.; Cui, J.; Wu, X.; Yan, Y.; Huo, P. Bioinspired Synthesis of Photocatalytic Nanocomposite Membranes Based on Synergy of Au-TiO2 and Polydopamine for Degradation of Tetracycline under Visible Light. ACS Appl. Mater. Interfaces 2017, 9, 23687–23697. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Enzymatic degradation of bisphenol-A with immobilized laccase on TiO2 sol-gel coated PVDF membrane. J. Memb. Sci. 2014, 469, 19–30. [Google Scholar] [CrossRef]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with different dosage of nano-TiO2. J. Memb. Sci. 2012, 389, 522–531. [Google Scholar] [CrossRef]
- Ngang, H.P.; Ooi, B.S.; Ahmad, A.L.; Lai, S.O. Preparation of PVDF-TiO2 mixed-matrix membrane and its evaluation on dye adsorption and UV-cleaning properties. Chem. Eng. J. 2012, 197, 359–367. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.J.; Chou, H.H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG-TiO 2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Memb. Sci. 2012, 405–406, 48–56. [Google Scholar] [CrossRef]
- Tahiri Alaoui, O.; Nguyen, Q.T.; Mbareck, C.; Rhlalou, T. Elaboration and study of poly(vinylidene fluoride)-anatase TiO2 composite membranes in photocatalytic degradation of dyes. Appl. Catal. A Gen. 2009, 358, 13–20. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Stability study of PVDF/TiO2 dual layer hollow fibre membranes under long-term UV irradiation exposure. J. Water Process Eng. 2015. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Zhang, Y.; Zhao, S. Comparing the antifouling effects of activated carbon and TiO2 in ultrafiltration membrane development. J. Colloid Interface Sci. 2018, 515. [Google Scholar] [CrossRef] [PubMed]
- Méricq, J.-P.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Yatim, N.S.M.; Karim, K.A.; Seng, O.B. Performance of Chemically Modified TiO2 -poly (vinylidene fluoride) DCMD for Nutrient Isolation and Its Antifouling Properties. J. Membr. Sci. Res. 2016, 2, 163–168. [Google Scholar]
- Lee, E.J.; An, A.K.; Hadi, P.; Lee, S.; Woo, Y.C.; Shon, H.K. Advanced multi-nozzle electrospun functionalized titanium dioxide/polyvinylidene fluoride-co-hexafluoropropylene (TiO2/PVDF-HFP) composite membranes for direct contact membrane distillation. J. Memb. Sci. 2017. [Google Scholar] [CrossRef]
- Kim, K.M.; Park, N.G.; Ryu, K.S.; Chang, S.H. Characteristics of PVdF-HFP/TiO2 composite membrane electrolytes prepared by phase inversion and conventional casting methods. Electrochim. Acta 2006, 51, 5636–5644. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, H.P.; Zhang, P.; Li, G.C.; Wu, Y.P.; Zhou, X.D. Effects of the porous structure on conductivity of nanocomposite polymer electrolyte for lithium ion batteries. J. Memb. Sci. 2008, 322, 416–422. [Google Scholar] [CrossRef]
- Martins, P.M.; Miranda, R.; Marques, J.; Tavares, C.J.; Botelho, G.; Lanceros-Mendez, S. Comparative efficiency of TiO2 nanoparticles in suspension vs. immobilization into P(VDF-TrFE) porous membranes. RSC Adv. 2016, 6, 12708–12716. [Google Scholar] [CrossRef]
- Kuang, X.; Gao, Q.; Zhu, H. Effect of calcination temperature of TiO2 on the crystallinity and the permittivity of PVDF-TrFE/TiO2 composites. J. Appl. Polym. Sci. 2013, 129, 296–300. [Google Scholar] [CrossRef]
- Almeida, N.A.; Martins, P.M.; Teixeira, S.; Lopes da Silva, J.A.; Sencadas, V.; Kühn, K.; Cuniberti, G.; Lanceros-Mendez, S.; Marques, P.A.A.P. TiO2/graphene oxide immobilized in P(VDF-TrFE) electrospun membranes with enhanced visible-light-induced photocatalytic performance. J. Mater. Sci. 2016, 51, 6974–6986. [Google Scholar] [CrossRef]
- Mandzy, N.; Grulke, E.; Druffel, T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 2005, 160, 121–126. [Google Scholar] [CrossRef]
- Veronovski, N.; Andreozzi, P.; La Mesa, C.; Sfiligoj-Smole, M. Stable TiO2 dispersions for nanocoating preparation. Surf. Coat. Technol. 2010, 204, 1445–1451. [Google Scholar] [CrossRef]
- Kim, K. Do; Han, D.N.; Kim, H.T. Optimization of experimental conditions based on the Taguchi robust design for the formation of nano-sized silver particles by chemical reduction method. Chem. Eng. J. 2004, 104, 55–61. [Google Scholar] [CrossRef]
- Liufu, S.; Xiao, H.; Li, Y. Adsorption of poly(acrylic acid) onto the surface of titanium dioxide and the colloidal stability of aqueous suspension. J. Colloid Interface Sci. 2005, 281, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, A.; Madaeni, S.S.; Mansourpanah, Y. The effect of anionic, non-ionic and cationic surfactants on morphology and performance of polyethersulfone ultrafiltration membranes for milk concentration. J. Memb. Sci. 2007, 296, 110–121. [Google Scholar] [CrossRef]
- Wu, G.; Chen, A. Direct growth of F-doped TiO2 particulate thin films with high photocatalytic activity for environmental applications. J. Photochem. Photobiol. A Chem. 2008, 195, 47–53. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Madaeni, S.S.; Rahimpour, A.; Farhadian, A.; Taheri, A.H. Formation of appropriate sites on nanofiltration membrane surface for binding TiO2 photo-catalyst: Performance, characterization and fouling-resistant capability. J. Memb. Sci. 2009, 330, 297–306. [Google Scholar] [CrossRef]
- Bae, T.H.; Kim, I.C.; Tak, T.M. Preparation and characterization of fouling-resistant TiO2 self-assembled nanocomposite membranes. J. Memb. Sci. 2006, 275, 1–5. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Rosselli, A.; Drioli, E. Preparation of hollow fibre membranes from PVDF/PVP blends and their application in VMD. J. Memb. Sci. 2010, 364, 219–232. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Simone, S.; MacEdonio, F.; Al-Jlil, S.A.; Al Shabonah, F.S.; Al-Romaih, H.S.; Al-Harbi, O.; Figoli, A.; Criscuoli, A. Novel PVDF hollow fiber membranes for vacuum and direct contact membrane distillation applications. Sep. Purif. Technol. 2013, 115, 27–38. [Google Scholar] [CrossRef]
- Falbo, F.; Santoro, S.; Galiano, F.; Simone, S.; Davoli, M.; Drioli, E.; Figoli, A. Organic/organic mixture separation by using novel ECTFE polymeric pervaporation membranes. Polymer 2016, 98, 110–117. [Google Scholar] [CrossRef]
- Figoli, A.; Simone, S.; Criscuoli, A.; Al-Jlil, S.A.; Al Shabouna, F.S.; Al-Romaih, H.S.; Di Nicolò, E.; Al-Harbi, O.A.; Drioli, E. Hollow fibers for seawater desalination from blends of PVDF with different molecular weights: Morphology, properties and VMD performance. Polymer 2014, 55, 1296–1306. [Google Scholar] [CrossRef]
- Figoli, A.; Ursino, C.; Galiano, F.; Di Nicolò, E.; Campanelli, P.; Carnevale, M.C.; Criscuoli, A. Innovative hydrophobic coating of perfluoropolyether (PFPE) on commercial hydrophilic membranes for DCMD application. J. Memb. Sci. 2017, 522, 192–201. [Google Scholar] [CrossRef]
- “Lighting.Philips”. Available online: http://www.lighting.philips.com/main/prof/lamps/fluorescent-lamps-and-starters/tl-d/tl-d-blacklight-blue/928048010805_EU/product (accessed on 9 December 2015).
- Simone, S.; Galiano, F.; Faccini, M.; Boerrigter, M.; Chaumette, C.; Drioli, E.; Figoli, A. Preparation and Characterization of Polymeric-Hybrid PES/TiO2 Hollow Fiber Membranes for Potential Applications in Water Treatment. Fibers 2017, 5, 14. [Google Scholar] [CrossRef]
- International Organization for Standardization Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. Available online: http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm%3Fcsnumber=46019 (accessed on 20 May 2012).
- Viana, R.B.; Da Silva, A.B.F.; Pimentel, A.S. Infrared spectroscopy of anionic, cationic, and zwitterionic surfactants. Adv. Phys. Chem. 2012, 2012. [Google Scholar] [CrossRef]
- Wongchitphimon, S.; Wang, R.; Jiraratananon, R.; Shi, L.; Loh, C.H. Effect of polyethylene glycol (PEG) as an additive on the fabrication of polyvinylidene fluoride-co-hexafluropropylene (PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Memb. Sci. 2011, 369, 329–338. [Google Scholar] [CrossRef]
- Hasan Nia, M.; Rezaei-Tavirani, M.; Nikoofar, A.R.; Masoumi, H.; Nasr, R.; Hasanzadeh, H.; Jadidi, M.; Shadnush, M. Stabilizing and dispersing methods of TiO2 nanoparticles in biological studies. J. Paramed. Sci. Spring 2015, 6, 8686. [Google Scholar]
- Li, J.-F.; Xu, Z.-L.; Yang, H.; Yu, L.-Y.; Liu, M. Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl. Surf. Sci. 2009, 255, 4725–4732. [Google Scholar] [CrossRef]
- Jeon, J.-D.; Kim, M.-J.; Kwak, S.-Y. Effects of addition of TiO2 nanoparticles on mechanical properties and ionic conductivity of solvent-free polymer electrolytes based on porous P(VdF-HFP)/P(EO-EC) membranes. J. Power Sources 2006, 162, 1304–1311. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, P.; Zheng, Q.; Li, J. The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Memb. Sci. 2007, 288, 231–238. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Effect of TiO2 nanoparticles on the performance of polyphenylsulfone biomaterial for orthopaedic implants. J. Mater. Chem. B 2014. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Zheng, Q. Preparation and properties of polysulfone/TiO2 composite ultrafiltration membranes. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 879–887. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Houas, A. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Riga, A.; Soutsas, K.; Ntampegliotis, K.; Karayannis, V.; Papapolymerou, G. Effect of system parameters and of inorganic salts on the decolorization and degradation of Procion H-exl dyes. Comparison of H2O2/UV, Fenton, UV/Fenton, TiO2/UV and TiO2/UV/H2O2 processes. Desalination 2007, 211, 72–86. [Google Scholar] [CrossRef]







| TiO2 Type | Membrane Preparation and Particle Immobilization Technique | Main Results/Application | Reference |
|---|---|---|---|
| Nanoparticles on Membrane Surface | |||
| 85% anatase and 15% rutile TiO2 (20 nm, TitanPE Technologies) | NIPS+ (NPs in coagulation bath) | Improved membrane hydrophilicity and roughness. Superior retention properties (98.28%) of humic acid. | [18] |
| TiO2 NPs synthesized from Tetrabutyl titanate (TBOT) | Pre-treated PVDF film immersed in the TiO2 suspension | Improved membrane hydrophilicity and permeability, anti-fouling properties. Tests for adsorption of Cu2+ (removal of heavy metals via solid-phase extraction); decreased adsorption capacity of BSA. | [19] |
| --- | Poly(acrylic acid) (PAA) plasma-grafted on commercial PVDF followed by dipping in aqueous TiO2 suspension. | Improved membrane hydrophilicity and permeability, anti-fouling properties. Tests for photodegradation of Reactive Black 5 (RB5) dye (wastewater treatment and re-use processes). | [20] |
| TiO2 NPs were synthesized from titanium (IV) iso-propoxide (TTIP) | Coating of TiO2 NPs onto PVDF membrane. | Super-hydrophobic PVDF membrane (for membrane distillation), improved rejection to NaCl and anti-fouling properties. Fouling tests with humic acid and CaCl2. | [21] |
| TiO2 (20 nm Degussa) | NIPS+ Coating of TiO2 NPs onto PVDF/SPES membrane. | Improved hydrophilicity, less tendency to fouling, improved BSA rejection. Membranes anti-bacterial properties tested on E. Coli via inhibition zone method. | [22] |
| 85% anatase and 15% rutile TiO2 (20 nm, TitanPE Technologies) | NIPS+ (NPs in coagulation bath) | NPs had a significant effect on the membrane anti-fouling property. By increasing TiO2 content, membrane surface increased. Sufficient electrostatic repulsion appears between highly charged PVDF-TiO2 MMMs and HA aggregates, alleviating the adsorption phenomenon. | [23] |
| TiO2 NPs were synthesized from titanium (IV) iso-propoxide (TTIP) | NIPS+ A two-step modification methodology (polydopamine (pDA) coating method and vacuum filtration process) | Au-TiO2/pDA/PVDF nanocomposite membranes were tested for the degradation of tetracycline under visible light irradiation. | [24] |
| TiO2 NPs were synthesized from titanium (IV) iso-propoxide (TTIP) | Commercial PVDF membrane (Millipore Pty. Ltd.) | Laccase covalently immobilized on the TiO2 sol–gel coated PVDF membranes. Bio-catalytic membranes exhibited good Bisphenol A degradation efficiency over repeated use. | [25] |
| TiO2 (20 nm Degussa) | TiO2 coated on modified PAA-PVDF membrane | Reduced fouling tendency of PVDF membranes using whey solutions as foulant. | [10] |
| TiO2 NPs synthesized from Titanium tetrachloride | PVDF membrane coated by atomic layer deposition (ALD) | The deposition of TiO2 enhanced the hydrophilicity and fouling resistance of the PVDF membranes, which was more evident at higher ALD cycle numbers. | [11] |
| Nanoparticles in Dope Solution | |||
| Anatase TiO2 (20 nm, Meidilin Nanometer Material) | TIPS | Microfiltration membranes showing uniform polymer spherulites, improved membrane performance. | [26] |
| Anatase TiO2 (20 nm, TitanPE Technologies) | NIPS | Improved membrane hydrophilicity and permeability, anti-fouling properties. Tests of photo-degradation of methylene blue (MB). | [27] |
| TiO2 (20 nm Degussa) | NIPS | Ultrafiltration membranes with improved membrane hydrophilicity and permeability, anti-fouling properties. Photodegradation experiments carried out on RB5; anti-fouling properties tested using BSA. | [28] |
| TiO2 (20–30 nm Degussa) | NIPS | Ultrafiltration membranes with good combination of flux and rejection and no particles aggregation. Foulants’ photocatalytic degradation was tested using Humic Acid (HA) (Natural organic matter, NOM, removal). | [29] |
| 98% anatase TiO2 (20 nm Degussa) | NIPS | Membranes for treatment of colored wastewaters from textile or dye industry. Membrane wetting and dyes (Brilliant Green, BG, and Indigo Carmin, IC) photodegradation improved via ethanol membrane-pretreatment. | [30] |
| __ | NIPS | PVDF–TiO2/PVDF dual layer hollow fiber membranes were prepared by the co-extrusion technique. The technique allows the nanoparticle distributed uniformly inside the membrane. The stability of dual layer hollow fiber membranes under UV changed in the surface during the whole operational period | [31] |
| TiO2 (20–30 nm Degussa) | NIPS | TiO2 NP improved the surface hydrophilicity and water permeation flux of the membrane. Anti-fouling properties tested using BSA | [32] |
| TiO2 Aeroxide P25 (85% anatase-15% rutile, ~20 nm) | NIPS | Under UV irradiation membrane super-hydrophilicity allowed to suppress pure water permeate flux decline and to reach higher fluxes. Fouled membranes after BSA filtration cleaned using water and UV irradiation. Permeate flux completely recovered after this cleaning. | [33] |
| TiO2 Aeroxide P25 (85% anatase-15% rutile, ~20 nm) | NIPS | Antibacterial activity against B. Subtilis which was enhanced by incorporating acid/alkali modified titania NPs into the polymer matrix. | [34] |
| TiO2 (20 nm) | TiO2/PVDF-HFP membranes prepared via electrospinning | The obtained membranes were tested in direct contact membrane distillation (DCMD), showing fluxes higher than those of commercial membranes. | [35] |
| TiO2 PC-101 (Japan Titan Kogyo, anatase type, 20 nm) | NIPS | Composite polymer electrolyte membranes exhibited excellent ionic conductivity, interfacial and electrochemical stability. | [36] |
| TiO2 synthesized in situ via Ti(OC4H9)4 hydrolysis | TiO2/PVDF-HFP membranes produced via NIPS | Enhanced porosity, ion conductivity; reduced activation energy for ion transport. | [37] |
| TiO2 (P25 EVONIK Industries) | TiO2/PVDF-TrFE and TiO2/zeolites (NaY)/PVDF-TrFE membranes prepared via Evaporation Induced Phase Separation (EIPS) | High membrane porosity which promoted MB degradation under UV light irradiation | [38] |
| TiO2 synthesized from tetrabutyl titanate (TBOT) by sol-gel method | TiO2/PVDF-TrFE membranes prepared via EIPS | The fabricated composite membranes manifested increased permittivity | [39] |
| TiO2 Aeroxide P25 (85% anatase-15% rutile, ~20 nm) | TiO2/PVDF-TrFE and TiO2/graphene oxide (GO)/PVDF-TrFE membranes produced by electrospinning | The presence of titania and GO improved the photocatalytic efficiency of the nanocomposite membranes towards the degradation of MB | [40] |
| Chemical | Type 1 | Type 2 | ||
|---|---|---|---|---|
| HF 1-0 | HF 1-0.5 | HF 2-0 | HF 2-0.5 | |
| PVDF 6012 | 18 | 18 | 19 | 19 |
| PVP k17 | 15 | 15 | 16 | 16 |
| PEG400 | - | - | 10 | 10 |
| H2O | 5 | 5 | - | - |
| NMP | 62 | 61.5 | 55 | 54.5 |
| TiO2 | - | 0.5 | - | 0.5 |
| Spinning Condition | |
|---|---|
| Dope temperature | 80 °C |
| Dope flow rate | 11–12 g/min |
| Bore fluid composition and flow rate | NMP 30%; 13 g/min |
| Bore fluid temperature | 50 °C |
| Outer coagulant | Tap water at room temperature |
| Air gap | 24 cm |
| Spinneret dimension | O.D.–I.D. 1.6–0.6 mm |
| Fiber | O.D. (mm) | I.D. (mm) | THICKNESS (mm) | POROSITY (%) |
|---|---|---|---|---|
| HF 1-0 | 1.85 ± 0.03 | 1.19 ± 0.02 | 0.33 ± 0.03 | 85.45 ± 0.18 |
| HF 1-0.5 | 1.77 ± 0.01 | 1.37 ± 0.02 | 0.20 ± 0.02 | 84.99 ± 0.29 |
| HF 2-0 | 2.22 ± 0.03 | 1.53 ± 0.02 | 0.34 ± 0.03 | 81.99 ± 1.88 |
| HF 2-0.5 | 2.05 ± 0.12 | 1.55 ± 0.11 | 0.25 ± 0.16 | 83.06 ± 0.64 |
| Fiber | Mechanical Tests | |
|---|---|---|
| Young Modulus (N/mm2) | ε Break (%) | |
| HF 1-0 | 41 ± 3 | 183 ± 15 |
| HF 1-0.5 | 92 ± 2 | 168 ± 21 |
| HF 2-0 | 54 ± 1 | 138 ± 8 |
| HF 2-0.5 | 69 ± 1 | 169 ± 2 |
| Measurement | HF 1-0 | HF 1-0.5 | HF 2-0 | HF 2-0.5 |
|---|---|---|---|---|
| Bubble point pressure (bar) | 0.77 ± 0.1 | 0.83 ± 0.15 | 0.83 ± 0.18 | 0.90 ± 0.12 |
| Smallest detected pore diameter (at 90% cff) | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 |
| Mean flow pore diameter (μm) | 0.13 ± 0.01 | 0.13 ± 0.03 | 0.14 ± 0.02 | 0.13 ± 0.03 |
| Largest detected pore diameter (μm) | 0.41 ± 0.08 | 0.55 ± 0.06 | 0.61 ± 0.07 | 0.51 ± 0.07 |
| HF | Reaction Rate kapp (min−1) | Area (m2) | SDR (m2∙min)−1 |
|---|---|---|---|
| HF 2-0 | 0.0033 | 0.0036 | 0.916 |
| HF 2-0.5 | 0.012 | 0.0036 | 3.33 |
| Membrane Materials | Main Results/Application | Reference |
|---|---|---|
| PVDF-g-PAA/TiO2 | TiO2-modified membranes assured a dye (RB5) removal in the range between 30% and 42% depending on titania concentration within 120 min UV lamp (254 nm, 15 W) operation | [20] |
| pDA/PVDF/Au-TiO2 | Au-TiO2/pDA/PVDF nanocomposite membranes led to a degradation ratio of 92% of tetracycline within 120 min under visible light irradiation (300 W xenon light source placed at a distance of 10 cm and provided with a filter in order to block the light in a wavelength of less than 420 nm). | [24] |
| PVDF/TiO2 | Membrane performance was evaluated in terms of pure water flux recovery under UV-A light (40 W, light intensity of 2.5 ± 0.2 mW/m2). The registered flux recovery ratios were about 100% within 60 min irradiation. | [27] |
| PVDF/TiO2 | PVDF/TiO2 membranes allowed to achieve >99% of RB5 dye removal after 60 min irradiation (UV-C, 15 W). | [28] |
| PVDF/TiO2 | HA-fouled membranes immerged in distilled water and irradiated with UV light (6 W, light intensity 0.04 mW/cm2). Water flux recovery increased by 11% when photocatalysis time was from 300 to 480 min. | [29] |
| PVDF/TiO2 | BG photocatalytic degradation ratio was ~81% after 450 min UV light irradiation; IC photocatalytic degradation ratio was ~89% after 300 min UV light irradiation. | [30] |
| PVDF/TiO2 | Pure water flux, measured during irradiation with a UV-lamp (9 W) reached 140 L/h m2 at 1 bar, preventing permeate flux decline observed in dark conditions. | [33] |
| PVDF-TrFE/TiO2 | MB aqueous solution (10−5 M, Ph 6.8) was irradiated by means of 6 UV-A lamps (8 W, light intensity ~3.7 mW/cm2). The prepared TiO2/PVDF-TrFE membranes, containing 3 wt % of titania, led to a MB degradation of 77% after 90 min irradiation. TiO2/NaY/P(VDF–TrFE) membranes prepared with 8 wt % of titania and 8 wt % zeolites allowed to degrade 96% MB after 40 min irradiation. | [38] |
| PVDF-TrFE/TiO2 and PVDF-TrFE/TiO2/GO | P(VDF-TrFE)/TiO2 and P(VDF)-TrFE/TiO2/GO membranes (20 wt % titania in both membrane types) in contact with MB aqueous solution (10−5 M) were irradiated with a high-power UV-A LED source (light intensity 4 mW/cm2), leading to a MB removal of 92–93% within 110 min irradiation. | [40] |
| PVDF/TiO2 | PVDF/TiO2 containing 0.5 wt % titania nanoparticles led to 97% MB degradation after 270 min irradiation with UV-A source (18 W, light intensity 2.7 mW/cm2). | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galiano, F.; Song, X.; Marino, T.; Boerrigter, M.; Saoncella, O.; Simone, S.; Faccini, M.; Chaumette, C.; Drioli, E.; Figoli, A. Novel Photocatalytic PVDF/Nano-TiO2 Hollow Fibers for Environmental Remediation. Polymers 2018, 10, 1134. https://doi.org/10.3390/polym10101134
Galiano F, Song X, Marino T, Boerrigter M, Saoncella O, Simone S, Faccini M, Chaumette C, Drioli E, Figoli A. Novel Photocatalytic PVDF/Nano-TiO2 Hollow Fibers for Environmental Remediation. Polymers. 2018; 10(10):1134. https://doi.org/10.3390/polym10101134
Chicago/Turabian StyleGaliano, Francesco, Xue Song, Tiziana Marino, Marcel Boerrigter, Omar Saoncella, Silvia Simone, Mirko Faccini, Christiane Chaumette, Enrico Drioli, and Alberto Figoli. 2018. "Novel Photocatalytic PVDF/Nano-TiO2 Hollow Fibers for Environmental Remediation" Polymers 10, no. 10: 1134. https://doi.org/10.3390/polym10101134
APA StyleGaliano, F., Song, X., Marino, T., Boerrigter, M., Saoncella, O., Simone, S., Faccini, M., Chaumette, C., Drioli, E., & Figoli, A. (2018). Novel Photocatalytic PVDF/Nano-TiO2 Hollow Fibers for Environmental Remediation. Polymers, 10(10), 1134. https://doi.org/10.3390/polym10101134










