Core-Shell Morphology of Redispersible Powders in Polymer-Cement Waterproof Mortars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses
2.3. Characterization of Polymer Dispersions
2.4. Spray-Drying
2.5. Polymer-Cement Mortars
2.6. Crack-Bridging Ability of the Polymer-Cement Mortar
3. Results and Discussion
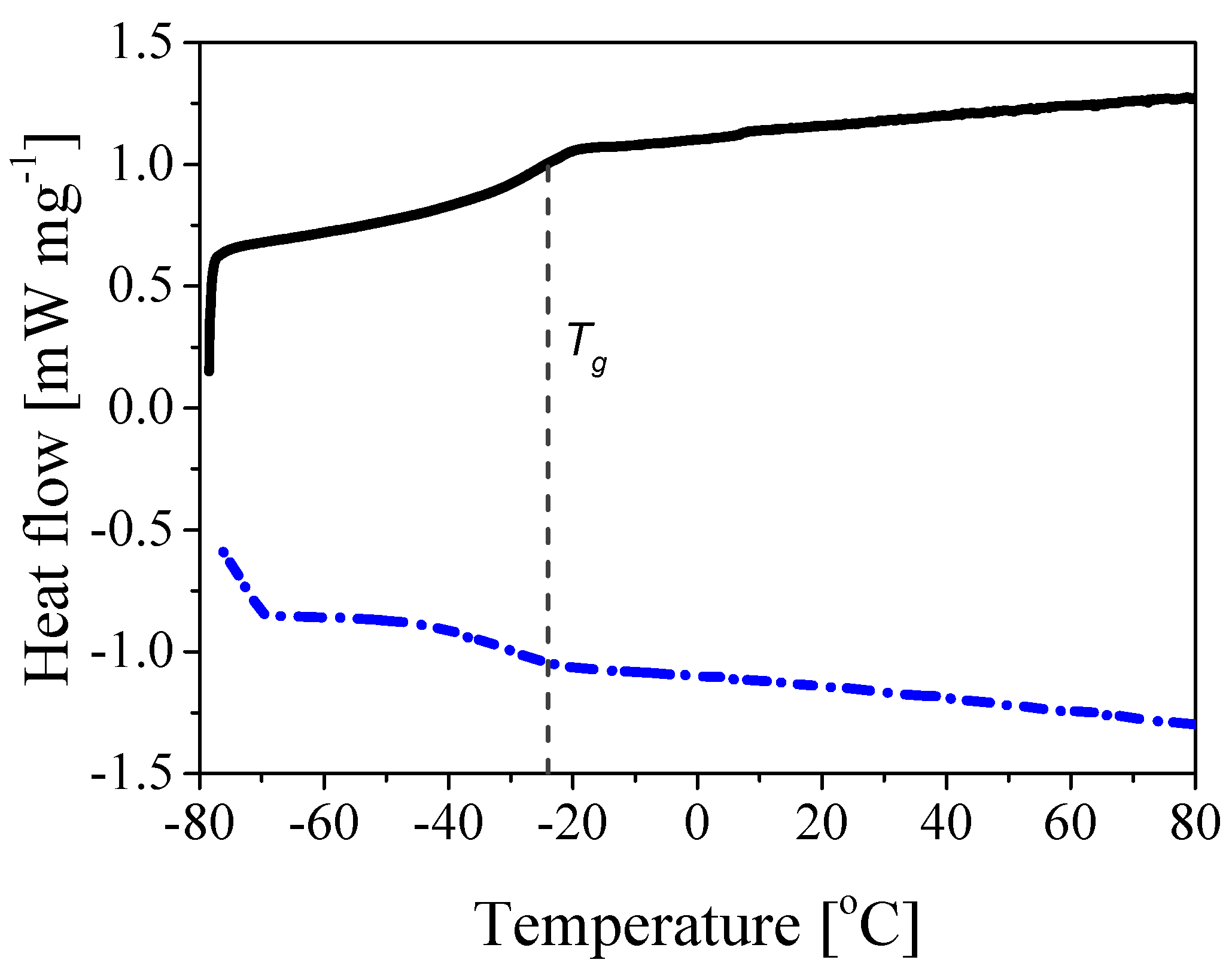
3.1. Synthesis of Core-Shell Polymer Nanoparticles
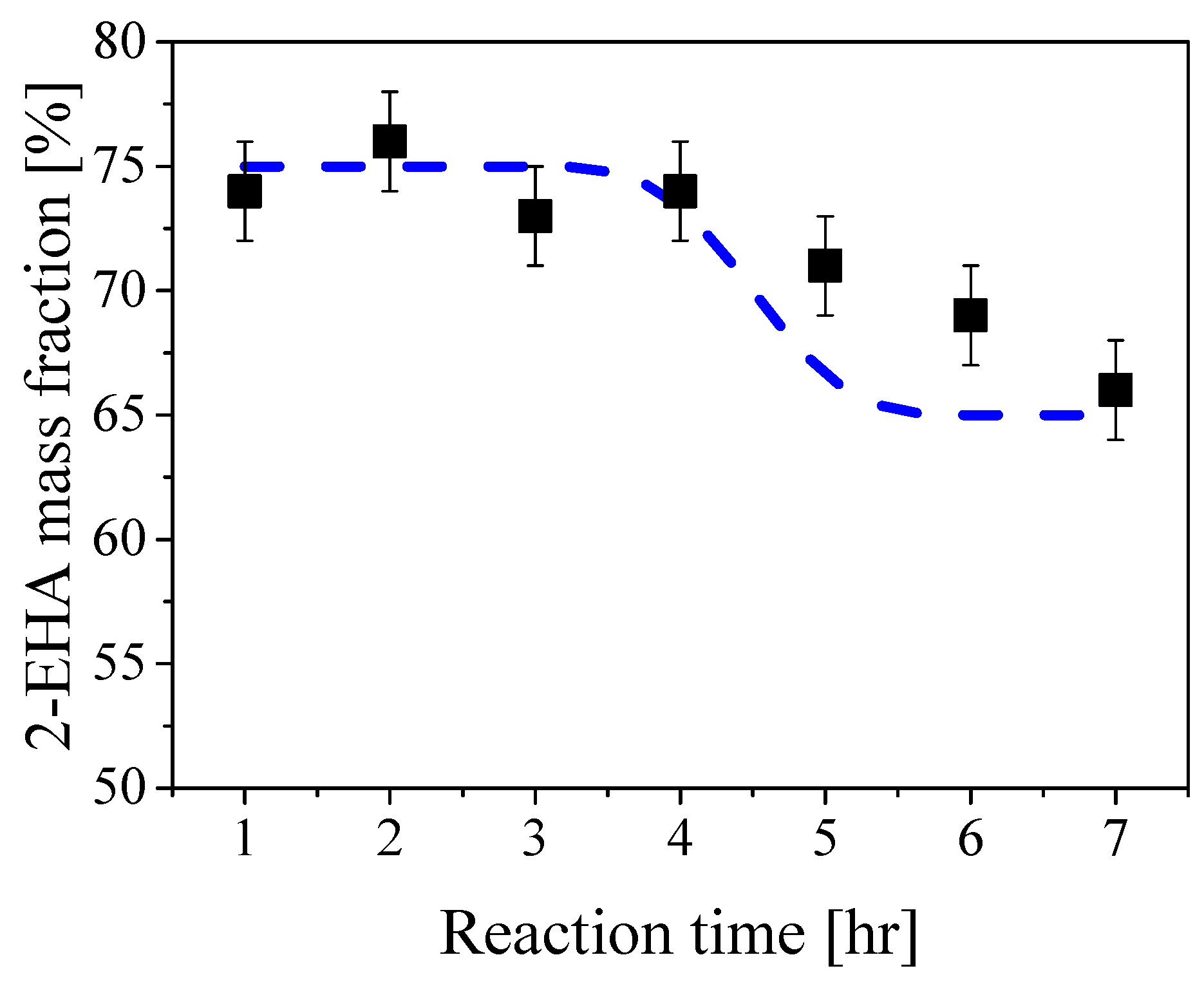
3.2. Spray Drying and Redispersibility
3.3. Crack-Bridging and Polymer-Cement Compatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohama, Y. Polymer-based admixtures. Cem. Concr. Compos. 1998, 20, 189–212. [Google Scholar] [CrossRef]
- Maranhão, F.L.; John, V.M. Bond strength and transversal deformation aging on cement-polymer adhesive mortar. Constr. Build. Mater. 2009, 23, 1022–1027. [Google Scholar] [CrossRef]
- Bayer, R.; Lutz, H. Dry mortars. Ullmann’s Encycl. Ind. Chem. [CrossRef]
- Wang, R.; Wang, P.M. Action of redispersible vinyl acetate and versatate copolymer powder in cement mortar. Constr. Build. Mater. 2011, 25, 4210–4214. [Google Scholar] [CrossRef]
- Mirza, J.; Mirza, M.; Lapointe, R. Laboratory and field performance of polymer-modified cement-based repair mortars in cold climates. Constr. Build. Mater. 2002, 16, 365–374. [Google Scholar] [CrossRef]
- Ohama, Y. Handbook of Polymer-Modified Concrete and Mortars: Properties and Process Technology; William Andrew: Norwich, NY, USA, 1995. [Google Scholar]
- Miller, M. Polymers in Cementitious Materials; iSmithers Rapra Publishing: Shawbury, UK, 2005. [Google Scholar]
- Jenni, A.; Holzer, L.; Zurbriggen, R.; Herwegh, M. Influence of polymers on microstructure and adhesive strength of cementitious tile adhesive mortars. Cem. Concr. Res. 2005, 35, 35–50. [Google Scholar] [CrossRef]
- Afridi, M.U.K.; Chaudhary, Z.U.; Ohama, Y.; Demura, K.; Iqbal, M.Z. Strength and elastic properties of powdered and aqueous polymer-modified mortars. Cem. Concr. Res. 1994, 24, 1199–1213. [Google Scholar] [CrossRef]
- Wang, R.; Ma, D.; Wang, P.; Wang, G. Study on waterproof mechanism of polymer-modified cement mortar. Mag. Concr. Res. 2015, 67, 972–979. [Google Scholar] [CrossRef]
- Fan, X.; Niu, L. Performance of redispersible polymer powders in wall coatings. J. Adhes. Sci. Technol. 2015, 29, 296–307. [Google Scholar] [CrossRef]
- Niu, L.; Lei, L.; Xia, Z. Redispersible polymer powder functionalized with NMA and its adhesive properties in dry-mixed coatings. J. Adhes. Sci. Technol. 2013, 27, 1432–1445. [Google Scholar] [CrossRef]
- Franks, F. Freeze-drying of bioproducts: Putting principles into practice. Eur. J. Pharm. Biopharm. 1998, 45, 221–229. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Christensen, K.; Pedersen, G.; Kristensen, H. Preparation of redispersible dry emulsions by spray drying. Int. J. Pharm. 2001, 212, 187–194. [Google Scholar] [CrossRef]
- Baumann, R.; Hofmann, S.D.; Kuehn, H.; Perello, M. Redispersible Polymer Powder from Polyolefin Dispersions and the Use Thereof in Construction Applications. U.S. Patent 8,802,767, 31 December 2014. [Google Scholar]
- Bett, B.; Richard, J. Water-Redispersible Powders of Film-Forming Polymers with a “Core/Shell” Structure. U.S. Patent 5,872,189, 16 February 1999. [Google Scholar]
- Zhang, X.; Pei, Y.; Xie, D.; Chen, H. Modeling spray drying of redispersible polyacrylate powder. Dry. Technol. 2014, 32, 222–235. [Google Scholar] [CrossRef]
- Masters, K. Spray Drying Handbook; Godwin: London, UK, 1985. [Google Scholar]
- Du Chesne, A.; Bojkova, A.; Gapinski, J.; Seip, D.; Fischer, P. Film formation and redispersion of waterborne latex coatings. J. Colloid Interface Sci. 2000, 224, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Halima, N.B. Poly (vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Nampi, P.P.; Kume, S.; Hotta, Y.; Watari, K.; Itoh, M.; Toda, H.; Matsutani, A. The effect of polyvinyl alcohol as a binder and stearic acid as an internal lubricant in the formation, and subsequent sintering of spray-dried alumina. Ceram. Int. 2011, 37, 3445–3450. [Google Scholar] [CrossRef]
- Jang, D.J.; Jeong, E.J.; Lee, H.M.; Kim, B.C.; Lim, S.J.; Kim, C.K. Improvement of bioavailability and photostability of amlodipine using redispersible dry emulsion. Eur. J. Pharm. Sci. 2006, 28, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Saija, L.M.; Uminski, M. Water-redispersible low-Tg acrylic powders for the modification of hydraulic binder compositions. J. Appl. Polym. Sci. 1999, 71, 1781–1787. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, B.; Shen, J. Morphologies of Polymer Grains During Spray Drying. Dry. Technol. 2013, 31, 433–438. [Google Scholar] [CrossRef]
- Calvo, L.M.; Garuti, J., Jr. Compositions for Use in Construction and Methods of Applying the Same. U.S. Patent 8,187,375, 29 May 2012. [Google Scholar]
- Xia, Z.; Zhang, X.; Situ, Y.; Chen, H.; Mei, C. Dry-Powder Latex Coating for Internal Wall and Its Production. CN Patent 101,481,584A, 15 July 2009. [Google Scholar]
- Hong, L.; Chen, L.; Ladika, M.; Li, Y.; Kim-Habermehl, L.; Bergman, R. Impact of particle size and surface charge density on redispersibility of spray-dried powders. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 274–281. [Google Scholar] [CrossRef]
- Wang, F.; Luo, Y.; Li, B.G.; Zhu, S. Synthesis and redispersibility of poly (styrene-block-n-butyl acrylate) core–shell latexes by emulsion polymerization with RAFT agent–surfactant design. Macromolecules 2015, 48, 1313–1319. [Google Scholar] [CrossRef]
- Rauh, A.; Rey, M.; Barbera, L.; Zanini, M.; Karg, M.; Isa, L. Compression of hard core–soft shell nanoparticles at liquid–liquid interfaces: influence of the shell thickness. Soft Matter 2017, 13, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Li, J.J.; Williams, G.R.; Zhao, M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J. Control. Release 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Yang, Y.Y.; Yu, D.G.; Du, Q.; Yang, X.L. Fast dissolving drug delivery membrane based on the ultra-thin shell of electrospun core-shell nanofibers. Eur. J. Pharm. Sci. 2018, 122, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Yu, D.G.; Zhu, M.J.; Zhao, M.; Williams, G.R. Tunable zero-order drug delivery systems created by modified triaxial electrospinning. Chem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Raines, C.C.; Starmer, P.H. Free Flowing Particles of an Emulsion Polymer Having SiO2 Incorporated Therein. U.S. Patent 5,017,630, 21 May 1991. [Google Scholar]
- Gonzalez-Leon, J.A.; Ryu, S.W.; Hewlett, S.A.; Ibrahim, S.H.; Mayes, A.M. Core-Shell Polymer Nanoparticles for Baroplastic Processing. Macromolecules 2005, 38, 8036–8044. [Google Scholar] [CrossRef]
- Caimi, S.; Cingolani, A.; Jaquet, B.; Siggel, M.; Lattuada, M.; Morbidelli, M. Tracking of Fluorescently Labeled Polymer Particles Reveals Surface Effects during Shear-Controlled Aggregation. Langmuir 2017, 33, 14038–14044. [Google Scholar] [CrossRef] [PubMed]
- Yamak, H.B. Emulsion polymerization: effects of polymerization variables on the properties of vinyl acetate based emulsion polymers. In Polymer Science; InTech: London, UK, 2013. [Google Scholar]
- Asua, J.M. Emulsion polymerization: from fundamental mechanisms to process developments. J. Polym. Sci. A Polym. Chem. 2004, 42, 1025–1041. [Google Scholar] [CrossRef]
- Pungor, E.; Horvai, G. A Practical Guide to Instrumental Analysis; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- European Standardization Organizations. Liquid-Applied Water Impermeable Products for Use Beneath Ceramic Tiling Bonded with Adhesives—Requirements, Test Methods, Evaluation of Conformity, Classification and Designation Standard; European Standards DIN EN 14891/2017; European Standardization Organizations: Brussels, Belgium, 2017. [Google Scholar]
- Fox, T.G. Influence of Diluent and of Copolymer Composition on the Glass Temperature of a Polymer System. Bull. Am. Phys. Soc. 1956, 1, 123. [Google Scholar]
- Hiemenz, P.C.; Lodge, T.P. Polymer Chemistry; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Lu, Z.; Kong, X.; Zhang, C.; Cai, Y.; Zhang, Q.; Zhang, Y. Effect of polymer latexes with varied glass transition temperature on cement hydration. J. Appl. Polym. Sci. 2017, 134, 45264. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, H.; Wiseman, N. Emulsifier-free emulsion copolymerization of styrene with quaternary ammonium cationic monomers. J. Appl. Polym. Sci. 2000, 76, 1129–1140. [Google Scholar] [CrossRef]
- Bohórquez, S.J.; Asua, J.M. Particle nucleation in high solids batch miniemulsion polymerization stabilized with a polymeric surfactant. J. Polym. Sci. A Polym. Chem. 2008, 46, 6407–6415. [Google Scholar] [CrossRef]
- Gauthier, C.; Sindt, O.; Vigier, G.; Guyot, A.; Schoonbrood, H.; Unzue, M.; Asua, J. Reactive surfactants in heterophase polymerization. XVII. Influence of the surfactant on the mechanical properties and hydration of the films. J. Appl. Polym. Sci. 2002, 84, 1686–1700. [Google Scholar] [CrossRef]
- Guyot, A. Advances in reactive surfactants. Adv. Colloid Interface Sci. 2004, 108, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Jing, Y.; Lin, Z. The application research of environment-friendly reactive surfactants in acrylate emulsion pressure sensitive adhesives. Int. J. Adhes. Adhes. 2017, 73, 1–7. [Google Scholar] [CrossRef]
- Mulvihill, J.; Toussaint, A.; Wilde, M.D. Onset, follow up and assessment of coalescence. Prog. Org. Coat. 1997, 30, 127–139. [Google Scholar] [CrossRef]
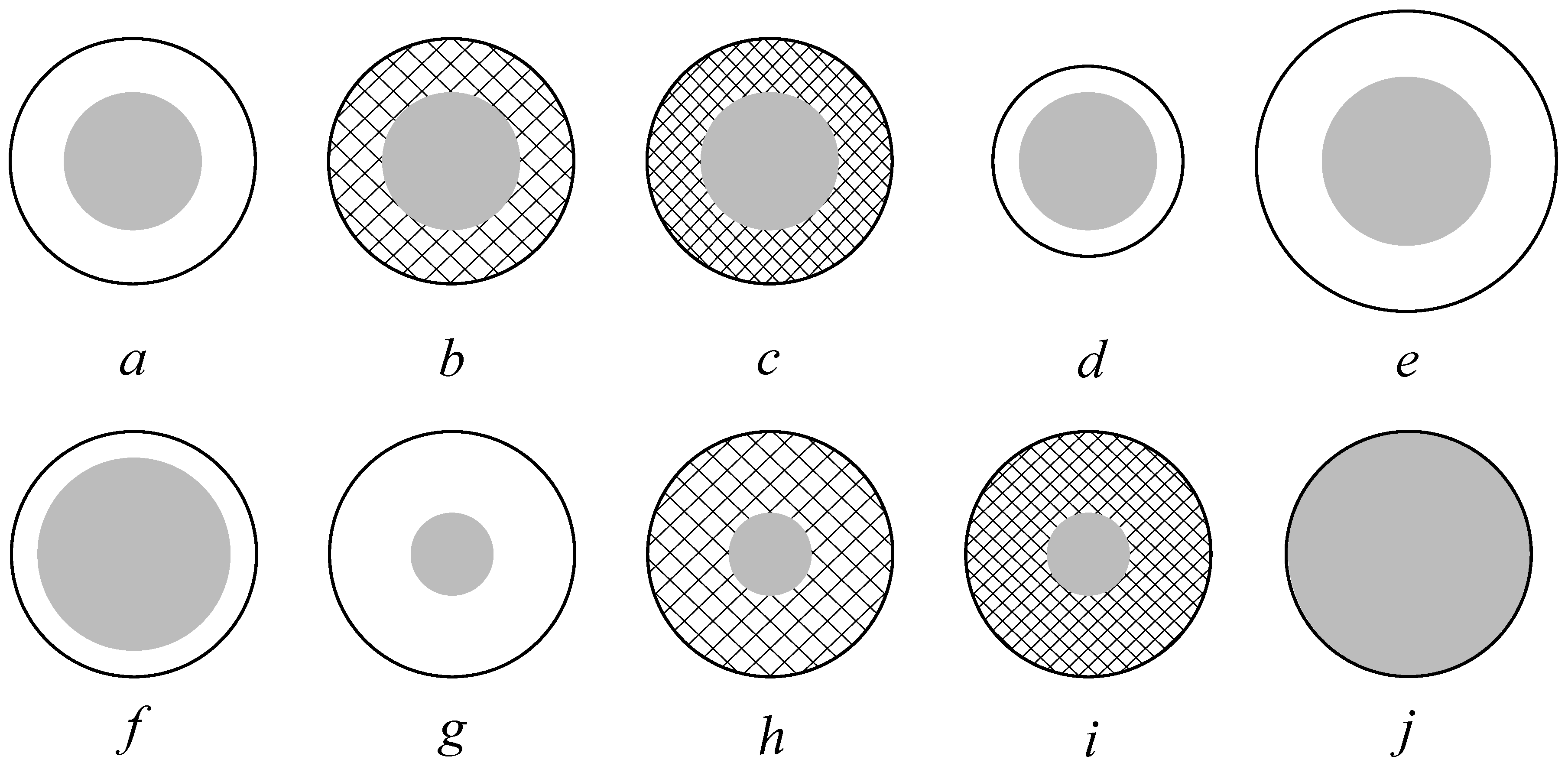




| Sample | a | b | c | d | e | f | g | h | i | j |
|---|---|---|---|---|---|---|---|---|---|---|
| Core size [nm] | 280 | 278 | 284 | 276 | 279 | 285 | 255 | 258 | 260 | 296 |
| NPs size [nm] | 302 | 296 | 301 | 286 | 311 | 294 | 293 | 294 | 303 | |
| PDI | 0.01 | 0.03 | 0.02 | 0.06 | 0.04 | 0.02 | 0.07 | 0.06 | 0.01 | 0.02 |
| Core [C] | −24.0 | −27.6 | −24.2 | −25.6 | −25.0 | −24.4 | −21.5 | −25.2 | −22.7 | −24.7 |
| Shell [C] | 65.8 | 74.2 | 86.7 | 65.8 | 65.8 | 65.8 | 65.8 | 74.2 | 86.7 |
| Sample | a | b | c | d | e | f | g | h | i |
|---|---|---|---|---|---|---|---|---|---|
| Grain size | mp | mp | mp | cp | fp | mp | fp | fp | fp |
| Free-flowing properties | ff | ff | ff | ca | ff | ff | ff | ff | ff |
| Redispersibility | gr | mr | mr | mr | gr | gr | mr | br | br |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caimi, S.; Timmerer, E.; Banfi, M.; Storti, G.; Morbidelli, M. Core-Shell Morphology of Redispersible Powders in Polymer-Cement Waterproof Mortars. Polymers 2018, 10, 1122. https://doi.org/10.3390/polym10101122
Caimi S, Timmerer E, Banfi M, Storti G, Morbidelli M. Core-Shell Morphology of Redispersible Powders in Polymer-Cement Waterproof Mortars. Polymers. 2018; 10(10):1122. https://doi.org/10.3390/polym10101122
Chicago/Turabian StyleCaimi, Stefano, Elias Timmerer, Michela Banfi, Giuseppe Storti, and Massimo Morbidelli. 2018. "Core-Shell Morphology of Redispersible Powders in Polymer-Cement Waterproof Mortars" Polymers 10, no. 10: 1122. https://doi.org/10.3390/polym10101122
APA StyleCaimi, S., Timmerer, E., Banfi, M., Storti, G., & Morbidelli, M. (2018). Core-Shell Morphology of Redispersible Powders in Polymer-Cement Waterproof Mortars. Polymers, 10(10), 1122. https://doi.org/10.3390/polym10101122






