Mechanochemically Carboxylated Multilayer Graphene for Carbon/ABS Composites with Improved Thermal Conductivity
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Production of Carbon-Based Nanofillers
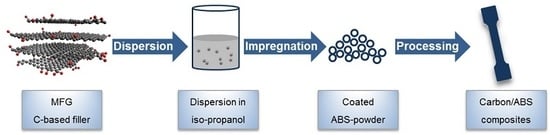
2.3. Preparation of Carbon/ABS Nanocomposites
2.4. Instrumental Analysis
3. Results and Discussion
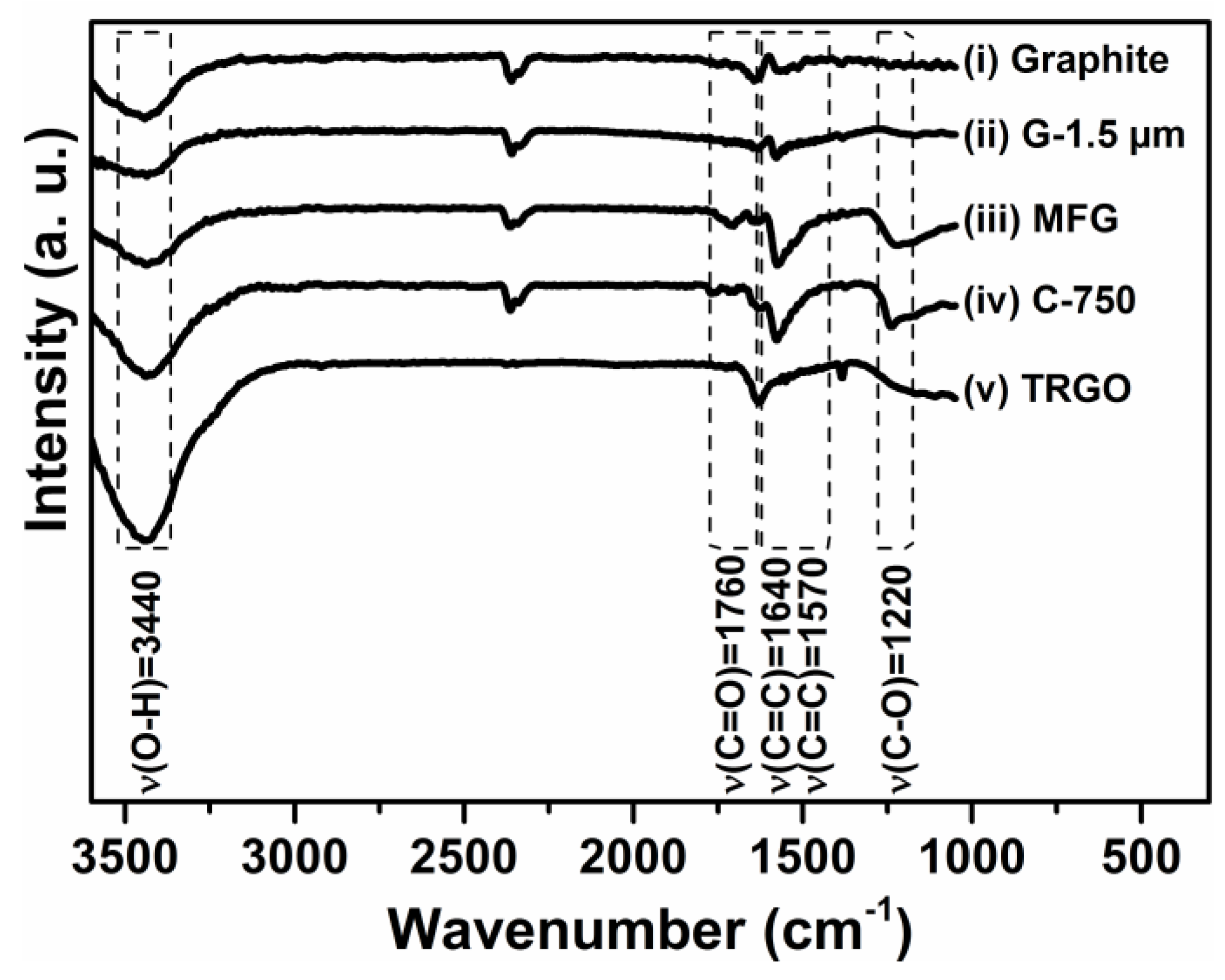
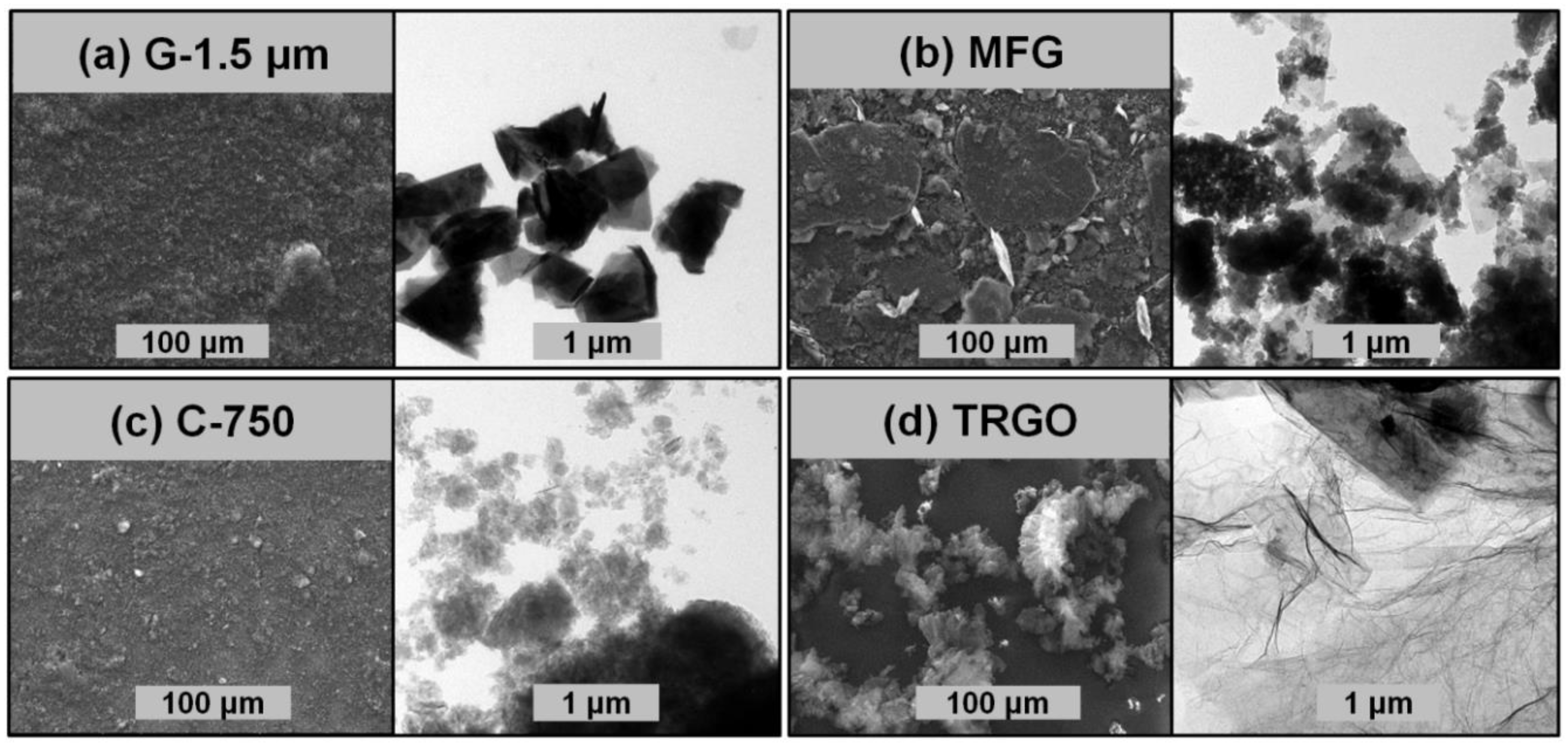
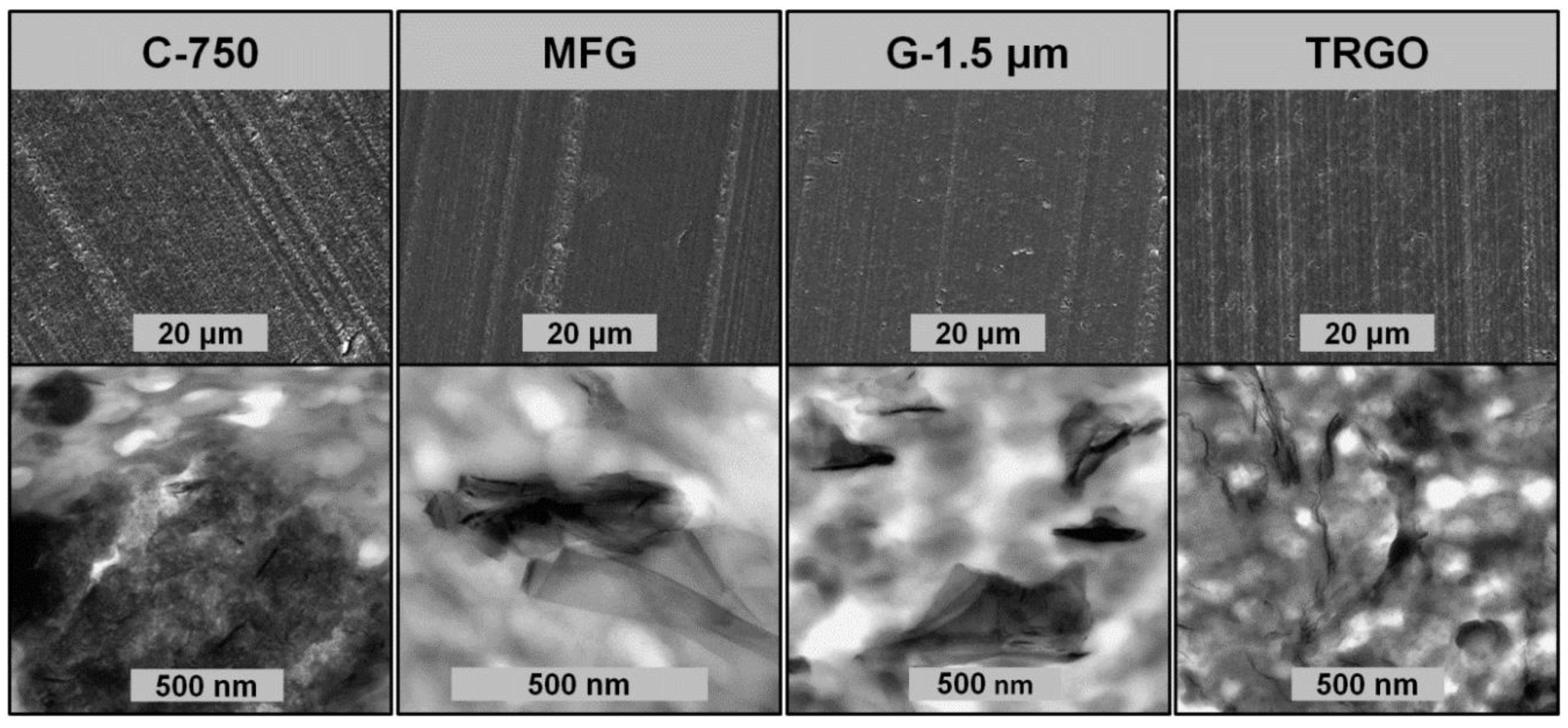
3.1. Carbon Fillers Derived from Graphite
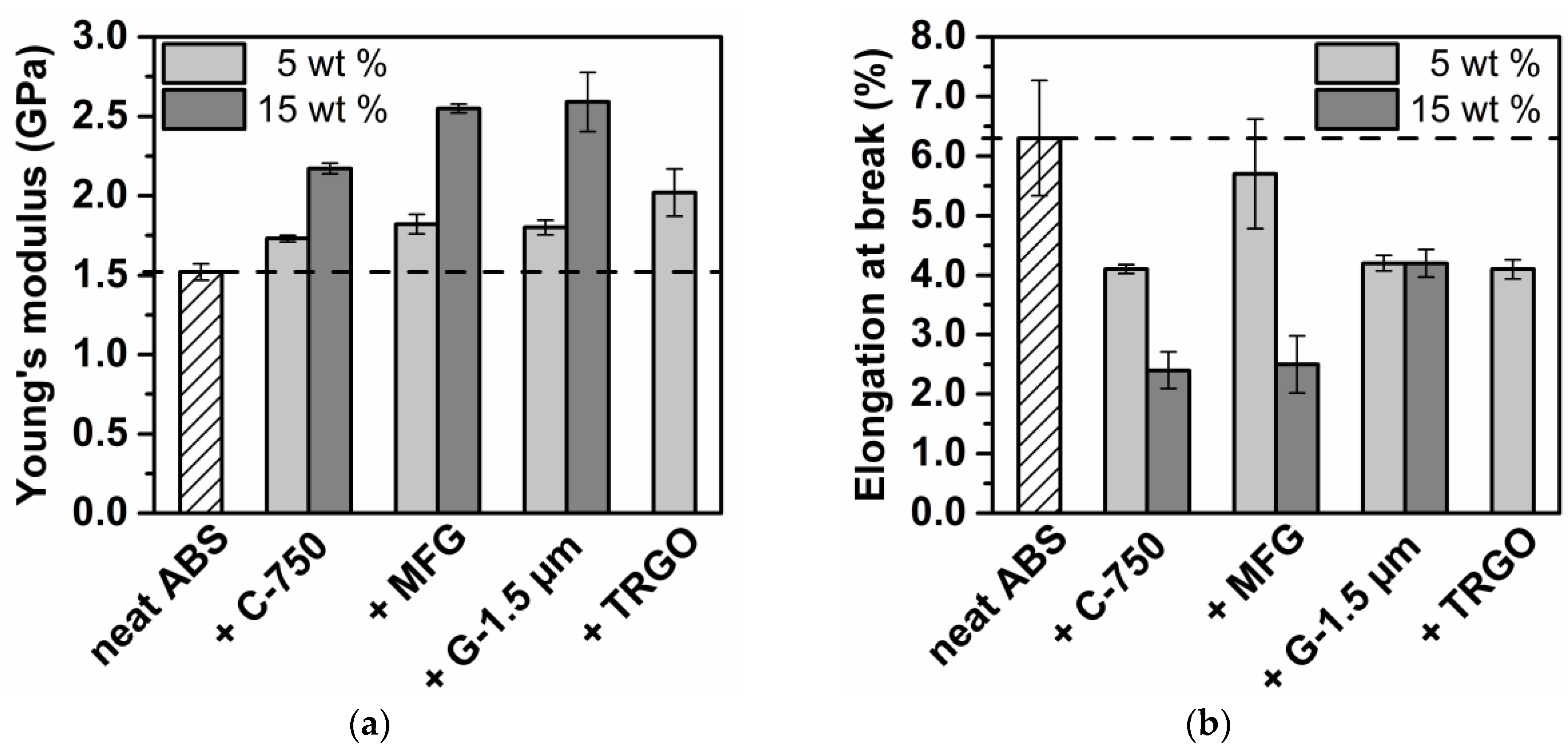
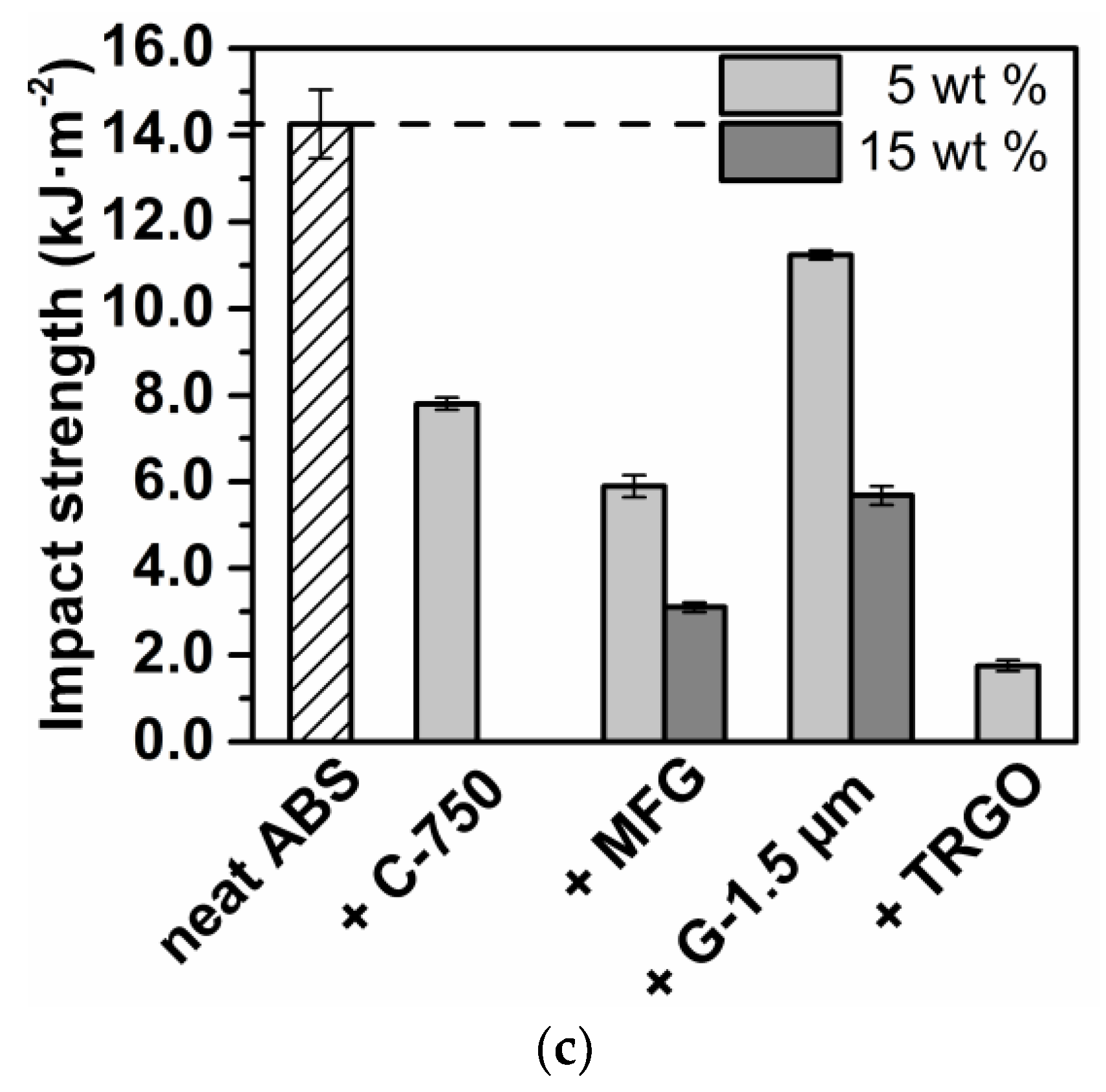
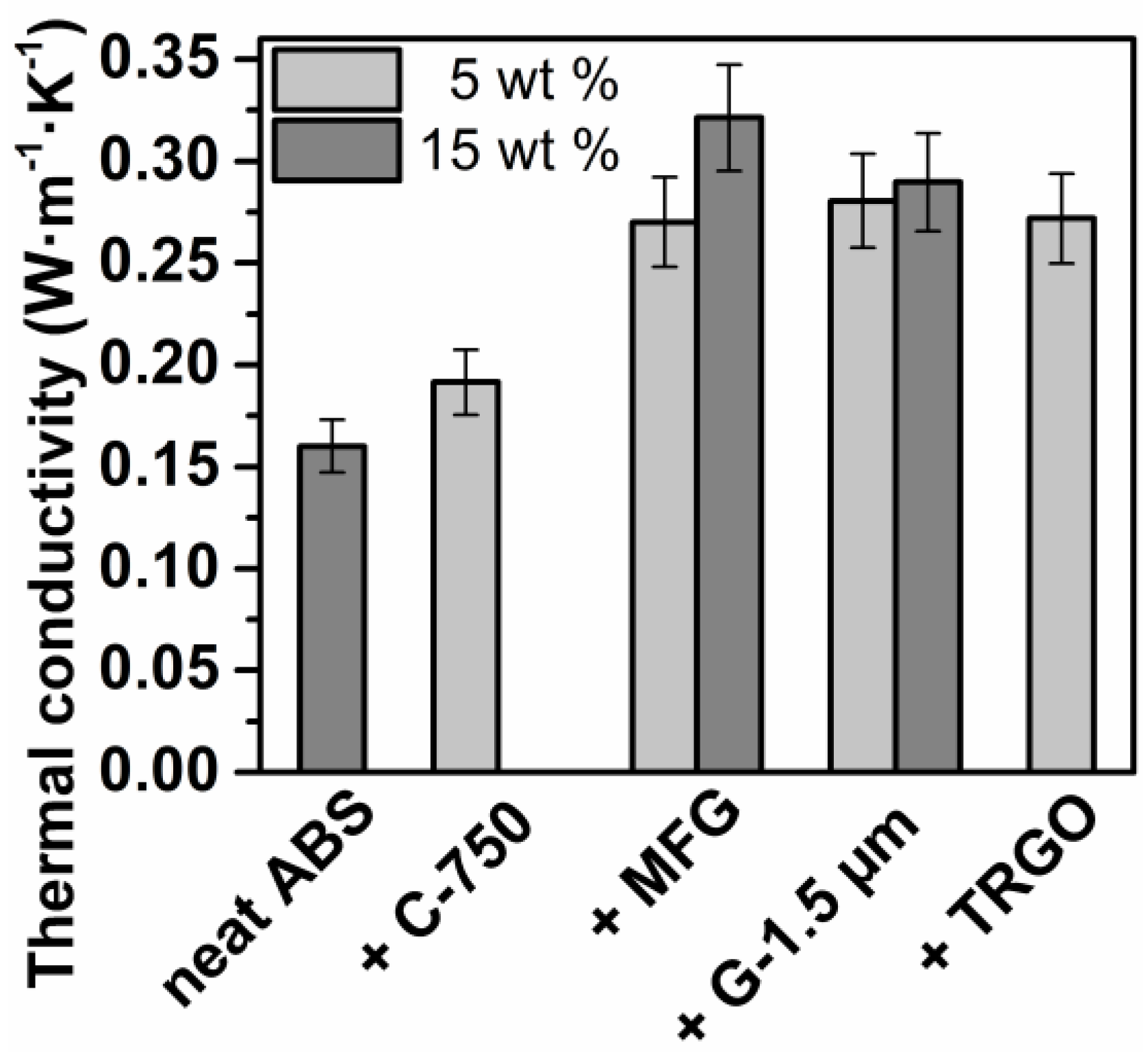
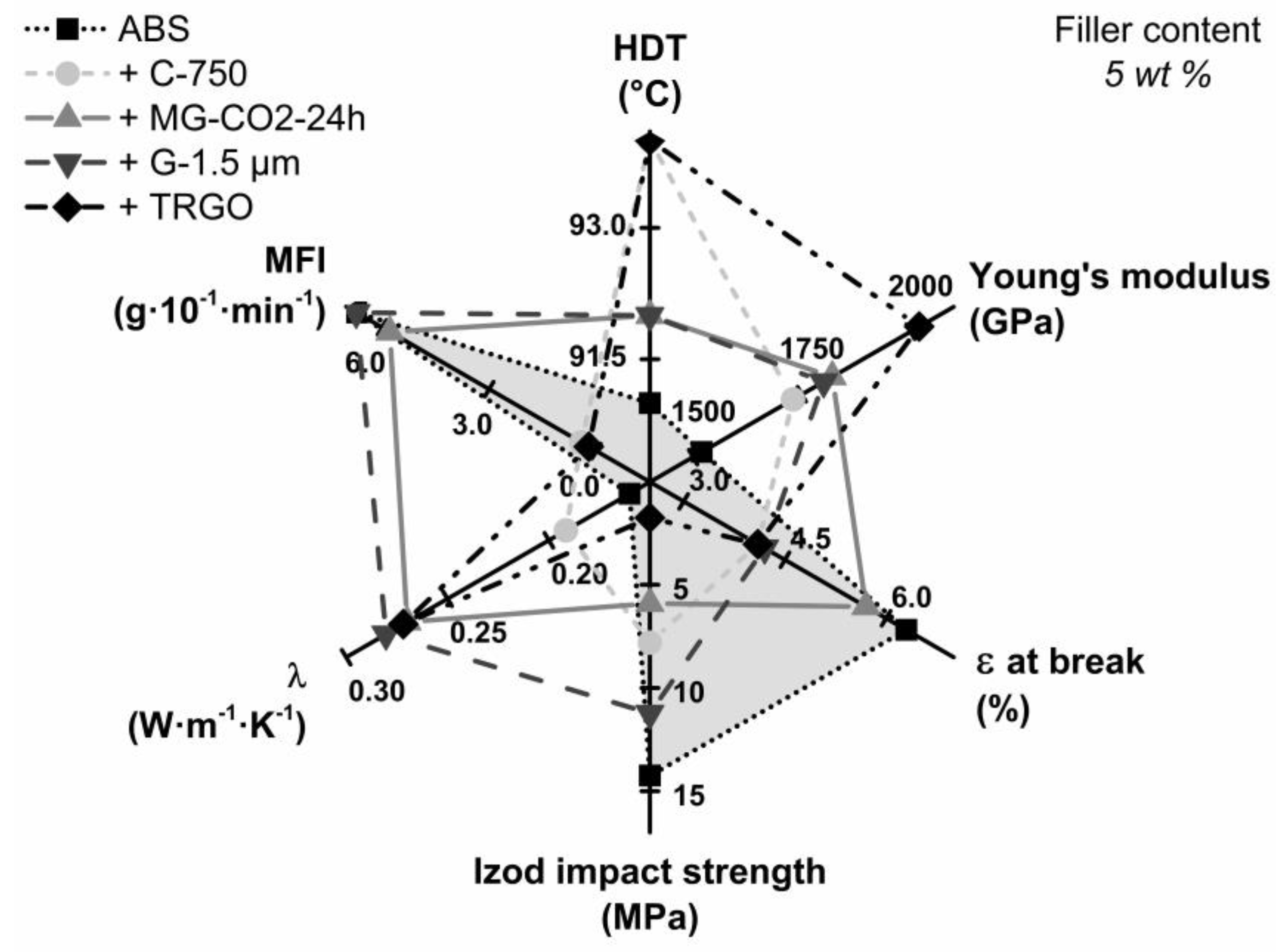
3.2. Carbon/ABS Nanocomposites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Chen, J.; Zhou, J.; Li, B. Thermal Conductivity of Polymers and Their Nanocomposites. Adv. Mater. 2018, 30, e1705544. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Krupka, J.; Strupinski, W. Measurements of the sheet resistance and conductivity of thin epitaxial graphene and SiC films. Appl. Phys. Lett. 2010, 96, 082101. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Holleman, A.F.; Wiberg, E.; Wiberg, N. Lehrbuch der Anorganischen Chemie; Auflage: 102., stark umgearb. u. verb.; De Gruyter: Berlin, Germany, 2007; ISBN 3110177706. [Google Scholar]
- Ramanathan, T.; Stankovich, S.; Dikin, D.A.; Liu, H.; Shen, H.; Nguyen, S.T.; Brinson, L.C. Graphitic nanofillers in PMMA nanocomposites—An investigation of particle size and dispersion and their influence on nanocomposite properties. J. Polym. Sci. B Polym. Phys. 2007, 45, 2097–2112. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Plath, A.; Beckert, F.; Tölle, F.J.; Sturm, H.; Mülhaupt, R. Stable aqueous dispersions of functionalized multi-layer graphene by pulsed underwater plasma exfoliation of graphite. J. Phys. D Appl. Phys. 2016, 49, 45301. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Luo, F.; Wu, H.; Liu, Y.; Zhang, C.; Chen, J. One-Step Ionic-Liquid-Assisted Electrochemical Synthesis of Ionic-Liquid-Functionalized Graphene Sheets Directly from Graphite. Adv. Funct. Mater. 2008, 18, 1518–1525. [Google Scholar] [CrossRef]
- Su, C.-Y.; Lu, A.-Y.; Xu, Y.; Chen, F.-R.; Khlobystov, A.N.; Li, L.-J. High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 2011, 5, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Wu, Z.-S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Müllen, K. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, A.M.; Vallés, C.; Cooper, A.J.; Kinloch, I.A.; Dryfe, R.A.W. Alkali reduction of graphene oxide in molten halide salts: Production of corrugated graphene derivatives for high-performance supercapacitors. ACS Nano 2014, 8, 11225–11233. [Google Scholar] [CrossRef] [PubMed]
- MacCrum, N.G.; Buckley, C.P.; Bucknall, C.B. Principles of Polymer Engineering, 2nd ed.; Oxford University Press: Oxford, UK, 2003; ISBN 0-19-856526-7. [Google Scholar]
- Fukushima, H.; Drzal, L.T.; Rook, B.P.; Rich, M.J. Thermal conductivity of exfoliated graphite nanocomposites. J. Therm. Anal. Calorim. 2006, 85, 235–238. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. Multifunctional polypropylene composites produced by incorporation of exfoliated graphite nanoplatelets. Carbon 2007, 45, 1446–1452. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Tölle, F.J.; Fabritius, M.; Mülhaupt, R. Emulsifier-Free Graphene Dispersions with High Graphene Content for Printed Electronics and Freestanding Graphene Films. Adv. Funct. Mater. 2012, 22, 1136–1144. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Navarro, C.; Burghard, M.; Kern, K. Elastic properties of chemically derived single graphene sheets. Nano Lett. 2008, 8, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
- Schwamb, T.; Burg, B.R.; Schirmer, N.C.; Poulikakos, D. An electrical method for the measurement of the thermal and electrical conductivity of reduced graphene oxide nanostructures. Nanotechnology 2009, 20, 405704. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Bae, S.-Y.; Seo, J.-M.; Baek, J.-B. Scalable Production of Edge-Functionalized Graphene Nanoplatelets via Mechanochemical Ball-Milling. Adv. Funct. Mater. 2015, 25, 6961–6975. [Google Scholar] [CrossRef]
- Ong, T.S.; Yang, H. Effect of atmosphere on the mechanical milling of natural graphite. Carbon 2000, 38, 2077–2085. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Shin, Y.-R.; Sohn, G.-J.; Choi, H.-J.; Bae, S.-Y.; Mahmood, J.; Jung, S.-M.; Seo, J.-M.; Kim, M.-J.; Wook Chang, D.; et al. Edge-carboxylated graphene nanosheets via ball milling. Proc. Natl. Acad. Sci. USA 2012, 109, 5588–5593. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Jeon, I.-Y.; Seo, J.-M.; Dai, L.; Baek, J.-B. Graphene phosphonic acid as an efficient flame retardant. ACS Nano 2014, 8, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. A new compounding method for exfoliated graphite–polypropylene nanocomposites with enhanced flexural properties and lower percolation threshold. Compos. Sci. Technol. 2007, 67, 2045–2051. [Google Scholar] [CrossRef]
- Caradonna, A.; Colucci, G.; Giorcelli, M.; Frache, A.; Badini, C. Thermal behavior of thermoplastic polymer nanocomposites containing graphene nanoplatelets. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Waheed, Q.; Khan, A.N.; Jan, R. Investigating the reinforcement effect of few layer graphene and multi-walled carbon nanotubes in acrylonitrile-butadiene-styrene. Polymer 2016, 97, 496–503. [Google Scholar] [CrossRef]
- Pour, R.H.; Hassan, A.; Soheilmoghaddam, M.; Bidsorkhi, H.C. Mechanical, thermal, and morphological properties of graphene reinforced polycarbonate/acrylonitrile butadiene styrene nanocomposites. Polym. Compos. 2016, 37, 1633–1640. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Zhang, B.B.; Hong, R.Y.; Kumar, M.R.; Xie, C.R. Enhanced electrical conductivity and mechanical properties of ABS/EPDM composites filled with graphene. Compos. Part B Eng. 2015, 83, 66–74. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, S.; Wang, F.; Wen, B.; Han, C.; Ding, Y.; Yang, M. Graphene networks with low percolation threshold in ABS nanocomposites: Selective localization and electrical and rheological properties. ACS Appl. Mater. Interfaces 2014, 6, 12252–12260. [Google Scholar] [CrossRef] [PubMed]
- Dul, S.; Fambri, L.; Pegoretti, A. Fused deposition modelling with ABS–graphene nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 85, 181–191. [Google Scholar] [CrossRef]
- Memarian, F.; Fereidoon, A.; Ghorbanzadeh Ahangari, M. The shape memory, and the mechanical and thermal properties of TPU/ABS/CNT: A ternary polymer composite. RSC Adv. 2016, 6, 101038–101047. [Google Scholar] [CrossRef]
- Beckert, F.; Bodendorfer, S.; Zhang, W.; Thomann, R.; Mülhaupt, R. Mechanochemical Route to Graphene-Supported Iron Catalysts for Olefin Polymerization and in Situ Formation of Carbon/Polyolefin Nanocomposites. Macromolecules 2014, 47, 7036–7042. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spektroskopische Methoden der Organichen Chemie, 7th ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2005. [Google Scholar]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.B. Introduction to Organic Spectroscopy; Macmillan: New York, NY, USA, 1987; ISBN 0023673001. [Google Scholar]
- Steurer, P.; Wissert, R.; Thomann, R.; Mülhaupt, R. Functionalized Graphenes and Thermoplastic Nanocomposites Based upon Expanded Graphite Oxide. Macromol. Rapid Commun. 2009, 30, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dékány, I. Evolution of Surface Functional Groups in a Series of Progressively Oxidized Graphite Oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
| Sample | C (wt %) a | H (wt %) a | O (wt %) b | σ (S∙cm−1) c | BET (m2∙g−1) e |
|---|---|---|---|---|---|
| Graphite | 99.6 | 0.2 | 1.2 | 3440 d | 1 |
| G-1.5 µm | 96.7 | 0.2 | 5.4 | 1.2 | 50 |
| MFG | 92.1 | 0.6 | 8.7 | 2.1∙10−1 | 261 |
| C-750 | 88.8 | 1.1 | 8.8 | 0.3∙10−1 | 770 |
| TRGO | 82.4 | 0.8 | 15.7 | 16.0 | 520 |
| Filler Type (wt %) | G-1.5 µm (g∙10−1∙min−1) | MFG (g∙10−1∙min−1) | C-750 (g∙10−1∙min−1) | TRGO (g∙10−1∙min−1) |
|---|---|---|---|---|
| 5 | 6.7 | 5.8 | 0,4 | 0.2 |
| 15 | 1.7 | 0.6 | n.d. b | n.d. b |
| Sample Code | Young’s Modulus b (MPa) | Elongation at Break b (%) | Impact Strength b (kJ∙m−2) | Electrical Conductivity (S∙cm−1) | Thermal Conductivity b (W∙m−1∙k−1) | HDT a (°C) |
|---|---|---|---|---|---|---|
| ABS | 1520 ± 50 | 6.3 ± 0.8 | 14.3 ± 0.8 | n. d. | 0.160 ± 0.013 | 91 |
| +5% C-750 | 1720 ± 20 | 4.1 ± 0.1 | 7.8 ± 0.1 | n. d. | 0.192 ± 0.016 | 94 |
| +15% C-750 | 2170 ± 40 | 2.4 ± 0.3 | n. d. | n. d. | n. d. | - |
| +5% MFG | 1790 ± 30 | 5.7 ± 0.6 c | 5.9 ± 0.3 | n. d. | 0.270 ± 0.022 | 92 |
| +15% MFG | 2550 ± 30 | 2.5 ± 0.3 | 3.1 ± 0.1 | n. d. | 0.321 ± 0.026 | 99 |
| +5% G-1.5 µm | 1850 ± 20 | 4.3 ±.0.1 | 11.2 ± 0.1 | n. d. | 0.280 ± 0.023 | 92 |
| +15% G-1.5 µm | 2740 ± 80 | 4.1 ± 0.2 | 5.7 ± 0.2 | n. d. | 0.290 ± 0.024 | 93 |
| +5% TRGO | 1930 ± 20 | 3.8 ± 0.2 | 1.8 ± 0.1 | 2 ∙ 10−7 | 0.272 ± 0.022 | 94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burk, L.; Gliem, M.; Lais, F.; Nutz, F.; Retsch, M.; Mülhaupt, R. Mechanochemically Carboxylated Multilayer Graphene for Carbon/ABS Composites with Improved Thermal Conductivity. Polymers 2018, 10, 1088. https://doi.org/10.3390/polym10101088
Burk L, Gliem M, Lais F, Nutz F, Retsch M, Mülhaupt R. Mechanochemically Carboxylated Multilayer Graphene for Carbon/ABS Composites with Improved Thermal Conductivity. Polymers. 2018; 10(10):1088. https://doi.org/10.3390/polym10101088
Chicago/Turabian StyleBurk, Laura, Matthias Gliem, Fabian Lais, Fabian Nutz, Markus Retsch, and Rolf Mülhaupt. 2018. "Mechanochemically Carboxylated Multilayer Graphene for Carbon/ABS Composites with Improved Thermal Conductivity" Polymers 10, no. 10: 1088. https://doi.org/10.3390/polym10101088
APA StyleBurk, L., Gliem, M., Lais, F., Nutz, F., Retsch, M., & Mülhaupt, R. (2018). Mechanochemically Carboxylated Multilayer Graphene for Carbon/ABS Composites with Improved Thermal Conductivity. Polymers, 10(10), 1088. https://doi.org/10.3390/polym10101088