Synthesis of Three-Dimensional Hierarchical Urchinlike Tungsten Trioxide Microspheres for High-Performance Supercapacitor Electrode
Abstract
1. Introduction
2. Materials and Methods
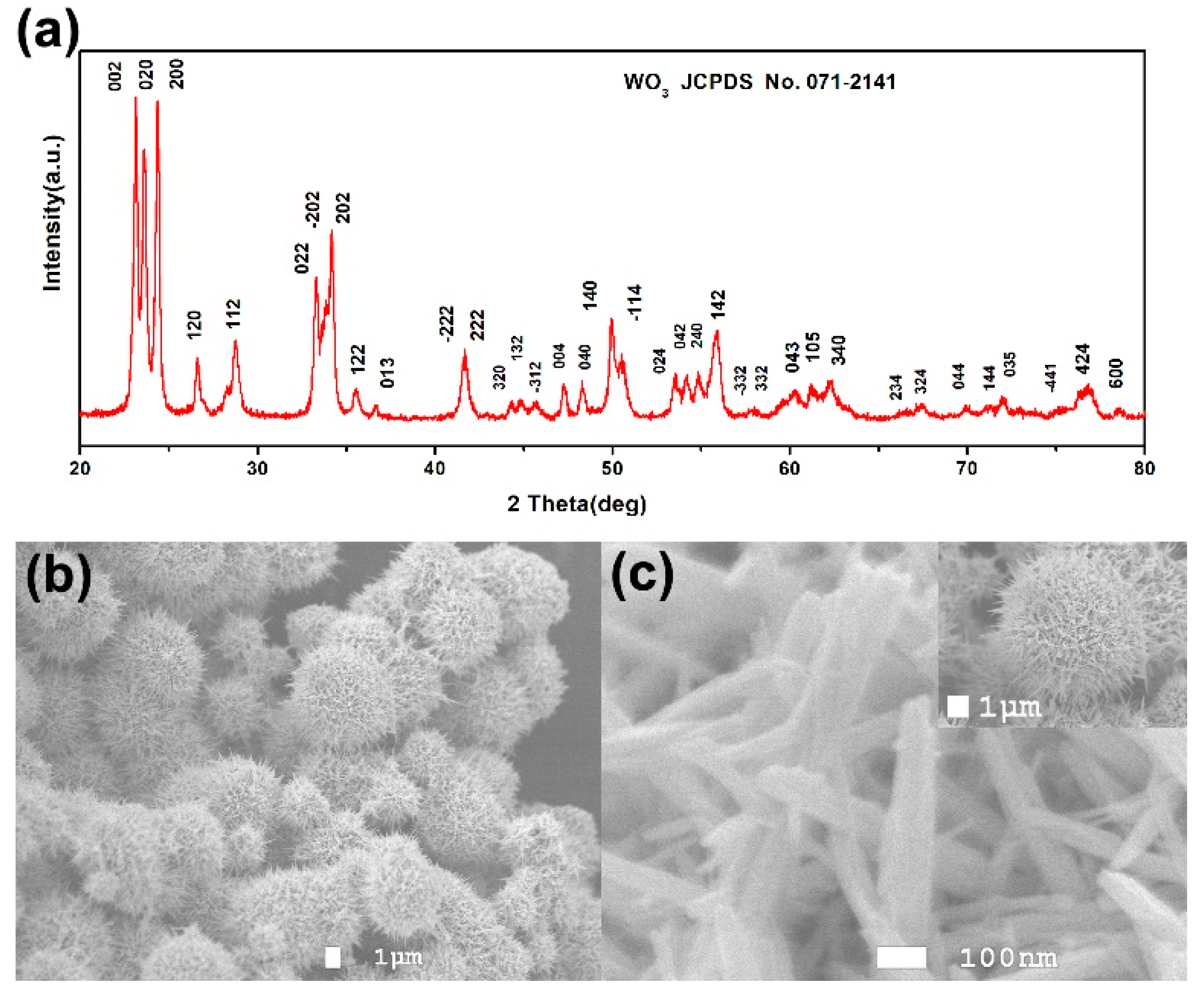
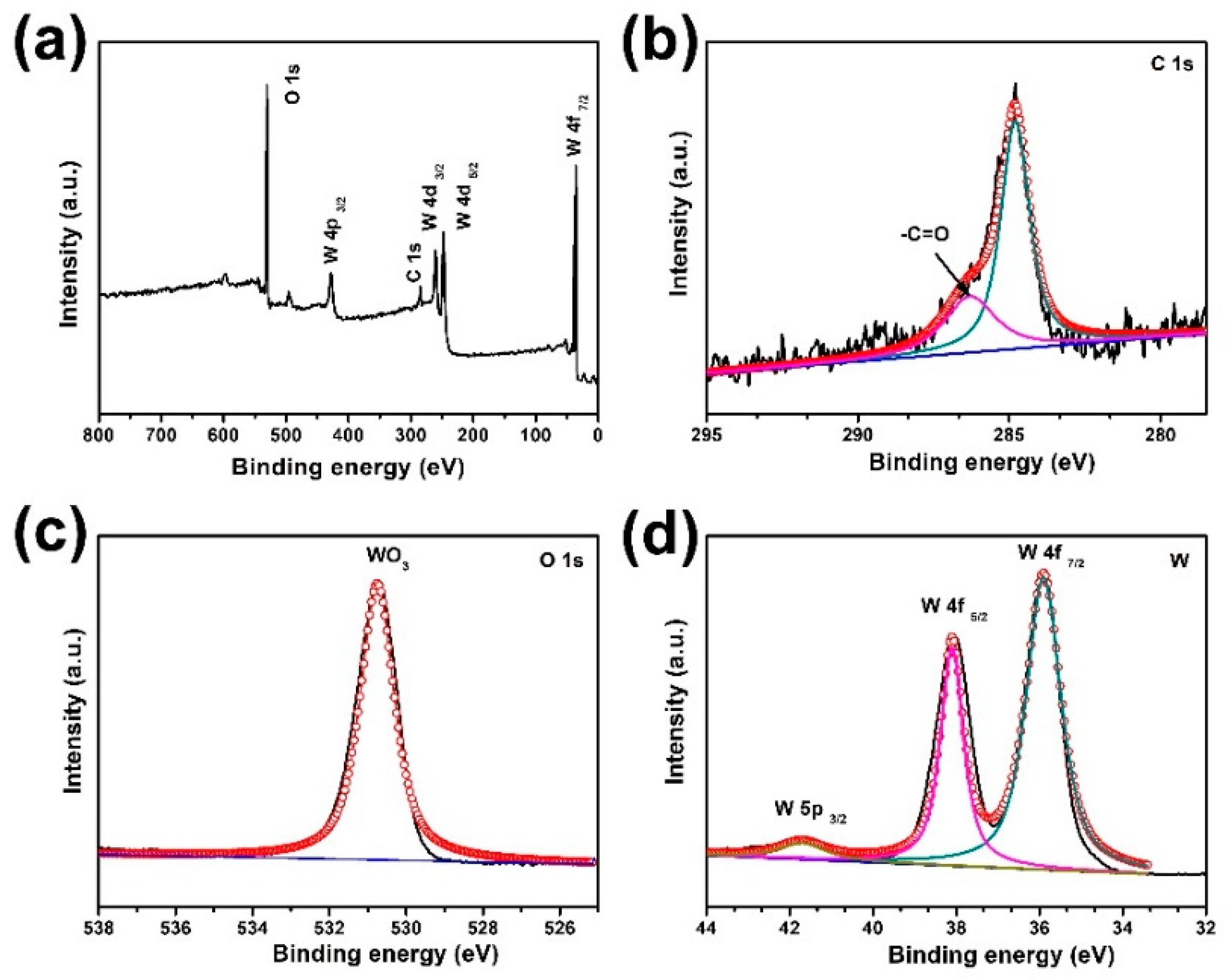
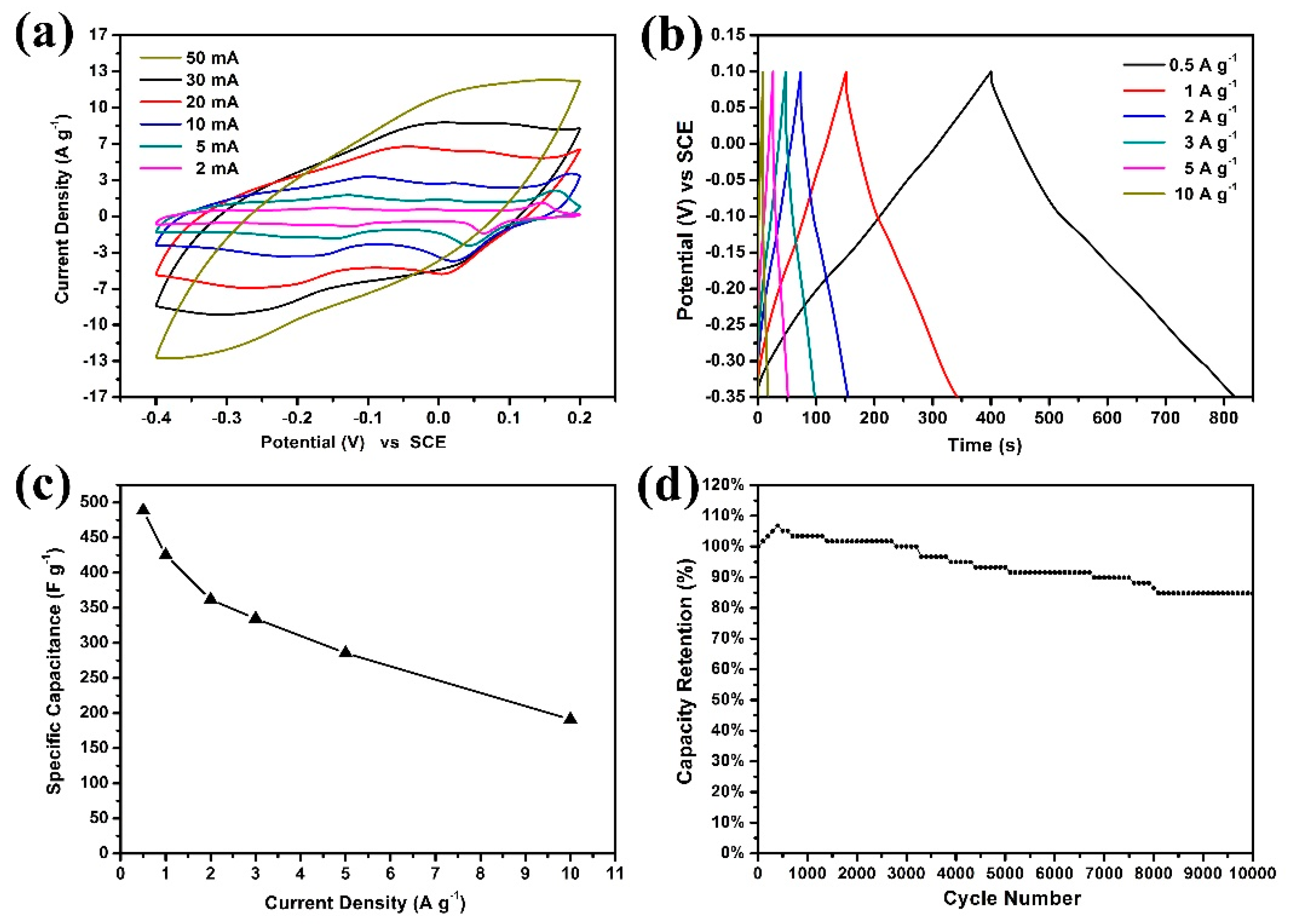
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kate, R.S.; Khalate, S.A.; Deokate, R.J. Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: A review. J. Alloy. Compd. 2018, 734, 89–111. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Wang, Y.; Liu, C.; Xing, S. Achieving MnO2 nanosheets through surface redox reaction on nickel nanochains for catalysis and energy storage. Chemistry 2017, 23, 5557–5564. [Google Scholar] [CrossRef] [PubMed]
- Lo, I.H.; Wang, J.Y.; Huang, K.Y.; Huang, J.H.; Kang, W.P. Synthesis of Ni (OH)2 nanoflakes on ZnO nanowires by pulse electrodeposition for high-performance supercapacitors. J. Power Sources 2016, 308, 29–36. [Google Scholar] [CrossRef]
- Shao, Y.L.; El-Kady, M.F.; Sun, J.Y.; Li, Y.G.; Zhang, Q.H.; Zhu, M.F.; Wang, H.Z.; Dunn, B.; Kaner, R.B. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gomez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, L.L.; Jia, S.Y.; Liu, X.C.; Wang, Y.H.; Xing, S.X. Facile route to achieve hierarchical hollow MnO2 nanostructures. Electrochimica Acta 2016, 203, 59–65. [Google Scholar] [CrossRef]
- Xiao, T.; Li, J.; Zhuang, X.Y.; Zhang, W.; Wang, S.L.; Chen, X.L.; Xiang, P.; Jiang, L.H.; Tan, X.Y. Wide potential window and high specific capacitance triggered via rough NiCo2S4 nanorod arrays with open top for symmetric supercapacitors. Electrochimica Acta 2018, 269, 397–404. [Google Scholar] [CrossRef]
- Zhang, P.; Guan, B.Y.; Yu, L.; Lou, X.W.D. Formation of Double-Shelled Zinc–Cobalt Sulfide Dodecahedral Cages from Bimetallic Zeolitic Imidazolate Frameworks for Hybrid Supercapacitors. Angew. Chem. Int. Ed. Engl. 2017, 56, 7141–7145. [Google Scholar] [CrossRef]
- Yao, Y.; Yin, M.; Yan, J.; Liu, S.F. P-type sub-tungsten-oxide based urchin-like nanostructure for superior room temperature alcohol sensor. Appl. Surf. Sci. 2018, 441, 277–284. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Hu, M.; Huang, R.; Huang, C. Hydrothermal Synthesis of Urchin-like WVO Nanostructures with Excellent Catalytic Performance. Inorg. Chem. 2018, 57, 14758–14763. [Google Scholar] [CrossRef]
- Xu, J.; Ding, T.T.; Wang, J.; Zhang, J.; Wang, S.; Chen, C.Q.; Fang, Y.Y.; Wu, Z.H.; Huo, K.F.; Dai, J.N. Tungsten oxide nanofibers self-assembled mesoscopic microspheres as high-performance electrodes for supercapacitor. Electrochimica Acta 2015, 174, 728–734. [Google Scholar] [CrossRef]
- Yoon, S.; Kang, E.; Kim, J.K.; Lee, C.W.; Lee, J. Development of high-performance supercapacitor electrodes using novel ordered mesoporous tungsten oxide materials with high electrical conductivity. Chem. Commun. 2011, 47, 1021–1023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodríguez-Moreno, J.; Navarrete-Astorga, E.; Dalchiele, E.A.; Schrebler, R.; Ramos-Barrado, J.R.; Martín, F. Vertically aligned ZnO@ CuS@ PEDOT core@ shell nanorod arrays decorated with MnO2 nanoparticles for a high-performance and semi-transparent supercapacitor electrode. Chem. Commun. 2014, 50, 5652–5655. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.K.; Dubal, D.P.; Ghodake, G.S.; Kim, D.Y.; Fulari, V.J. Nanoflower-like CuO/Cu (OH)2 hybrid thin films: Synthesis and electrochemical supercapacitive properties. J. Electroanal. Chem. 2014, 732, 80–85. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Holze, R.; Jadhav, H.S.; Lokhande, C.D.; Park, C.J. Surfactant-assisted morphological tuning of hierarchical CuO thin films for electrochemical supercapacitors. Dalton Trans. 2013, 42, 6459–6467. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Zhou, S.; Zhao, N.; Wong, C.P. Facile and scalable fabrication of three-dimensional Cu (OH)2 nanoporous nanorods for solid-state supercapacitors. J. Mater. Chem. A 2015, 3, 17385–17391. [Google Scholar] [CrossRef]
- Poongodi, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Meena, P.; Masuda, Y.; Lee, C. Electrodeposition of WO3 nanostructured thin films for electrochromic and H2S gas sensor applications. J. Alloy. Compd. 2017, 719, 71–81. [Google Scholar] [CrossRef]
- Sarma, D.D.; Rao, C.N.R. XPES studies of oxides of second-and third-row transition metals including rare earths. J. Electron Spectrosc. Relat. Phenom. 1980, 20, 25–45. [Google Scholar] [CrossRef]
- Jiang, Y.; Leng, X.J.; Jia, Z.L.; Chen, H.X.; Suo, H.; Zhao, C. In situ growth of NiO nanostructures directly on nickel foam and its electrochemical property. J. Mater. Sci. Mater. Electron. 2015, 26, 2995–3000. [Google Scholar] [CrossRef]
- Gao, L.N.; Wang, X.F.; Xie, Z.; Song, W.F.; Wang, L.J.; Wu, X.; Qu, F.Y.; Chen, D.; Shen, G.Z. High-performance energy-storage devices based on WO3 nanowire arrays/carbon cloth integrated electrodes. J. Mater. Chem. A 2013, 1, 7167–7173. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Tang, J.; Kamachi, Y.; Nakato, T.; Kim, J.H.; Yamauchi, Y. Asymmetric supercapacitors using 3D nanoporous carbon and cobalt oxide electrodes synthesized from a single metal—Organic framework. Acs Nano 2015, 9, 6288–6296. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.Y.; Qu, F.Y.; Wang, G.; Wu, X. Facile hydrothermal synthesis of WO3 nanorods for photocatalysts and supercapacitors. J. Alloy. Compd. 2017, 724, 695–702. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, A.C.; Chodankar, N.R.; Patil, A.M.; Kim, J.H.; Lokhande, C.D. Temperature dependent surface morphological modifications of hexagonal WO3 thin films for high performance supercapacitor application. Electrochimica Acta 2017, 224, 397–404. [Google Scholar] [CrossRef]
- Zheng, F.; Gong, H.Q.; Li, Z.; Yang, W.G.; Xu, J.H.; Hu, P.F.; Li, Y.; Gong, Y.; Zhen, Q. Tertiary structure of cactus-like WO3 spheres self-assembled on Cu foil for supercapacitive electrode materials. J. Alloy. Compd. 2017, 712, 345–354. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, A.C.; Patil, A.M.; Lokhande, C.D. Facile synthesis of self-assembled WO3 nanorods for high-performance electrochemical capacitor. J. Alloy. Comp. 2019, 770, 1130–1137. [Google Scholar] [CrossRef]
- Shinde, N.M.; Jagadale, A.D.; Kumbhar, V.S.; Rana, T.R.; Kim, J.H.; Lokhande, C.D. Wet chemical synthesis of WO3 thin films for supercapacitor application. Korean J. Chem. Eng. 2015, 32, 974. [Google Scholar] [CrossRef]
- Zheng, F.; Song, S.; Lu, F.; Li, R.; Bu, N.; Liu, J.; Li, Y.; Hud, P.; Zhen, Q. Hydrothermal preparation, growth mechanism and supercapacitive properties of WO3 nanorod arrays grown directly on a Cu substrate. CrystEngComm 2016, 18, 3891. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, P.; Shen, L.; Wang, K.; Song, S.; Yu, X.; Tan, S.; Zhao, C.; Mai, W. WO3 nanoflowers with excellent pseudo-capacitive performance and the capacitance contribution analysis. J. Mater. Chem. A 2016, 4, 7266. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, W.; Huang, Y.; Huang, Y.; Zhi, C. Proton-insertion-enhanced pseudocapacitance based on the assembly structure of tungsten oxide. ACS Appl. Mater. Interfaces 2014, 6, 18901–18910. [Google Scholar] [CrossRef]
- Chang, K.H.; Hu, C.C.; Huang, C.M.; Liu, Y.L.; Chang, C.I. Microwave-assisted hydrothermal synthesis of crystalline WO3–WO3·0.5 H2O mixtures for pseudocapacitors of the asymmetric type. J. Power Sources 2011, 196, 2387. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Wan, J.; He, D.; Yang, X.; Suo, H.; Zhao, C. Synthesis of Three-Dimensional Hierarchical Urchinlike Tungsten Trioxide Microspheres for High-Performance Supercapacitor Electrode. Crystals 2019, 9, 485. https://doi.org/10.3390/cryst9090485
He X, Wan J, He D, Yang X, Suo H, Zhao C. Synthesis of Three-Dimensional Hierarchical Urchinlike Tungsten Trioxide Microspheres for High-Performance Supercapacitor Electrode. Crystals. 2019; 9(9):485. https://doi.org/10.3390/cryst9090485
Chicago/Turabian StyleHe, Xu, Junning Wan, Dong He, Xiaotian Yang, Hui Suo, and Chun Zhao. 2019. "Synthesis of Three-Dimensional Hierarchical Urchinlike Tungsten Trioxide Microspheres for High-Performance Supercapacitor Electrode" Crystals 9, no. 9: 485. https://doi.org/10.3390/cryst9090485
APA StyleHe, X., Wan, J., He, D., Yang, X., Suo, H., & Zhao, C. (2019). Synthesis of Three-Dimensional Hierarchical Urchinlike Tungsten Trioxide Microspheres for High-Performance Supercapacitor Electrode. Crystals, 9(9), 485. https://doi.org/10.3390/cryst9090485




