Crystal Growth in Gels from the Mechanisms of Crystal Growth to Control of Polymorphism: New Trends on Theoretical and Experimental Aspects
Abstract
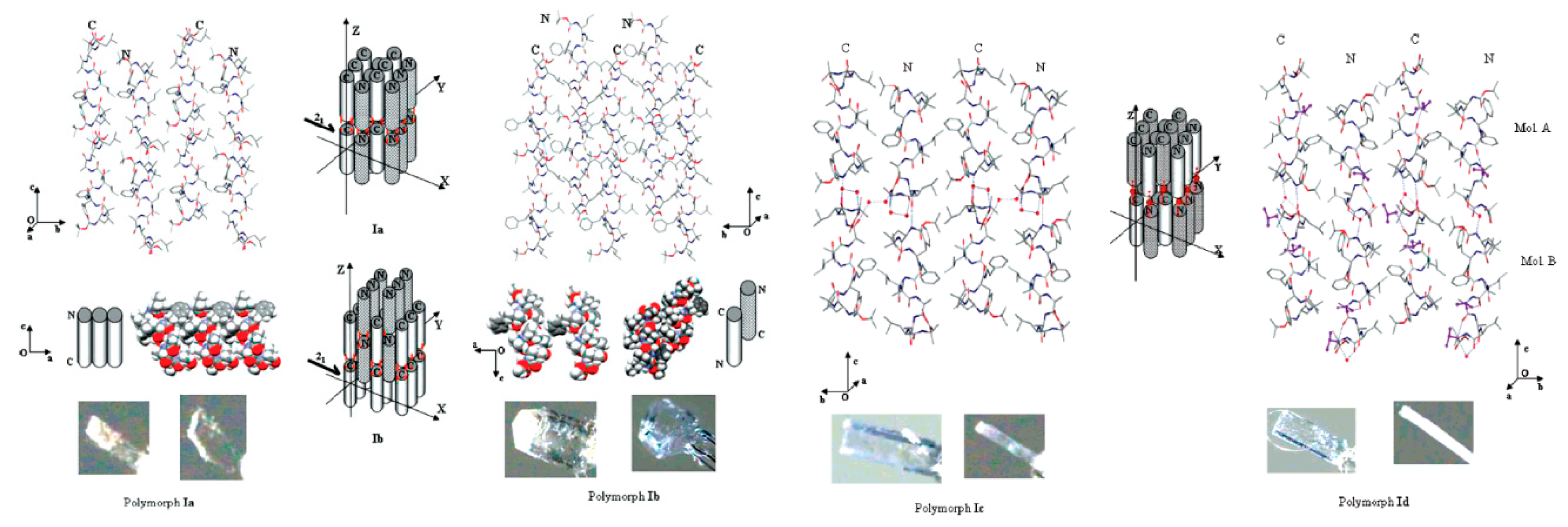
1. Polymorphism
1.1. Definition
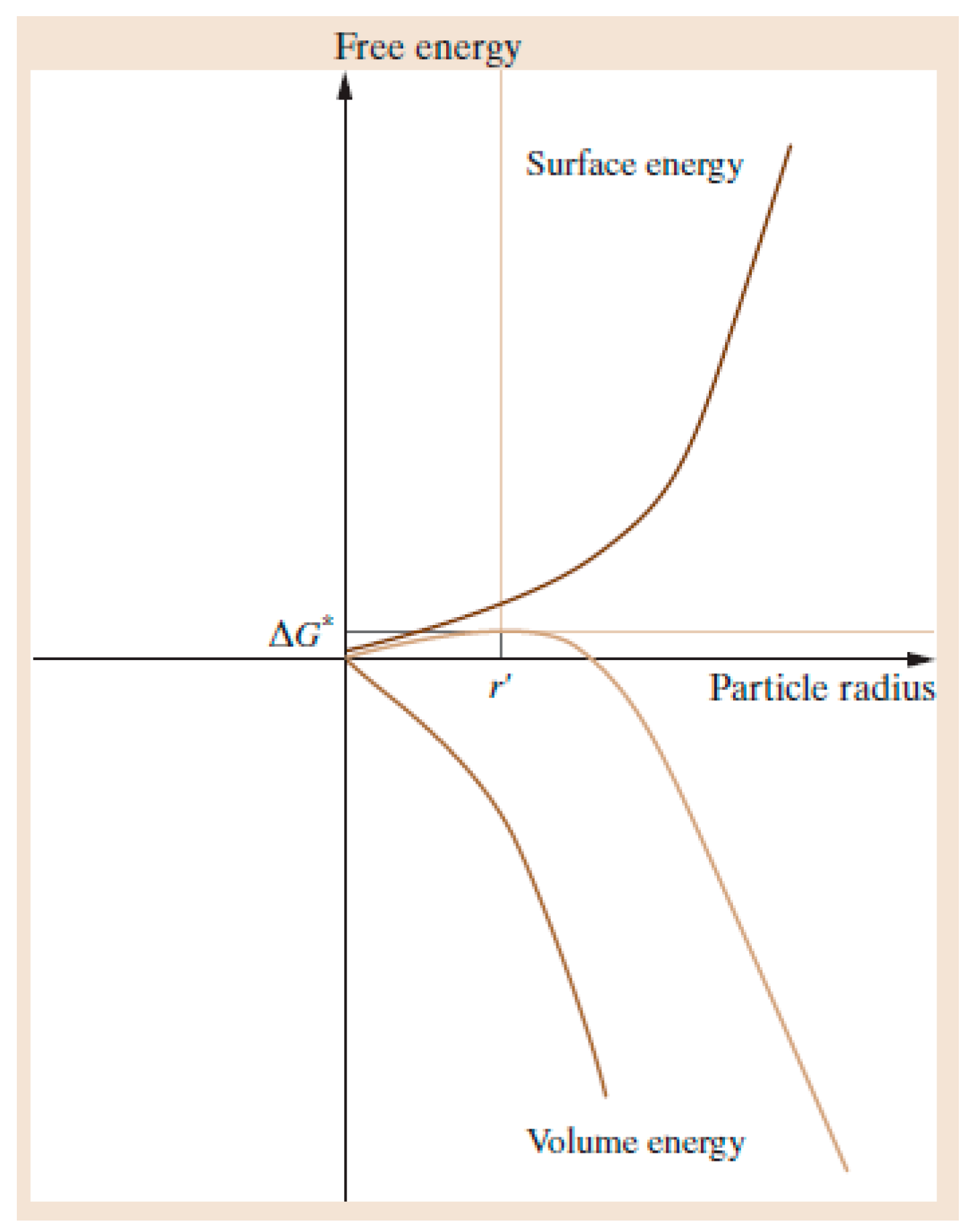
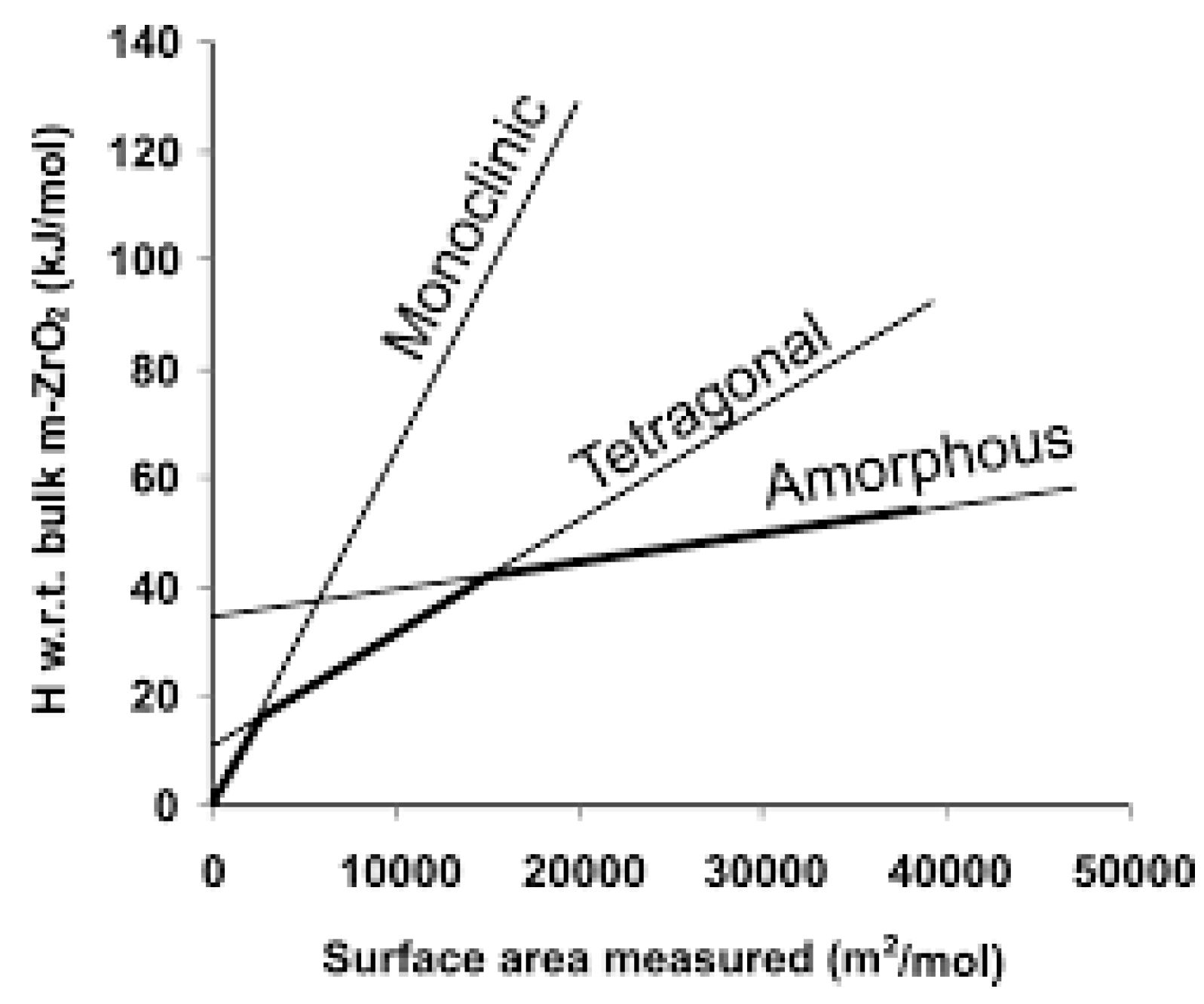
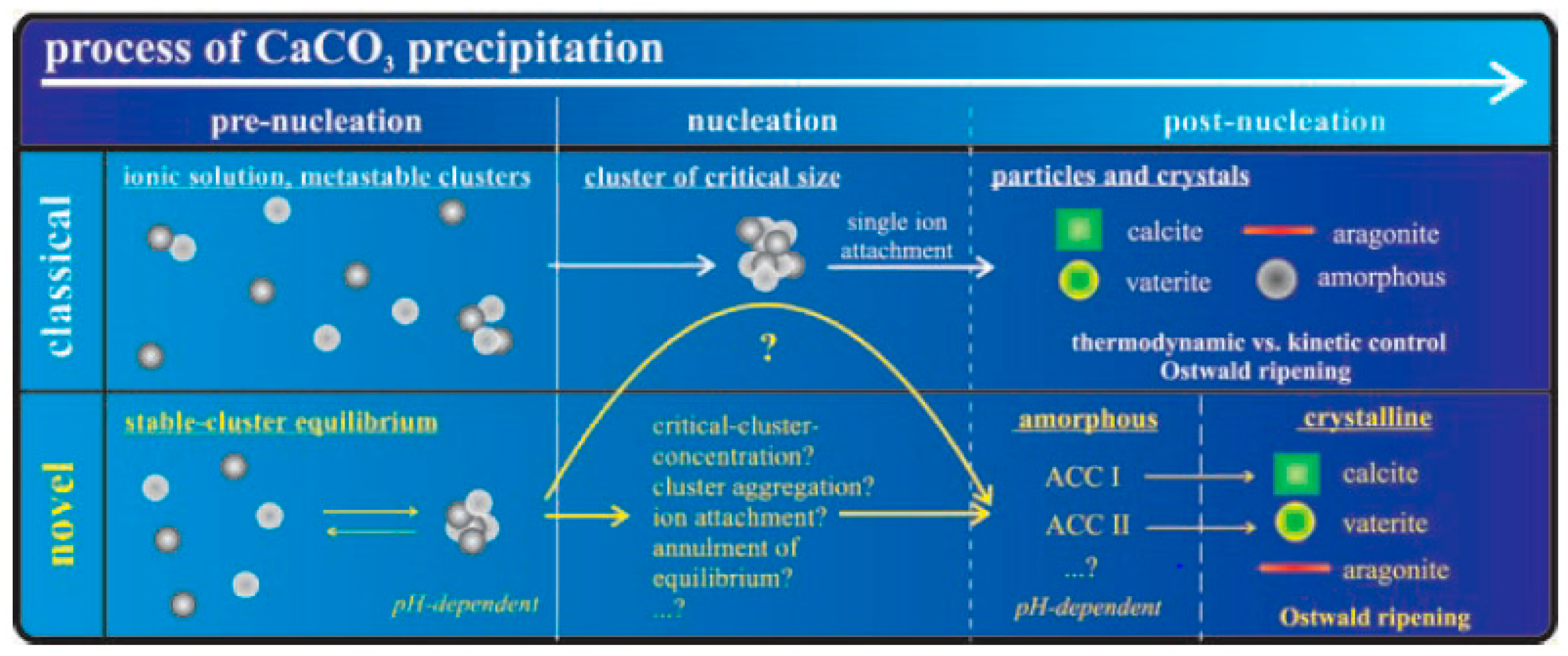
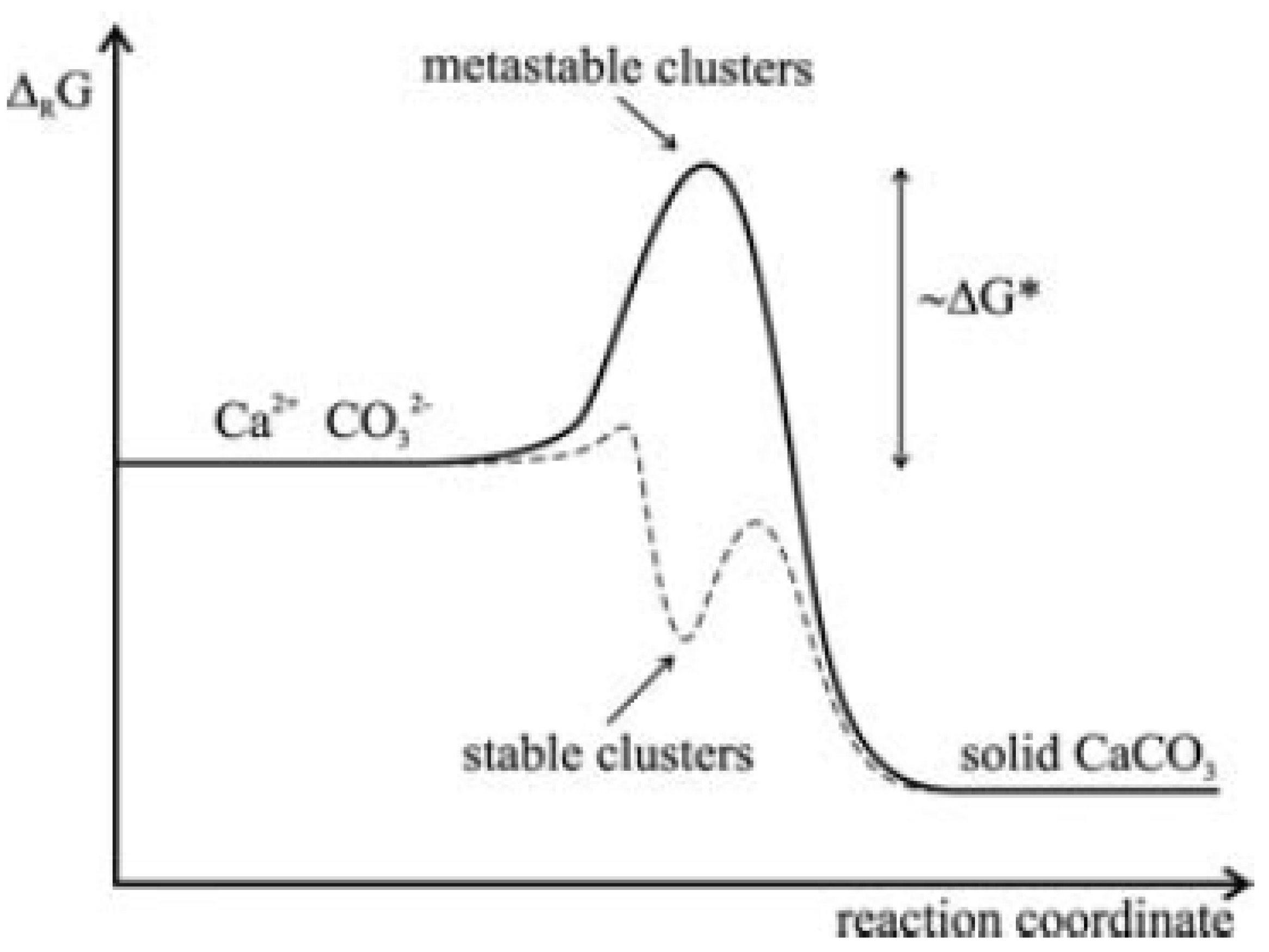
1.2. Nucleation and Polymorphism
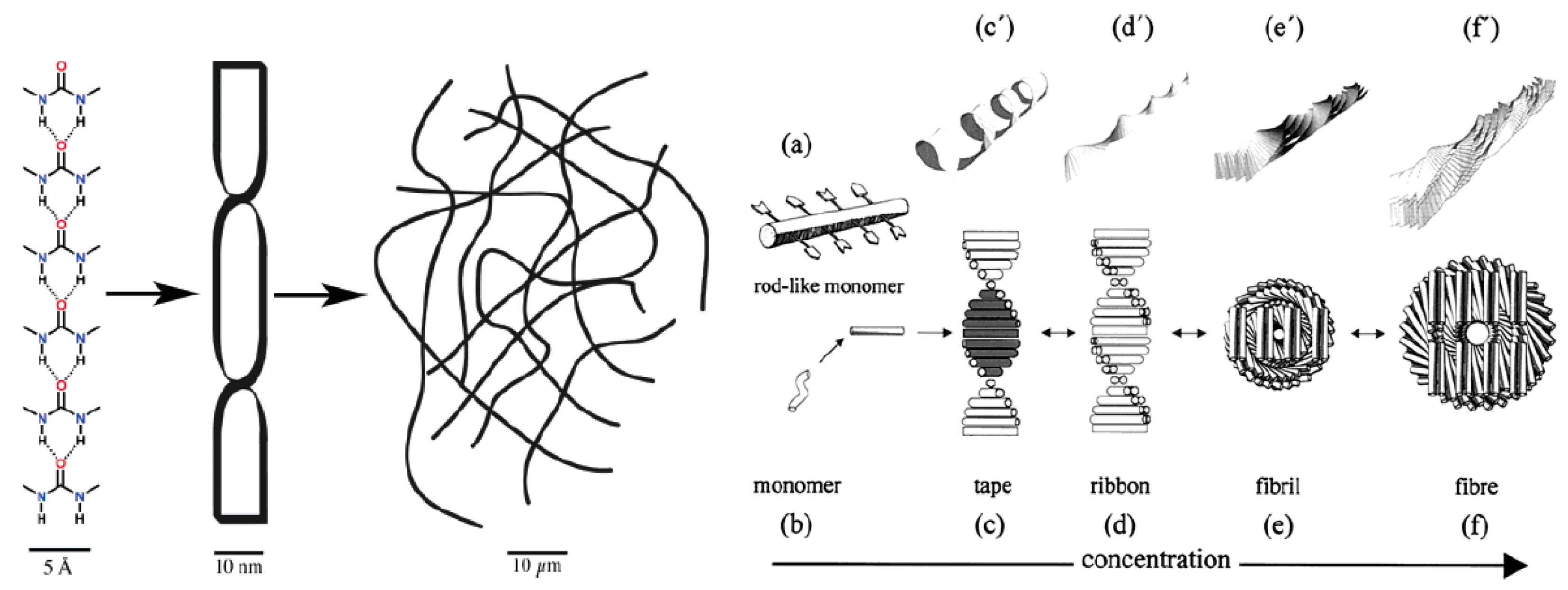
2. Gels
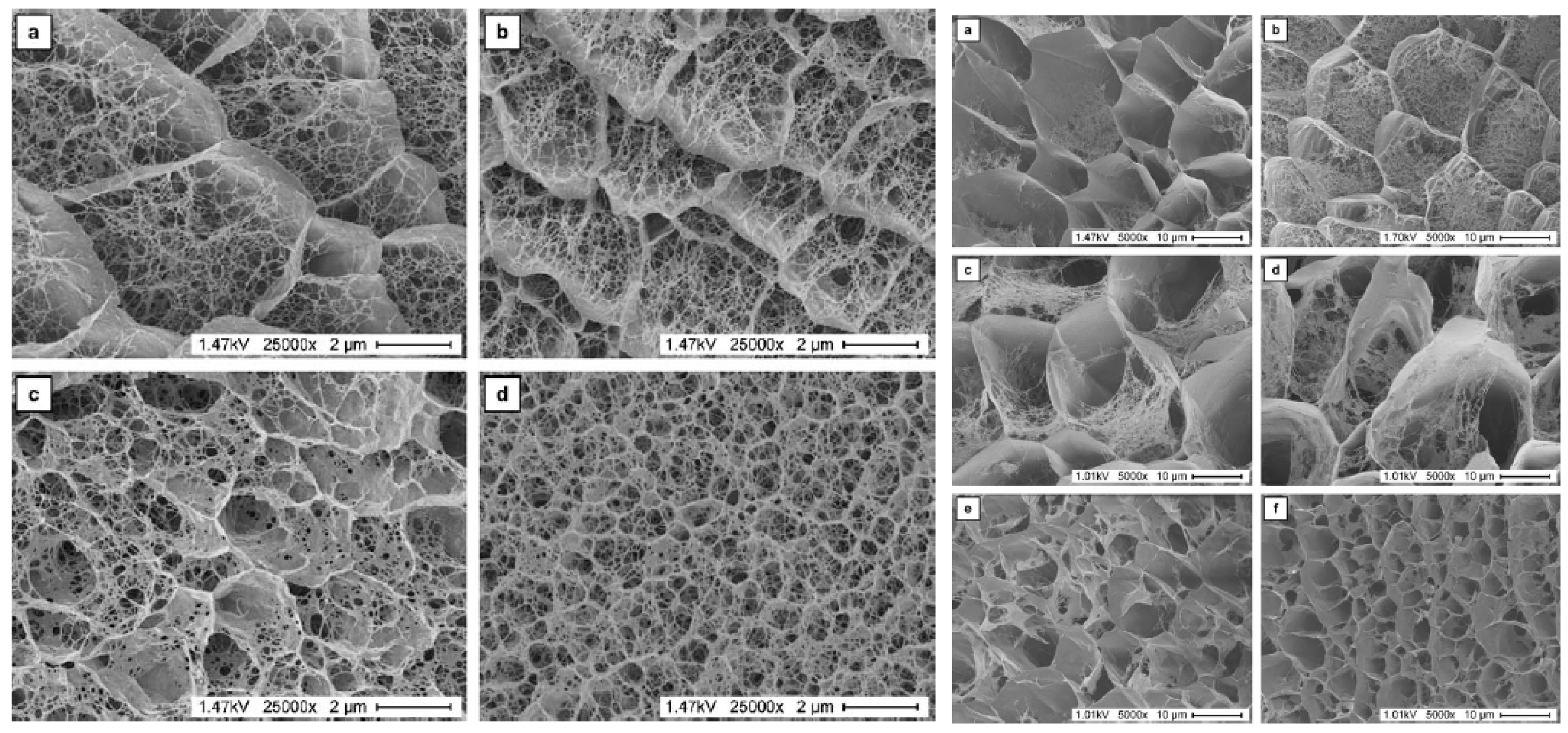
2.1. Properties of Gels Used for Crystallization
2.2. Studies on Crystal Growth in Gels
3. Protein Crystallization in Gels
3.1. Particular Characteristics of Protein Crystal Growth
3.2. New Trends of Crystal Growth in Gels and the Use of Magnetic Fields: All-Inclusive Method
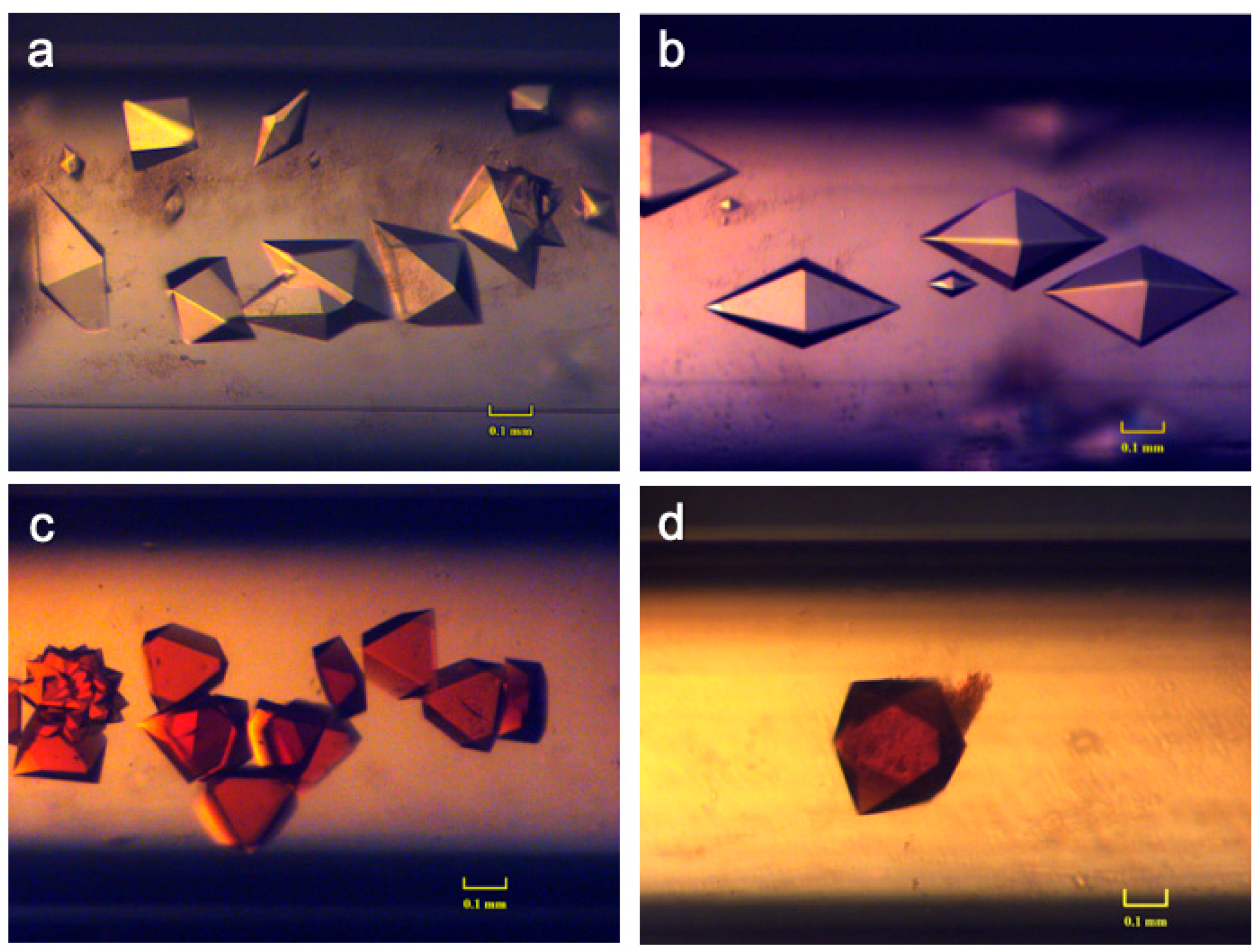
3.2.1. Case Studies Using Ferritin and Thaumatin Grown in Gels and under the Presence of a Strong Magnetic Field
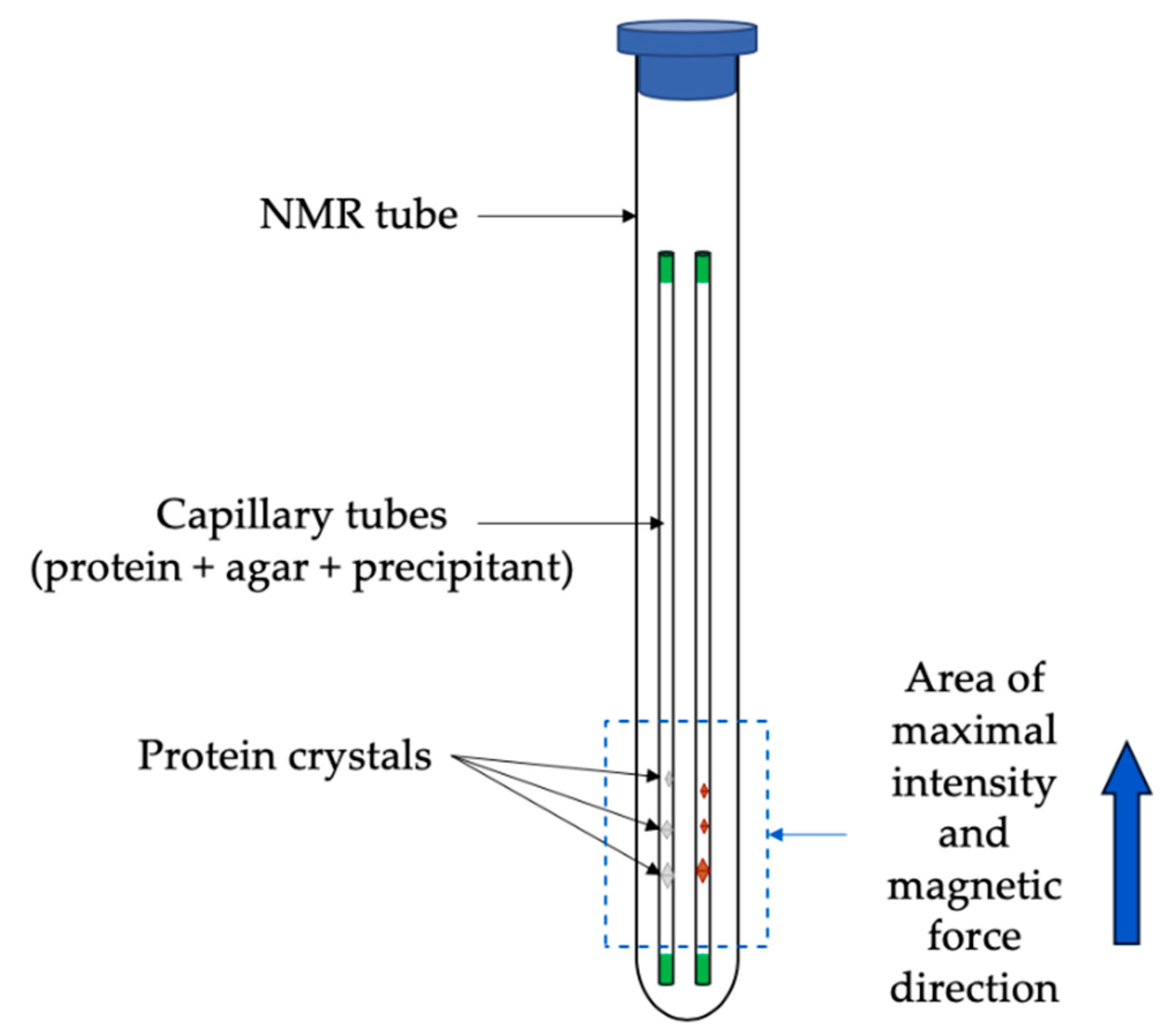
3.2.2. Crystallization Conditions and Experimental Setup
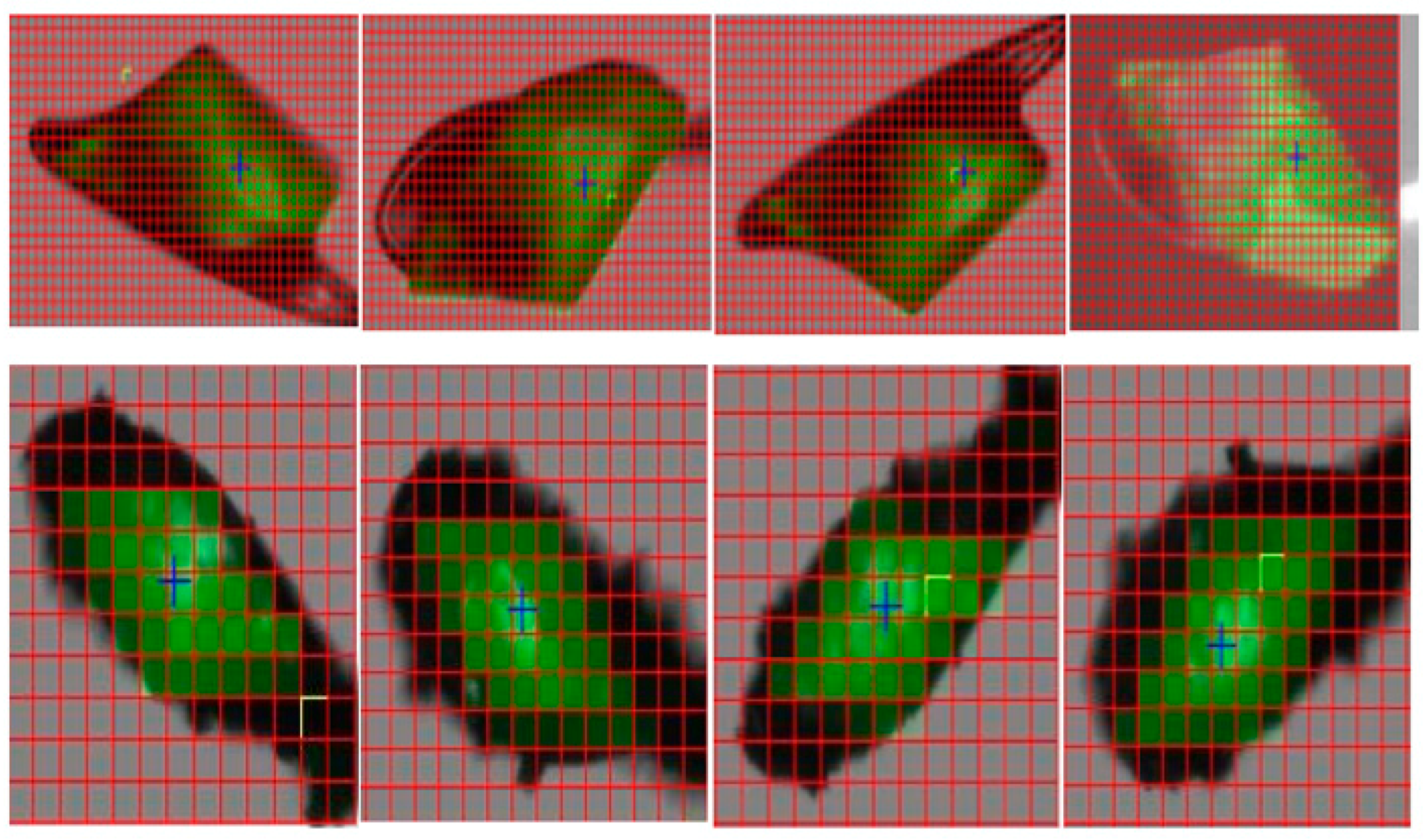
3.2.3. Results and Discussions
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burger, A. The Relevance of Polymorphism. Pharm. Int. 1983, 4, 186. [Google Scholar]
- Lee, E.H. A Practical Guide to Pharmaceutical Polymorph Screening & Selection. Asian J. Pharm. Sci. 2014, 9, 163–175. [Google Scholar]
- Hernández-Paredes, J.; Olvera-Tapia, A.L.; Arenas-García, J.I.; Höpfl, H.; Morales-Rojas, H.; Herrera-Ruiz, D.; Gonzaga-Morales, A.I.; Rodríguez-Fragoso, L. On Molecular Complexes Derived from Amino Acids and Nicotinamides in Combination with Boronic Acids. CrystEngComm 2015, 17, 5166–5186. [Google Scholar] [CrossRef]
- Long, S.; Zhou, P.; Theiss, K.L.; Siegler, M.A.; Li, T. Solid-State Identity of 2-Hydroxynicotinic Acid and Its Polymorphism. CrystEngComm 2015, 17, 5195–5205. [Google Scholar] [CrossRef]
- Tenorio Clavijo, J.C.; Guimarães, F.F.; Ellena, J.; Martins, F.T. Isostructurality and the Conformational Role of the 2′,3′-Moieties in the Diversity of Lamivudine Crystal Forms Probed in Halide Salts. CrystEngComm 2015, 17, 5187–5194. [Google Scholar] [CrossRef]
- Abramov, Y.A. Virtual Hydrate Screening and Coformer Selection for Improved Relative Humidity Stability. CrystEngComm 2015, 17, 5216–5224. [Google Scholar] [CrossRef]
- Upadhyay, P.P.; Bond, A.D. Crystallization and Disorder of the Polytypic A1 and A2 Polymorphs of Piroxicam. CrystEngComm 2015, 17, 5266–5272. [Google Scholar] [CrossRef]
- Maddileti, D.; Nangia, A. Polymorphism in Anti-Hyperammonemic Agent N-Carbamoyl-l-Glutamic Acid. CrystEngComm 2015, 17, 5252–5265. [Google Scholar] [CrossRef]
- Hean, D.; Gelbrich, T.; Griesser, U.J.; Michael, J.P.; Lemmerer, A. Structural Insights into the Hexamorphic System of an Isoniazid Derivative. CrystEngComm 2015, 17, 5143–5153. [Google Scholar] [CrossRef]
- Horstman, E.M.; Bertke, J.A.; Kim, E.H.; Gonzalez, L.C.; Zhang, G.G.Z.; Gong, Y.; Kenis, P.J.A. Crystallization and Characterization of Cocrystals of Piroxicam and 2,5-Dihydroxybenzoic Acid. CrystEngComm 2015, 17, 5299–5306. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and Fictions about Polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef] [PubMed]
- Falini, G.; Fermani, S.; Gazzano, M.; Ripamonti, A. Polymorphism and Architectural Crystal Assembly of Calcium Carbonate in Biologically Inspired Polymeric Matrices. J. Chem. Soc. Dalt. Trans. 2000, 3983–3987. [Google Scholar] [CrossRef]
- Tohse, H.; Saruwatari, K.; Kogure, T.; Nagasawa, H.; Takagi, Y. Control of Polymorphism and Morphology of Calcium Carbonate Crystals by a Matrix Protein Aggregate in Fish Otoliths. Cryst. Growth Des. 2009, 9, 4897–4901. [Google Scholar] [CrossRef]
- Fratzl, P.; Aizenberg, J.; Addadi, L.; Falini, G.; Reggi, M.; Fermani, S.; Sparla, F.; Goffredo, S.; Dubinsky, Z.; Levi, O.; et al. Control of Aragonite Deposition in Colonial Corals by Intra-Skeletal Macromolecules. J. Struct. Biol. 2013, 183, 226–238. [Google Scholar]
- Vasudev, P.G.; Shamala, N.; Balaram, P. Nucleation, Growth, and Form in Crystals of Peptide Helices. J. Phys. Chem. B 2008, 112, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Van Driessche, A.E.S.; Van Gerven, N.; Bomans, P.H.H.; Joosten, R.R.M.; Friedrich, H.; Gil-Carton, D.; Sommerdijk, N.A.J.M.; Sleutel, M. Molecular Nucleation Mechanisms and Control Strategies for Crystal Polymorph Selection. Nature 2018, 556, 89–94. [Google Scholar] [CrossRef]
- McCrone, W.C. Polymorphism. Phys. Chem. Org. Solid State 1965, 2, 726–767. [Google Scholar]
- Saridakis, E.; Dierks, K.; Moreno, A.; Dieckmann, M.W.M.; Chayen, N.E. Separating Nucleation and Growth in Protein Crystallization Using Dynamic Light Scattering. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002, 58, 1597–1600. [Google Scholar] [CrossRef]
- Gibbs, J.W. Equilibrium of Heterogenous Substances. Am. J. Sci. 1878, 16, 441–458. [Google Scholar] [CrossRef]
- Frenkel, J. A General Theory of Heterophase Fluctuations and Pretransition Phenomena. J. Chem. Phys. 1939, 7, 538–547. [Google Scholar] [CrossRef]
- Gutiérrez-Quezada, A.E.; Arreguín-Espinosa, R.; Moreno, A. Protein Crystal Growth Methods. In Springer Handbook of Crystal Growth; Dhanaraj, G., Byrappa, K., Prasad, V., Dudley, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1583–1605. [Google Scholar]
- Sand, K.K.; Rodriguez-Blanco, J.D.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in Water-Alcohol Mixtures: Spherulitic Growth, Polymorph Stabilization, and Morphology Change. Cryst. Growth Des. 2012, 12, 842–853. [Google Scholar] [CrossRef]
- Chen, S.F.; Yu, S.H.; Hang, J.; Li, F.; Liu, Y. Polymorph Discrimination of CaCO3 Mineral in an Ethanol/Water Solution: Formation of Complex Vaterite Superstructures and Aragonite Rods. Chem. Mater. 2006, 18, 115–122. [Google Scholar] [CrossRef]
- Nicolaï, B.; Espeau, P.; Céolin, R.; Perrin, M.A.; Zaske, L.; Giovannini, J.; Leveiller, F. Polymorph Formation from Solvate Desolvation: Spironolactone Forms i and II from the Spironolactone-Ethanol Solvate. J. Therm. Anal. Calorim. 2007, 90, 337–339. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Meekes, H.; Ter Horst, J.H. Polymorphism Control through a Single Nucleation Event. Cryst. Growth Des. 2014, 14, 1493–1499. [Google Scholar] [CrossRef]
- Garg, R.K.; Sarkar, D. Polymorphism Control of P-Aminobenzoic Acid by Isothermal Anti-Solvent Crystallization. J. Cryst. Growth 2016, 454, 180–185. [Google Scholar] [CrossRef]
- Lai, T.T.C.; Cornevin, J.; Ferguson, S.; Li, N.; Trout, B.L.; Myerson, A.S. Control of Polymorphism in Continuous Crystallization via Mixed Suspension Mixed Product Removal Systems Cascade Design. Cryst. Growth Des. 2015, 15, 3374–3382. [Google Scholar] [CrossRef]
- Padrela, L.; Zeglinski, J.; Ryan, K.M. Insight into the Role of Additives in Controlling Polymorphic Outcome: A CO2-Antisolvent Crystallization Process of Carbamazepine. Cryst. Growth Des. 2017, 17, 4544–4553. [Google Scholar] [CrossRef]
- Hatakka, H.; Alatalo, H.; Louhi-Kultanen, M.; Lassila, I.; Hæggström, E. Closed-Loop Control of Reactive Crystallization Part II: Polymorphism Control of L-Glutamic Acid by Sonocrystallization and Seeding. Chem. Eng. Technol. 2010, 33, 751–756. [Google Scholar] [CrossRef]
- Stolar, T.; Lukin, S.; Tireli, M.; Sović, I.; Karadeniz, B.; Kereković, I.; Matijašić, G.; Gretić, M.; Katančić, Z.; Dejanović, I.; et al. Control of Pharmaceutical Cocrystal Polymorphism on Various Scales by Mechanochemistry: Transfer from the Laboratory Batch to the Large-Scale Extrusion Processing. ACS Sustain. Chem. Eng. 2019, 7, 7102–7110. [Google Scholar] [CrossRef]
- Zbačnik, M.; Nogalo, I.; Cinčić, D.; Kaitner, B. Polymorphism Control in the Mechanochemical and Solution-Based Synthesis of a Thermochromic Schiff Base. CrystEngComm 2015, 17, 7870–7877. [Google Scholar] [CrossRef]
- Cinčić, D.; Brekalo, I.; Kaitner, B. Solvent-Free Polymorphism Control in a Covalent Mechanochemical Reaction. Cryst. Growth Des. 2012, 12, 44–48. [Google Scholar] [CrossRef]
- Freund, F.; Williams, S.C.; Johnson, R.D.; Coldea, R.; Gegenwart, P.; Jesche, A. Single Crystal Growth from Separated Educts and Its Application to Lithium Transition-Metal Oxides. Sci. Rep. 2016, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.Y.; Chia, C.H.; Zakaria, S. Polymorphs Calcium Carbonate on Temperature Reaction. AIP Conf. Proc. 2014, 1614, 52–56. [Google Scholar]
- Rungsimanon, T.; Yuyama, K.I.; Sugiyama, T.; Masuhara, H.; Tohnai, N.; Miyata, M. Control of Crystal Polymorph of Glycine by Photon Pressure of a Focused Continuous Wave Near-Infrared Laser Beam. J. Phys. Chem. Lett. 2010, 1, 599–603. [Google Scholar] [CrossRef]
- Miura, K.; Fujiwara, K.; Tsukazaki, A. Growth Control of Corundum-Derivative MnSnO3 Thin Films by Pulsed-Laser Deposition. AIP Adv. 2019, 9. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Liu, L.; Gong, X.; Wang, M. Alumina Hydrate Polymorphism Control in Al-Water Reaction Crystallization by Seeding to Change the Metastable Zone Width. Cryst. Growth Des. 2016, 16, 1056–1062. [Google Scholar] [CrossRef]
- Little, L.J.; King, A.A.K.; Sear, R.P.; Keddie, J.L. Controlling the Crystal Polymorph by Exploiting the Time Dependence of Nucleation Rates. J. Chem. Phys. 2017, 147. [Google Scholar] [CrossRef]
- Violante, A.; Huang, P.M. Formation Mechanism of Aluminum Hydroxide Polymorphs. Clays Clay Miner. 1993, 41, 590–597. [Google Scholar] [CrossRef]
- Ogino, T.; Suzuki, T.; Sawada, K. The Formation and Transformation Mechanism of Calcium Carbonate in Water. Geochim. Cosmochim. Acta 1987, 51, 2757–2767. [Google Scholar] [CrossRef]
- McNaught, A.D.; Blackwell, W.; IUPAC. Compendium of Chemical Terminology; Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slyozov, V. The Kinetics of Precipitation from Supersaturated Solid Solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Mizuno, K.; Mura, T.; Uchida, S. Control of Polymorphisms and Functions in All-Inorganic Ionic Crystals Based on Polyaluminum Hydroxide and Polyoxometalates. Cryst. Growth Des. 2016, 16, 4968–4974. [Google Scholar] [CrossRef]
- Navrotsky, A. Energetic Clues to Pathways to Biomineralization: Precursors, Clusters, and Nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 12096–12101. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Wi, H.S.; Jo, W.; Cho, Y.C.; Lee, H.H.; Jeong, S.-Y.; Kim, Y.-I.; Lee, G.W. Multiple Pathways of Crystal Nucleation in an Extremely Supersaturated Aqueous Potassium Dihydrogen Phosphate (KDP) Solution Droplet. Proc. Natl. Acad. Sci. USA 2016, 113, 13618–13623. [Google Scholar] [CrossRef] [PubMed]
- Desgranges, C.; Delhommelle, J. Molecular Mechanism for the Cross-Nucleation between Polymorphs. J. Am. Chem. Soc. 2006, 128, 10368–10369. [Google Scholar] [CrossRef] [PubMed]
- Desgranges, C.; Delhommelle, J. Insights into the Molecular Mechanism Underlying Polymorph Selection. J. Am. Chem. Soc. 2006, 128, 15104–15105. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; Molinero, V. Cross-Nucleation between Clathrate Hydrate Polymorphs: Assessing the Role of Stability, Growth Rate, and Structure Matching. J. Chem. Phys. 2014, 140. [Google Scholar] [CrossRef] [PubMed]
- Bonn, D.; Shahidzadeh, N. Multistep Crystallization Processes: How Not to Make Perfect Single Crystals. Proc. Natl. Acad. Sci. USA 2016, 113, 13551–13553. [Google Scholar] [CrossRef]
- Vekilov, P.G. Two-Step Mechanism for the Nucleation of Crystals from Solution. J. Cryst. Growth 2005, 275, 65–76. [Google Scholar] [CrossRef]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable Prenucleation Calcium Carbonate Clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef]
- Li, K.; Wu, S.; Xu, S.; Du, S.; Zhao, K.; Lin, L.; Yang, P.; Yu, B.; Hou, B.; Gong, J. Oiling out and Polymorphism Control of Pyraclostrobin in Cooling Crystallization. Ind. Eng. Chem. Res. 2016, 55, 11631–11637. [Google Scholar] [CrossRef]
- Muschol, M.; Rosenberger, F. Liquid-Liquid Phase Separation in Supersaturated Lysozyme Solutions and Associated Precipitate Formation/Crystallization. J. Chem. Phys. 1997, 107, 1953–1962. [Google Scholar] [CrossRef]
- Mattei, A.; Li, T. Polymorph Formation and Nucleation Mechanism of Tolfenamic Acid in Solution: An Investigation of Pre-Nucleation Solute Association. Pharm. Res. 2012, 29, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Xing, R.; Yan, X. Peptide Self-Assembly: Thermodynamics and Kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [Google Scholar] [CrossRef] [PubMed]
- Capetti, E.; Ferretti, A.M.; Santo, V.D.; Ponti, A. Surfactant-Controlled Composition and Crystal Structure of Manganese(II) Sulfide Nanocrystals Prepared by Solvothermal Synthesis. Beilstein J. Nanotechnol. 2015, 6, 2319–2329. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.P.; Kwon, S.J.; Jazbinsek, M.; Choubey, A.; Losio, P.A.; Gramlich, V.; Günter, P. Morphology and Polymorphism Control of Organic Polyene Crystals by Tailor-Made Auxiliaries. Cryst. Growth Des. 2006, 6, 2327–2332. [Google Scholar] [CrossRef]
- Ruiz-Arellano, R.R.; Medrano, F.J.; Moreno, A.; Romero, A. Structure of Struthiocalcin-1, an Intramineral Protein from Struthio Camelus Eggshell, in Two Crystal Forms. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 809–818. [Google Scholar] [CrossRef]
- Reyes-Grajeda, J.P.; Marín-García, L.; Stojanoff, V.; Moreno, A. Purification, Crystallization and Preliminary X-Ray Analysis of Struthiocalcin 1 from Ostrich (Struthio Camelus) Eggshell. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2007, 63, 987–989. [Google Scholar] [CrossRef]
- Marín-García, L.; Frontana-Uribe, B.A.; Reyes-Grajeda, J.P.; Stojanoff, V.; Serrano-Posada, H.J.; Moreno, A. Chemical Recognition of Carbonate Anions by Proteins Involved in Biomineralization Processes and Their Influence on Calcite Crystal Growth. Cryst. Growth Des. 2008, 8, 1340–1345. [Google Scholar] [CrossRef]
- Falini, G.; Albeck, S.; Weiner, S.; Addadi, L. Control of Aragonite or Calcite Polymorphism by Mollusk Shell Macromolecules. Science 1996, 271, 67–69. [Google Scholar] [CrossRef]
- Zou, Z.; Bertinetti, L.; Politi, Y.; Fratzl, P.; Habraken, W.J.E.M. Control of Polymorph Selection in Amorphous Calcium Carbonate Crystallization by Poly(Aspartic Acid): Two Different Mechanisms. Small 2017, 13, 1603100. [Google Scholar] [CrossRef]
- Mandal, D.; Yoon, S.; Kim, K.J. Prepration of Silver Nanoparticles Doped Pvdf: Formation of Piezoelectric Polymorph. In Proceedings of the 18th International Conference on Composite Materials (ICCM), Jeju Island, Korea, 21–26 August 2011; pp. 1–4. [Google Scholar]
- Kim, K.; Centrone, A.; Hatton, T.A.; Myerson, A.S. Polymorphism Control of Nanosized Glycine Crystals on Engineered Surfaces. CrystEngComm 2011, 13, 1127–1131. [Google Scholar] [CrossRef]
- Rusa, C.C.; Wei, M.; Bullions, T.A.; Rusa, M.; Gomez, M.A.; Porbeni, F.E.; Wang, X.; Shin, I.D.; Balik, C.M.; White, J.L.; et al. Controlling the Polymorphic Behaviors of Semicrystalline Polymers with Cyclodextrins. Cryst. Growth Des. 2004, 4, 1431–1441. [Google Scholar] [CrossRef]
- Tulli, L.G.; Moridi, N.; Wang, W.; Helttunen, K.; Neuburger, M.; Vaknin, D.; Meier, W.; Shahgaldian, P. Polymorphism Control of an Active Pharmaceutical Ingredient beneath Calixarene-Based Langmuir Monolayers. Chem. Commun. 2014, 50, 3938–3940. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, M.H.; Chou, W.Y.; Lee, Y.C.; Cheng, H.L.; Chung, H.Y.; Chang, C.C.; Chiu, C.Y.; Ho, T.Y. Polymorphic Transformation Induced by Nanoimprinted Technology in Pentacene-Film Early-Stage Growth. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Demirci, U. Gels Handbook: Fundamentals, Properties and Applications; World Scientific Pub Co Inc.: Toh Tuck, Singapore, 2016. [Google Scholar]
- Aizawa, M.; Suzuki, S. Properties of Water in Macromoleular Gels. III. Dilatometric Studies of the Properties of Water in Macromolecular Gels. Bull. Chem. Soc. Jpn. 2006, 44, 2967–2971. [Google Scholar] [CrossRef]
- Aizawa, M.; Suzuki, S.; Suzuki, T.; Toyama, H. The Properties of Water in Macromolecular Gels. VI. The Relationship between the Rheological Properties and the States of Water in Macromolecular Gels. Bull. Chem. Soc. Jpn. 2006, 46, 1638–1640. [Google Scholar] [CrossRef]
- Aizawa, M.; Mizuguchi, J.; Suzuki, S.; Hayashi, S.; Suzuki, T.; Mitomo, N.; Toyama, H. Properties of Water in Macromolecular Gels. IV. Proton Magnetic Resonance Studies of Water Iu Macromolecular Gels. Bull. Chem. Soc. Jpn. 2006, 45, 3031–3034. [Google Scholar] [CrossRef]
- Rogovina, L.Z.; Vasil’ev, V.G.; Braudo, E.E. Definition of the Concept of Polymer Gel. Polym. Sci. Ser. C 2008, 50, 85–92. [Google Scholar] [CrossRef]
- Tuvikene, R.; Truus, K.; Kollist, A.; Volobujeva, O.; Mellikov, E.; Pehk, T. Gel-Forming Structures and Stages of Red Algal Galactans of Different Sulfation Levels. J. Appl. Phycol. 2008, 527–535. [Google Scholar] [CrossRef]
- Blank, Z.; Reimschuessel, A.C. Structural Studies of Organic Gels by SEM. J. Mater. Sci. 1974, 9, 1815–1822. [Google Scholar] [CrossRef]
- Robert, M.C.; Lefaucheux, F. Crystal Growth in Gels: Principle and Applications. J. Cryst. Growth 1988, 90, 358–367. [Google Scholar] [CrossRef]
- García-Ruiz, J.M. The Uses of Crystal Growth in Gels and Other Diffusing-Reacting Systems. Key Eng. Mater. 2009, 58, 87–106. [Google Scholar] [CrossRef]
- Henisch, H.K.; García-Ruiz, J.M. Crystal Growth in Gels and Liesegang Ring Formation I. Diffusion Relationships. J. Cryst. Growth 1986, 75, 195–200. [Google Scholar] [CrossRef]
- Henisch, H.K.; García-Ruiz, J.M. Crystal Growth in Gels and Liesegang Ring Formation. II. Crystallization Criteria and Successive Precipitation. J. Cryst. Growth 1986, 75, 203–211. [Google Scholar] [CrossRef]
- Liesegang, R.E. Chemische Reaktionen in Gallerten; Naturwiss Wschr: Leipzig, Germany, 1924. (In German) [Google Scholar]
- Henisch, H.K.; Dennis, J.; Hanoka, J.I. Crystal Growth in Gels. J. Phys. Chem. Solids 1965, 26, 1–9. [Google Scholar] [CrossRef]
- McCaurEyl, J.; Roy, R. Controlled Nucleation and Crystal Growth of Various CaCO3 Phases by the Silica Gel Technique. Am. Mineral. 1974, 59, 947–963. [Google Scholar]
- Franke, W.A.; Mehran, N.-A. Crystal Growth in Gels at Elevated Pressures: The Upper Limit of Temperature for Metastable Formation of Aragonite. Cryst. Res. Technol. 1992, 27, 295–299. [Google Scholar] [CrossRef]
- Diao, Y.; Helgeson, M.E.; Myerson, A.S.; Hatton, T.A.; Doyle, P.S.; Trout, B.L. Controlled Nucleation from Solution Using Polymer Microgels Ying. J. Am. Chem. Soc. 2011, 132, 3756–3759. [Google Scholar] [CrossRef] [PubMed]
- Myerson, A.S.; Woldeyes, M.A.; Doyle, P.S.; Whaley, K.E.; Hatton, T.A.; Helgeson, M.E.; Diao, Y.; Trout, B.L. Gel-Induced Selective Crystallization of Polymorphs. J. Am. Chem. Soc. 2011, 134, 673–684. [Google Scholar]
- Bernard, Y.; Degoy, S.; Lefaucheux, F.; Robert, M.C. A Gel-Mediated Feeding Technique for Protein Crystal Growth from Hanging Drops. Acta Crystallogr. Sect. D Biol. Crystallogr. 1994, 50, 504–507. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Moreno, A. Investigations on Protein Crystal Growth by the Gel Acupuncture Method. Acta Crystallogr. Sect. D Biol. Crystallogr. 1994, 50, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Juárez-Martínez, G.; Hernández-Pérez, T.; Batina, N.; Mundo, M.; McPherson, A. Physical and Chemical Properties of Gels Application to Protein Nucleation Control in the Gel Acupuncture Technique. J. Cryst. Growth 1999, 205, 375–381. [Google Scholar] [CrossRef]
- Grassmann, O.; Müller, G.; Löbmann, P. Organic-Inorganic Hybrid Structure of Calcite Crystalline Assemblies Grown in a Gelatin Hydrogel Matrix: Relevance to Biomineralization. Chem. Mater. 2002, 14, 4530–4535. [Google Scholar] [CrossRef]
- Grassmann, O.; Neder, R.B.; Putnis, A.; Löbmann, P. Biomimetic Control of Crystal Assembly by Growth in an Organic Hydrogel Network. Am. Mineral. 2003, 88, 647–652. [Google Scholar] [CrossRef]
- Grassmann, O.; Löbmann, P. Biomimetic Nucleation and Growth of CaCO3 in Hydrogels Incorporating Carboxylate Groups. Biomaterials 2004, 25, 277–282. [Google Scholar] [CrossRef]
- Moreno, A.; Quiroz-García, B.; Yokaichiya, F.; Stojanoff, V.; Rudolph, P. Protein Crystal Growth in Gels and Stationary Magnetic Fields. Cryst. Res. Technol. 2007, 42, 231–236. [Google Scholar] [CrossRef]
- Estroff, L.A.; Hamilton, A.D. Water Gelation by Small Organic Molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef]
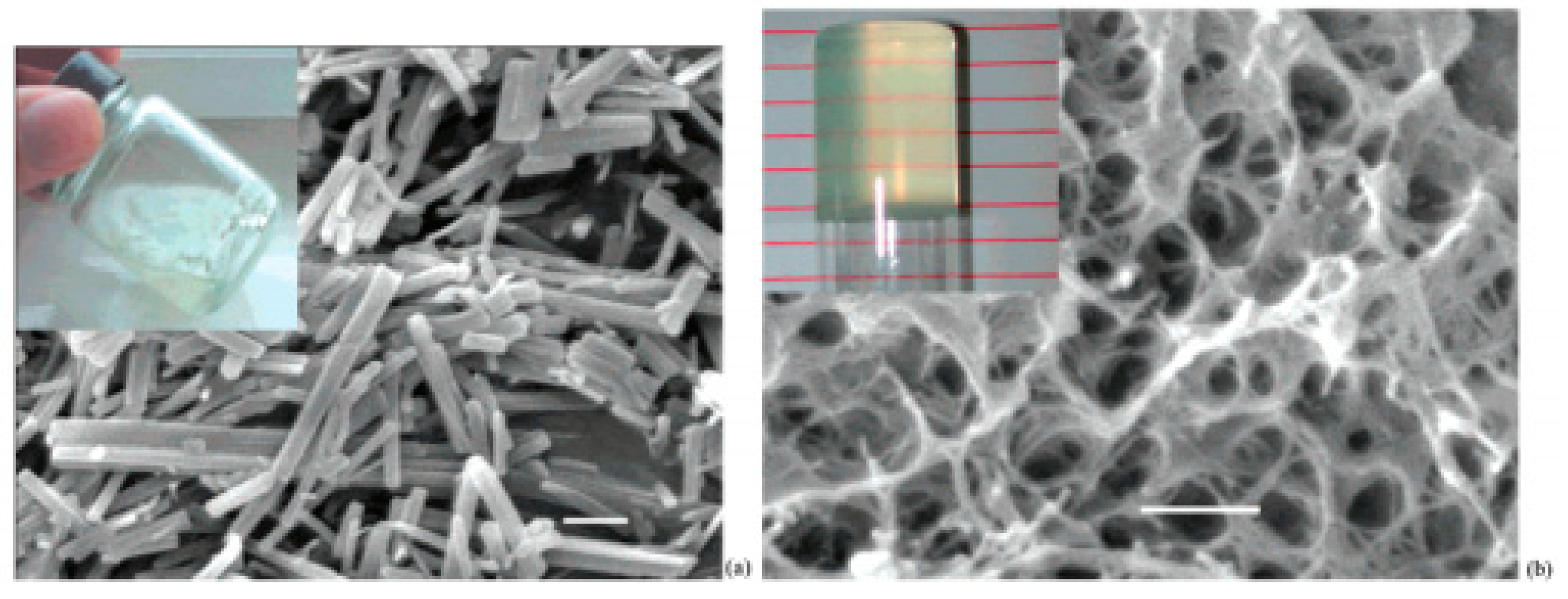
- Foster, J.A.; Piepenbrock, M.O.M.; Lloyd, G.O.; Clarke, N.; Howard, J.A.K.; Steed, J.W. Anion-Switchable Supramolecular Gels for Controlling Pharmaceutical Crystal Growth. Nat. Chem. 2010, 2, 1037–1043. [Google Scholar] [CrossRef]
- Liu, X.Y.; Sawant, P.D.; Tan, W.B.; Noor, I.B.M.; Pramesti, C.; Chen, B.H. Creating New Supramolecular Materials by Architecture of Three-Dimensional Nanocrystal Fiber Networks. J. Am. Chem. Soc. 2002, 124, 15055–15063. [Google Scholar] [CrossRef]
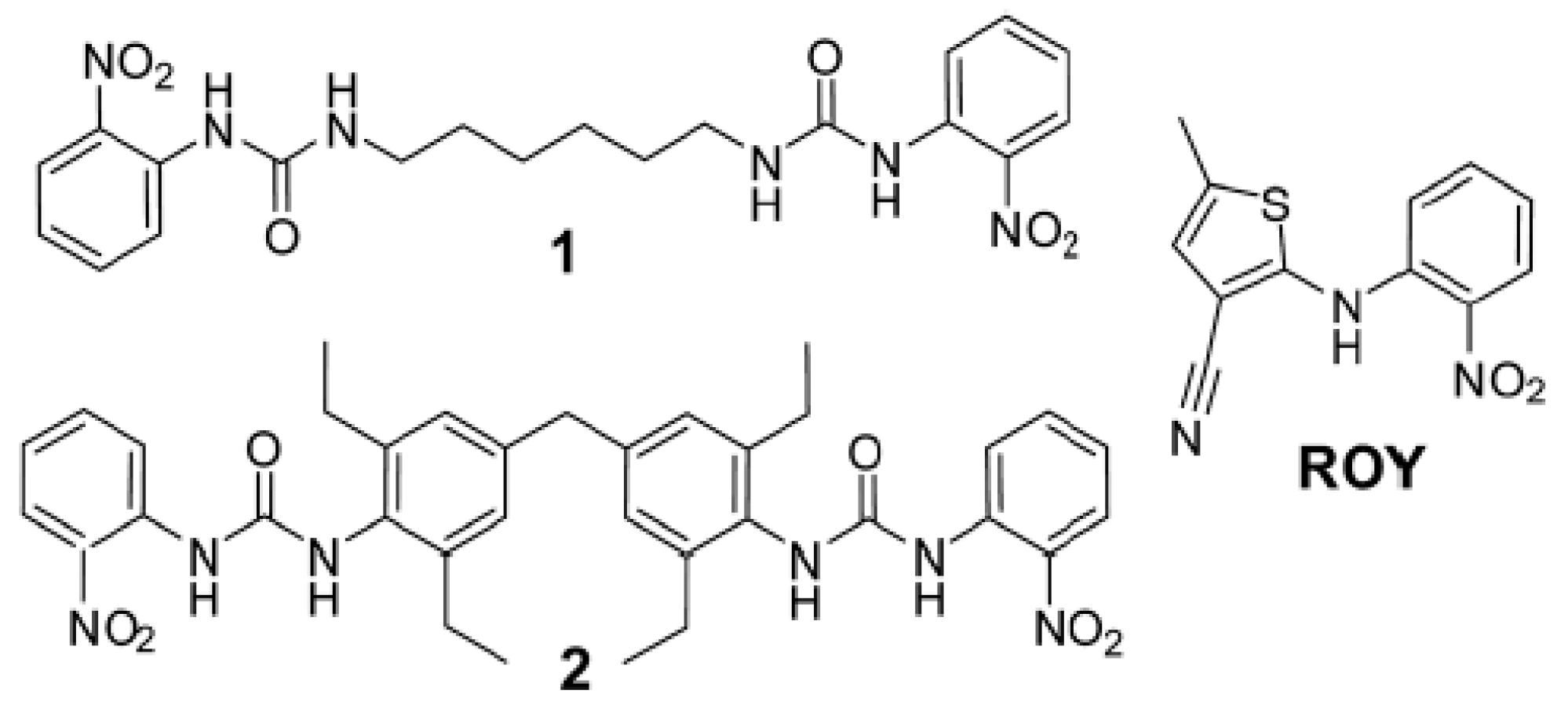
- Foster, J.A.; Damodaran, K.K.; Maurin, A.; Day, G.M.; Thompson, H.P.G.; Cameron, G.J.; Bernal, J.C.; Steed, J.W. Pharmaceutical Polymorph Control in a Drug-Mimetic Supramolecular Gel. Chem. Sci. 2016, 8, 78–84. [Google Scholar] [CrossRef]
- Galkin, O.; Chen, K.; Nagel, R.L.; Hirsch, R.E.; Vekilov, P.G. Liquid-Liquid Separation in Solutions of Normal and Sickle Cell Hemoglobin. Proc. Natl. Acad. Sci. USA 2002, 99, 8479–8483. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, G.; Curcio, E.; Drioli, E. Trypsin Crystallization by Membrane-Based Techniques. J. Struct. Biol. 2005, 150, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, G.; Polino, M.; Nicoletta, F.P.; Belviso, B.D.; Caliandro, R.; Fontananova, E.; De Filpo, G.; Curcio, E.; Drioli, E. Tailored Hydrogel Membranes for Efficient Protein Crystallization. Adv. Funct. Mater. 2014, 24, 1582–1590. [Google Scholar] [CrossRef]
- Belviso, B.D.; Caliandro, R.; Salehi, S.M.; Di Profio, G.; Caliandro, R. Protein Crystallization in Ionic-Liquid Hydrogel Composite Membranes. Crystals 2019, 9, 253. [Google Scholar] [CrossRef]
- Pareja-Rivera, C.; Cuéllar-Cruz, M.; Esturau-Escofet, N.; Demitri, N.; Polentarutti, M.; Stojanoff, V.; Moreno, A. Recent advances in the understanding of the influence of electric and magnetic fields on protein crystal growth. Cryst. Growth Des. 2017, 17, 135–145. [Google Scholar] [CrossRef]
- Messerschmidt, A. X-Ray Crystallography of Biomacromolecules; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2007. [Google Scholar]
- Yan, E.; Zhang, C.-Y.; He, J.; Yin, D.-C. An overview of hardware for protein crystallization in a magnetic field. Int. J. Mol. Sci. 2016, 17, 1906. [Google Scholar] [CrossRef] [PubMed]
- Ataka, M.; Wakayama, N.-C. Effects of a magnetic field and magnetization force on protein crystal growth. Why does a magnet improve the quality of some crystals? Acta. Crystallogr. 2002, D58, 1708–1710. [Google Scholar] [CrossRef]
- Sazaki, G. Crystal quality enhancement by magnetic fields. Prog. Biophys. Mol. Biol. 2009, 101, 45–55. [Google Scholar] [CrossRef]
- Surade, S.; Ochi, T.; Nietlispach, D.; Chirgadze, D.; Moreno, A. Investigations into protein crystallization in the presence of a strong magnetic field. Cryst. Growth Des. 2010, 10, 691–699. [Google Scholar] [CrossRef]
- Yin, D.-C. Protein crystallization in a magnetic field. Progress in Crystal Growth and Characterization of Materials. Prog. Cryst. Growth Charact. Mater. 2015, 61, 1–26. [Google Scholar] [CrossRef]
- Gil-Alvaradejo, G.; Ruiz-Arellano, R.R.; Owen, C.; Rodríguez-Romero, A.; Rudiño-Piñera, E.; Antwi, M.K.; Stojanoff, V.; Moreno, A. Novel protein crystal growth electrochemical cell for applications in X-ray diffraction and atomic force microscopy. Cryst. Growth Des. 2011, 11, 3917–3922. [Google Scholar] [CrossRef]
- Lorber, B.; Sauter, C.; Théobald-Dietrich, A.; Moreno, A.; Schellenberger, P.; Robert, M.-C.; Capelle, B.; Sanglier, S.; Potier, N.; Giegé, R. Crystal growth of proteins, nucleic acids, and viruses in gels. Prog. Biophys. Mol. Biol. 2009, 101, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Romero, A.; Esturau-Escofet, N.; Pareja-Rivera, C.; Moreno, A. Crystal Growth of High-Quality Protein Crystals under the Presence of an Alternant Electric Field in Pulse-Wave Mode, and a Strong Magnetic Field with Radio Frequency Pulses Characterized by X-ray Diffraction. Crystals 2017, 7, 179. [Google Scholar] [CrossRef]
- Harrison, P.M. The structure of apoferritin: molecular size, shape and symmetry from x-ray data. J. Mol. Biol. 1963, 6, 404–422. [Google Scholar] [CrossRef]
- Granick, S.; Hahn, P.F. Ferritin VIII—speed and uptake of iron by liver and its conversion to ferritin iron. J. Biol. Chem. 1994, 155, 661–669. [Google Scholar]
- Van Der Wel, H.; Loeve, K. Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. Eur. J. Biochem. 1972, 31, 221–225. [Google Scholar] [PubMed]
- Most, B.H.; Summerfield, R.J.; Boxall, M. Tropical plants with sweetening properties physiological and agronomic problems of protected cropping 2. Tha uma tococcus daniellii. Econ. Bot. 1978, 32, 321–335. [Google Scholar] [CrossRef]
- Lorber, B.; Sauter, C.; Robert, M.C.; Capelle, B.; Giegé, R. Crystallization within agarose gel in microgravity improves the quality of thaumatin crystals. Acta Crystallogr. 1999, 55, 1491–1494. [Google Scholar] [CrossRef]
- Wlodawer, A.; Minor, W.; Dauter, Z.; Jaskolski, M. Protein crystallography for non-crystallographers, or how to get the best (but not more) from published macromolecular structures. FEBS J. 2008, 275, 1–21. [Google Scholar] [CrossRef]
- Sanishvili, R.; Nagarajan, V.; Yoder, D.; Becker, M.; Xu, S.H.; Corcoran, S.; Akey, D.L.; Smith, J.L.; Fischetti, R.F. A 7µ mini-beam improves diffraction data from small or imperfect crystals of macromolecules. Acta Crystallogr. 2008, D64, 425–435. [Google Scholar]
- Diederichs, K.; Karplus, P.A. Improved R-factors for diffraction data analysis in macromolecular crystallography. Nat. Struct. Biol. 1997, 4, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Mendoza, M.E. Crystallization in Gels. In Handbook of Crystal Growth, 2nd ed.; Rudolph, P., Nishinaga, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume II, pp. 1277–1315. [Google Scholar]
- Gavira, J.A. Current trends in protein crystallization. Arch. Biochem. Biophys. 2016, 602, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Rosales-Hoz, M. Crystal growth of inorganic, organic, and biological macromolecules in gels. Prog. Crystal Growth Charact. Mater. 2017, 3, 63–71. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez-González, O.; Campos-Escamilla, C.; Flores-Ibarra, A.; Esturau-Escofet, N.; Arreguin-Espinosa, R.; Stojanoff, V.; Cuéllar-Cruz, M.; Moreno, A. Crystal Growth in Gels from the Mechanisms of Crystal Growth to Control of Polymorphism: New Trends on Theoretical and Experimental Aspects. Crystals 2019, 9, 443. https://doi.org/10.3390/cryst9090443
Velásquez-González O, Campos-Escamilla C, Flores-Ibarra A, Esturau-Escofet N, Arreguin-Espinosa R, Stojanoff V, Cuéllar-Cruz M, Moreno A. Crystal Growth in Gels from the Mechanisms of Crystal Growth to Control of Polymorphism: New Trends on Theoretical and Experimental Aspects. Crystals. 2019; 9(9):443. https://doi.org/10.3390/cryst9090443
Chicago/Turabian StyleVelásquez-González, Omar, Camila Campos-Escamilla, Andrea Flores-Ibarra, Nuria Esturau-Escofet, Roberto Arreguin-Espinosa, Vivian Stojanoff, Mayra Cuéllar-Cruz, and Abel Moreno. 2019. "Crystal Growth in Gels from the Mechanisms of Crystal Growth to Control of Polymorphism: New Trends on Theoretical and Experimental Aspects" Crystals 9, no. 9: 443. https://doi.org/10.3390/cryst9090443
APA StyleVelásquez-González, O., Campos-Escamilla, C., Flores-Ibarra, A., Esturau-Escofet, N., Arreguin-Espinosa, R., Stojanoff, V., Cuéllar-Cruz, M., & Moreno, A. (2019). Crystal Growth in Gels from the Mechanisms of Crystal Growth to Control of Polymorphism: New Trends on Theoretical and Experimental Aspects. Crystals, 9(9), 443. https://doi.org/10.3390/cryst9090443








