Synthesis, X-Ray Crystal Structure, Hirshfeld Surface Analysis, and Molecular Docking Study of Novel Hepatitis B (HBV) Inhibitor: 8-Fluoro-5-(4-fluorobenzyl)-3-(2-methoxybenzyl)-3,5-dihydro-4H-pyrimido[5,4-b]indol-4-one
Abstract
:1. Introduction
2. Results and Discussion
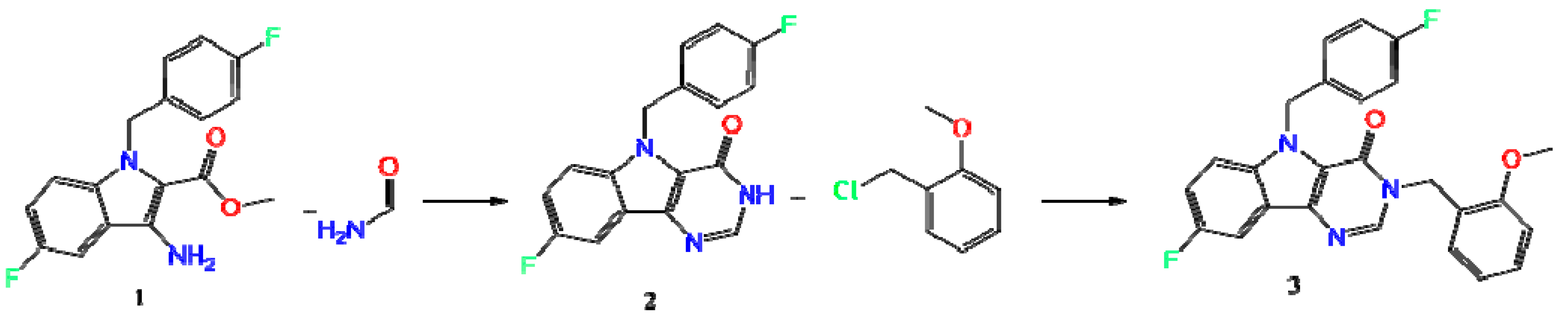
2.1. Synthesis
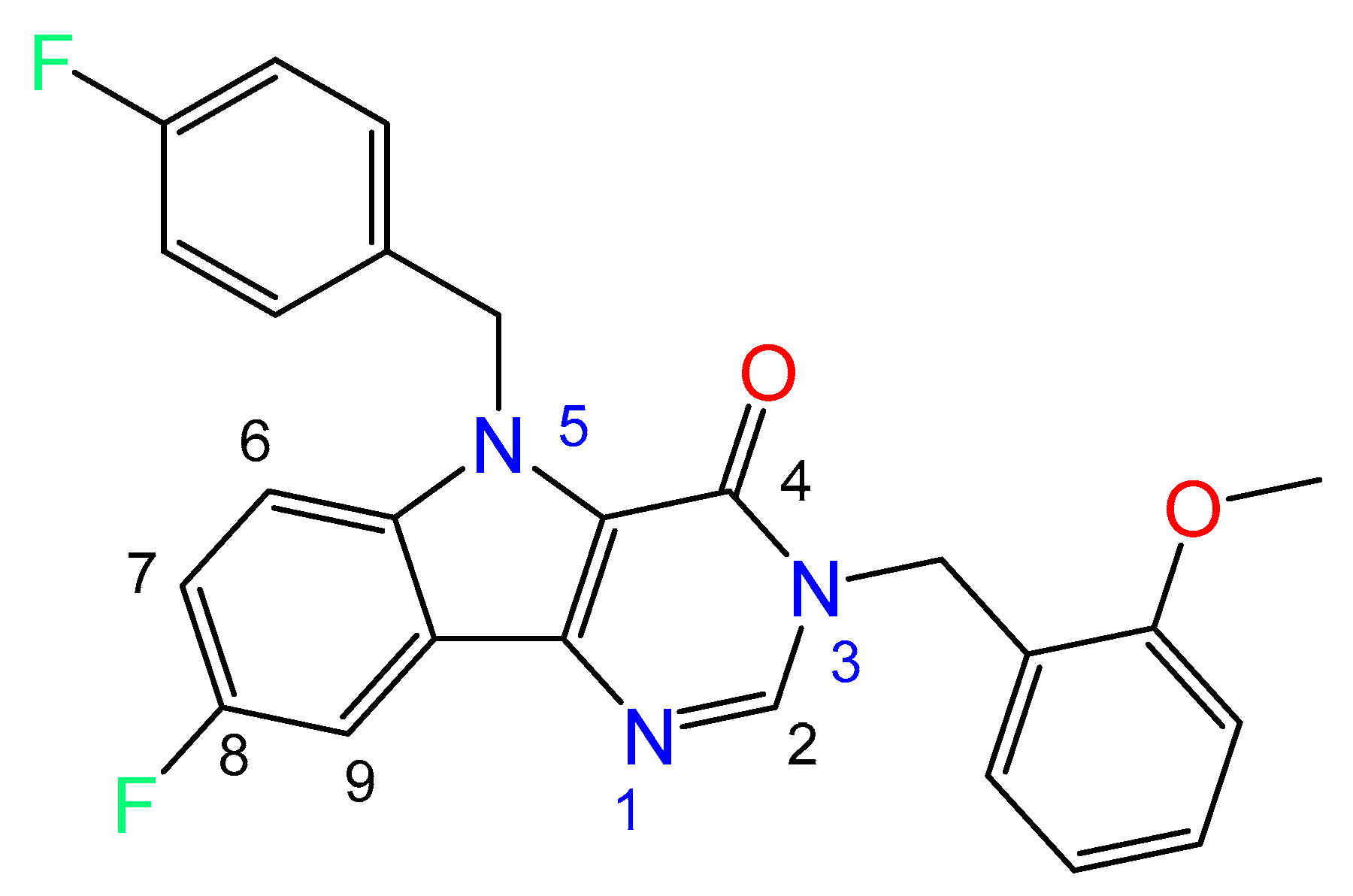
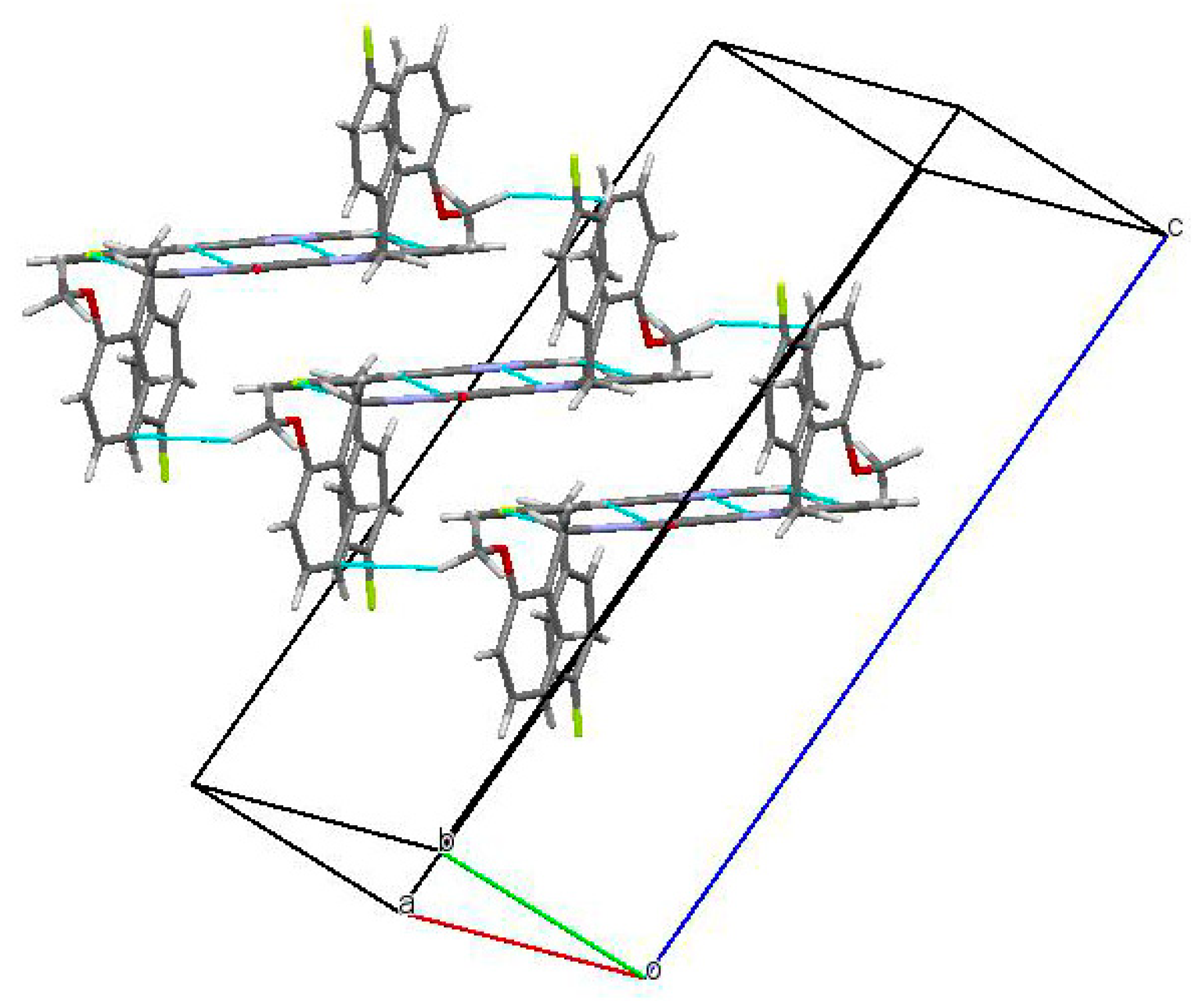
2.2. Crystal and Molecular Structure Analysis
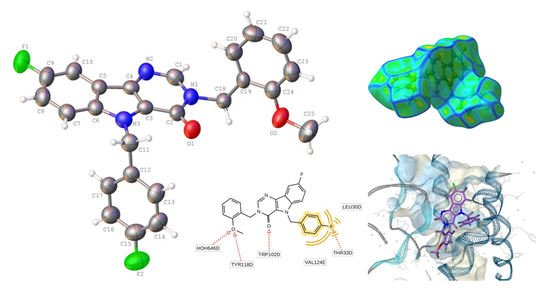
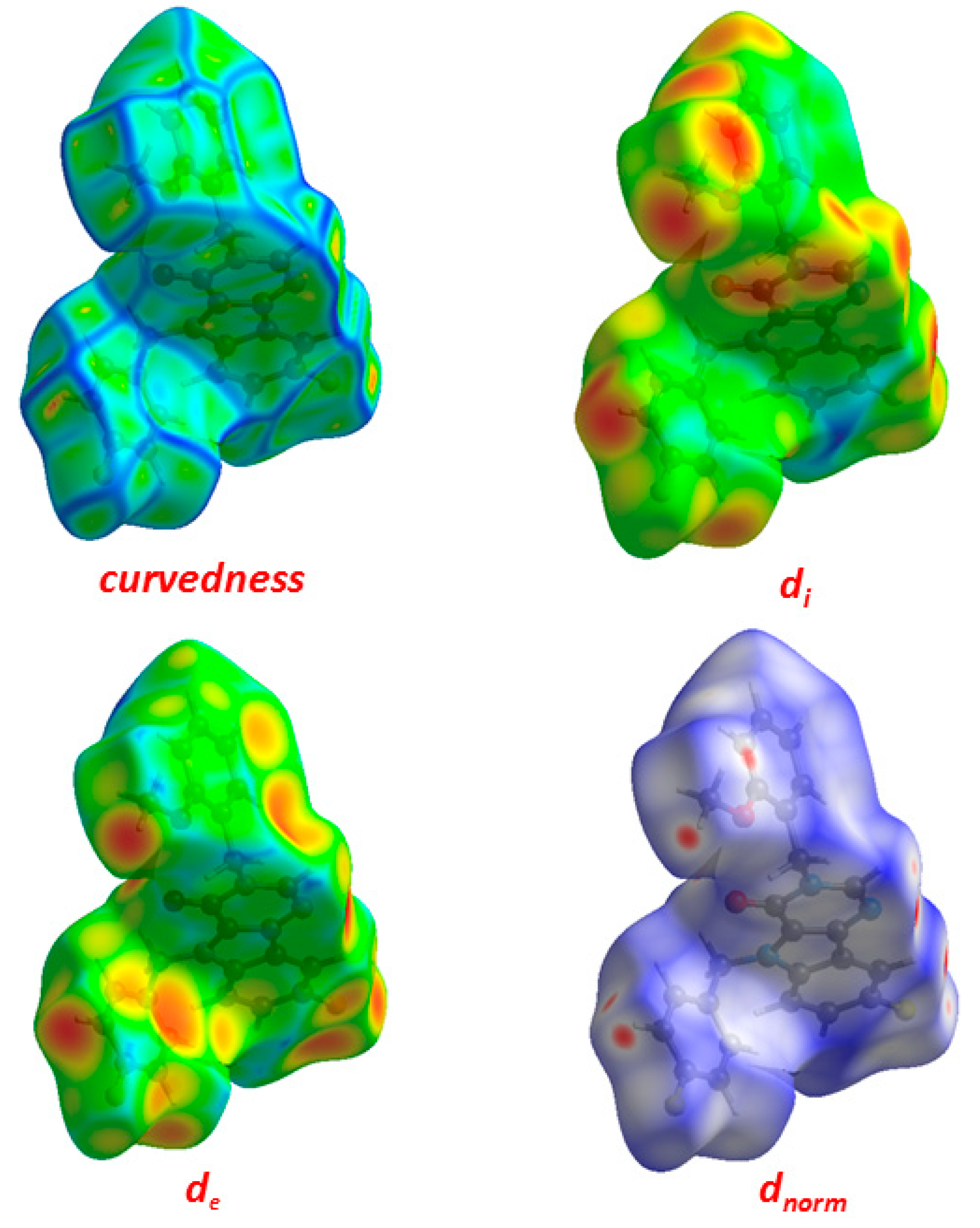
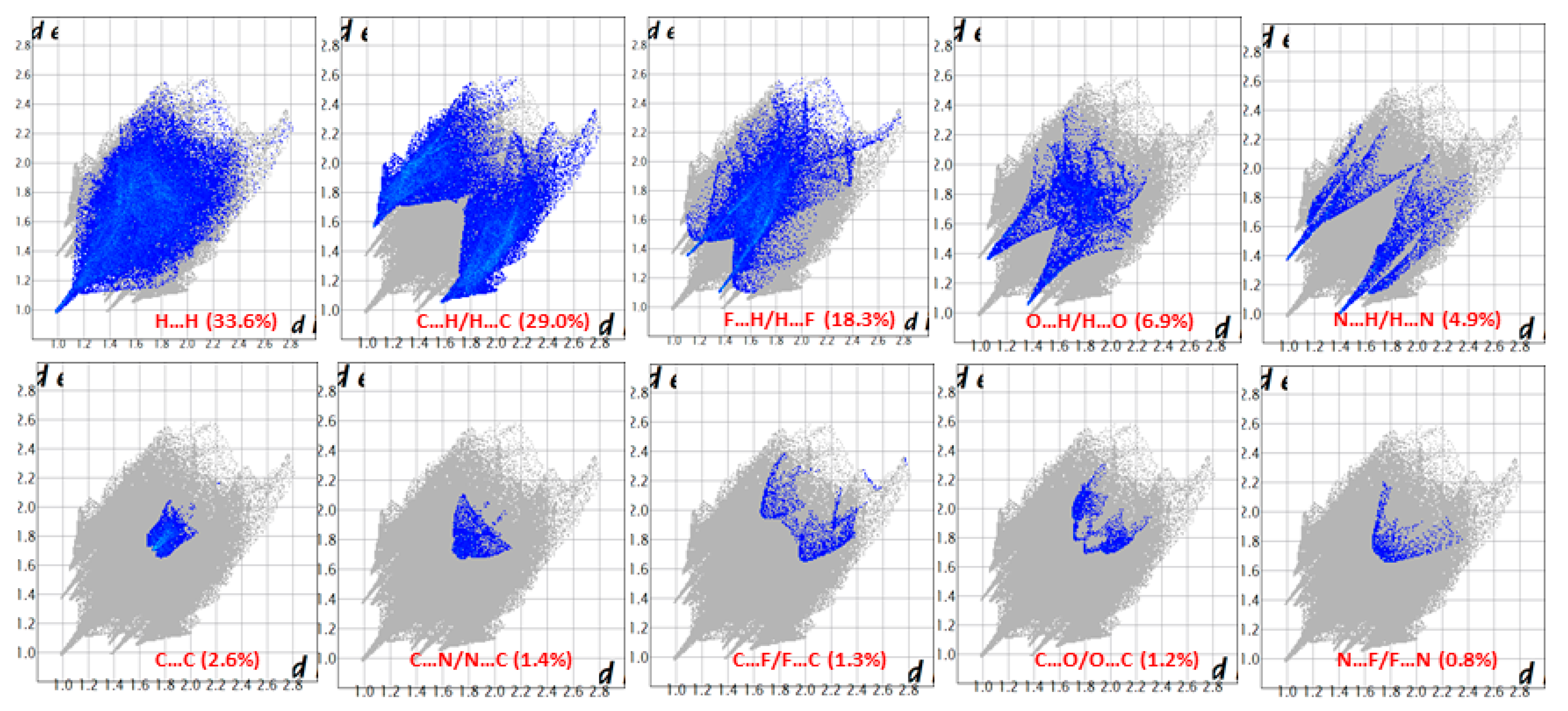
2.3. Hirshfeld Surface Analysis
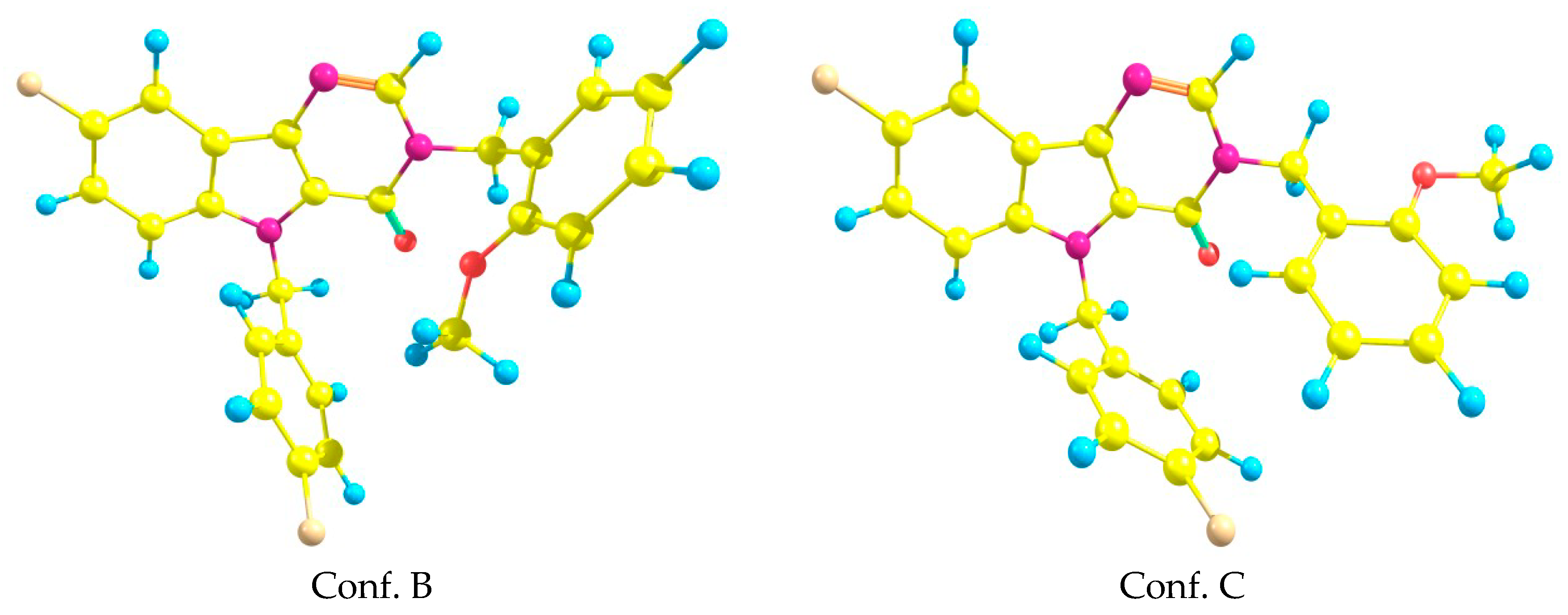
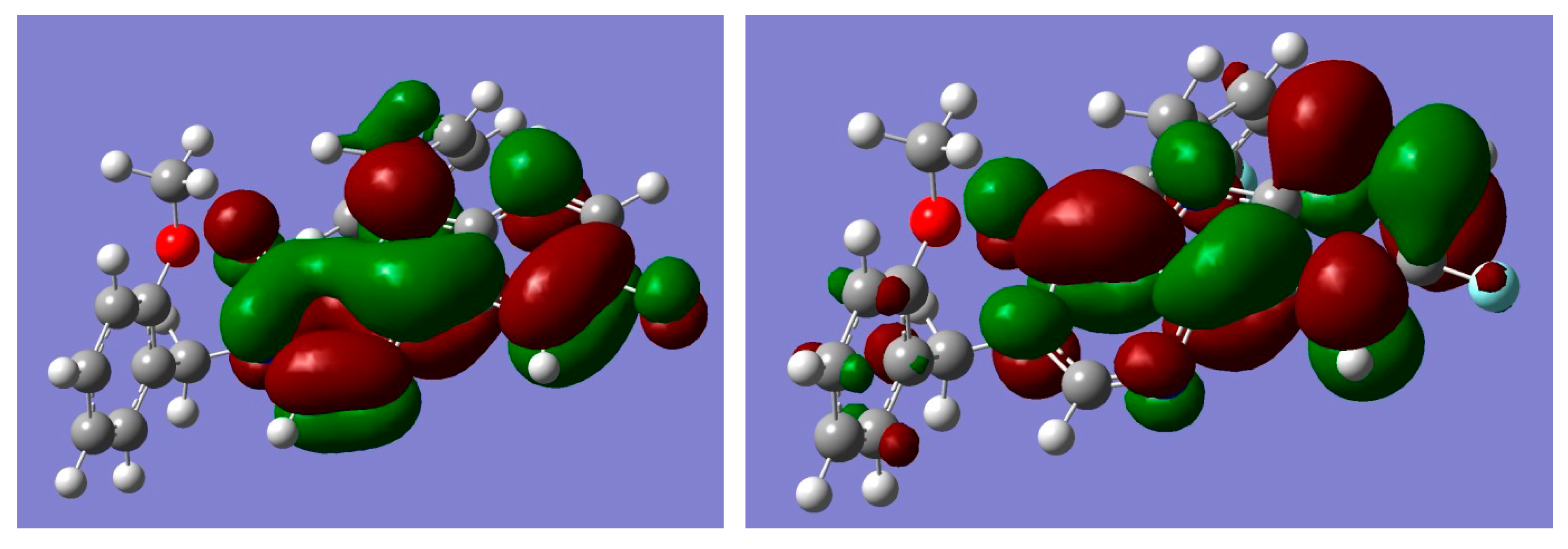
2.4. Quantum Chemistry Calculations of Geometry and Electronic Structure
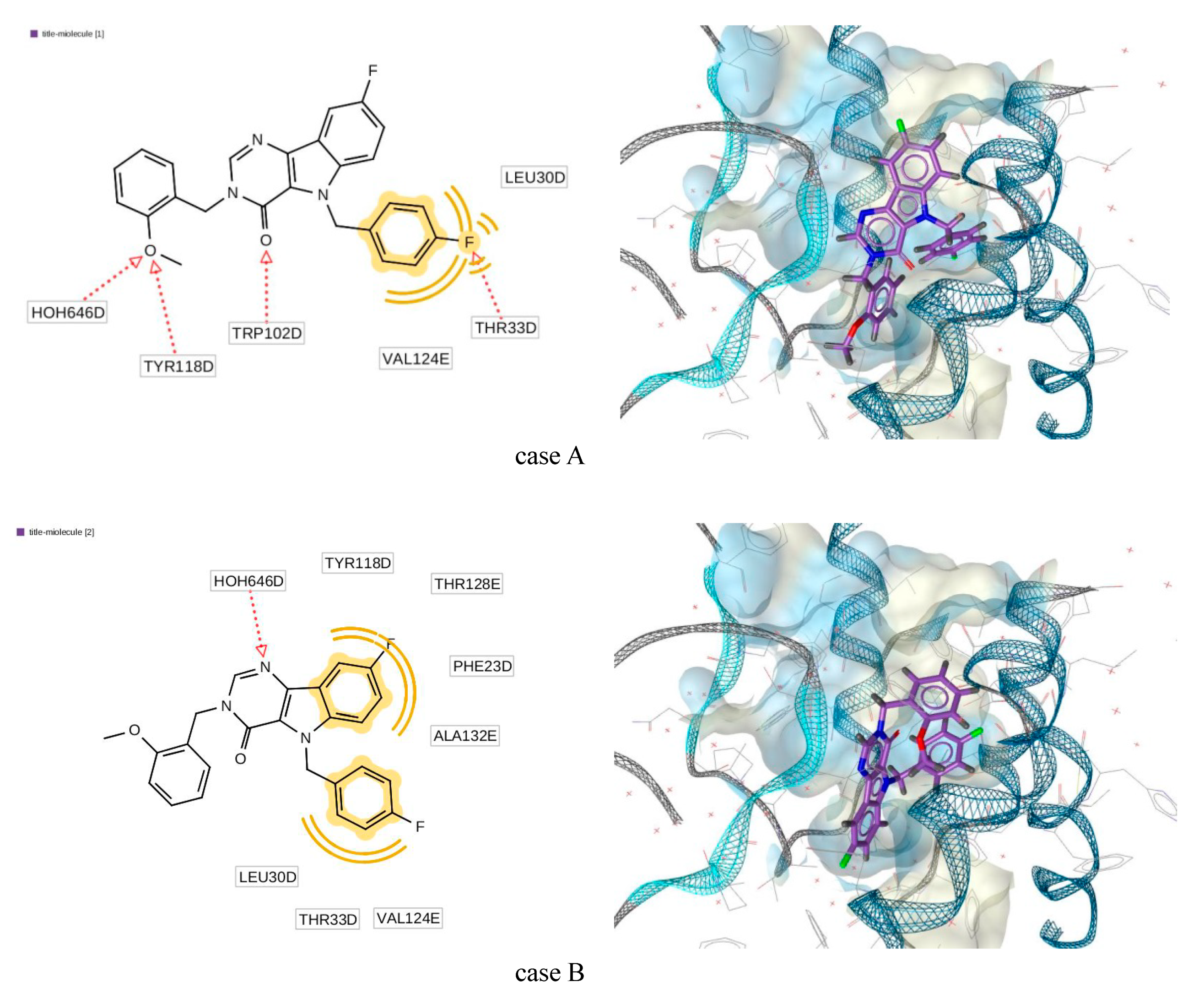
2.5. Molecular Docking Simulations
2.6. Anti-Hepatitis B Virus (HBV) Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. Synthesis and Crystallization of Compound (3)
3.2. X-ray Diffraction Study
3.3. Theoretical Calculations
3.4. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Hepatitis B Fact Sheet N204; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Elguero, J.; Goya, P.; Jagerovic, N.; Silva, A.M.S. Pyrazoles as Drugs: Facts and Fantasies in Targets in Heterocyclic Systems. Ital. Soc. Chem. 2002, 6, 52. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 2nd ed.; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Deiters, A.; Martin, S.F. Synthesis of Oxygen- and Nitrogen-Containing Heterocycles by Ring-Closing Metathesis. Chem. Rev. 2004, 104, 2199. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Cämmerer, S.; Filali, S.; Fröhner, W.; Knöll, J.; Krahl, M.P.; Reddy, K.R.; Knölker, H.-J. Novel Routes to Pyrroles, Indoles and Carbazoles - Applications in Natural Product Synthesis. Curr. Org. Chem. 2005, 9, 1601. [Google Scholar] [CrossRef]
- Gataullin, R.R. Synthesis of compounds containing a cycloalka[b]indole fragment. Russ. J. Org. Chem. 2009, 45, 321. [Google Scholar] [CrossRef]
- Bolton, D.; Forbes, I.T.; Hayward, C.J.; Piper, D.C.; Thomas, D.R.; Thompson, M.; Upton, N. Synthesis and potential anxiolytic activity of 4-amino-pyrido [2, 3-b] indoles. Bioorg. Med. Chem. Lett. 1993, 3, 1941. [Google Scholar] [CrossRef]
- Love, B.E. Synthesis of carbolines possessing antitumor activity. Top. Heterocycl. Chem. 2006, 2, 93. [Google Scholar]
- Somei, U.; Basha, A. Indole Alkaloids; Hawood Academic Publishers: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875. [Google Scholar] [CrossRef]
- Sundberg, R.J. Indoles, 1st ed.; Academic Press: London, UK, 1996. [Google Scholar]
- Sundberg, R.J. Comprehensive Heterocyclic Chemistry II, 2nd ed.; Katritzky, A.R., Ress, C.W., Scriven, E.F.V., Bird, C.W., Eds.; Pergamon Press: Oxford, UK, 1996; Volume 2, p. 119. [Google Scholar]
- Okamoto, A.; Tanaka, K.; Saito, I. Rational design of a DNA wire possessing an extremely high hole transport ability. J. Am. Chem. Soc. 2003, 125, 5066. [Google Scholar] [CrossRef]
- Okamoto, A.; Tanaka, K.; Saito, I.J. DNA logic gates. Am. Chem. Soc. 2004, 126, 9458. [Google Scholar] [CrossRef]
- Showalter, D.H.H.; Bridges, J.A.; Zhou, H.; Sercel, D.A.; McMichael, A.; Fry, D.W.J. Tyrosine Kinase Inhibitors. 16. 6,5,6-Tricyclic Benzothieno[3,2-d]pyrimidines and Pyrimido[5,4-b]- and -[4,5-b]indoles as Potent Inhibitors of the Epidermal Growth Factor Receptor Tyrosine Kinase. Med. Chem. 1999, 42, 5464. [Google Scholar] [CrossRef]
- Bundy, G.L.; Banitt, L.S.; Dobrowoski, P.L.; Palmer, J.R.; Schwartz, T.M.; Zimmermann, D.C.; Lipton, M.F.; Mauragis, M.A.; Veley, M.F.; Appell, R.B.; et al. Synthesis of 2, 4-Di-1-pyrrolidinyl-9H-pyrimido [4, 5-b] indoles, including antiasthma clinical candidate PNU-142731A. Org. Process Res. Dev. 2001, 5, 144. [Google Scholar] [CrossRef]
- Barnes, P.J.; Chung, K.F.; Page, C.P. Inflammatory mediators of asthma: An update. Pharmacol. Rev. 1998, 50, 515. [Google Scholar] [PubMed]
- Howarth, P.H. The airway inflammatory response in allergic asthma and its relationship to clinical disease. Allergy 1995, 50, 13. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.E.; Hide, I.; Daly, J.; Rothenhausler, K.; Eger, K. 7-Deaza-2-phenyladenines: structure-activity relationships of potent A1 selective adenosine receptor antagonists. J. Med. Chem. 1990, 33, 2822. [Google Scholar] [CrossRef] [PubMed]
- Darrow, J.W.; Maynard, G.D.; Horvath, R.F. Substituted 9h-pyridino[2,3-b]indole and 9h-pyrimidino[4,5-b]indole Derivatives: Selective Neuropeptide y Receptor Ligands. International Patent Application Publication No. WO 9951598, 14 October 1999. [Google Scholar]
- Ivachtchenko, A.V.; Ivachtchenko, A.A.; Savchuk, N.P.; Rogovoj, B.; Bychko, V.V. Hepatitis B Virus (HBV) Inhibitor. International Patent Application Publication No. WO 2019017814, 24 January 2019. [Google Scholar]
- Ivachtchenko, A.V.; Ivachtchenko, A.A.; Savchuk, N.P.; Rogovoj, B.; Bychko, V.V.; Khvat, A. Hepatitis B virus (HBV) Penetration Inhibitor and Pharmaceutical Composition for Hepatitis Treatment. International Patent Application Publication No. RU 2662161, 24 July 2018. [Google Scholar]
- Barker, A.J.; Kettle, J.G.; Faull, A.W. Preparation of Substituted Indoles for Treatment of A Disease or Condition Mediated by Monocyte Chemoattractant Protein-1 (MCP-1). International Patent Application Publication No. WO 9907351 A2, 18 February 1999. [Google Scholar]
- Zefirov, Y.V.; Zorky, P.M. New applications of van der Waals radii in chemistry. Russ. Chem. Rev. 1995, 64, 415–428. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17; The University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Endres, D.; Miyahara, M.; Moisant, P.; Zlotnick, A. A reaction landscape identifies the intermediates critical for self-assembly of virus capsids and other polyhedral structures. Protein Sci. 2005, 14, 1518–1525. [Google Scholar] [CrossRef]
- Stray, S.J.; Zlotnick, A. BAY 41-4109 has multiple effects on Hepatitis B virus capsid assembly. J. Mol. Recognit. 2006, 19, 542–548. [Google Scholar] [CrossRef]
- Choi, I.G.; Yu, Y.G. Interaction and assembly of HBV structural proteins: Novel target sites of anti-HBV agents. Infect. Disord. Drug Targets 2007, 7, 251–256. [Google Scholar] [CrossRef]
- Firdayani, A.; Arsianti, C.; Yanuar, A. Molecular Docking and Dynamic Simulation Benzoylated Emodin into HBV Core Protein. J. Young Pharm. 2018, 10, S20–S24. [Google Scholar] [CrossRef]
- Wu, G.; Liu, B.; Zhang, Y.; Li, J.; Arzumanyan, A.; Clayton, M.M.; Schinazi, R.F.; Wang, Z.; Goldmann, S.; Ren, Q.; et al. Preclinical characterization of GLS4, an inhibitor of hepatitis B virus core particle assembly. Antimicrob. Agents Chemother. 2013, 57, 5344–5354. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wei, Z.M.; Wu, G.Y.; Wang, J.H.; Zhang, Y.J.; Li, J.; Zhang, H.H.; Xie, X.W.; Wang, X.; Wang, Z.H.; et al. In vitro inhibition of HBV replication by a novel compound, GLS4, and its efficacy against adefovir-dipivoxil-resistant HBV mutations. Antivir. Ther. 2012, 17, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank Nucleic Acids Research. 2000, Volume 28, pp. 235–242. Available online: http://www.rcsb.org/ (accessed on 24 July 2019).
- Wolber, G.; Langer, T. LigandScout: 3-D Pharmacophores Derived from Protein-Bound Ligands and Their Use as Virtual Screening Filters. J. Chem. Inf. Model. 2005, 45, 160–169. Available online: http://www.inteligand.com/ligandscout/ (accessed on 24 July 2019). [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian-09; Revision A.02, Gaussian, Inc.: Wallingford, CT, USA, 2009.
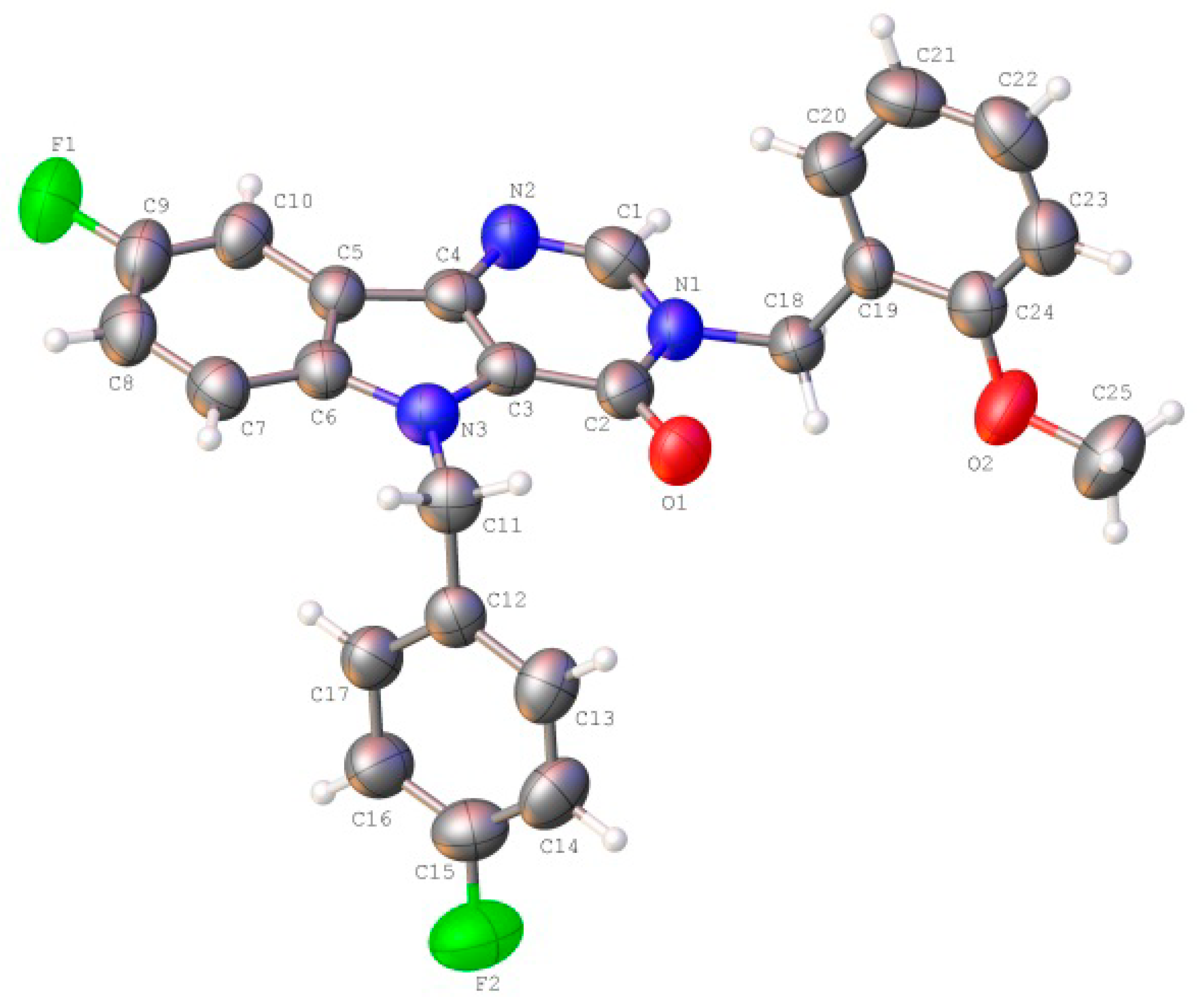
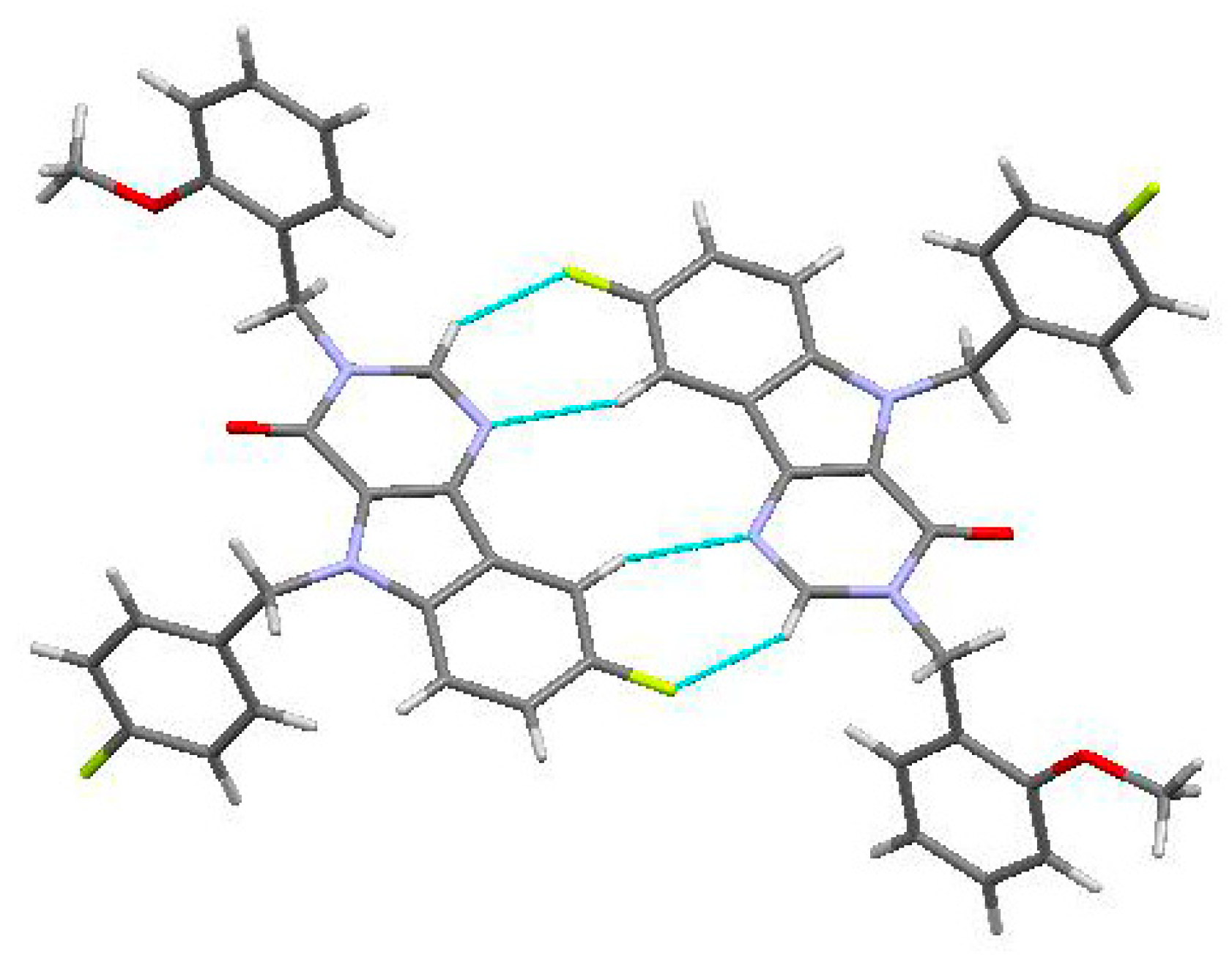
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| C1—H1···F1 i | 0.93 | 2.60 | 3.433(5) | 149.0 |
| C10—H10···N2 i | 0.93 | 2.53 | 3.417(6) | 159.8 |
| C14—H14···O1 ii | 0.93 | 2.56 | 3.346(5) | 143.1 |
| C20—H20···C1 | 0.93 | 2.79 | 3.381(6) | 122.5 |
| C23—H23···C9 iii | 0.93 | 2.87 | 3.668(6) | 144.3 |
| C25-H25A…C21 iiii | 0.93 | 2.74 | 3.572(3) | 144.9 |
| Bond/Torsion Angle | Experimental Value (X-ray Study) | Calculated Value, (Vacuum) |
|---|---|---|
| F1–C9 | 1.358(5) | 1.345 |
| F2—C15 | 1.366(5) | 1.342 |
| O1—C2 | 1.221(5) | 1.227 |
| O2—C24 | 1.362(5) | 1.354 |
| O2—C25 | 1.425(5) | 1.411 |
| N1—C1 | 1.369(5) | 1.377 |
| N1—C2 | 1.400(5) | 1.404 |
| N1—C18 | 1.460(4) | 1.472 |
| N2—C1 | 1.289(5) | 1.292 |
| N2—C4 | 1.375(5) | 1.371 |
| N3—C3 | 1.385(5) | 1.377 |
| N3—C6 | 1.395(5) | 1.381 |
| N3—C11 | 1.449(5) | 1.459 |
| C1–N1–C12–C19 | 93.6 | 58.5 |
| N1–C18–C19–C20 | −29.1 | −116.1 |
| C25–O2–C24–C23 | 8.7 | 1.7 |
| C3–N3–C11–C12 | −78.3 | −94.6 |
| N3–C11–C12–C17 | −50.7 | −106 |
| PDB | Molecule | Affinity (kcal/mol) | Binding Affinity Score |
|---|---|---|---|
| 5E0I | NVR10-001E2 | −21.6 | −28.6 |
| molecule (3) | −17.9 | −33.5 | |
| 5GMZ | 4-methyl heteroaryldihydropyrimidine | −16.18 | −25.95 |
| molecule (3) | −16.30 | −31.02 | |
| 5WRE | HAP-R01 | −21.46 | −38.45 |
| molecule (3) | −15.16 | −23.39 | |
| 5T2P | SBA-R01 | −15.81 | −26.70 |
| molecule (3) | −17.14 | −44.42 |
| Torsion Angle | Docking Case A | ab Initio Conf. C | Docking Case B | ab Initio Conf. B |
|---|---|---|---|---|
| N3–C11–C12–C17 | −3.6 | 53.6 | 30.9 | 19 |
| N1–C18–C19–C24 | −176.1 | −170 | 63.2 | 60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivashchenko, A.V.; Mitkin, O.D.; Kravchenko, D.V.; Kuznetsova, I.V.; Kovalenko, S.M.; Bunyatyan, N.D.; Langer, T. Synthesis, X-Ray Crystal Structure, Hirshfeld Surface Analysis, and Molecular Docking Study of Novel Hepatitis B (HBV) Inhibitor: 8-Fluoro-5-(4-fluorobenzyl)-3-(2-methoxybenzyl)-3,5-dihydro-4H-pyrimido[5,4-b]indol-4-one. Crystals 2019, 9, 379. https://doi.org/10.3390/cryst9080379
Ivashchenko AV, Mitkin OD, Kravchenko DV, Kuznetsova IV, Kovalenko SM, Bunyatyan ND, Langer T. Synthesis, X-Ray Crystal Structure, Hirshfeld Surface Analysis, and Molecular Docking Study of Novel Hepatitis B (HBV) Inhibitor: 8-Fluoro-5-(4-fluorobenzyl)-3-(2-methoxybenzyl)-3,5-dihydro-4H-pyrimido[5,4-b]indol-4-one. Crystals. 2019; 9(8):379. https://doi.org/10.3390/cryst9080379
Chicago/Turabian StyleIvashchenko, Aleksandr V., Oleg D. Mitkin, Dmitry V. Kravchenko, Irina V. Kuznetsova, Sergiy M. Kovalenko, Natalya D. Bunyatyan, and Thierry Langer. 2019. "Synthesis, X-Ray Crystal Structure, Hirshfeld Surface Analysis, and Molecular Docking Study of Novel Hepatitis B (HBV) Inhibitor: 8-Fluoro-5-(4-fluorobenzyl)-3-(2-methoxybenzyl)-3,5-dihydro-4H-pyrimido[5,4-b]indol-4-one" Crystals 9, no. 8: 379. https://doi.org/10.3390/cryst9080379
APA StyleIvashchenko, A. V., Mitkin, O. D., Kravchenko, D. V., Kuznetsova, I. V., Kovalenko, S. M., Bunyatyan, N. D., & Langer, T. (2019). Synthesis, X-Ray Crystal Structure, Hirshfeld Surface Analysis, and Molecular Docking Study of Novel Hepatitis B (HBV) Inhibitor: 8-Fluoro-5-(4-fluorobenzyl)-3-(2-methoxybenzyl)-3,5-dihydro-4H-pyrimido[5,4-b]indol-4-one. Crystals, 9(8), 379. https://doi.org/10.3390/cryst9080379