Growth and Property Investigations of Two Organic–Inorganic Hybrid Molecular Crystals with High Thermal Stability: 4-Iodoanilinium perchlorate 18-crown-6 and 4-Iodoanilinium Borofluorate 18-crown-6
Abstract
1. Introduction
2. Materials and Methods
3. Results
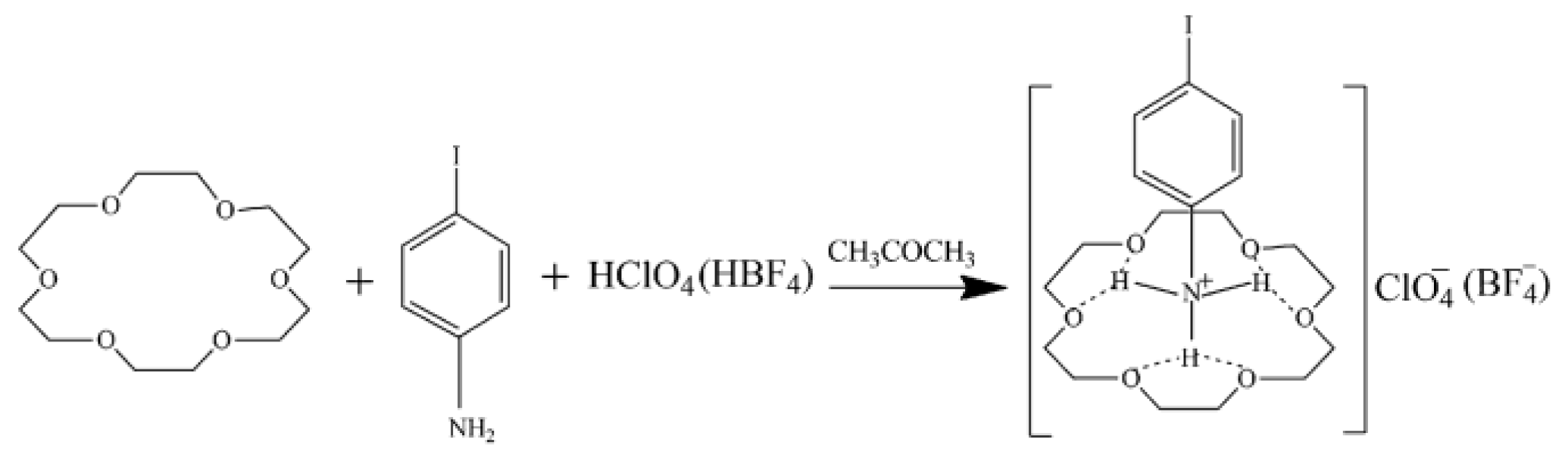
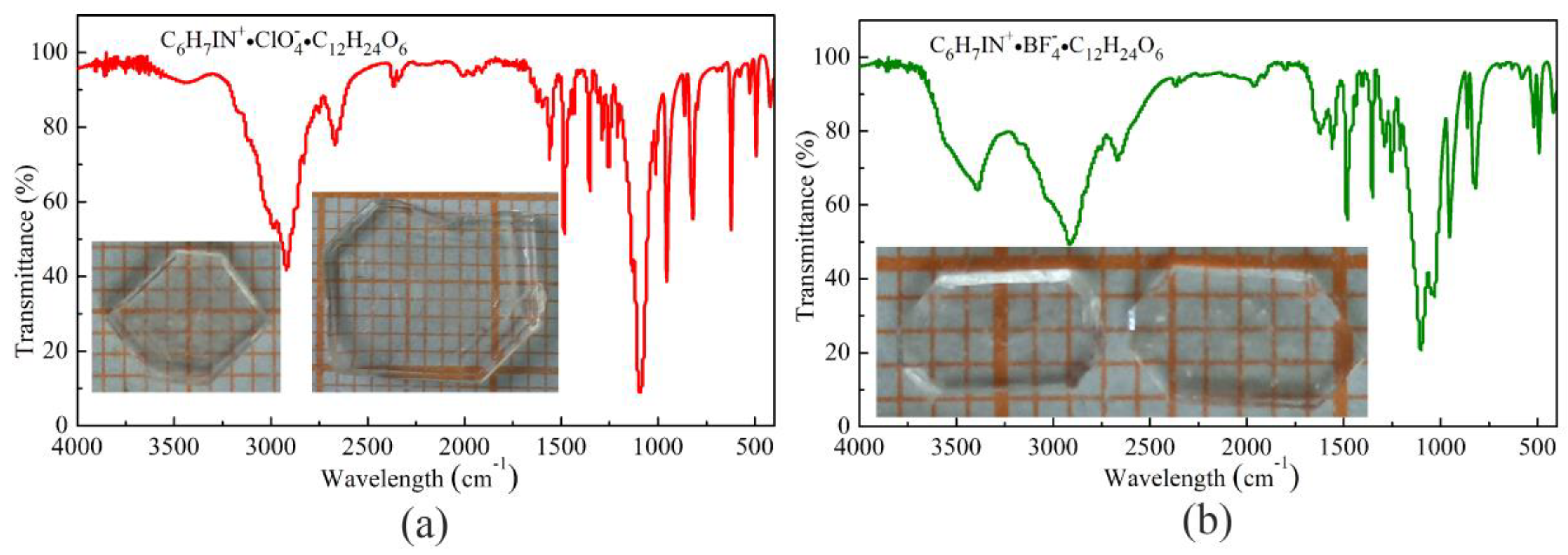
3.1. Crystal Growth
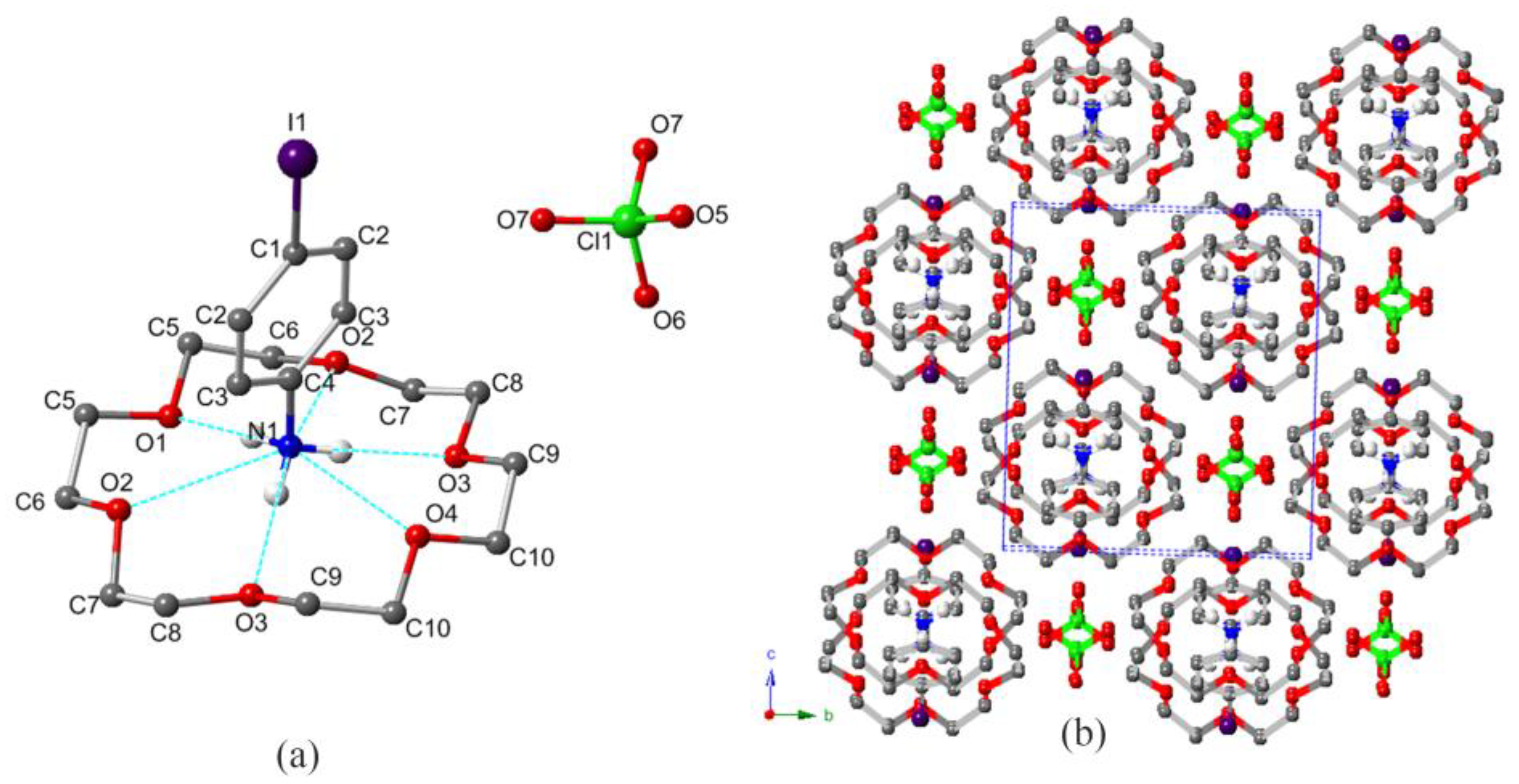
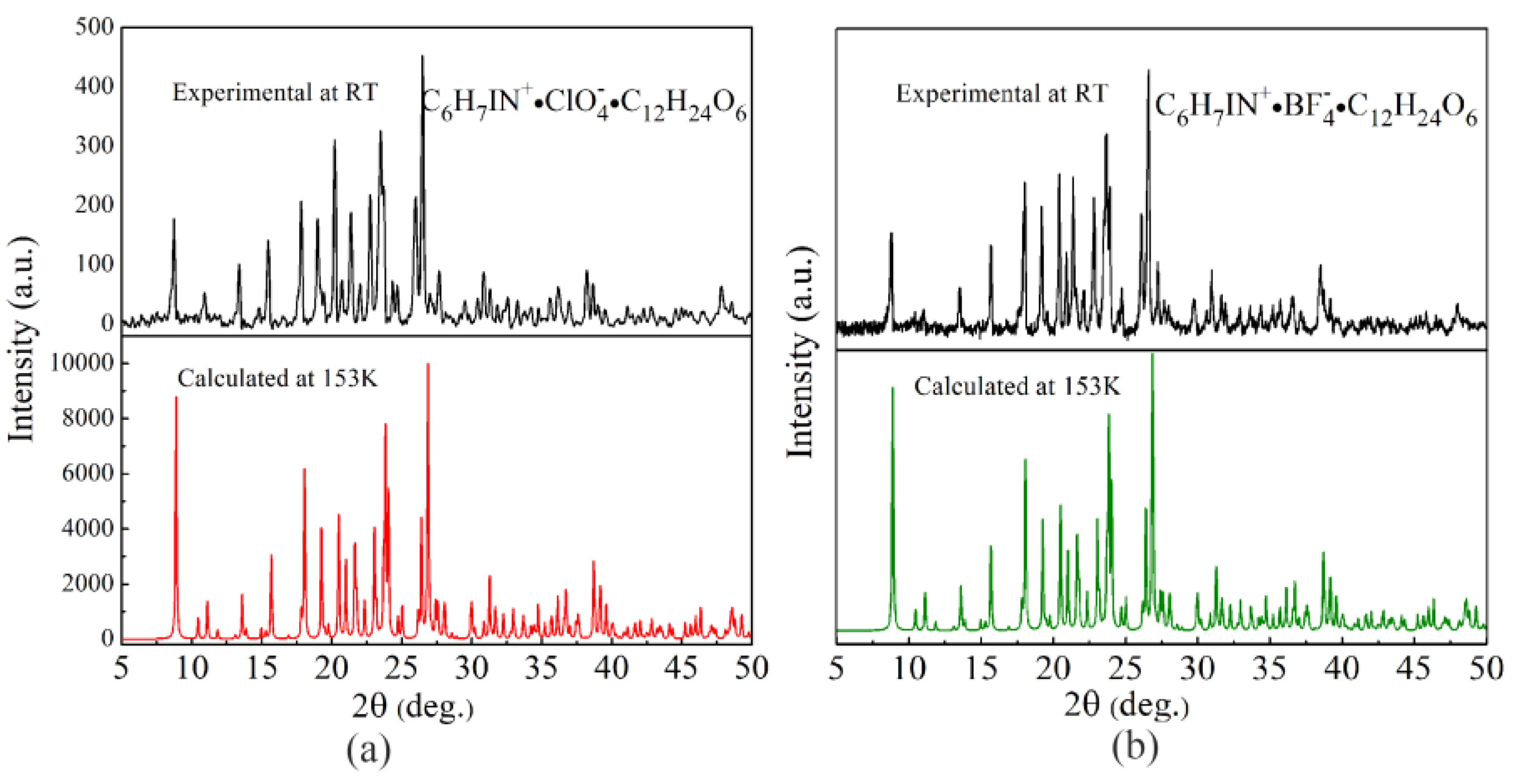
3.2. Crystal Structure and XRD
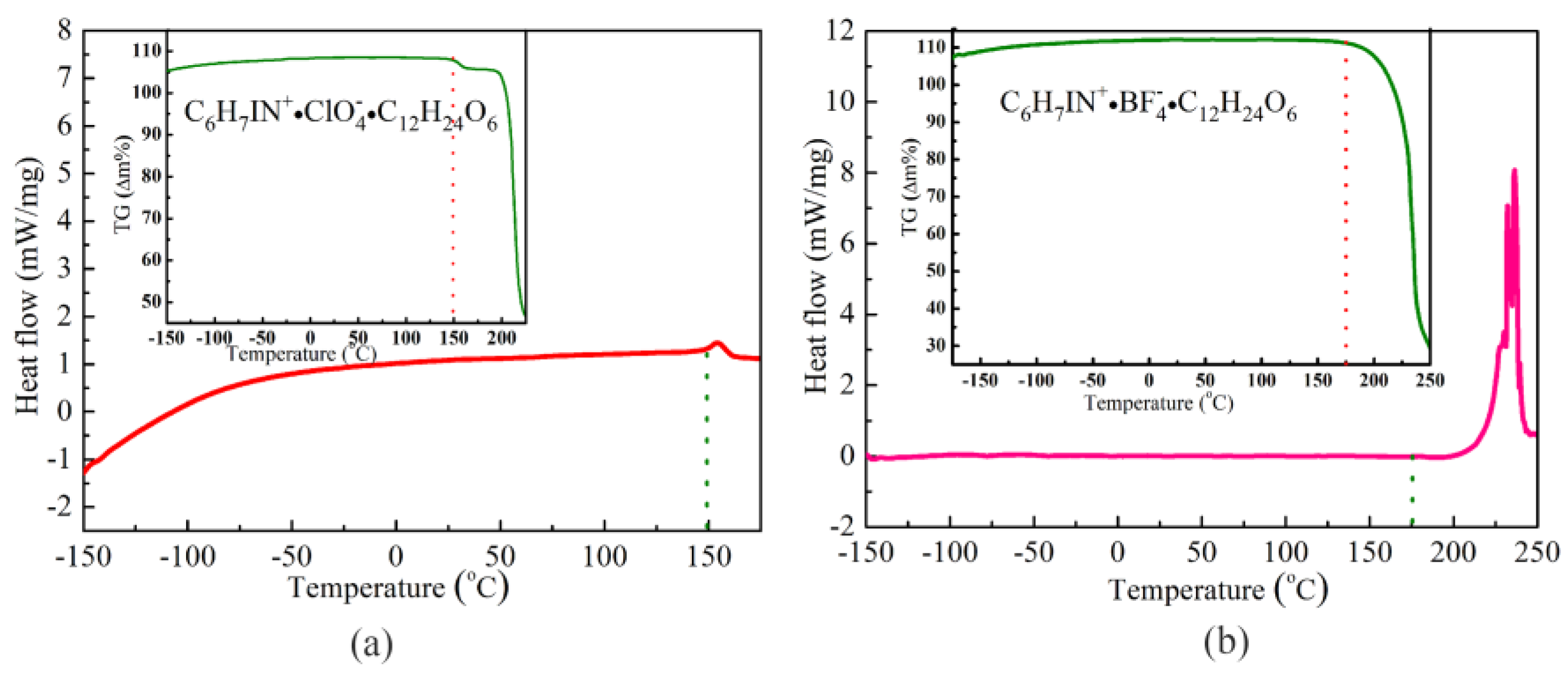
3.3. Thermal Measurements
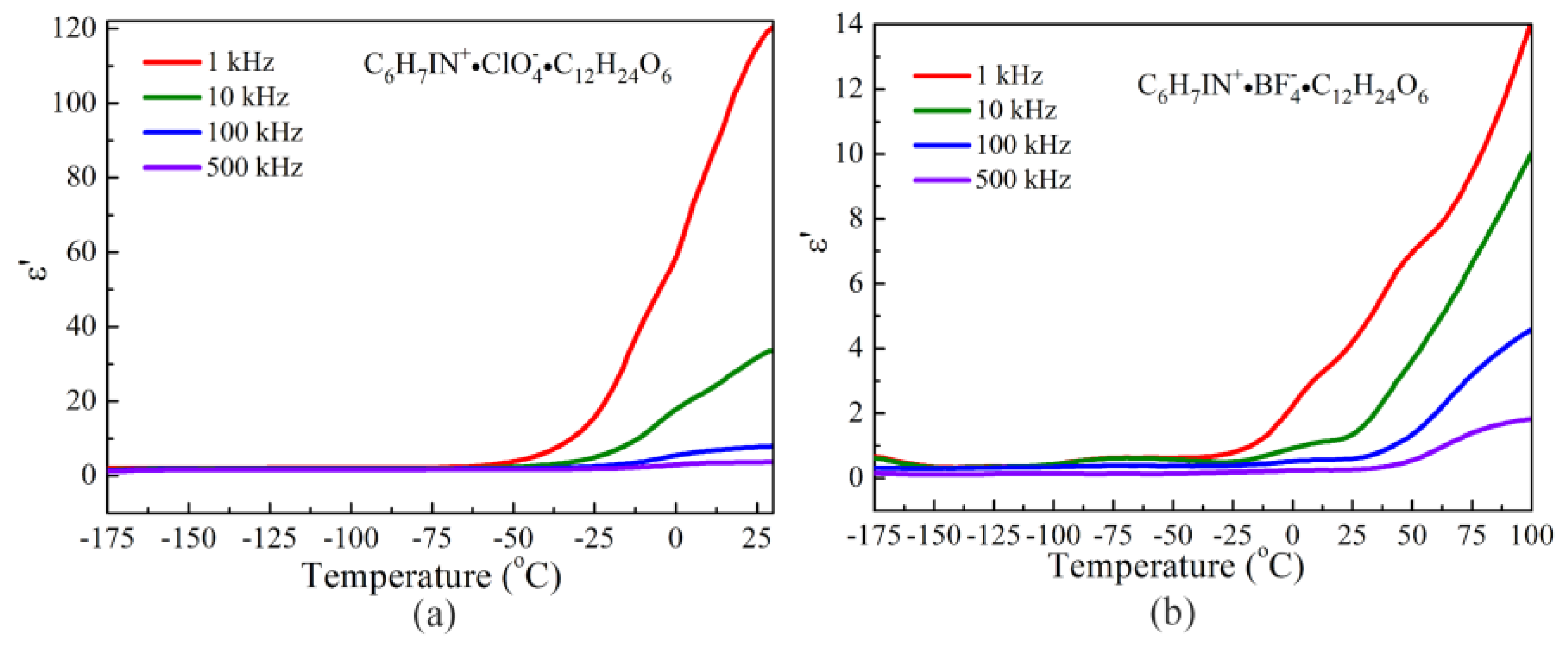
3.4. Dielectric Properties
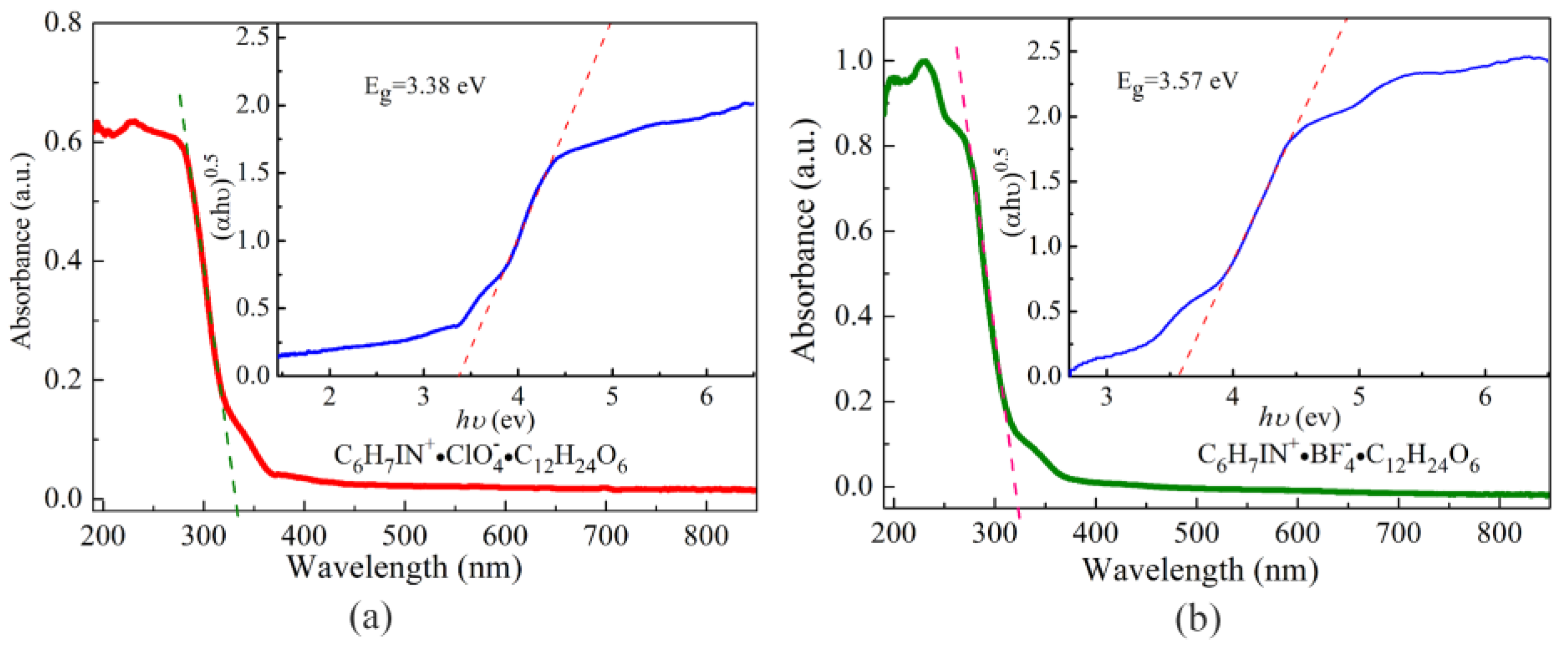
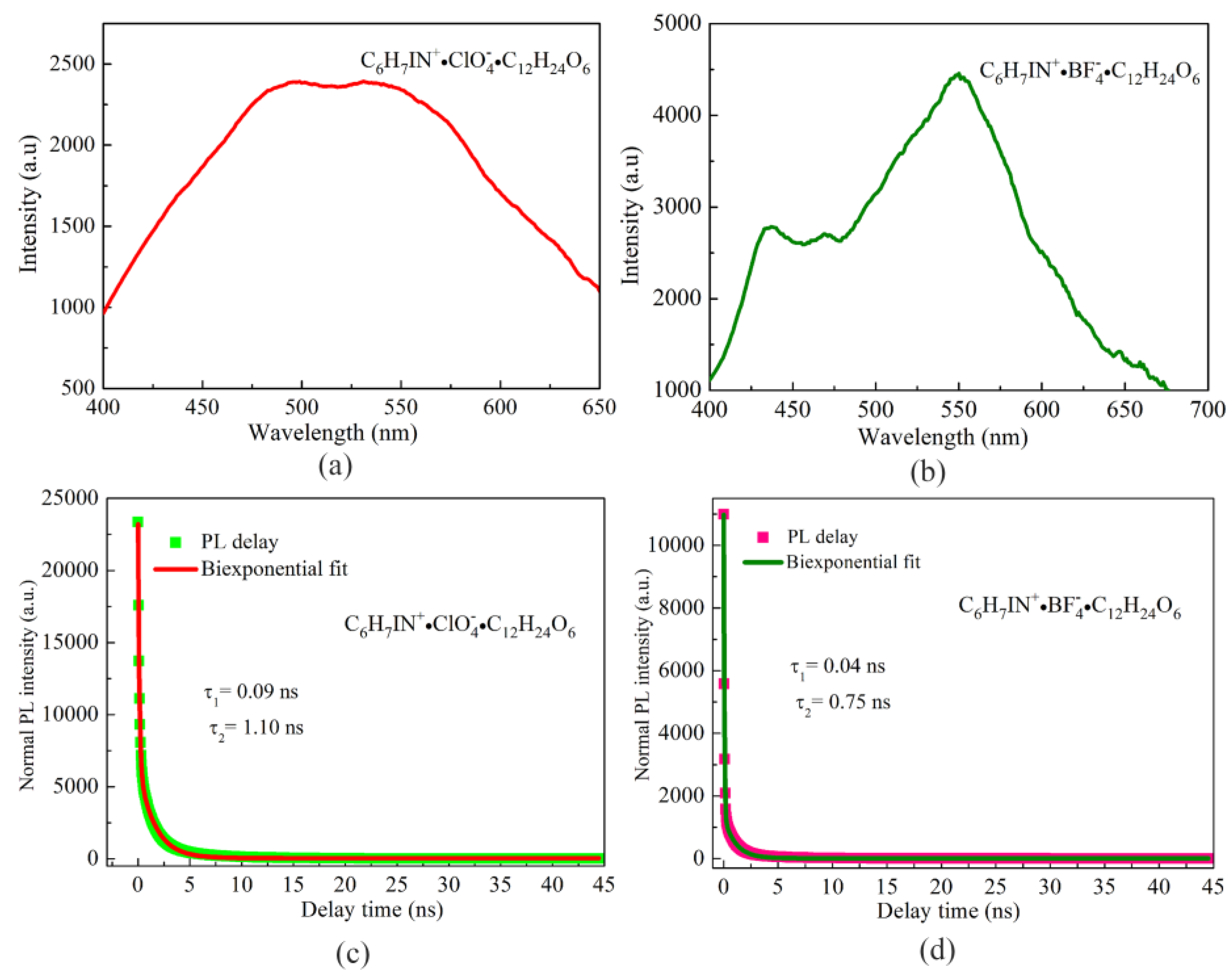
3.5. Optical Properties
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ye, H.-Y.; Tang, Y.-Y.; Li, P.-F.; Liao, W.-Q.; Gao, J.-X.; Hua, X.-N.; Cai, H.; Shi, P.-P.; You, Y.-M.; Xiong, R.-G. Metal-free three-dimensional perovskite ferroelectrics. Science 2018, 361, 151–155. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-M.; Liao, W.-Q.; Zhao, D.; Ye, H.-Y.; Zhang, Y.; Zhou, Q.; Niu, X.; Wang, J.; Li, P.-F.; Fu, D.-W.; et al. An organic-inorganic perovskite ferroelectric with large piezoelectric response. Science 2017, 357, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-Q.; Zhang, Y.; Hu, C.-L.; Mao, J.-G.; Ye, H.-Y.; Li, P.-F.; Huang, S.D.; Xiong, R.-G. A lead-halide perovskite molecular ferroelectric semiconductor. Nat. Commun. 2015, 6, 7338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Y.; Ye, Q.; Fu, D.-W.; Xiong, R.-G. Optoelectronic duple bistable switches: A bulk molecular single crystal and unidirectional ultraflexible thin film based on imidazolium fluorochromate. Adv. Funct. Mater. 2017, 27, 1603945. [Google Scholar] [CrossRef]
- Bermúdez-García, J.M.; Sánchez-Andújar, M.; Yáñez-Vilar, S.; Castro-García, S.; Artiaga, R.; López-Beceiro, J.; Botana, L.; Alegríade, A.; Señarís-Rodríguez, M.A. Multiple phase and dielectric transitions on a novel multi-sensitive [TPrA][M(dca)3] (M: Fe2+, Co2+ and Ni2+) hybrid inorganic-organic perovskite family. J. Mater. Chem. C 2016, 4, 4889–4898. [Google Scholar]
- Duong, T.-M.H.; Nobusue, S.; Tada, H. Face-shared structures of one-dimensional organic–inorganic lead iodide perovskites. Appl. Phys. Express 2018, 11, 115502. [Google Scholar] [CrossRef]
- Uchida, H.; Oota, K.; Minami, T.; Takeya, K.; Kawase, K. Generation of single-cycle terahertz pulse using Cherenkov phase matching with 4-dimethylamino-N-methyl-4-stilbazolium tosylate crystal. Appl. Phys. Express 2017, 10, 062601. [Google Scholar] [CrossRef]
- Jaffe, A.; Lin, Y.; Mao, W.L.; Karunadasa, H.I. Pressure-induced metallization of the halide perovskite (CH3NH3)PbI3. J. Am. Chem. Soc. 2017, 139, 4330–4333. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Matsushita, T.; Uchida, S.; Kondo, T.; Segawa, H. Quantitative fraction analysis of coexisting phases in a polycrystalline CH3NH3PbI3 perovskite. Appl. Phys. Express 2018, 11, 101401. [Google Scholar] [CrossRef]
- Zhou, P.; Sun, Z.; Zhang, S.; Ji, C.; Zhao, S.; Xiong, R.-G.; Luo, J. A sequentially switchable molecular dielectric material tuned by the stepwise ordering in diisopropylammonium trifluoromethanesulfonate. J. Mater. Chem. C 2014, 2, 2341–2346. [Google Scholar] [CrossRef]
- Tokizane, Y.; Nawata, K.; Han, Z.; Koyama, M.; Notake, T.; Takida, Y.; Minamide, H. Tunable terahertz waves from 4-dimethylamino-N-methyl-4-stibazolium tosylate pumped with dual-wavelength injection-seeded optical parametric generation. Appl. Phys. Express 2017, 10, 022101. [Google Scholar] [CrossRef]
- Hoja, J.; Ko, H.-Y.; Neumann, M.A.; Car, R.; DiStasio, R.A., Jr.; Tkatchenko, A. Reliable and practical computational description of molecular crystal polymorphs. Sci. Adv. 2019, 5, eaau3338. [Google Scholar] [CrossRef] [PubMed]
- Filippetti, A.; Caddeo, C.; Delugas, P.; Mattoni, A. Appealing perspectives of hybrid lead-Iodide perovskites as thermoelectric materials. J. Phys. Chem. C 2016, 120, 28472–28479. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, Y.; Zhang, S.; Ji, C.; Chen, T.; Hong, M.; Luo, J. Ultrahigh pyroelectric figures of merit associated with distinct bistable dielectric phase transition in a new molecular compound: di-n-Butylaminium trifluoroacetate. Adv. Mater. 2015, 27, 4795–4801. [Google Scholar] [CrossRef]
- Wang, D.; Shen, C.; Lan, J.; Huang, P.; Cui, Z.; Kang, T.; Niu, Y.; Wang, S.; Wang, J.; Boughton, R.I. Exploration of the correlation between weak absorption and thermal-stress for KDP and 70%-DKDP crystals. J. Alloys Compd. 2019, 790, 212–220. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, H.; Huang, B.; Yuan, X.; Zhang, J.; Zhang, J.; Jiang, L.; Xiong, T.; Zeng, G. State-of-the-art advances and challenges of iron-based metal organic frameworks from attractive features, synthesis to multifunctional applications. Small 2018, 15, 1803088. [Google Scholar] [CrossRef]
- Shen, C.; Wang, D.; Zhang, J.; Zhang, H.; Wang, J.; Boughton, R.I. The growth and investigations of electromechanical properties of Fresnoite Ba2Si2TiO8 crystal as a function of orientation. J. Cryst. Growth 2018, 487, 17–22. [Google Scholar] [CrossRef]
- Mączka, M.; Gągor, A.; Ptak, M.; Paraguassu, W.; da Silva, T.A.; Sieradzki, A.; Pikul, A. Phase transitions and coexistence of magnetic and electric orders in the methylhydrazinium metal formate frameworks. Chem. Mater. 2017, 29, 2264–2275. [Google Scholar] [CrossRef]
- Shen, C.; Wang, D.; Zhang, H.; Wang, J.; Boughton, R.I. Fresnoite Ba2TiSi2O8: A novel stimulated Raman scattering active crystal. Appl. Phys. Express 2016, 9, 122402. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Yu, C.-H.; Yao, Y.-F.; Zhang, W. Geometric isotope effect of deuteration in a hydrogen-bonded host-guest crystal. Nat. Commun. 2018, 9, 481. [Google Scholar] [CrossRef]
- Mączka, M.; Costa, N.L.M.; Gągor, A.; Paraguassu, W.; Sieradzkic, A.; Hanuzad, J. Structural, thermal, dielectric and phonon properties of perovskite-like imidazolium magnesium formate. Phys. Chem. Chem. Phys. 2016, 18, 13993–14000. [Google Scholar] [CrossRef]
- Dufrois, Q.; Daran, J.-C.; Vendier, L.; Dinoi, C.; Etienne, M. Triangles and squares for a unique molecular crystal structure: unsupported two-coordinate lithium cations and CC agostic interactions in cyclopropyllithium derivatives. Angew. Chem. 2018, 130, 1804–1809. [Google Scholar] [CrossRef]
- Fu, D.-W.; Cai, H.-L.; Li, S.-H.; Ye, Q.; Zhou, L.; Zhang, W.; Zhang, Y.; Deng, F.; Xiong, R.-G. 4-Methoxyanilinium perrhenate 18-Crown-6: A new ferroelectric with order originating in swing like motion slowing down. PRL 2013, 110, 257601. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-W.; Zhang, W.; Cai, H.-L.; Zhang, Y.; Ge, J.-Z.; Xiong, R.-G.; Huang, S.D. Supramolecular bola-like ferroelectric: 4-methoxyanilinium tetrafluoroborate-18-crown-6. J. Am. Chem. Soc. 2011, 133, 12780–12786. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, D.; Xu, H.; Pan, Z.; Zhang, H.; Wang, J.; Boughton, R.I. Bulk crystal growth and thermal, spectroscopic and laser properties of disordered Melilite Nd:Ca2Ga2SiO7 single crystal. J. Alloys Compd. 2017, 727, 8–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, H.-Y.; Fu, D.-W.; Xiong, R.-G. An order-disorder ferroelectric host-guest inclusion compound. Angew. Chem. Int. Ed. 2014, 53, 2114–2118. [Google Scholar] [CrossRef]
- Ye, H.-Y.; Li, S.-H.; Zhang, Y.; Zhou, L.; Deng, F.; Xiong, R.-G. Solid state molecular dynamic investigation of an inclusion ferroelectric: [(2,6-Diisopropylanilinium)([18]crown-6)]BF4. J. Am. Chem. Soc. 2014, 136, 10033–10040. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, S.; Zhang, S.; Deng, F.; Hong, M.; Luo, J. Second-order nonlinear optical switch of a new hydrogen-bonded supramolecular crystal with a high laser-induced damage threshold. Adv. Opt. Mater. 2014, 2, 1199–1205. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Gao, B.; Zhu, C.; Liu, Z. Inorganic anions regulate the phase transition in two organic cation salts containing [(4-nitroanilinium)(18-crown-6)]+ supramolecules. Crystals 2017, 7, 224. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Liu, Y.; Chen, Y.; Zhao, W.-Q.; Fang, W.-N. Synthesis, characterization, and phase transition of an inorganic-organic hybrid compound, [(3-nitroanilinium+)(18-crown-6)][IO4−](CH3OH). Chinese Chem. Lett. 2017, 28, 297–301. [Google Scholar] [CrossRef]
- Tang, Y.-Z.; Yu, Y.-M.; Xiong, J.-B.; Tan, Y.-H.; Wen, H.-R. Unusual high-temperature reversible phase-transition behavior, structures, and dielectric–ferroelectric properties of two new crown ether clathrates. J. Am. Chem. Soc. 2015, 137, 13345–13351. [Google Scholar] [CrossRef]
- Ge, J.-Z.; Fu, X.-Q.; Hang, T.; Ye, Q.; Xiong, R.-G. Reversible phase transition of the 1:1 complexes of 18-crown-6 with 4-ethoxyanilinium perchlorate. Cryst. Growth Des. 2010, 3632–3637. [Google Scholar] [CrossRef]
- Ge, J.-Z.; Zhao, M.-M. 4-Methylanilinium tetrafluoroborate 18-crown-6 clathrate. Acta Cryst. E 2010, 66, o1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, M.-M. Accelerating ab initio phasing with de novo models. Acta Cryst. E 2011, 67, o596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Audebert, P.; Wei, Y.; Lauret, J.-S.; Galmichec, L.; Deleporte, E. Synthesis and optical properties of novel organic-inorganic hybrid UV (R-NH3)2PbCl4 semiconductors. J. Mater. Chem. 2011, 21, 466–474. [Google Scholar] [CrossRef]
- Ding, J.; Du, S.; Zhao, Y.; Zhang, X.; Zuo, Z.; Cui, H.; Zhan, X.; Gu, Y.; Sun, H. High-quality inorganic-organic perovskite CH3NH3PbI3 single crystals for photo-detector applications. J. Mater. Sci. 2017, 52, 276–284. [Google Scholar] [CrossRef]
| Empirical Formula | C18H31ClINO10 | C18H31BF4INO6 |
|---|---|---|
| formula weight/g·mol−1 | 583.79 | 571.15 |
| temperature/K | 153 | 153 |
| wavelength/Å | 0.71073 | 0.71073 |
| crystal colour | Colourless | Colourless |
| crystal system | Orthorhombic | Orthorhombic |
| space group | Pnma | Pnma |
| a/Å | 15.975(3) | 15.901(3) |
| b/Å | 11.418(2) | 11.289(2) |
| c/Å | 12.743(3) | 12.735(3) |
| α/° | 90 | 90 |
| β/° | 90 | 90 |
| γ/° | 90 | 90 |
| volume/Å3 | 2324.4(8) | 2286.0(8) |
| Z | 4 | 4 |
| density/mg·m−3 | 1.668 | 1.660 |
| Absorption coefficient (mm−1) | 1.545 | 1.467 |
| F(000) | 1184 | 1152 |
| Measured theta range (o) | 3.01~27.45 | 3.02~27.48 |
| Absorption correction | Semi-empirical from equivalents | |
| Data/restraints/parameters | 2787/0/154 | 2753/0/155 |
| GOF on F2 | 1.004 | 1.012 |
| R, wR [I > 2sigma(I)] | 0.0380, 0.1025 | 0.0268, 0.0804 |
| R, wR [all data] | 0.0400, 0.1042 | 0.0287, 0.0818 |
| CCDC | 1883445 | 1883437 |
| D–H⋅⋅⋅A | d(D–H) | d(H⋅⋅⋅A) | d(D⋅⋅⋅A) | ∠(D–H⋅⋅⋅A) | |
|---|---|---|---|---|---|
| 1 | N1–H1B⋅⋅⋅O1 | 0.890 | 1.98 | 2.872 | 177.6 |
| N1–H1A⋅⋅⋅O2 | 0.890 | 2.56 | 2.9354 | 106.1 | |
| N1–H1A⋅⋅⋅O3 | 0.890 | 1.99 | 2.872 | 174.5 | |
| N1–H1A⋅⋅⋅O4 | 0.890 | 2.56 | 2.872 | 101.3 | |
| C6-H6B…O5 | 0.990 | 2.65 | 3.449 | 137.73 | |
| C7-H7A…O5 | 0.990 | 2.58 | 3.469 | 149.16 | |
| C8-H8B…O7 | 0.990 | 2.67 | 3.603 | 156.76 | |
| C10-H10A…O7 | 0.990 | 2.71 | 3.691 | 172.15 | |
| 2 | N1–H1B⋅⋅⋅O1 | 0.890 | 1.98 | 2.864 | 176.7 |
| N1–H1B⋅⋅⋅O2 | 0.890 | 2.58 | 2.9336 | 104.5 | |
| N1–H1A⋅⋅⋅O3 | 0.890 | 1.99 | 2.8724 | 174.2 | |
| N1–H1A⋅⋅⋅O4 | 0.890 | 2.57 | 2.878 | 101.4 | |
| C6-H6B…F1 | 0.990 | 2.609 | 3.395 | 136.38 | |
| C7-H7A…F1 | 0.990 | 2.530 | 3.408 | 147.54 | |
| C8-H8B…F3 | 0.990 | 2.606 | 3.533 | 155.85 |
| Crystals | Phase Transition Temperature (K) | Characteristic |
|---|---|---|
| 4-iodoanilinium perchlorate 18-crown-6 this work | 423 | High thermal stability; Narrow band gap (3.38 eV) |
| 4-iodoanilinium borofluorate 18-crown-6 this work | 448 | High thermal stability; Narrow band gap (3.57 eV) |
| Cyclohexyl ammonium 18-crown-6 tetrafluoroborate [31] | 397 | Ferroelectric Ps = 3.27 μC/cm2 |
| Cyclohexyl ammonium 18-crown-6 perchlorate [31] | 390 | Ferroelectric Ps = 3.78 μC/cm2 |
| [(2,6-diisopropylanilinium)([18]crown-6)]BF4] [27] | T1 = 305 T2 = 120 | Ferroelectric Ps = 0.3 μC/cm2 |
| (2,6-diisopropylanilinium)([18]crown-6)](ClO4) [26] | T1 = 278 T2 = 132 | Ferroelectric Ps = 0.35 μC/cm2 |
| (4-nitroanilinium)2(18-crown-6)2(PF6)2(CH3OH) [29] | 265 | ----- |
| (4-nitroanilinium)(18-crown-6)(HSO4 [29] | 255 | ----- |
| Bis(imidazolium hydrochlorate) dehydrate 18-crown-6 [28] | 220 | Superior nonlinear optical switchingcontrast (~12); High laser-induced damage threshold (∼8.9 GW/cm2) |
| [(3-nitroanilinium+)(18-crown-6)][IO4](CH3OH) [30] | 220 | ----- |
| 4-ethoxyanilinium perchlorate 18-crown-6 [32] | 163 | ----- |
| Methoxyanilinium perrhenate 18-Crown-6 [25] | 153 | Ferroelectric Ps = 1.2 μC/cm2 |
| 4-methoxyanilinium tetrafluoroborate-18-crown-6 [24] | 127 | Ferroelectric Ps = 0.54 μC/cm2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Kang, T.; Zhao, F.; Wang, D.; Shen, C.; Wang, J. Growth and Property Investigations of Two Organic–Inorganic Hybrid Molecular Crystals with High Thermal Stability: 4-Iodoanilinium perchlorate 18-crown-6 and 4-Iodoanilinium Borofluorate 18-crown-6. Crystals 2019, 9, 207. https://doi.org/10.3390/cryst9040207
Zhang L, Kang T, Zhao F, Wang D, Shen C, Wang J. Growth and Property Investigations of Two Organic–Inorganic Hybrid Molecular Crystals with High Thermal Stability: 4-Iodoanilinium perchlorate 18-crown-6 and 4-Iodoanilinium Borofluorate 18-crown-6. Crystals. 2019; 9(4):207. https://doi.org/10.3390/cryst9040207
Chicago/Turabian StyleZhang, Lihui, Tiantian Kang, Fanghua Zhao, Duanliang Wang, Chuanying Shen, and Jiyang Wang. 2019. "Growth and Property Investigations of Two Organic–Inorganic Hybrid Molecular Crystals with High Thermal Stability: 4-Iodoanilinium perchlorate 18-crown-6 and 4-Iodoanilinium Borofluorate 18-crown-6" Crystals 9, no. 4: 207. https://doi.org/10.3390/cryst9040207
APA StyleZhang, L., Kang, T., Zhao, F., Wang, D., Shen, C., & Wang, J. (2019). Growth and Property Investigations of Two Organic–Inorganic Hybrid Molecular Crystals with High Thermal Stability: 4-Iodoanilinium perchlorate 18-crown-6 and 4-Iodoanilinium Borofluorate 18-crown-6. Crystals, 9(4), 207. https://doi.org/10.3390/cryst9040207




