Abstract
Interleukin 17A (IL-17A) is a proinflammatory cytokine produced by Th17 cells. Antibody BCD-085 (netakimab) against human IL-17A is one of the new inhibitors of this cytokine. In netakimab, the VH domain is replaced by the VHH domain of Lama glama possessing a long complementarity determining region (CDR-H3) in its heavy chain. Here we demonstrate the high affinity of IL-17A to the Fab fragment of netakimab and to its integral part, the VHH domain. We have determined the crystal structure of the Fab fragment of netakimab at 1.9 Å resolution. High variability in the orientation of light and heavy chains of the Fab fragment of netakimab was shown, which is determined by the peculiarity of the structural organization of the CDR-H3. As the high conformational plasticity of the molecule hampers modeling the Fab fragment of netakimab complexed to IL-17A, we have carried out modeling the complex between the antigen and the integral part of the Fab fragment, the VHH domain. We explain the high netakimab Fab fragment affinity for IL-17A by a large number of protein–protein contacts due to additional interactions between CDR-H3 and the cytokine dimer.
1. Introduction
Cytokine IL-17A plays a key role in protecting an organism against extracellular bacterial and fungal infections [1]. However, excessive production of this protein is associated with immune-mediated inflammatory and autoimmune diseases (for instance, psoriasis, psoriatic arthritis, rheumatoid arthritis, systemic lupus erythematosus, etc.) [2]. By now, substantial progress has been achieved in the therapy of such diseases, which was associated with the widespread introduction of therapeutic preparations based on anti-IL-17 antibodies [3].
Along with classical antibodies, preparations based on non-canonical immunoglobulins (HCAb) are being extensively developed. These antibodies do not contain the light chain. HCAbs were first discovered in dromedary [4]. Later it was shown that HCAbs, as well as classical antibodies, are present in the blood of cartilaginous fish and mammals of the Camelidae family. HCAbs were also found in the blood of humans exposed to gamma radiation, though, in this case, they were non-functional.
The overall configuration of variable domains is similar in a classical antibody (VH domain) and noncanonical one (VHH domain). Three variable regions (complementarity determining regions, CDRs) often stabilized by disulfide bonds are located in loops between two β-sheets. Variable regions in the VHH domain are longer than those in canonical antibodies [5], they form extended loops. This enables HCAbs to recognize epitopes with a concave surface (for instance, active centers of enzymes) [6], which are often inaccessible to classical antibodies. HCAbs also feature other useful properties, including reduced immunogenicity, increased solubility, and high thermal and pH stability, and they easily penetrate into various tissues. In spite of their high potential and unique specificity compared to classical IgG, using VHH domains (also named single-domain antibodies or nanobodies) in the therapy of various diseases is limited due to rapid plasma clearance [7,8,9]. There are two basic approaches to extend the half-life of recombinant single-domain antibodies in the plasma: increasing the hydrodynamic radius of the molecule (conjugation with PEG and other polymers), obtaining a VHH linked to the Fc fragment [10], modification of the Fc regions of antibodies and conjugation with albumin [11]. Success was also achieved in a strategy of nanoantibody dimerization. In this case, one antibody was directed against an antigen while the other one against albumin [12].
Designing a Fab fragment of the classical antibody in which the VHH domain is substituted for the VH domain, is yet another promising solution [13,14]. In this work, we present the crystal structure of the netakimab Fab fragment, which is directed against the IL-17A, kinetic analysis of the interaction between IL-17A with the Fab fragment and its integral part, the VHH domain, and modeling the interactions in the IL-17A/VHH domain complex.
Netakimab is a humanized monoclonal anti-IL-17 antibody that belongs to the IgG1/κ type. The light chain belongs to the VK3 structural family and contains domains CL and VL. The heavy chain consists of constant domains CH3, CH2, and CH1; the variable domain VH is substituted with a variable domain of a non-canonical antibody, VHH. The netakimab Fab fragment demonstrates functional activity, which is higher than that of the widely applied therapeutic preparation secukinumab.
Presently, netakimab in a highly concentrated subcutaneous form is being tested in a phase III clinical trial (Identifier NCT03390101 for ClinicalTrials.gov) in patients with moderate to severe vulgar psoriasis. The netakimab preparation demonstrates high efficiency (over 90% of patients reached PASI (Psoriasis Area and Severity Index) 75 at week 12), high solubility (up to 60 mg/mL), and a high safety profile [15], which indicates its physicochemical and biological stability upon therapeutic application. We suggested that tighter contact between the VHH domain of the netakimab Fab fragment and the IL-17A region that interact with the IL-17 RA receptor provides high functional activity of the preparation.
2. Materials and Methods
2.1. Production and Purification of the Fab Fragment and IL-17A
For transient expression of the gene for the netakimab Fab fragment (the Fab fragment), Chinese hamster ovary CHO-K1-S line at a density of 2 × 106 cells/mL was transfected with a pTT-5-BCD-085-Fab plasmid, which carries genes of light and heavy chains of the Fab fragment, in the presence of polyethyleneimine (PEI, Polysciences, Inc., Warrington, PA, USA). DNA/PEI ratio was 1:3 to 1:10. Cells were cultured in the FreeStyle CHO (GIBCO) in a suspension in flasks in a shaker-incubator (Multitron) for seven days. Cultures were centrifuged at 2000× g for 20 min and filtered through a 0.22 μm filter (Millipore).
The recombinant Fab fragment, which contained a hexa-histidine tag at the C-terminus of the heavy chain, was purified from the culture medium using Profinity IMAC Ni-charged resin (Bio-Rad). Culture medium was successively supplemented with NiCl2 to a concentration of 1 mM and 5 ml of the resin, mixed in a shaker for one hour at room temperature. The resin was then transferred to a 5 mL column (Thermo Scientific), washed with five column volumes of PBS. The Fab fragment was eluted with a buffer A (300 mM imidazole, 150 mM NaCl, Na-phosphate buffer pH 8.0). Fractions containing the purified protein were concentrated and dialyzed against a buffer B (150 mM NaCl, 50 mM sodium acetate, pH 5.5). The obtained preparation was further purified using the size exclusion chromatography on the Superdex G-75 resin (GE Healthcare) equilibrated in buffer B (Supplementary Figure S1A). At every step of purification, protein preparations were controlled by gel electrophoresis under denaturing conditions.
2.2. Production and Purification of the VHH Domain
Gene of the single-domain anti-IL-17A antibody (VHH domain) was (PCR-amplified using primers VHH_For, 5′-GCTGGAATTCGAAGTTCAACTGGTGCAG-3′, and VHH_Rev, 5′-CAGGGGAAGCTTTTATGAGGAGACGGTGACCAG-3′ and pTT-5-VHH anti-IL-17A plasmid as a template) cloned in the pET-28a vector between EcoRI and HindIII cloning sites. The obtained genetic construction was validated by sequencing and used for transformation of the Escherichia coli strain BL21(DE3)/Rosetta. The transformants were grown in a liquid LB medium supplemented with kanamycin (50 μg/mL) and chloramphenicol (25 μg/mL) at 310 K with rotation (150 revolutions per minute). Synthesis of the VHH domain was induced at A260 = 0.6 by adding IPTG (0.3 mM) and cultures were grown for another three hours under the same conditions. Cells were harvested by the centrifugation at 8000× g for 15 min.
The obtained cell mass was suspended in buffer C (500 mM NaCl, 50 mM Tris-HCl, pH 7.0, 10 mM imidazole). Cells were disrupted by sonication at 277 K. Cell debris was removed by the centrifugation at 15,000× g for 30 min at 277 K. The supernatant was loaded onto a Ni-NTA agarose (GE Healthcare) column equilibrated with buffer C. The VHH domain was eluted using a step gradient of imidazole concentration (20 mM, 40 mM, and 150 mM) in buffer C. Fractions containing the protein were concentrated and dialyzed against buffer B. The obtained preparation was further purified by the size exclusion chromatography on the Superdex G-75 resin equilibrated with buffer B (Supplementary Figure S1B). At every stage, preparations were analyzed by gel electrophoresis under denaturing conditions.
2.3. Circular Dichroism Measurements
CD measurements were performed on a Chirascan Spectrometer (Applied Photophysics, UK) equipped with a Julabo F25 computer-controlled thermostat. Spectra were registered using cuvettes with 0.1 mm path length. CD spectra of VHH are typical for that of an ordered globular protein (Supplementary Figure S2).
2.4. Production and Purification of IL-17A
The human IL-17A gene was overexpressed using the method identical to that for obtaining the Fab fragment. The pTT-5-IL-17A plasmid carrying genes of intact human IL-17A was used. Purification of IL-17A from culture liquid was performed using the Profinity IMAC Ni-charged (Bio-Rad) resin as described for the purification of the Fab fragment. Fractions containing pure protein were concentrated and dialyzed against buffer B.
2.5. Analysis of the Interaction of the Fab Fragment and the VHH domain with IL-17A by Surface Plasmon Resonance Approach (SPR)
Measurements were carried out using OCTET RED96 apparatus (Pall Forte Bio) in a running buffer (PBS supplemented with 0.1% BSA and 0.1% Tween-20) at 303 K. Biosensors AR2G (Pall Forte Bio) were rehydrated for one hour in deionized water and activated in 20 mM 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC)/10 mM N-hydroxy(sulfo) succinimide (Sulfo-NHS). IL-17A at a concentration of 25 μg/mL in 10 mM sodium acetate (pH 4.0) was nonspecifically (through the amino groups) immobilized on the surface of the biosensor. After the neutralization with 1 M Ethanolamine (pH 8.5), sensors were immersed into in wells containing solutions of the Fab fragment at concentrations ranging from 2.5 nM to 20 nM or with VHH domain solutions at concentrations ranging from 1.25 nM to 10 nM. Sensors were then immersed into the running buffer for the subsequent dissociation stage; duration of association stage was 1000 s, and the dissociation stage was 3600 s. The curves obtained were analyzed using the Octet System Data Analysis software and 1:1 Langmuir binding model. The measurements were repeated 3 times.
2.6. Crystallization of the Fab Fragment
Crystallization experiments were performed at 298 K using the hanging-drop vapor-diffusion method on siliconized glass cover slides in Linbro plates. Drops were made by mixing a protein solution at 20 mg/mL in 50 mM sodium acetate pH 5.5, 150 mM NaCl with a well solution—1.5 M (NH4)2SO4, 150 mM sodium citrate pH 5.5 (condition No. 15 of Stura FootPrint Screen I from Molecular Dimensions) at 1:1 volume ratio. Crystals appeared in 20 days and grew to maximum dimensions of 0.2 mm × 0.05 mm × 0.2 mm. Before freezing in liquid nitrogen, the crystals were transferred to a solution composed of 1.6 M (NH4)2SO4, 20% glycerol, 80 mM sodium acetate trihydrate pH 4.6.
2.7. Diffraction Data Collection and Determining the Structure of the Fab Fragment
Diffraction data were collected to 1.9 Å resolution on the ID23-1 beamline at the European Synchrotron Radiation Facility (ESRF), Grenoble, France [16] equipped with a Pilatus 6M detector. Data collection was controlled by the MxCuBE system [17] and the strategy was calculated by BEST [18]. Data were processed and scaled with the XDS package [19]. The crystals of the Fab fragment BCD-085 belonged to space group P212121 with unit cell parameters a = 90.56, b = 111.92, c = 165.43 Å, and four heterodimer molecules in the asymmetric unit.
The structure was solved by molecular replacement with Phaser [20] using the structure of the light chain from the crystal structure of human germline antibody IGHV5-51/IGKV3-20 (PDB entry 5I1K) and for the heavy chain structure of the broadly neutralizing human anti-hepatitis C virus (HCV) glycoprotein E2 Fab fragment HC33.4 (PDB entry 5FGB) as a search model. Water molecules and metal ions were removed from the model. The initial model was subjected to crystallographic refinement with REFMAC5 [21]. Manual rebuilding of the model was carried out in Coot [22]. The final model of the Fab fragment, refined to an R factor of 16.8% and Rfree of 22.8% at 1.9 Å resolution. Data and refinement statistics are summarized in Table 1. The coordinates and structure factors have been deposited in the Protein Data Bank (PDB entry 6QKD). Figures were prepared using PyMOL [23].

Table 1.
Data collection and refinement statistics.
2.8. Docking and Molecular Dynamics Simulations
For complex modeling, we use a combination of information from IL-17A/ IL-17A RA complex and best structures constructed from VHH and IL-17A dimer by docking at HDOCK web server [24]. The molecular dynamics simulations of the VHH domain and IL-17A complex were performed using the Gromacs 4.5.3 program [25] with Charmm27 all-atom force fields [26,27]. The lengths of covalent bonds involving hydrogen atoms were constrained using the LINCS algorithm [28], which enabled us to use the integration step of 2fs. Electrostatic interactions were estimated using the particle mesh Ewald (PME) technique [29,30]. Van der Waals interactions were described using the switch functions in the range of 10–12 Å. A constant temperature (300 K) was maintained with the velocity rescale (v-rescale) thermostat [25]. The Berendsen barostat was applied to maintain the pressure of the system at 1 atm [31].
The molecular dynamics simulations were performed using the three-dimensional structure of the VHH domain of the Fab fragment in a complex with IL-17A as the starting model. To check the quality of the parameters used in the calculations, the stereochemical parameters of the models obtained after 2ns molecular dynamics simulations were compared with the experimental structures of the corresponding molecules. Three 200ns molecular dynamics trajectories were calculated at a constant temperature of 300 K and a pressure of 1 atm. All molecular dynamics trajectories were calculated using resources of the Joint Supercomputer Center of the Russian Academy of Sciences (JSCC RAS, www.jscc.ru). The PISA (protein interfaces, surfaces, and assemblies) service at the European Bioinformatics Institute [32] was used for calculating contact areas and bond lengths [33].
3. Results
3.1. Crystal Structure of the Fab Fragment of Netakimab at 1.9 Å Resolution
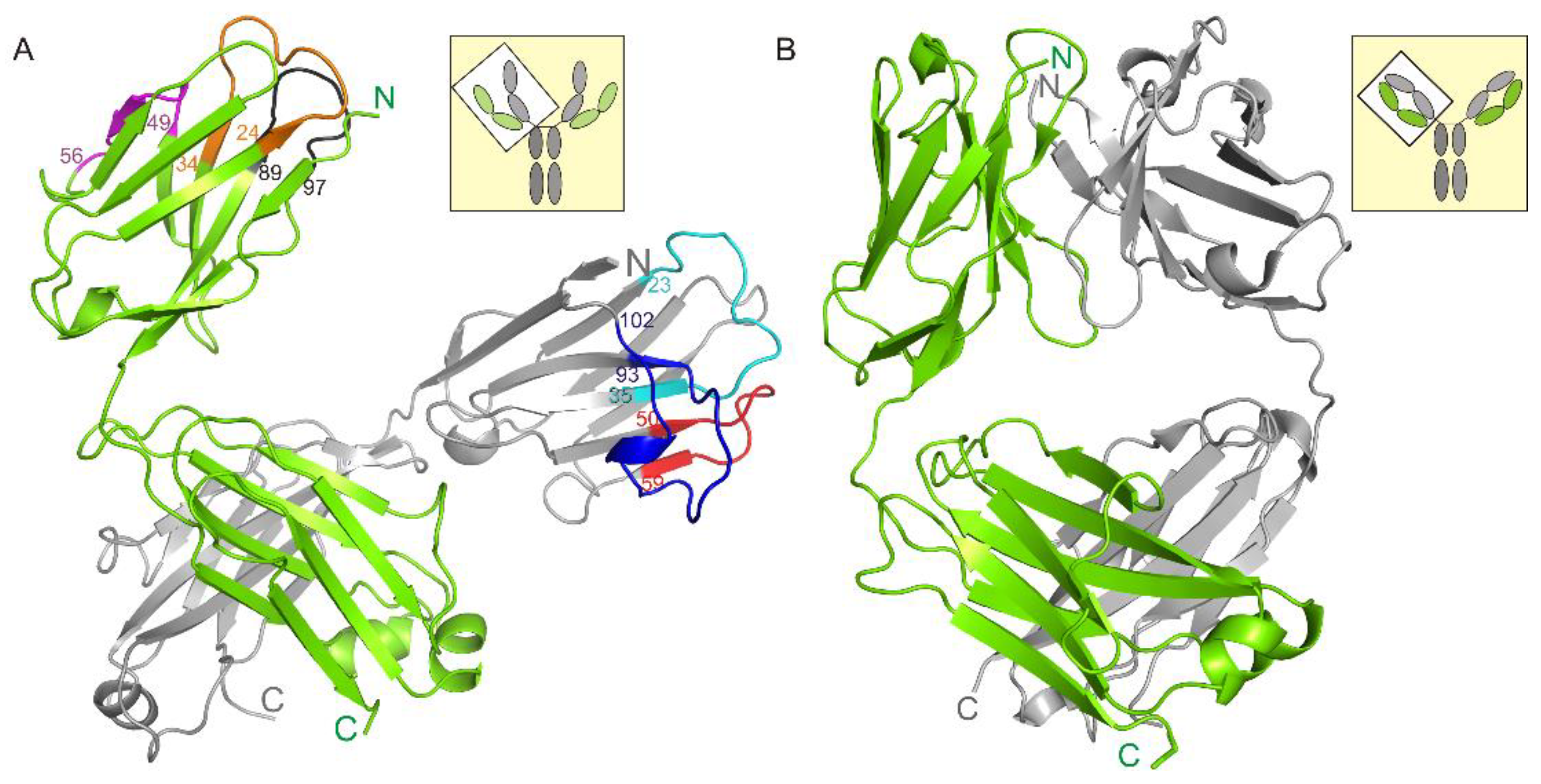
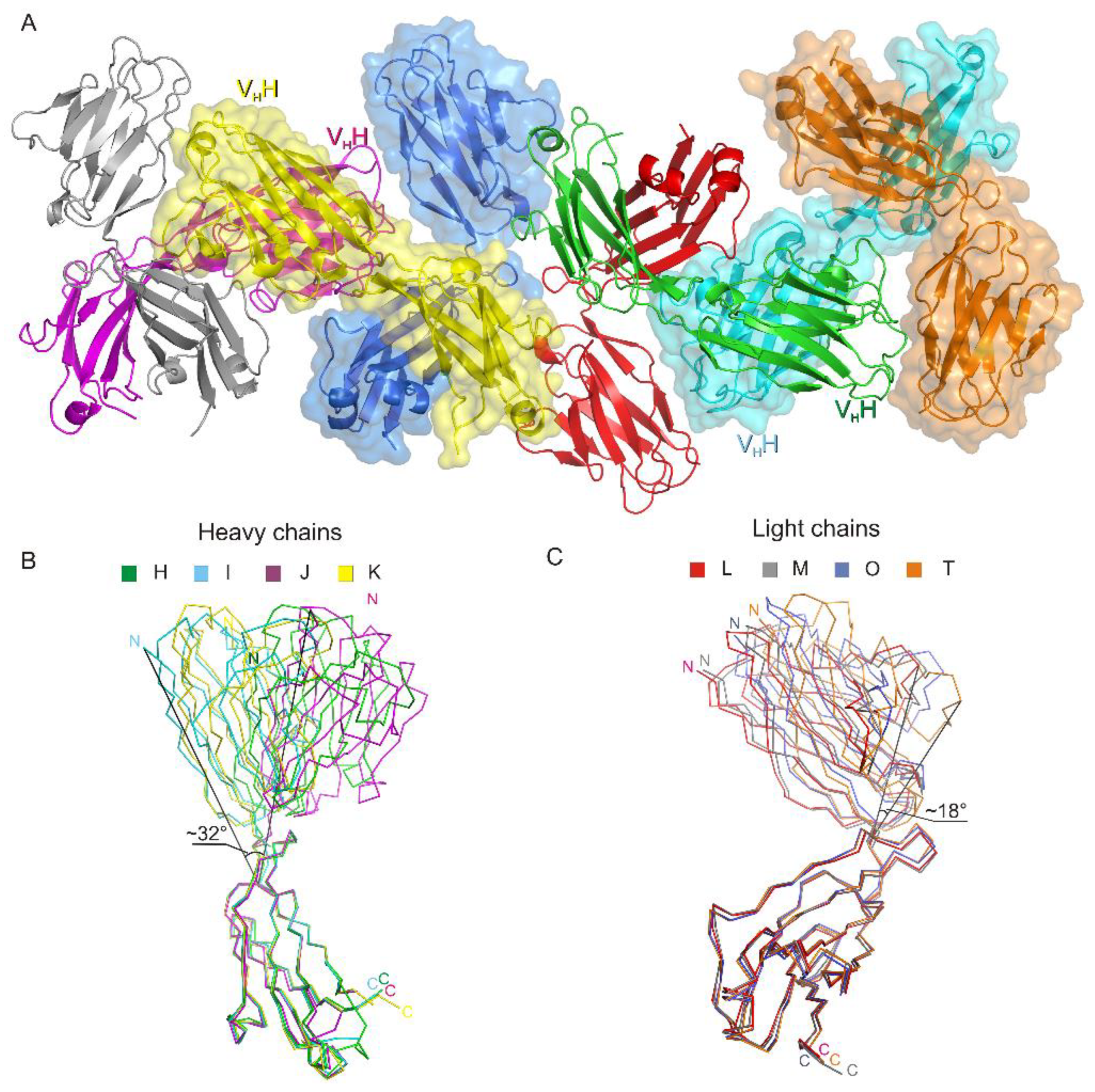
The asymmetric unit contains four netakimab Fab fragment molecules. C-terminal (constant) domains of heavy and light chains interact with each other in a classic way, whereas N-terminal ones do not, only small contacts with neighboring heterodimer molecules are observed. (Figure 1A, and Figure 2A). Therefore, the netakimab Fab fragment molecule acquires a V-shaped form, which differs from the form of classical Fab fragments (Figure 1B). High diversity in conformations of loops in the four netakimab Fab fragment molecules is observed that causes variations in the regions of interactions between nearby molecules. We suggested that these interactions between constant domains of heavy and light chains in the asymmetric unit do not have an impact on the functional properties of the netakimab Fab fragment. Protein–protein contacts between the antibody variable domains of heavy and light chains, as well as contacts between variable domains of heavy and light chains in different molecules in the asymmetric unit vary considerably. Therefore, these chains are located at different angles and on different distances in the asymmetric unit (Figure 2A). The angle between extreme positions of heavy chain domains is about 32°, whereas the angle between the extreme positions of the light chains is about 18° (Figure 2B). Such variability in orientations of light and heavy chains of the Fab fragment within the crystal unit suggests high levels of mobility.
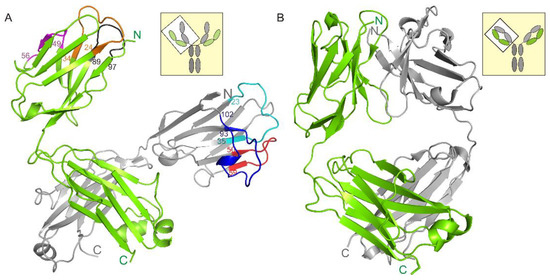
Figure 1.
The structure of the netakimab Fab fragment. (A) The netakimab Fab fragment, light chain is colored green, heavy chain–gray. CDR-H1–cyan, CDR-H2–red, CDR-H3–blue. CDR-L1–brown, CDR-L2–violet, CDR-L3–black. Position numbers are shown for amino acid residues at the beginning and the end of CDRs. (B) The crystal structure of the human germline antibody IGHV5-51/IGKV3-20 (PDB entry 5I1K), light chain–green, heavy chain–gray.
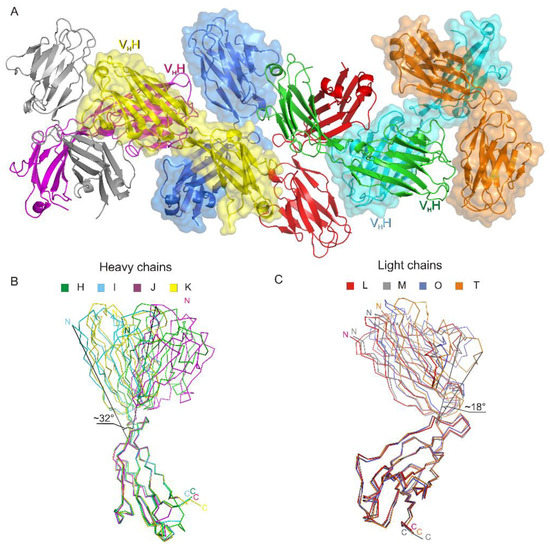
Figure 2.
The crystal packing of the netakimab Fab fragment. (A) Crystal packing of the four Fab fragment molecules in the asymmetric unit. Heavy and light chains are indicated with different colors, surfaces of even molecules are colored. (B) Structural alignment of the different conformations obtained for the heavy chains (H, I, J, K). (C) Structural alignment of the different conformations obtained for the light chains. Extreme fluctuation angles for variable domains relatively to constant domains are indicated (the angle vertices are at the last matching Cα atoms of the main chains).
3.2. Kinetic Analysis
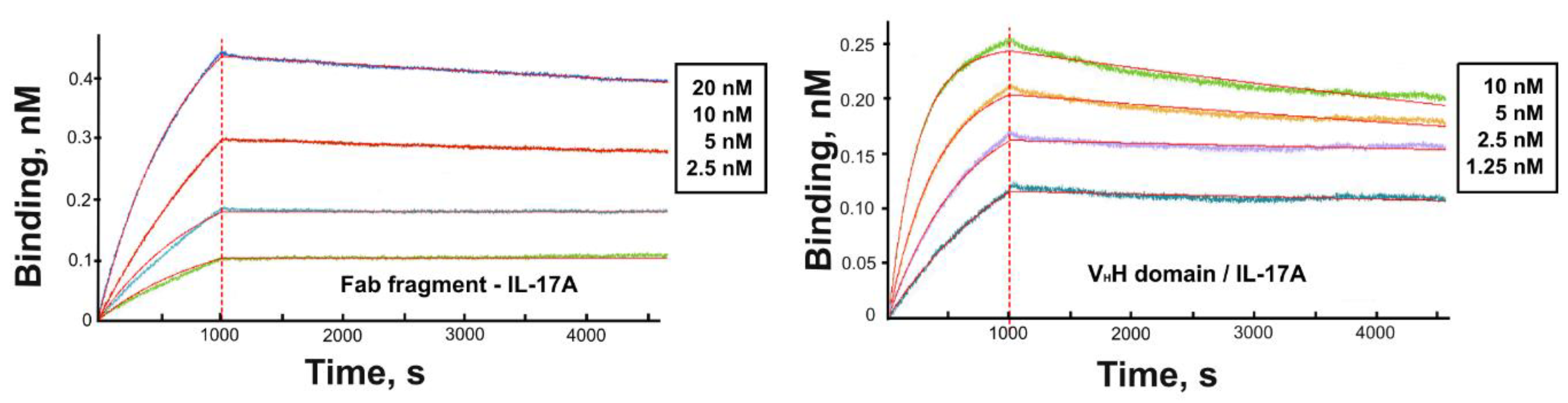
The affinity of the VHH domain and the VHH-based Fab fragment for IL-17A was assessed using SPR (Figure 3). Kinetic constants and equilibrium dissociation constants (KD) of complexes of the Fab fragment and the VHH domain with IL-17A were determined using global fitting of the four curves and 1:1 Langmuir interaction model (Table 2). Kinetic analysis of binding the Fab fragment with IL-17A and results of gel-filtration chromatography (not shown) evidences that the Fab fragment forms a stable complex with the IL-17A dimer at a molar ratio of 1:1.
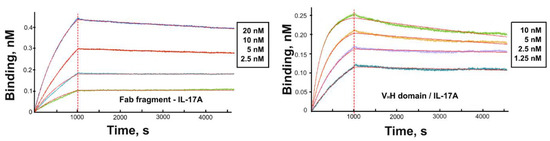
Figure 3.
Sensograms of the interaction of the VHH domain (alone or as a part of the Fab fragment) with IL-17A. The experimental curves are shown in different colors while fitted curves are in red.

Table 2.
Kinetic Constants of Interactions of IL-17A with the Fab Fragment and the VHH Domain.
As we did not succeed in obtaining crystals of VHH alone or with IL-17A or complexed with IL-17A suitable for the X-ray analysis, we simulated the interactions between the IL-17A dimer and the VHH domain as a part of the Fab fragment.
3.3. Modeling the VHH Domain/IL-17A Complex
As reliable modeling interactions between the entire Fab fragment and IL-17A do not seem to be possible due to high conformational plasticity of the molecule, we performed modeling of the VHH domain as a part of the Fab fragment with the IL-17A dimer. IL-17A model was used from the structure of the IL-17A/ IL-17A RA complex (PDB entry 4HSA), as it is currently the most complete IL-17A model, in which the N-terminal region including the β0 strand and flexible loop between β0 and main β sheet stabilized by IL-17A RA [34]. Possible intermolecular hydrogen bonds and hydrophobic interactions upon the complex formation were determined.
4. Discussion
A fraction of functional antibodies in Camelidae lacks light chains. Their variable domains represent single-domain antibodies with a molecular weight of 15 kDa. Due to their small size, the nanoantibodies are rapidly excreted from the organism by the kidneys. In general, the half-life of nanoantibodies in human blood does not exceed 2 hours, which is significantly shorter than the half-life of classical monoclonal antibodies [35]. Therefore, design and medical application of chimeric antibodies containing VHH domains is highly relevant. However, the structure of such chimeric Fab fragments has not yet been determined. We have determined the structure of the netakimab Fab fragment containing the anti-IL17A VHH domain and performed modeling of the interaction between the anti-IL-17A VHH domain and IL-17A.
4.1. Structure of the Netakimab Fab Fragment
CDR loops of the Fab fragment light and heavy chains that link structural elements of β-sheets were classified according to the work [36] based on their belonging to canonical clusters (Table 3). This classification is similar to the one published by Martin and coworkers [37], but CDR-H1 and CDR-H3 begin right after cysteine residues and CDR-L2 includes Tyr at the N-terminal part.

Table 3.
Comparison of H- and L-loops of the netakimab Fab fragment with the canonical types (clustering according to [36]).
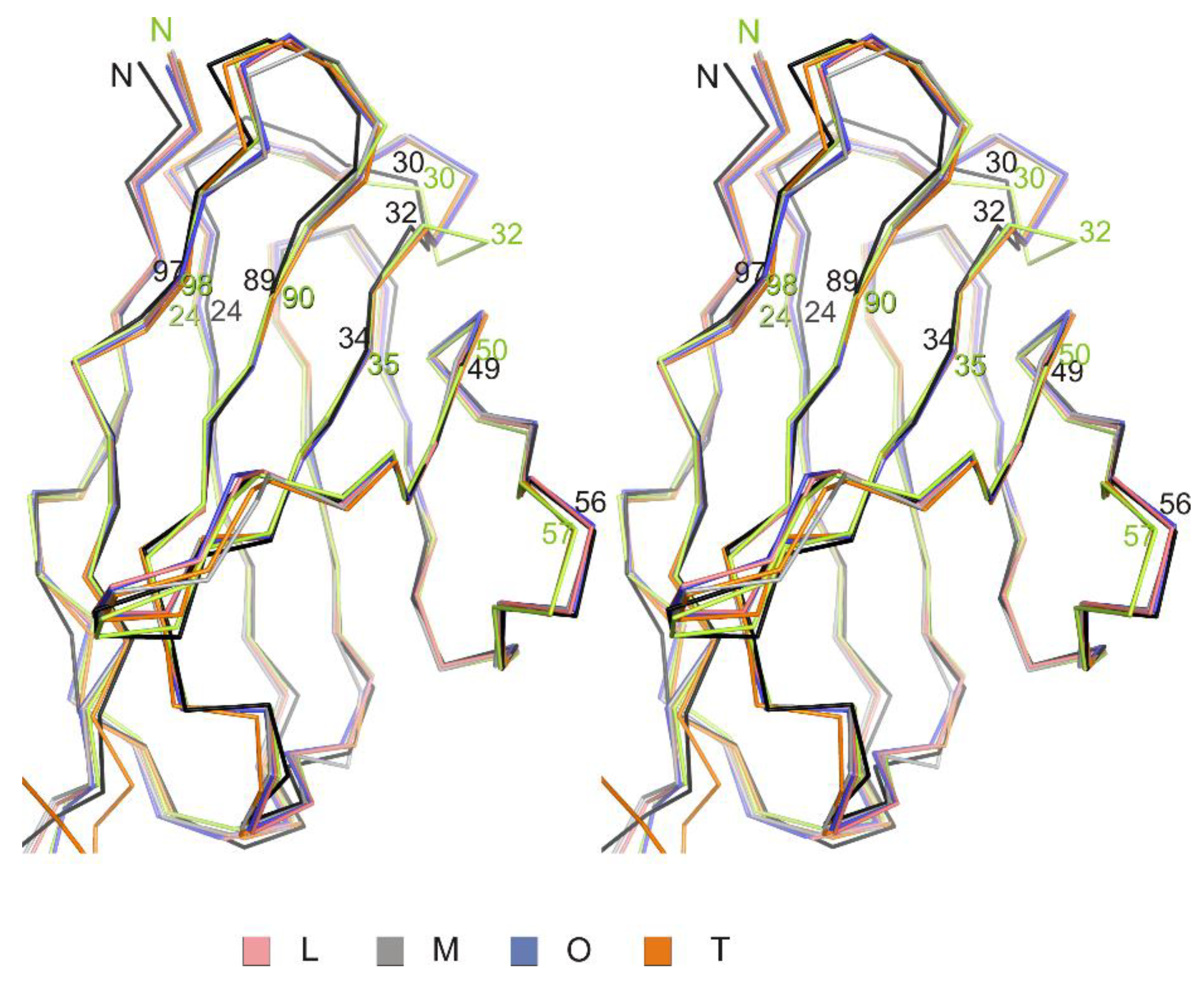
CDR regions of the netakimab Fab fragment variable domains of light chains have conformations similar to that of canonical type clusters (Table 3, Figure 4). Variable domains of the netakimab Fab fragment heavy chains demonstrate lower (as compared to corresponding domains of light chains) structural similarity to the canonical structures.
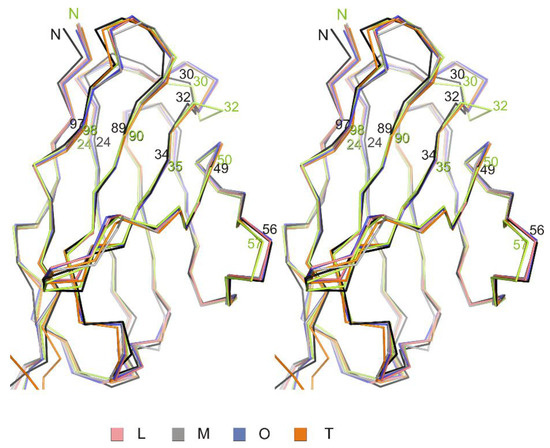
Figure 4.
Structural alignment of variable domains of light chains. A stereo view of structural alignment of Cα chains of variable domains of the four netakimab Fab molecules (L,M,O,T) and corresponding regions of light chains of antibodies, whose structures have similar structural organization of the CDR regions (green: human germline antibody IGHV5-51/IGKV3-20; black: FV from human IGM immunoglobulin).
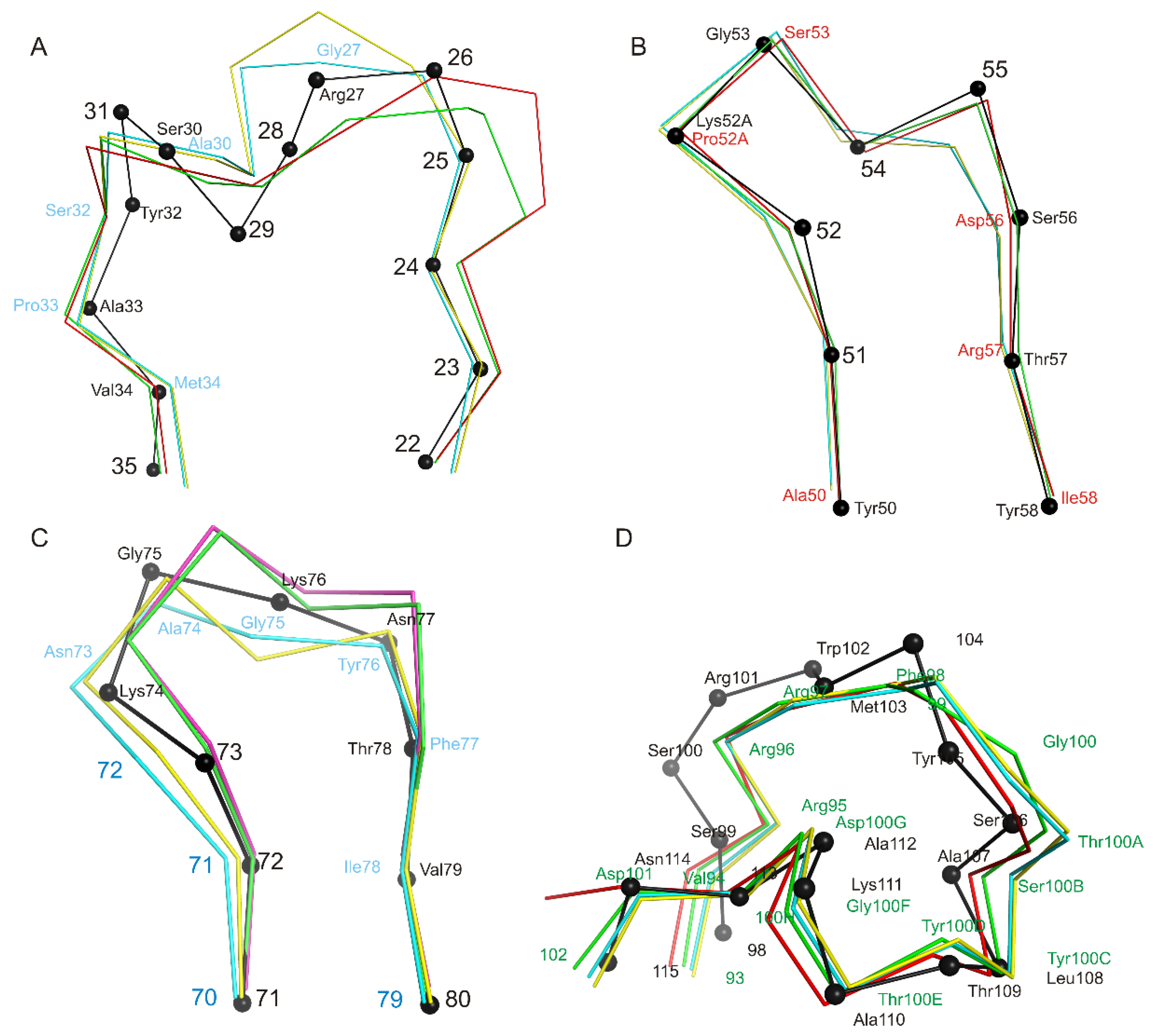
We have shown that the CDR-H1 (13 amino acids long) of the Fab fragment (Figure 5A) belongs to the H1-13-3 cluster (the most similar structure is PDB entry 1SEQ). In the region between residues 24 and 31, there is relatively high chain mobility in different molecules in an asymmetrical unit. However, the main chain conformation in molecules in a crystallographic unit is preserved. CDR-H2 (10 amino acids long) belongs to the H2-10-2 cluster (Table 3, Figure 5B), differences from the most similar structure are only marginal. It is worth mentioning that there are differences in the structure of the loop (70-79 aa), which belongs to the framework part of HFR3 (Figure 5C).
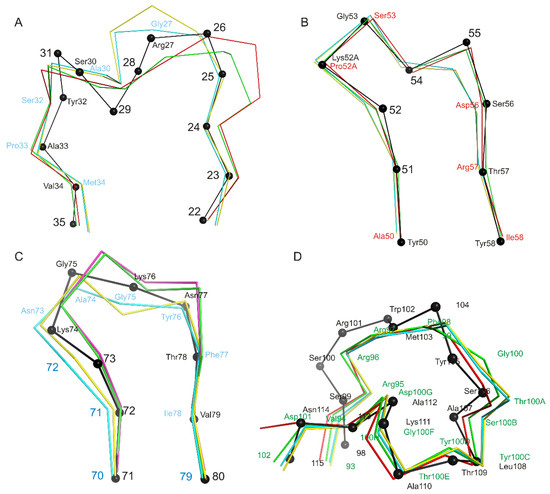
Figure 5.
Structural alignment of CDR loops of heavy chains and HFR3. Structural alignment of Cα atoms of CDR loops of heavy chains and HFR3 of four netakimab Fab fragment molecules and Cα atoms (black) of the most similar structures listed in Table 3 (for CDR loops) and PDB entry 2P45 (for HFR3). Cα atoms of the Fab fragment are colored variously. If amino acids coincide, only position number is provided, whereas both name and position are indicated if they differ. (A) CDR-H1, (B) CDR-H2, (C) HFR3, (D) CDR-H3.
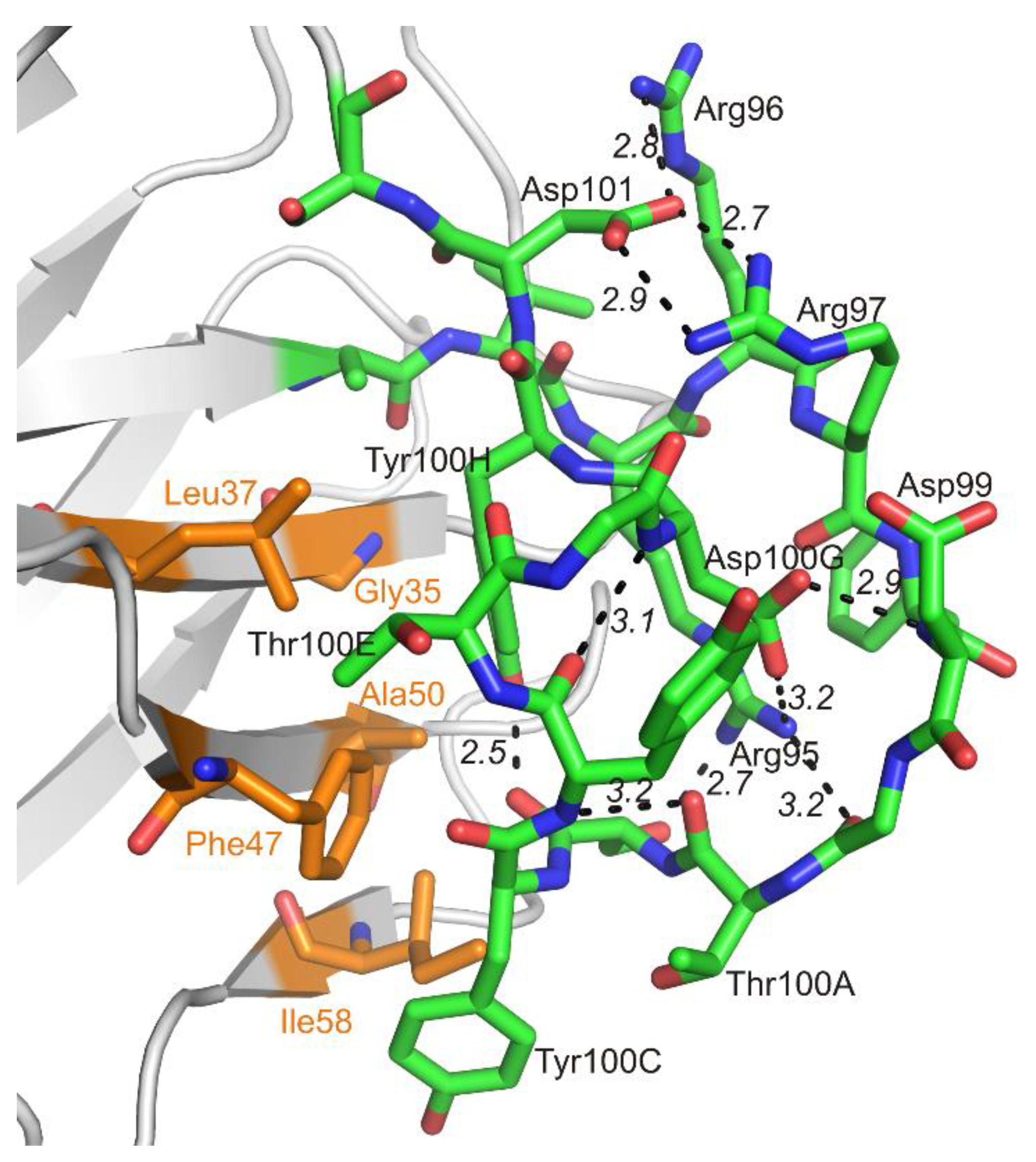
The netakimab Fab fragment CDR-H3 loop is relatively long (18 residues between positions 94 and 102 according to Kabat Database). Unusual V-shaped conformation of the Fab fragment molecule is determined by the specificity of the CDR-H3 VHH domain sequence. Elongated CDR-H3 region forming “beak” causes a change of the surface (VL side) of the VHH domain within the Fab fragment, which prevents its interaction with the VL chain. In addition to aromatic residues (three tyrosines and one phenylalanine), the CDR-H3 loop features high content of charged residues: three arginines and three aspartates. CDR-H3 structure is stabilized by at least ten hydrogen bonds (Figure 6). Interaction of CDR-H3 with the VHH domain is realized by means of hydrophobic interactions of the loop residues Tyr100C, Thr100E and Tyr100H with Gly35, Leu37, and Phe47 belonging to HFR2, and with Ala50 and Ile58 located at the beginning and at the end of CDR-H2.
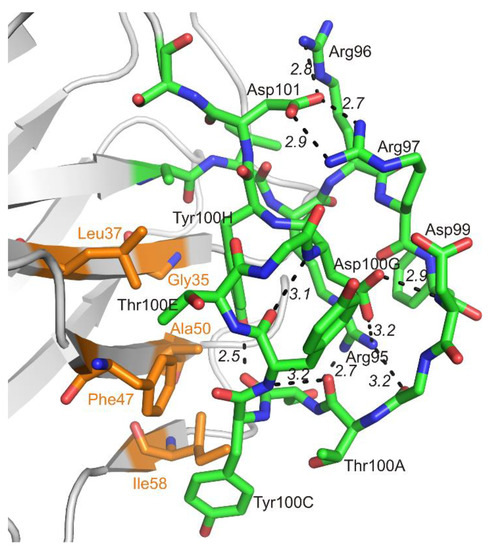
Figure 6.
Interaction within the CDR-H3 loop (green) and its contacts with amino acid residues of the VHH domain as part of the Fab fragment (orange).
High variability in the orientation of light and heavy chains of the netakimab Fab fragment in the asymmetric unit may indicate their high mobility in vivo. Comparative analysis of structures of Fab antibody fragments from the PDB reveals high interdomain mobility in molecules of various Fab fragments alone [38] and complexed [39]. In our case, there is a high range of Fab conformations between the constant and variable domains in a single crystal structure.
4.2. Modeling the VHH Domain/IL-17A Complex
As the position of variable domains of light and heavy chains of the netakimab Fab fragment molecules varies in the asymmetric unit (Figure 2), modeling its complex with IL-17A is complicated. Therefore, to assess interactions of the cytokine with the netakimab Fab fragment, we used a part of the structure containing the VHH domain. The affinity of the VHH domain to IL-17A (KD = 0.09 nM) is comparable to that of the netakimab Fab fragment for the cytokine (KD = 0.3 nM) (Table 2), which suggests that protein-protein contacts in the VHH/IL-17A complex are decisive for the Fab fragment binding to IL-17A. The functional activity of the VHH domain (the ability to inhibit production of interleukin 6 in the cell line HT1080 [ATCC:CCL-121]) is also preserved in the structure of the Fab fragment (Table 4).

Table 4.
Anti-IL-17A antibodies. KD for antibody-cytokine complexes and half-maximal inhibitory concentrations.
Currently, the VHH/IL-17A complex is one of the most stable complexes of this cytokine. Antigen affinity for the IL-17RA receptor [34] and the Fab fragment of the CAT 2200 antibody [40] is one order of magnitude lower (KD = 0.3 and 2.1 nM, respectively, Table 4). Modeling the VHH/IL-17A complex allowed suggesting reasons for such strong binding.
For modeling the VHH/IL-17A complex we used the IL-17A structure from its complex with the receptor, as it is the most complete one. It is known that IL-17A forms a functional dimer (monomers A and B). Mobile N-terminal part of IL-17A is virtually invisible in the electron density maps of both individual molecule and bound to the CAT 2200 Fab fragment. However, in a complex with the IL-17RA receptor the N-terminal part of one of the IL-17A monomers is clearly visible.
The molecule of the VHH domain is capable of forming a complex with both IL-17A monomers forming eleven hydrogen bonds and five hydrophobic contacts (Table 5).

Table 5.
VHH domain/IL-17A direct interactions. The residue position includes a chain indicator (H: VHH domain; A: IL-17A monomer A; B: IL-17A monomer B). The distance cutoff used for hydrogen bonds is 3.5 Å, and for nonpolar interactions is 5.0 Å.
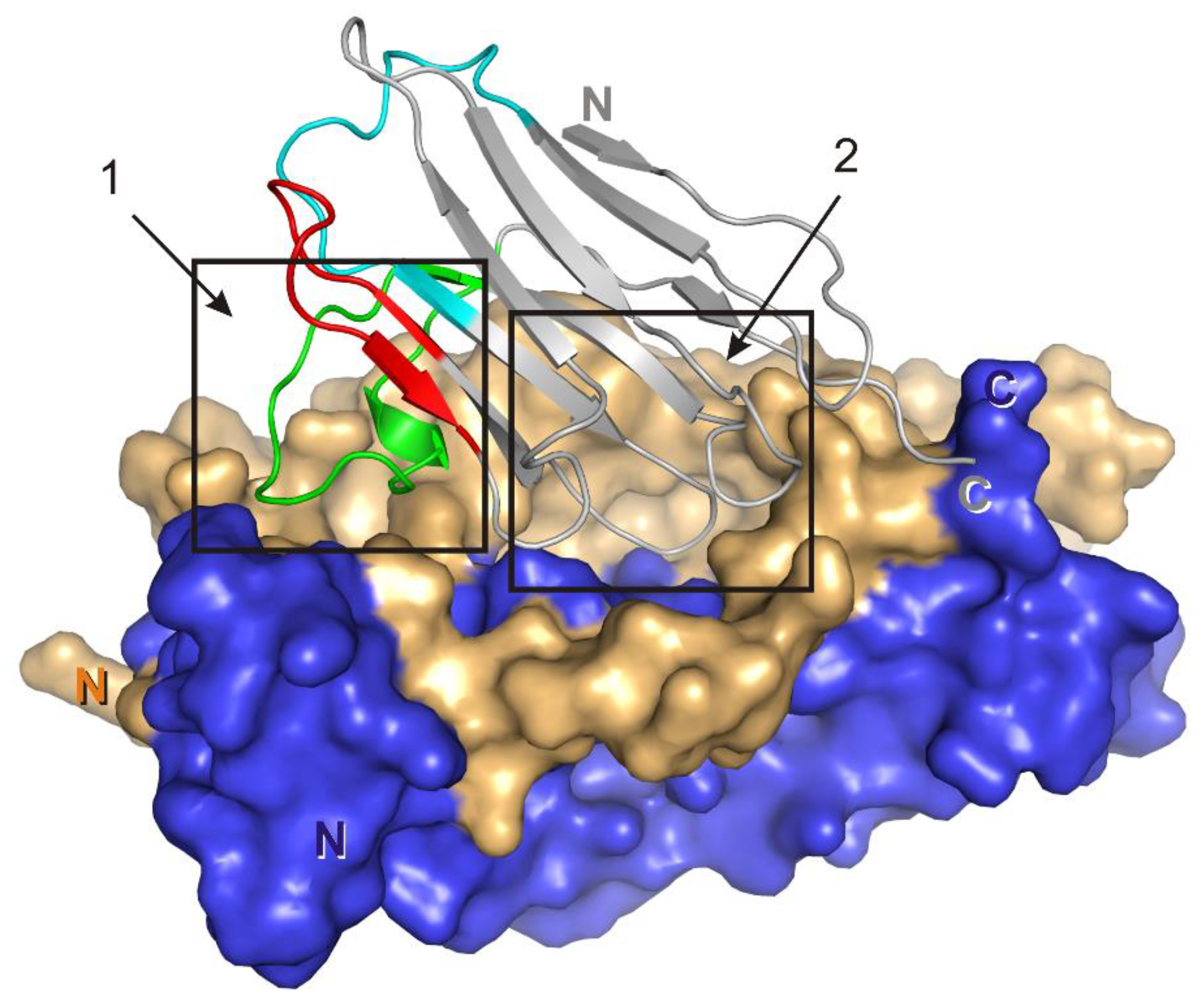
A region of the VHH domain due to a specific spatial organization of CDR-H3 (Figure 7), penetrates a hollow on the IL-17A surface forming multiple contacts. The IL-17A monomer A in the model of the complex forms three hydrogen bonds with the VHH domain (two with HFR2 and one with CDR-H3), whereas monomer B forms eight hydrogen bonds and five hydrophobic contacts, most of which are also located in CDR-H3. The surface of the intermolecular contact in the VHH/IL-17A complex may be divided into two regions (Figure 7), which correspond to two out of three regions of IL-17A interaction with the IL-17 RA receptor. Region 1 is formed by the N-terminal part of IL-17A, region 2 is formed by the central part of β-sheets of the dimer. We suggest that as the N-terminal part of IL-17A is essential for binding with IL-17A RA it could participate in binding with the VHH domain (hydrogen bind Lys38-Glu85, Table 5).
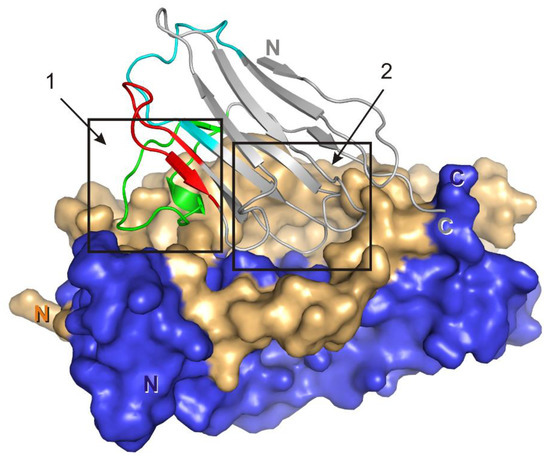
Figure 7.
VHH/IL-17A complex. Surface of the IL-17A dimer is shown (monomer A is dark blue, monomer B is yellow). Crystallographic model of the VHH domain is in gray. CDR-H1–cyan, CDR-H2–red, CDR-H3–green. The two contacting regions are indicated by frames.
Using the PISA service we compared contact areas in our VHH/IL-17A complex and a complex between IL-17A and the CAT 2200 antibody Fab fragment (PDB entry 2VXS) (Supplementary Figure S3B). Total contact area in the VHH/IL-17A complex was 1185 Å2 (963 Å2 with monomer B and 222 Å2 with monomer A). The contact area in the complex with the CAT 2200 antibody included seven hydrogen bonds and it was less extensive–833 Å2. However, most of the interactions were also recorded for monomer B (607 Å2). Moreover, interactions occurred with both heavy and light chains of the Fab fragment. Superposition of the structure of the netakimab Fab fragment and the structure of VHH in the model complex (Supplementary Figure S3A) did not show significant clashes of the light chain of the Fab fragment with the dimer IL-17A. Thus, comparative structural analysis of the IL-17A complexes can explain the increased the VHH domain affinity for IL-17A by enlarged area of protein-protein contacts due to additional interactions between CDR-H3 and the cytokine dimer.
Correlation is observed between the antibody affinity for the cytokine and its functional activity (Table 4). The VHH domain demonstrates full functional activity, which is 6.6-fold higher than that of the widely applied therapeutic preparation secukinumab. Presumably, tighter contact between the VHH domain and the IL-17A region that interact with the IL-17 RA receptor provides the high functional activity of the preparation. Thus, the netakimab Fab fragment carrying the VHH domain may become the most efficient therapeutic preparation for treating psoriasis, and the structural data obtained can be used for the further design of effective cytokine inhibitors.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/9/3/177/s1, Figure S1: Elution profiles of size-exclusion chromatography and SDS–PAGE gel of purified Fab (A) and VHH domain (B). Lane M – molecular-weight marker, Figure S2: Circular dichroism spectrum of VHH domain, Figure S3: (A) Netakimab Fab fragment /IL-17A complex. Surface of the IL-17A dimer is shown (monomer A is dark blue, monomer B is yellow). Crystal structure of Fab fragment (light chain is colored green, heavy chain–gray). (B) Fab fragment CAT 2200/ IL-17A complex (PDB ID 2VXS). Monomer A of IL-17A is dark blue, monomer B is yellow. Light chain Fab fragment CAT 2200 of is colored green, heavy chain–gray.
Author Contributions
Conceptualization: O.K., M.G., data curation: O.K., A.G., formal analysis: A.G., funding acquisition: O.K., A.G., investigation: S.E., A.U., methodology: O.K., I.K., V.E., S.E., A.G., project administration: S.T., M.G., resources: I.K., V.E., supervision: O.K., S.T., validation: A.U., O.K., visualization: A.G., S.E., writing: O.K., S.T., A.G. wrote the paper with input from all authors.
Funding
This work was supported by the Russian Science Foundation (grant number 177410156).
Acknowledgments
We acknowledge the Structural Biology Group of the European Synchrotron Radiation Facility for granting access to the synchrotron beamlines.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cypowyj, S.; Picard, C.; Maródi, L.; Casanova, J.-L.; Puel, A. Immunity to Infection in IL-17-Deficient Mice and Humans. Eur. J. Immunol. 2012, 42, 2246–2254. [Google Scholar] [CrossRef]
- Beringer, A.; Noack, M.; Miossec, P. IL-17 in Chronic Inflammation: From Discovery to Targeting. Trends Mol. Med. 2016, 22, 230–241. [Google Scholar] [CrossRef]
- Paek, S.Y.; Frieder, J.; Kivelevitch, D.; Menter, M.A. IL-17 Inhibitors for Psoriasis. Semin. Cutan. Med. Surg. 2018, 37, 148–157. [Google Scholar] [CrossRef]
- Wolfson, W. Ablynx Makes Nanobodies from Llama Bodies. Chem. Biol. 2006, 13, 1243–1244. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Single domain camel antibodies: Current status. J. Biotechnol. 2001, 74, 277–302. [Google Scholar] [CrossRef]
- Schmitz, K.R.; Bagchi, A.; Roovers, R.C.; van Bergen en Henegouwen, P.M.P.; Ferguson, K.M. Structural Evaluation of EGFR Inhibition Mechanisms for nanobodies/VHH Domains. Structure 2013, 21, 1214–1224. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V. Efficient Cancer Therapy with a Nanobody-Based Conjugate. Cancer Res. 2004, 64, 2853–2857. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V.; Lauwereys, M.; Hassanzadeh, G.; Gobert, M.; Conrath, K.; Muyldermans, S.; De Baetselier, P.; Revets, H. Efficient Tumor Targeting by Single-Domain Antibody Fragments of Camels. Int. J. Cancer 2002, 98, 456–462. [Google Scholar] [CrossRef]
- Harmsen, M.M.; Van Solt, C.B.; Fijten, H.P.D.; Van Setten, M.C. Prolonged in Vivo Residence Times of Llama Single-Domain Antibody Fragments in Pigs by Binding to Porcine Immunoglobulins. Vaccine 2005, 23, 4926–4934. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Meyers, A.J.; McLean, M.D.; Arbabi-Ghahroudi, M.; MacKenzie, R.; Hall, J.C. In Vivo Neutralization of α-Cobratoxin with High-Affinity Llama Single-Domain Antibodies (VHHs) and a VHH-Fc Antibody. PLoS ONE 2013, 8, e69495. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Strategies to Extend Plasma Half-Lives of Recombinant Antibodies. BioDrugs 2009, 23, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Hoefman, S.; Ottevaere, I.; Baumeister, J.; Sargentini-Maier, M. Pre-Clinical Intravenous Serum Pharmacokinetics of Albumin Binding and Non-Half-Life Extended Nanobodies®. Antibodies 2015, 4, 141–156. [Google Scholar] [CrossRef]
- Ulitin, A.; Evdokimov, S.; Soloviev, V.; Chernyh, Y.; Goncharova, O.; Korzhavin, D.; Chernovskaya, T.; Nemankin, T.; Ivanov, R.; Morozov, D.; et al. High Affinity and Aggregatively Stable Antibodies on the Basis of Variable Domains vl and a Derivative vhh. U.S. Patent 2016048188A1, 31 March 2016. [Google Scholar]
- International Nonproprietary Names for Pharmaceutical Substances (INN). INN: List 80; WHO Drug Information; WHO: Geneva, Switzerland, 2018; Volume 32, pp. 425–508. [Google Scholar]
- Samtsov, A.V.; Khairutdinov, V.R.; Bakulev, A.L.; Kubanov, A.A.; Karamova, A.E.; Artem’eva, A.V.; Korotaeva, T.V. Efficacy and Safety of BCD-085, a Novel Interleukin-17 Inhibitor. Results of Phase II Clinical Trial in Patients with Moderate-to-Severe Plaque Psoriasis. Vestn. Dermatol. Venerol. 2017, 5, 52–63. [Google Scholar] [CrossRef]
- Nurizzo, D.; Mairs, T.; Guijarro, M.; Rey, V.; Meyer, J.; Fajardo, P.; Chavanne, J.; Biasci, J.C.; McSweeney, S.; Mitchell, E. The ID23-1 Structural Biology Beamline at the ESRF. J. Synchrotron Radiat. 2006, 13 Pt 3, 227–238. [Google Scholar] [CrossRef]
- Gabadinho, J.; Beteva, A.; Guijarro, M.; Rey-Bakaikoa, V.; Spruce, D.; Bowler, M.W.; Brockhauser, S.; Flot, D.; Gordon, E.J.; Hall, D.R.; et al. MxCuBE: A Synchrotron Beamline Control Environment Customized for Macromolecular Crystallography Experiments. J. Synchrotron Radiat. 2010, 17, 700–707. [Google Scholar] [CrossRef]
- Bourenkov, G.P.; Popov, A.N. Optimization of Data Collection Taking Radiation Damage into Account. Acta Crystallogr. D Biol. Crystallogr. 2010, 66 Pt 4, 409–419. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66 Pt 2, 125–132. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser Crystallographic Software. J. Appl. Crystallogr. 2007, 40 Pt 4, 658–674. [Google Scholar] [CrossRef]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the Refinement of Macromolecular Crystal Structures. Acta Crystallogr. D Biol. Crystallogr. 2011, 67 Pt 4, 355–367. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and Development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66 Pt 4, 486–501. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System. Available online: https://pymol.org/2/ (accessed on 1 March 2019).
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S. HDOCK: A web server for protein—Protein and protein—DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017, 45, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef]
- Mackerell, A.D., Jr.; Feig, M.; Brooks, C.L., 3rd. Extending the Treatment of Backbone Energetics in Protein Force Fields: Limitations of Gas-Phase Quantum Mechanics in Reproducing Protein Conformational Distributions in Molecular Dynamics Simulations. J. Comput. Chem. 2004, 25, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Bekker, H.; Berendsen, H.; Johannes, G. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- PDBePISA (Proteins, Interfaces, Structures and Assemblies). Available online: http://www.ebi.ac.uk/pdbe/prot_int/pistart.html (accessed on 1 March 2019).
- Krissinel, E.; Henrick, K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- Liu, S.; Song, X.; Chrunyk, B.A.; Shanker, S.; Hoth, L.R.; Marr, E.S.; Griffor, M.C. Crystal Structures of Interleukin 17A and Its Complex with IL-17 Receptor A. Nat. Commun. 2013, 4, 1888. [Google Scholar] [CrossRef]
- Hassanzadeh-Ghassabeh, G.; Devooght, N.; Pauw, P.; Vincke, C.; Muyldermans, S. Nanobodies and their potential applications. Nanomedicine 2013, 8, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- North, B.; Lehmann, A.; Dunbrack, R.L., Jr. A New Clustering of Antibody CDR Loop Conformations. J. Mol. Biol. 2011, 406, 228–256. [Google Scholar] [CrossRef]
- Martin, A.C.; Thornton, J.M. Structural Families in Loops of Homologous Proteins: Automatic Classification, Modelling and Application to Antibodies. J. Mol. Biol. 1996, 263, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, R.L.; Zemla, A.; Wilson, I.A.; Rupp, B. Antibody Elbow Angles Are Influenced by Their Light Chain Class. J. Mol. Biol. 2006, 357, 1566–1574. [Google Scholar] [CrossRef]
- Bailey, L.J.; Sheehy, K.M.; Dominik, P.K.; Liang, W.G.; Rui, H.; Clark, M.; Jaskolowski, M.; Kim, Y.; Deneka, D.; Tang, W.-J.; et al. Locking the Elbow: Improved Antibody Fab Fragments as Chaperones for Structure Determination. J. Mol. Biol. 2018, 430, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, S.; Abbott, W.M.; Hargreaves, D.; Pauptit, R.A.; Davies, R.A.; Needham, M.R.C.; Langham, C.; Barker, W.; Aziz, A.; Snow, M.J.; et al. Structure of IL-17A in Complex with a Potent, Fully Human Neutralizing Antibody. J. Mol. Biol. 2009, 394, 905–921. [Google Scholar] [CrossRef]
- Ekimova, V.; Ulitin, A.; Evdokimov, S.; Sofronova, E.; Nemankin, T.; Solovyev, V.; Ustugov, I.; Nedorubov, A.; Chernykh, Y.; Goncharova, O.; et al. High Affinity Anti-Il-17a Monoclonal Antibody. In Proceedings of the Conference: PEGS, Boston, MA, USA, 4–8 May 2015. [Google Scholar]
- Padova, D.; Gram, H.; Hofstetter, H.; Jeschke, M.; Rondeau, J.; Van Den Berg, W. IL-17 Antagonistic Antibodies. U.S. Patent 7,807,155 B2, 5 October 2010. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).