Melting Diagrams of Adefovir Dipivoxil and Dicarboxylic Acids: An Approach to Assess Cocrystal Compositions
Abstract
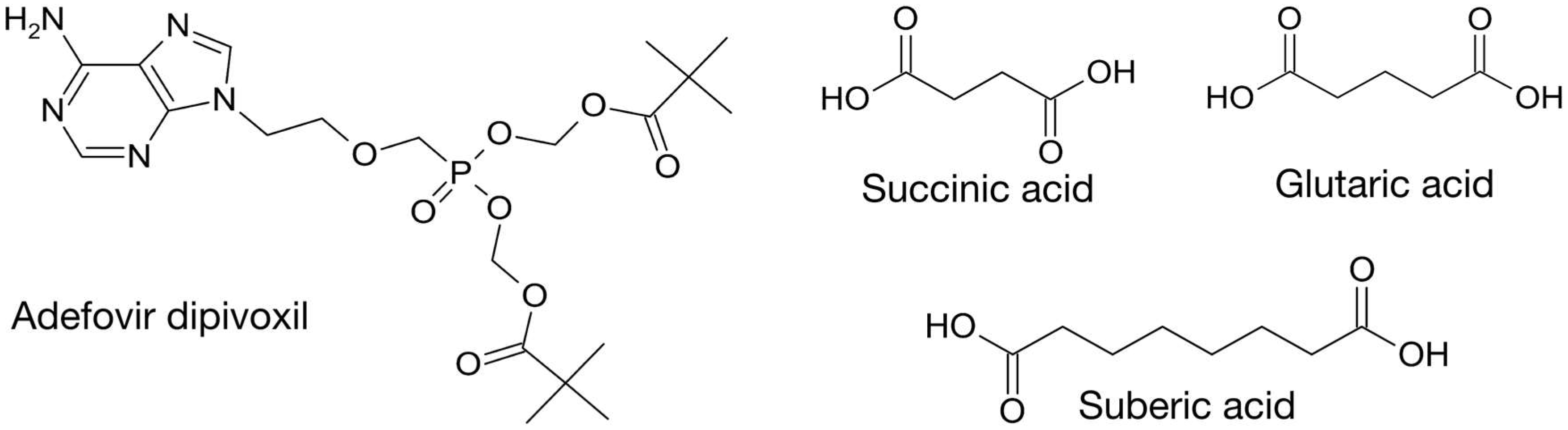
:1. Introduction
2. Materials and Methods
2.1. Cocrystallization
2.2. Characterization
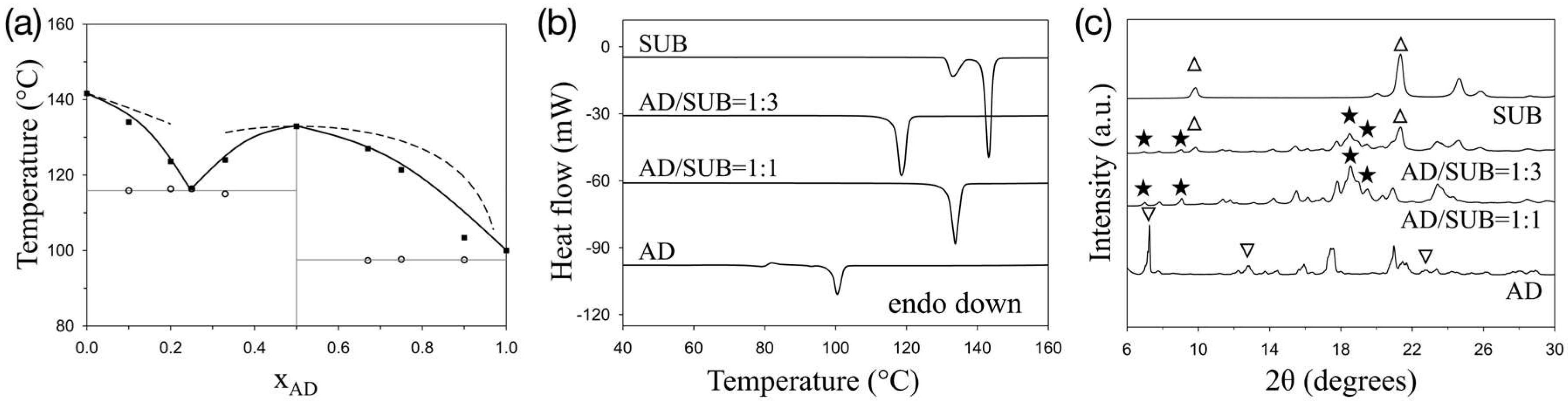
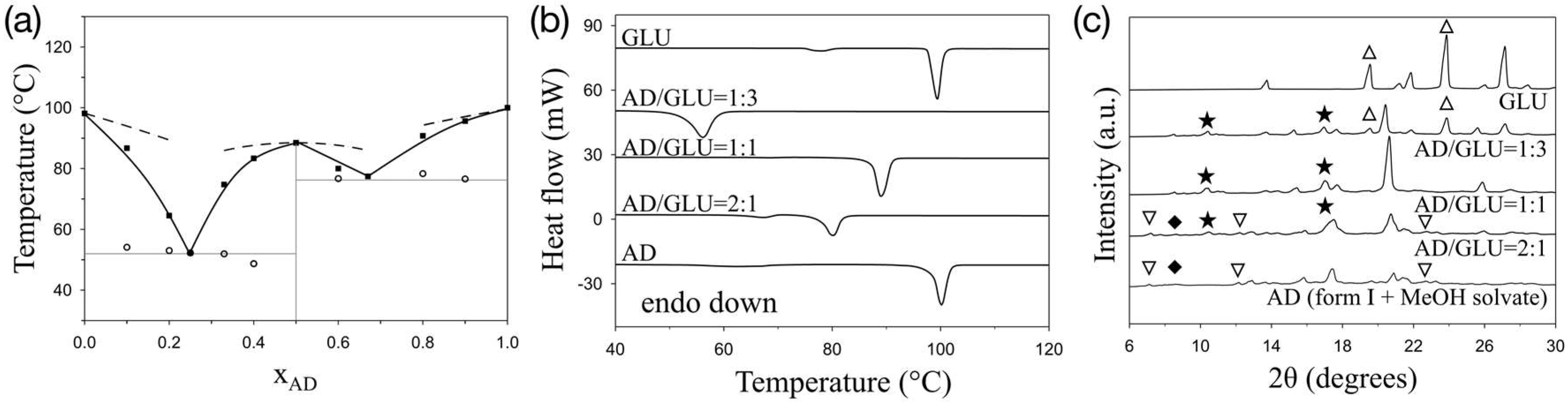
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Stahl, P.H.; Nakano, M. Pharmaceutical aspects of the drug salt form. In Handbook of Pharmaceutical Salts, 1st ed.; Stahl, P.H., Wermuth, C.G., Eds.; WILEY-VCH: Weinheim, Germany, 2008; pp. 83–116. ISBN 978-3-906390-58-1. [Google Scholar]
- Rao, V.M.; Sanghvi, R.; Zhu, H. Solubility of pharmaceutical solids. In Developing Solid Oral Dosage Forms, 1st ed.; Qiu, Y., Chen, Y., Zhang, G.G.Z., Liu, L., Porter, W.R., Eds.; Academic Press: Burlington, MA, USA, 2009; pp. 1–24. ISBN 978-0-444-53242-8. [Google Scholar]
- Jones, W.; Motherwell, W.D.S.; Trask, A.V. Pharmaceutical cocrystals: An emerging approach to physical property enhancement. MRS Bull. 2006, 31, 875–879. [Google Scholar] [CrossRef]
- Shan, N.; Zaworotko, M.J. The role of cocrystals in pharmaceutical science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hornedo, N.; Nehm, S.J.; Jayasankar, A. Cocrytals: Design, properties and formation mechanisms. In Encyclopedia of Pharmaceutical Technology, 3rd ed.; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; Volume 1, pp. 615–635. ISBN 978-0-8493-9396-9. [Google Scholar]
- Chow, S.F.; Chen, M.; Shi, L.; Chow, A.H.L.; Sun, C.C. Simultaneously improving the mechanical properties, dissolution performance, and hygroscopicity of ibuprofen and flurbiprofen by cocrystallization with nicotinamide. Pharm. Res. 2012, 29, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility advantage of pharmaceutical cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Physical stability enhancement of theophylline via cocrystallization. Int. J. Pharm. 2006, 320, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Friščić, T.; Fábián, L.; Laity, P.R.; Day, G.M.; Jones, W. Improving mechanical properties of crystalline solids by cocrystal formation: New compressible forms of paracetamol. Adv. Mat. 2009, 21, 3905–3909. [Google Scholar] [CrossRef]
- Wood, P.A.; Feeder, N.; Furlow, M.; Galek, P.T.A.; Groom, C.R.; Pidcock, E. Knowledge-based approaches to co-crystal design. CrystEngComm 2014, 16, 5839–5848. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Nanda, A. A review about regulatory status and recent patents of pharmaceutical co-crystals. Adv. Pharm. Bull. 2018, 8, 355–363. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Chow, P.S.; Tan, R.B.H. Operating regions in cooling cocrystallization of caffeine and glutaric acid in acetonitrile. Cryst. Growth Des. 2010, 10, 2382–2387. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; Rasmuson, Å.C. Thermodynamics and crystallization of a theophylline–salicylic acid cocrystal. CrystEngComm 2015, 17, 4125–4135. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Kaialy, W.; Buckton, G.; Gill, H.; Nokhodchi, A.; Velaga, S.P. Theophylline cocrystals prepared by spray drying: Physicochemical properties and aerosolization performance. AAPS PharmSciTech 2013, 14, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Jones, W. Recent advances in understanding the mechanism of cocrystal formation via grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friščić, T.; et al. Applying hot-stage microscopy to co-crystal screening: A study of nicotinamide with seven active pharmaceutical ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef]
- Shevchenko, A.; Bimbo, L.M.; Miroshnyk, I.; Haarala, J.; Jelínková, K.; Syrjänen, K.; van Veen, B.; Kiesvaara, J.; Santos, H.A.; Yliruusi, J. A new cocrystal and salts of itraconazole: Comparison of solid-state properties, stability and dissolution behavior. Int. J. Pharm. 2012, 436, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Fábián, L.; Friščić, T.; Jones, W. Powder X-ray diffraction as an emerging method to structurally characterize organic solids. Org. Lett. 2007, 9, 3133–3136. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.D.M.; Tremayne, M.; Kariuki, B.M. Contemporary advances in the use of powder X-ray diffraction for structure determination. Angew. Chem. Int. Ed. 2001, 40, 1626–1651. [Google Scholar] [CrossRef]
- Day, G.M.; van de Streek, J.; Bonnet, A.; Burley, J.C.; Jones, W.; Motherwell, W.D.S. Polymorphism of scyllo-inositol: Joining crystal structure prediction with experiment to elucidate the structures of two polymorphs. Cryst. Growth Des. 2006, 6, 2301–2307. [Google Scholar] [CrossRef]
- Friščić, T.; Halasz, I.; Strobridge, F.C.; Dinnebier, R.E.; Stein, R.S.; Fábián, L.; Curfs, C. A rational approach to screen for hydrated forms of the pharmaceutical derivative magnesium naproxen using liquid-assisted grinding. CrystEngComm 2011, 13, 3125–3129. [Google Scholar] [CrossRef]
- Évora, A.O.L.; Castro, R.A.E.; Maria, T.M.R.; Silva, M.R.; ter Horst, J.H.; Canotilho, J.; Eusébio, M.E.S. Co-crystals of diflunisal and isomeric pyridinecarboxamides—A thermodynamics and crystal engineering contribution. CrystEngComm 2016, 18, 4749–4759. [Google Scholar] [CrossRef]
- Jung, S.; Ha, J.-M.; Kim, I.W. Bis[(2,2-dimethylpropanoyloxy)methyl]{[2-(6-amino-9H-purin-9-yl)ethoxy]methyl}phosphonate–succinic acid (2/1). Acta Crystallogr. E 2012, 68, o809–o810. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, J.; Kim, I.W. Structures and physical properties of the cocrystals of adefovir dipivoxil with dicarboxylic acids. J. Cryst. Growth 2013, 373, 59–63. [Google Scholar] [CrossRef]
- Jung, S.; Ha, J.-M.; Kim, I.W. Phase transformation of adefovir dipivoxil/succinic acid cocrystals regulated by polymeric additives. Polymers 2014, 6, 1–11. [Google Scholar] [CrossRef]
- Jung, S.; Choi, I.; Kim, I.W. Liquid-assisted grinding to prepare a cocrystal of adefovir dipivoxil thermodynamically less stable than its neat phase. Crystals 2015, 5, 583–591. [Google Scholar] [CrossRef]
- Roux, M.V.; Temprado, M.; Chickos, J.S. Vaporization, fusion and sublimation enthalpies of the dicarboxylic acids from C4 to C14 and C16. J. Chem. Thermodyn. 2005, 37, 941–953. [Google Scholar] [CrossRef]
- Arimilli, M.N.; Kelly, D.E.; Lee, T.T.K.; Manes, L.V.; Munger, J.D., Jr.; Prisbe, E.J.; Schultze, L.M. Nucleotide Analog Compositions. U.S. Patent 6,451,340, 17 September 2002. [Google Scholar]
- Dheep, G.R.; Sreekumar, A. Investigation on thermal reliability and corrosion characteristics of glutaric acid as an organic phase change material for solar thermal energy storage applications. Appl. Therm. Eng. 2018, 129, 1189–1196. [Google Scholar] [CrossRef]
- Soustelle, M. Phase Transformations, 1st ed.; ISTE Ltd.: London, UK, 2015; pp. 75–112. ISBN 978-1-84821-868-0. [Google Scholar]
- Prigogine, I.; Defay, R. Chemical Thermodynamics, 1st ed.; Longmans: London, UK, 1954; pp. 357–380. ISBN 978-0582462830. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, H.; Choi, I.; Kim, I.W. Melting Diagrams of Adefovir Dipivoxil and Dicarboxylic Acids: An Approach to Assess Cocrystal Compositions. Crystals 2019, 9, 70. https://doi.org/10.3390/cryst9020070
An H, Choi I, Kim IW. Melting Diagrams of Adefovir Dipivoxil and Dicarboxylic Acids: An Approach to Assess Cocrystal Compositions. Crystals. 2019; 9(2):70. https://doi.org/10.3390/cryst9020070
Chicago/Turabian StyleAn, Hyunseon, Insil Choi, and Il Won Kim. 2019. "Melting Diagrams of Adefovir Dipivoxil and Dicarboxylic Acids: An Approach to Assess Cocrystal Compositions" Crystals 9, no. 2: 70. https://doi.org/10.3390/cryst9020070
APA StyleAn, H., Choi, I., & Kim, I. W. (2019). Melting Diagrams of Adefovir Dipivoxil and Dicarboxylic Acids: An Approach to Assess Cocrystal Compositions. Crystals, 9(2), 70. https://doi.org/10.3390/cryst9020070




