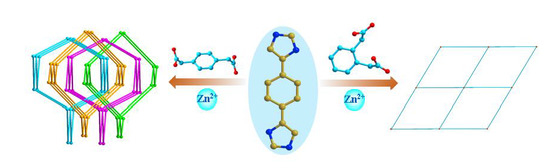
Coordination Assemblies of Zn(II) Coordination Polymers: Positional Isomeric Effect and Optical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Physical Techniques
2.2. Synthesis of [Zn(L)(pphda)] (1)
2.3. Synthesis of [Zn(L)(ophda)]·H2O (2)
2.4. Crystallographic Data Collection and Refinements
3. Results
3.1. Structural Descriptions
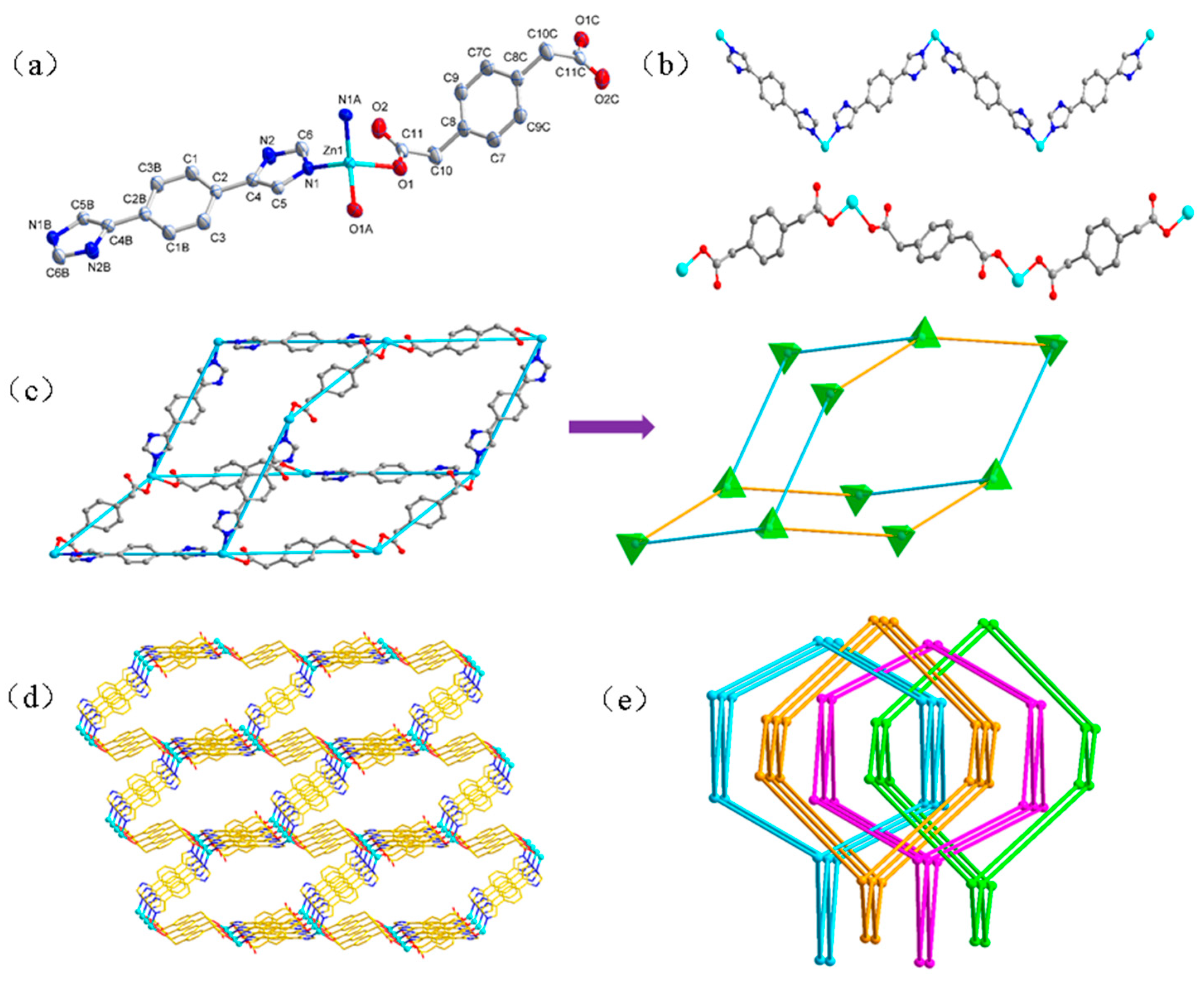
3.1.1. Structure of [Zn(L)(pphda)] (1)
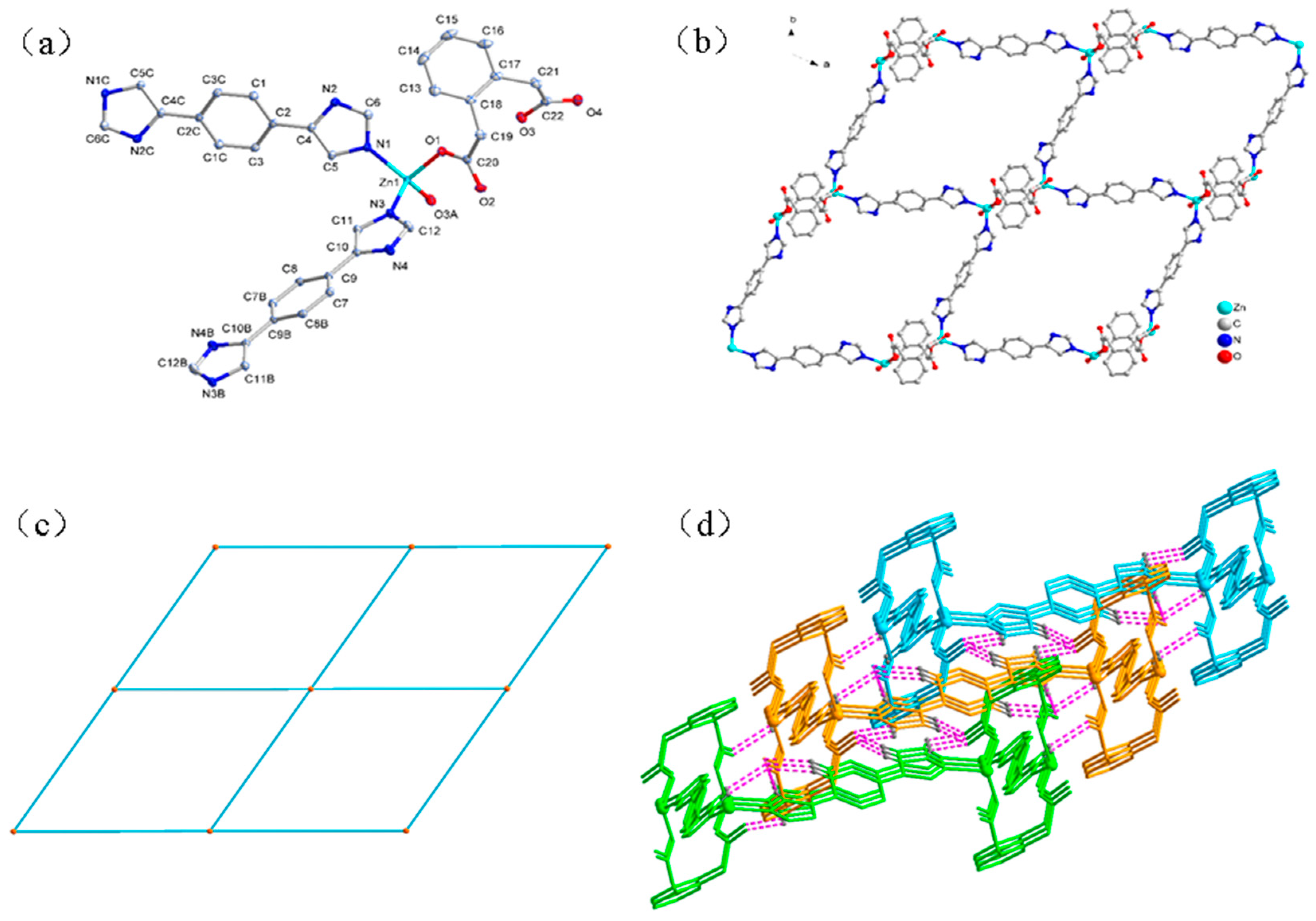
3.1.2. Structure of [Zn(L)(ophda)]·H2O (2)
3.2. Synthesis, Thermal Analyses and X-ray Powder Diffraction Analyses
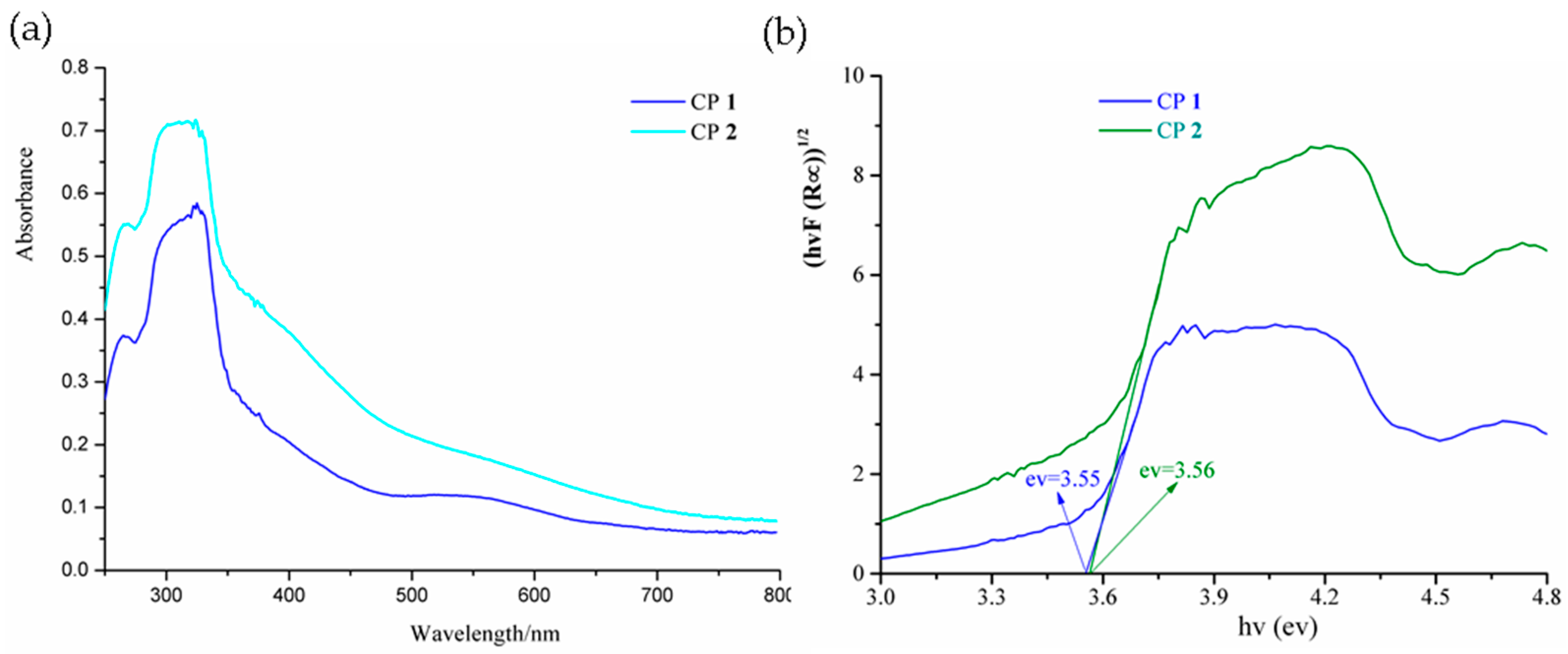
3.3. Diffuse Reflectance Spectra
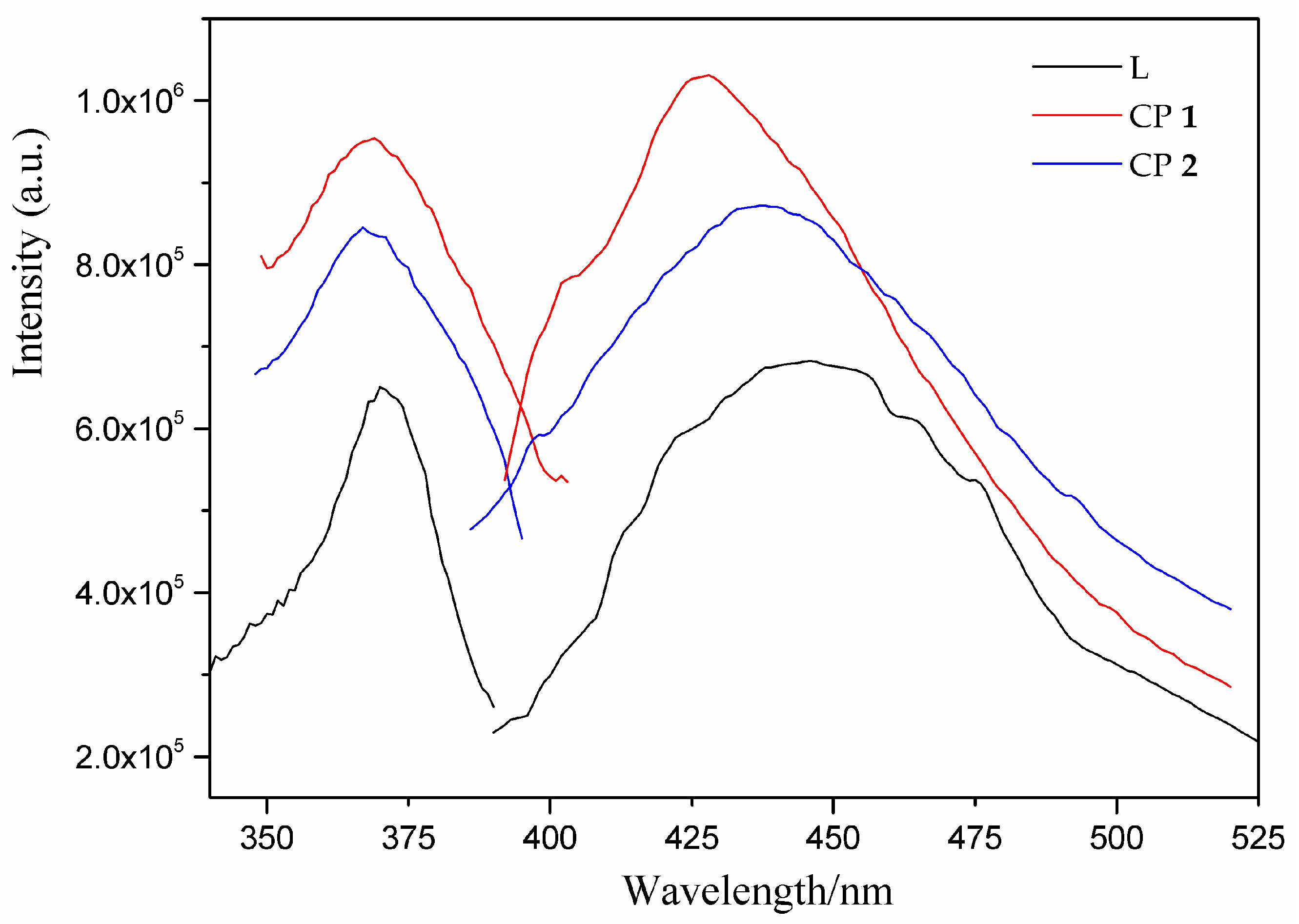
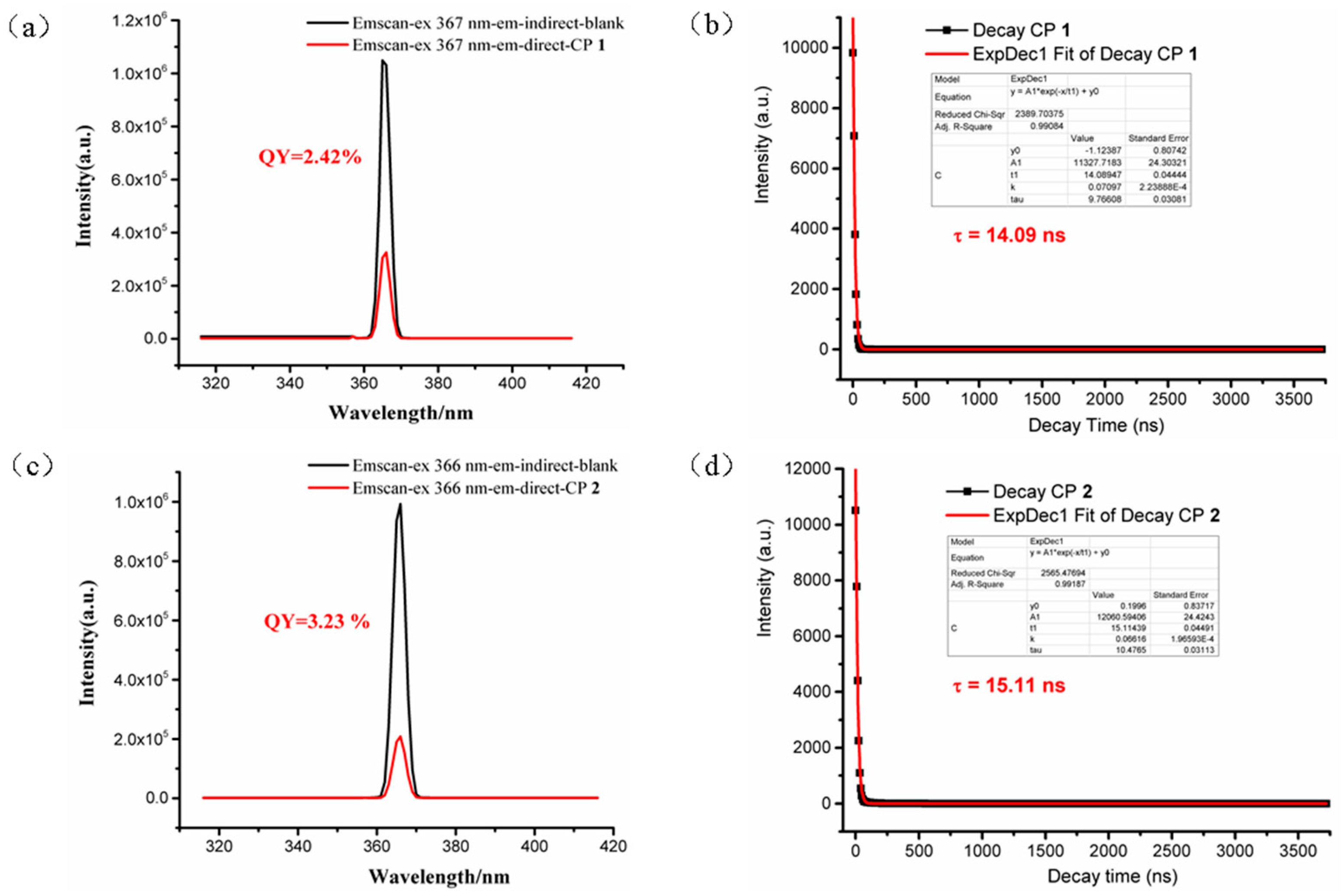
3.4. Photoluminescent Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, Y.S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.Y. Metal-organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.Q.; Jiang, H.L.; Sun, L.B. Metal-organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.C.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.G.; Ma, L.F.; Yan, D.P. Facile synthesis of 1D organic–inorganic perovskite micro-belts with high water stability for sensing and photonic applications. Chem. Sci. 2019, 10, 4567–4572. [Google Scholar] [CrossRef]
- Yang, X.G.; Zhai, Z.M.; Lu, X.M.; Zhao, Y.; Chang, X.H.; Ma, L.F. Room temperature phosphorescence of Mn(II) and Zn(II) coordination polymers for photoelectron response applications. Dalton Trans. 2019, 48, 10785–10789. [Google Scholar] [CrossRef]
- Guo, X.Z.; Li, J.L.; Shi, S.S.; Zhou, H.; Han, S.S.; Chen, S.S. Synthesis, structure and luminescent property of a Zn(II) complex with mixed multi-N donor and 2,5-dihydroxy-terephthalic acid ligands. Chin. J. Struct. Chem. 2018, 37, 1117–1124. [Google Scholar]
- Jin, J.C.; Wu, X.R.; Luo, Z.D.; Deng, F.Y.; Liu, J.Q.; Singh, A.; Kumar, A. Luminescent sensing and photocatalytic degradation properties of an uncommon (4,5,5)- connected 3D MOF based on 3,5-di(3′,5′-dicarboxylphenyl)benzoic acid. CrystEngComm 2017, 19, 4368–4377. [Google Scholar] [CrossRef]
- Han, M.L.; Chang, X.H.; Feng, X.; Ma, L.F.; Wang, L.Y. Temperature and pH driven self-assembly of Zn(II) coordination polymers: Crystal structures, supramolecular isomerism, and photoluminescence. CrystEngComm 2014, 16, 1687–1695. [Google Scholar] [CrossRef]
- Vleet, M.J.V.; Weng, T.; Li, X.; Schmidt, J.R. In situ, time-resolved, and mechanistic studies of metal-organic framework nucleation and growth. Chem. Rev. 2018, 118, 3681–3721. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.G.; Lu, X.M.; Yang, C.D.; Fan, N.N.; Yang, Z.T.; Wang, L.Y.; Ma, L.F. {Zn6} cluster based metal–organic framework with enhanced room-temperature phosphorescence and optoelectronic performances. Inorg. Chem. 2019, 58, 6215–6221. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, M.L.; Fu, H.R.; Ma, L.F.; Luo, F.; Li, D.S. Engineering design toward exploring the functional group substitution in 1D channels of Zn-organic frameworks upon nitro explosives and antibiotics detection. Dalton Trans. 2018, 47, 5359–5365. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhou, Y.L.; Zeng, M.H.; Kurmoo, M. The concept of mixed organic ligands in metal-organic frameworks: Design, tuning and functions. Dalton Trans. 2015, 44, 5258–5275. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.F.; Wang, L.Y.; Hu, J.L.; Wang, Y.Y. Syntheses, structures, and photoluminescence of a series of d10 coordination polymers with R-Isophthalate (R = −OH, −CH3, and −C(CH3)3). Cryst. Growth Des. 2009, 9, 5334–5342. [Google Scholar] [CrossRef]
- Hua, J.A.; Zhao, Y.; Liu, Q.; Zhao, D.; Chen, K.; Sun, W.Y. Zinc (II) coordination polymers with substituted benzenedicarboxylate and tripodal imidazole ligands: Syntheses, structures and properties. CrystEngComm 2014, 16, 7536–7546. [Google Scholar] [CrossRef]
- Du, M.; Li, C.P.; Liu, C.S.; Fang, S.M. Design and construction of coordination polymers with mixed-ligand synthetic strategy. Coord. Chem. Rev. 2013, 257, 1282–1305. [Google Scholar] [CrossRef]
- Yang, G.; Hou, L.; Ma, J.; Wang, Y.Y. Investigation on the prime factors influencing the formation of entangled metal–organic frameworks. CrystEngComm 2013, 15, 2561–2578. [Google Scholar] [CrossRef]
- Halder, A.; Bhattacharya, B.; Haque, F.; Ghoshal, D. Structural diversity in six mixed ligand Zn(II) metal−organic frameworks constructed by rigid and flexible dicarboxylates and different N,N′-donor ligands. Cryst. Growth Des. 2017, 17, 6613–6624. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.F.; Hou, L.; Wang, J.P.; Wang, Y.Y.; Zhu, Z.H. Structural diversity of cadmium(II) coordination polymers induced by tuning the coordination sites of isomeric ligands. Inorg. Chem. 2016, 55, 8871–8880. [Google Scholar] [CrossRef]
- Singh, R.; Bharadwaj, P.K. Coordination polymers built with a linear bis-imidazole and different dicarboxylates: Unusual entanglement and emission properties. Cryst. Growth Des. 2013, 13, 3722–3733. [Google Scholar] [CrossRef]
- Wang, S.; Yun, R.; Peng, Y.; Zhang, Q.; Lu, J.; Dou, J.; Bai, J.; Li, D.; Wang, D. A series of four-connected entangled metal_organic frameworks assembled from pamoic acid and pyridine-containing ligands: Interpenetrating, self-Penetrating, and supramolecular isomerism. Cryst. Growth Des. 2012, 12, 79–92. [Google Scholar] [CrossRef]
- Wang, S.L.; Hu, F.L.; Zhou, J.Y.; Zhou, Y.; Huang, Q.; Lang, J.P. Rigidity versus flexibility of ligands in the assembly of entangled coordination polymers based on Bi- and tetra carboxylates and N-donor ligands. Cryst. Growth Des. 2015, 15, 4087–4097. [Google Scholar] [CrossRef]
- Han, M.L.; Duan, Y.P.; Li, D.S.; Wang, H.B.; Zhao, J.; Wang, Y.Y. Positional isomeric tunable two Co(II) 6-connected 3-D frameworks with pentanuclear to binuclear units: Structures, ion-exchange and magnetic properties. Dalton Trans. 2014, 43, 15450–15456. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Chen, M.S.; Su, Z.; Sun, W.Y. Temperature dependent selective gas sorption of the microporous metal-imidazolate framework [Cu(L)] [H2L=1,4-di(1H-imidazol-4-yl)benzene]. Chem. Commun. 2011, 47, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Sheng, L.Q.; Zhao, Y.; Liu, Z.D.; Qiao, R.; Yang, S. Syntheses, structures, and properties of a series of polyazaheteroaromatic core-based Zn(II) coordination polymers together with carboxylate auxiliary ligands. Cryst. Growth Des. 2016, 16, 229–241. [Google Scholar] [CrossRef]
- Li, W.D.; Li, J.L.; Guo, X.Z.; Zhang, Z.Y.; Chen, S.S. Metal(II) coordination polymers derived from mixed 4-imidazole ligands and carboxylates: Syntheses, topological structures, and properties. Polymers 2018, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- SAINT, version 6.2; Bruker AXS, Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal structure fefinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Liu, T.; Lü, J.; Shi, L.; Guo, Z.; Cao, R. Conformation control of a flexible 1,4-phenylenediacetate ligand in coordination complexes: A rigidity-modulated strategy. CrystEngComm 2009, 11, 583–588. [Google Scholar] [CrossRef]
- Liu, L.; Huang, C.; Wang, Z.; Wu, D.; Hou, H.; Fan, Y. Entangled Zn(II)/Cd(II) coordination complexes based on a flexible bis(methylbenzimidazole) ligand and different dicarboxylates. CrystEngComm 2013, 15, 7095–7105. [Google Scholar] [CrossRef]
- Shi, Z.; Pan, Z.; Qin, L.; Zhou, J.; Zheng, H. Five new transition metal coordination polymers based on V-shaped bis-triazole ligand with aromatic dicarboxylates: Syntheses, structures, and properties. Cryst. Growth Des. 2017, 17, 2757–2766. [Google Scholar] [CrossRef]
- Yang, Y.J.; Wang, M.J.; Zhang, K.L. A novel photoluminescent Cd(ii)–organic framework exhibiting rapid and efficient multi-responsive fluorescence sensing for trace amounts of Fe3+ions and some NACs, especially for 4-nitroaniline and 2-methyl-4-nitroaniline. J. Mater. Chem. C 2016, 4, 11404–11418. [Google Scholar] [CrossRef]
- Han, M.L.; Wang, J.G.; Ma, L.F.; Guo, H.; Wang, L.Y. Construction of Cd(II) coordination polymers based on R-isophthalate (R = -CH3 or -OCH3) and flexible N-donor co-ligands: Syntheses, structures and photoluminescence. CrystEngComm 2012, 14, 2691–2701. [Google Scholar] [CrossRef]
- Su, J.; Yao, L.; Zhao, M.; Wang, H.; Zhang, Q.; Cheng, L.; Tian, Y. Structural induction effect of a zwitterion pyridiniumolate for metal–organic frameworks. Inorg. Chem. 2015, 54, 6169–6175. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhao, M.; Su, J.; Xu, S.; Hu, L.; Liu, H.; Zhang, Q.; Zhang, J.; Wu, J.; Tian, Y. Three coordination polymers based on a star-like geometry 4, 4’’, 4’’ -nitrilotribenzoic acid ligand and their framework dependent luminescent properties. J. Solid State Chem. 2018, 258, 328–334. [Google Scholar] [CrossRef]
- Sun, Y.X.; Sun, W.Y. Zinc(II)– and cadmium(II)–organic frameworks with 1-imidazole-containing and 1-imidazole-carboxylate ligands. CrystEngComm 2015, 17, 4045–4063. [Google Scholar] [CrossRef]
- Wang, C.C.; Ke, S.Y.; Cheng, C.W.; Wang, Y.W.; Chiu, H.S.; Ko, Y.C.; Sun, N.K.; Ho, M.L.; Chang, C.K.; Chuang, Y.C.; et al. Four mixed-ligand Zn(II) three-dimensional metal-organic frameworks: Synthesis, structural diversity, and photoluminescent property. Polymers 2017, 9, 644. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.F.; Okamura, T.-A.; Sun, W.Y.; Ueyama, N. Syntheses, structures, and photoluminescence properties of metal(II) halide complexes with pyridine-containing flexible tripodal ligands. Inorg. Chem. 2006, 45, 8523–8532. [Google Scholar] [CrossRef]
- Zhu, M.A.; Guo, X.Z.; Xiao, L.; Chen, S.S. A new Cd(II) coordination compound based on 4-(1,2,4-Triazol-4-yl)phenylacetic acid: Synthesis, structure and photoluminescence property. Chin. J. Struct. Chem. 2018, 37, 437–444. [Google Scholar]
- Cui, P.P.; Zhang, X.D.; Wang, P.; Zhao, Y.; Azam, M.; Al-Resayes, S.I.; Sun, W.Y. Zinc(II) and copper(II) hybrid frameworks via metal-ion metathesis with enhanced gas uptake and photoluminescence properties. Inorg. Chem. 2017, 56, 14157–14163. [Google Scholar] [CrossRef]
- Blatov, V.A. TOPOS, A Multipurpose Crystallochemical Analysis with the Program Package; Samara State University: Samara, Russia, 2009. [Google Scholar]
- Chen, S.S.; Li, J.L.; Li, W.D.; Guo, X.Z.; Zhao, Y. Four new transition metal coordination polymers based on mixed 4-imidazole and carboxylate–sulfonate ligands: Syntheses, structures, and properties. J. Solid State Chem. 2019, 277, 510–518. [Google Scholar] [CrossRef]
- Li, Y.W.; Ma, H.; Chen, Y.Q.; He, K.H.; Li, Z.X.; Bu, X.H. Structure modulation in Zn(II)–1,4-bis(imidazol-1-yl)benzene frameworks by varying dicarboxylate anions. Cryst. Growth Des. 2012, 12, 189–196. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, Y.J.; Lee, J.W.; Shin, W.H.; Kang, J.K. Tunability of electronic band gaps from semiconducting to metallic states via tailoring Zn ions in MOFs with Co ions. Phys. Chem. Chem. Phys. 2009, 11, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Feng, G.; Song, Z.; Zhou, Y.P.; Chao, H.Y.; Yuan, D.; Tan, T.T.; Guo, Z.; Hu, Z.; Tang, B.Z.; et al. Two-dimensional metal-organic framework with wide channels and responsive turn-on fluorescence for the chemical sensing of volatile organic compounds. J. Am. Chem. Soc. 2014, 136, 7241–7244. [Google Scholar] [CrossRef] [PubMed]
| Crystal Parameter | 1 | 2 |
|---|---|---|
| Empirical formula | C22H18N4O4Zn | C22H20N4O5Zn |
| Formula weight | 467.77 | 485.81 |
| Temperature / K | 296(2) | 296(2) |
| Crystal system | Orthorhombic | Triclinic |
| Space group | P nna | P-1 |
| a /Å | 12.3264(11) | 10.6434(11) |
| b /Å | 21.1865(17) | 10.7143(11) |
| c /Å | 7.7490(6) | 11.7601(12) |
| α /° | 109.8560(10) | |
| β /° | 102.1290(10) | |
| γ /° | 110.4050(10) | |
| Mu (mm−1) | 1.252 | 1.161 |
| V (Å3) | 2023.7(3) | 1097.0(2) |
| Z, Dcalc / (Mg/m3) | 4, 1.535 | 2, 1.471 |
| F(000) | 960 | 500 |
| θ range / ° | 1.92–27.54 | 1.98–26.00 |
| Reflections collected | 113,01 | 8830 |
| Independent reflections | 2324 | 4685 |
| Goodness-of-fit on F2 | 1.100 | 1.056 |
| Rint | 0.0145 | 0.0147 |
| R1 [I > 2σ (I)]a | 0.0324 | 0.0260 |
| wR2 [I > 2σ (I)]b | 0.0907 | 0.0752 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-J.; Zhang, T.-T.; Li, W.-D.; Wang, Y.-Y.; Chen, S.-S. Coordination Assemblies of Zn(II) Coordination Polymers: Positional Isomeric Effect and Optical Properties. Crystals 2019, 9, 664. https://doi.org/10.3390/cryst9120664
Liu C-J, Zhang T-T, Li W-D, Wang Y-Y, Chen S-S. Coordination Assemblies of Zn(II) Coordination Polymers: Positional Isomeric Effect and Optical Properties. Crystals. 2019; 9(12):664. https://doi.org/10.3390/cryst9120664
Chicago/Turabian StyleLiu, Chang-Jie, Tong-Tong Zhang, Wei-Dong Li, Yuan-Yuan Wang, and Shui-Sheng Chen. 2019. "Coordination Assemblies of Zn(II) Coordination Polymers: Positional Isomeric Effect and Optical Properties" Crystals 9, no. 12: 664. https://doi.org/10.3390/cryst9120664
APA StyleLiu, C.-J., Zhang, T.-T., Li, W.-D., Wang, Y.-Y., & Chen, S.-S. (2019). Coordination Assemblies of Zn(II) Coordination Polymers: Positional Isomeric Effect and Optical Properties. Crystals, 9(12), 664. https://doi.org/10.3390/cryst9120664