Effect of Ag/rGO on the Optical Properties of Plasmon-Modified SnO2 Composite and Its Application in Self-Powered UV Photodetector
Abstract
1. Introduction
2. Experimentals
2.1. Materials
2.2. Synthesis of Graphene Oxide
2.3. Synthesis of Ag/rGO Hybrid Composite
2.4. Fabrication of SnO2 Nanorods and Incorporation of Ag/rGO into SnO2 Nanorods
2.5. Device Fabrication
2.6. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ben Elkamel, I.; Hamdaoui, N.; Mezni, A.; Ajjel, R.; Beji, L. Synthesis and characterization of Cu doped ZnO nanoparticles for stable and fast response UV photodetector at low noise current. J. Mater. Sci. Mater. Electron. 2019, 30, 9444–9454. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, P.; Bai, Z. Self-powered UV–visible photodetectors based on ZnO/graphene/CdS/electrolyte heterojunctions. J. Alloys Compd. 2019, 776, 346–352. [Google Scholar] [CrossRef]
- Fang, H.; Zheng, C.; Wu, L.; Li, Y.; Cai, J.; Hu, M.; Fang, X.; Ma, R.; Wang, Q.; Wang, H. Solution-Processed Self-Powered Transparent Ultraviolet Photodetectors with Ultrafast Response Speed for High-Performance Communication System. Adv. Funct. Mater. 2019, 29, 1809013. [Google Scholar] [CrossRef]
- Singh, M.K.; Pandey, R.K.; Prakash, R. High-performance photo detector based on hydrothermally grown SnO2 nanowire/reduced graphene oxide (rGO) hybrid material. Org. Electron. 2017, 50, 359–366. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Z.; Lu, Z.; Li, G. High responsivity and low dark current nonpolar GaN-based ultraviolet photo-detectors. J. Mater. Chem. C 2018, 6, 6641–6646. [Google Scholar] [CrossRef]
- Sarkar, K.; Hossain, M.; Devi, P.; Rao, K.D.M.; Kumar, P. Self-Powered and Broadband Photodetectors with GaN: Layered rGO Hybrid Heterojunction. Adv. Mater. Interfaces 2019, 6, 1900923. [Google Scholar] [CrossRef]
- Shelke, N.T.; Karche, B.R. Hydrothermal synthesis of WS2/RGO sheet and their application in UV photodetector. J. Alloys Compd. 2015, 653, 298–303. [Google Scholar] [CrossRef]
- Huang, Z.; Han, W.; Tang, H.; Ren, L.; Chander, D.S.; Qi, X.; Zhang, H. Photoelectrochemical-type sunlight photodetector based on MoS2/graphene heterostructure. 2D Mater. 2015, 2, 035011. [Google Scholar] [CrossRef]
- Choi, H.; Seo, S.; Lee, J.H.; Hong, S.H.; Song, J.; Kim, S.; Yim, S.Y.; Lee, K.; Park, S.J.; Lee, S. Solution-processed ZnO/SnO2 bilayer ultraviolet phototransistor with high responsivity and fast photoresponse. J. Mater. Chem. C 2018, 6, 6014–6022. [Google Scholar] [CrossRef]
- Paria, D.; Jeong, H.H.; Vadakkumbatt, V.; Deshpande, P.; Fischer, P.; Ghosh, A.; Ghosh, A. Graphene-silver hybrid devices for sensitive photodetection in the ultraviolet. Nanoscale 2018, 10, 7685–7693. [Google Scholar] [CrossRef]
- Deka Boruah, B. Zinc oxide ultraviolet photodetectors: Rapid progress from conventional to self-powered photodetectors. Nanoscale Adv. 2019, 1, 2059–2085. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Y.; Chen, L.; Li, L. Self-Powered Nanoscale Photodetectors. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Gao, C.T.; Duan, H.G.; Lu, B.G.; Pan, X.J.; Xie, E.Q. Nanocrystalline TiO2 film based photoelectrochemical cell as self-powered UV-photodetector. Nano Energy 2012, 1, 640–645. [Google Scholar] [CrossRef]
- Lin, C.H.; Fu, H.C.; Lien, D.H.; Hsu, C.Y.; He, J.H. Self-powered nanodevices for fast UV detection and energy harvesting using core-shell nanowire geometry. Nano Energy 2018, 51, 294–299. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, J.; Liu, F.; Zhang, Y. Enhanced photoresponse performance of self-powered UV–visible photodetectors based on ZnO/Cu2O/electrolyte heterojunctions via graphene incorporation. J. Alloys Compd. 2017, 726, 803–809. [Google Scholar] [CrossRef]
- Abdalla, J.T.; Huang, Y.W.; Yu, Q.J.; Wang, J.Z.; Wang, J.N.; Yu, C.L.; Gao, S.Y.; Jiao, S.J.; Wang, D.B.; Alarabi, A.M.; et al. TiCl4 surface-treated SnO2 photoanodes for self-powered UV photodetectors and dye-sensitized solar cells. Mater. Technol. 2017, 32, 443–450. [Google Scholar] [CrossRef]
- Amany, A.; Wang, D.B.; Wang, J.Z.; Zeng, Z.; Jiao, S.J.; Hao, C.L.; Zhao, Y.C.; Xie, Y.; Gao, S.Y.; Ni, S.M.; et al. Enhanced the UV response of AlN coated ZnO nanorods photodetector. J. Alloys Compd. 2019, 776, 111–115. [Google Scholar] [CrossRef]
- Ni, S.M.; Guo, F.Y.; Wang, D.B.; Liu, G.; Xu, Z.K.; Kong, L.P.; Wang, J.Z.; Jiao, S.J.; Zhang, Y.; Yu, Q.J.; et al. Effect of MgO Surface Modification on the TiO2 Nanowires Electrode for Self-Powered UV Photodetectors. ACS Sustain. Chem. Eng. 2018, 6, 7265–7272. [Google Scholar] [CrossRef]
- Kar, A.; Kundu, S.; Patra, A. Surface Defect-Related Luminescence Properties of SnO2 Nanorods and Nanoparticles. J. Phys. Chem. C 2011, 115, 118–124. [Google Scholar] [CrossRef]
- Katoh, R.; Furube, A.; Yoshihara, T.; Hara, K.; Fujihashi, G.; Takano, S.; Murata, S.; Arakawa, H.; Tachiya, M. Efficiencies of electron injection from excited N3 dye into nanocrystalline semiconductor (ZrO2, TiO2, ZnO, Nb2O5, SnO2, In2O3) films. J. Phys. Chem. B 2004, 108, 4818–4822. [Google Scholar] [CrossRef]
- Gao, C.; Li, X.; Wang, Y.; Chen, L.; Pan, X.; Zhang, Z.; Xie, E. Titanium dioxide coated zinc oxide nanostrawberry aggregates for dye-sensitized solar cell and self-powered UV-photodetector. J. Power Sources 2013, 239, 458–465. [Google Scholar] [CrossRef]
- Gao, C.T.; Li, X.D.; Zhu, X.P.; Chen, L.L.; Wang, Y.Q.; Teng, F.; Zhang, Z.X.; Duan, H.G.; Xie, E.Q. High performance, self-powered UV-photodetector based on ultrathin, transparent, SnO2-TiO2 core-shell electrodes. J. Alloys Compd. 2014, 616, 510–515. [Google Scholar] [CrossRef]
- Wang, Z.R.; Ran, S.H.; Liu, B.; Chen, D.; Shen, G.Z. Multilayer TiO2 nanorod cloth/nanorod array electrode for dye-sensitized solar cells and self-powered UV detectors. Nanoscale 2012, 4, 3350–3358. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wei, L.; Li, Q.; Chen, Y.; Yan, S.; Jiao, J.; Liu, G.; Mei, L. High-performance self-powered UV photodetectors based on TiO2 nano-branched arrays. Nanotechnology 2014, 25, 075202. [Google Scholar] [CrossRef]
- Xie, Y.R.; Wei, L.; Li, Q.H.; Chen, Y.X.; Liu, H.; Yan, S.S.; Jiao, J.; Liu, G.L.; Mei, L.M. A high performance quasi-solid-state self-powered UV photodetector based on TiO2 nanorod arrays. Nanoscale 2014, 6, 9116–9121. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Jose, R.; Archana, P.S.; Chellappan, V.; Ramakrishna, S. Charge Transport through Electrospun SnO2 Nanoflowers and Nanofibers: Role of Surface Trap Density on Electron Transport Dynamics. J. Phys. Chem. C 2012, 116, 22112–22120. [Google Scholar] [CrossRef]
- Zamand, N.; Pour, A.N.; Housaindokht, M.R.; Izadyar, M. Size-controlled synthesis of SnO2 nanoparticles using reverse microemulsion method. Solid State Sci. 2014, 33, 6–11. [Google Scholar] [CrossRef]
- Vasquez, F.C.; Paraguay-Delgado, F.; Morales-Mendoza, J.E.; Antunez-Flores, W.; Lardizabal, D.; Alonso-Nunez, G.; Berhault, G. Shape and size controlled growth of SnO2 nano-particles by efficient approach. Superlattices Microstruct. 2016, 90, 274–287. [Google Scholar] [CrossRef]
- Sephra, P.J.; Baraneedharan, P.; Sivakumar, M.; Thangadurai, T.D.; Nehru, K. Size controlled synthesis of SnO2 and its electrostatic self-assembly over reduced graphene oxide for photocatalyst and supercapacitor application. Mater. Res. Bull. 2018, 106, 103–112. [Google Scholar] [CrossRef]
- Lee, J.W.; Nam, S.H.; Yu, J.N.; Kim, D.I.; Jeong, R.H.; Boo, J.H. Shape Control of Zn2SnO4/SnO2 Composites and Changes in Photocatalytic Efficiency. Sci. Adv. Mater. 2018, 10, 1045–1050. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Yue, J.; Moriceau, J.; Chen, C.Y.; Liu, M.S.; Yuan, Y.; Jiang, X.C.; Yu, A.B. Experimental and theoretical studies on noble metal decorated tin oxide flower-like nanorods with high ethanol sensing performance. Sens. Actuators B Chem. 2015, 219, 83–93. [Google Scholar] [CrossRef]
- Chew, C.K.T.; Salcianu, C.; Bishop, P.; Carmalt, C.J.; Parkin, I.P. Functional thin film coatings incorporating gold nanoparticles in a transparent conducting fluorine doped tin oxide matrix. J. Mater. Chem. C 2015, 3, 1118–1125. [Google Scholar] [CrossRef]
- Sevastyanov, E.Y.; Maksimova, N.K.; Novikov, V.A.; Rudov, F.V.; Sergeychenko, N.V.; Chernikov, E.V. Effect of Pt, Pd, Au additives on the surface and in the bulk of tin dioxide thin films on the electrical and gas-sensitive properties. Semiconductors 2012, 46, 801–809. [Google Scholar] [CrossRef]
- Hung-Low, F.; Ramirez, D.A.; Peterson, G.R.; Hikal, W.M.; Hope-Weeks, L.J. Development of a carbon-supported Sn-SnO2 photocatalyst by a new hybridized sol-gel/dextran approach. RSC Adv. 2016, 6, 21019–21025. [Google Scholar] [CrossRef]
- Rakibuddin, M.; Ananthakrishnan, R. Fabrication of graphene aerosol hybridized coordination polymer derived CdO/SnO2 heteronanostructure with improved visible light photocatalytic performance. Sol. Energy Mater. Sol. Cells 2017, 162, 62–71. [Google Scholar] [CrossRef]
- Zeng, Y.; Pan, X.; Dai, W.; Chen, Y.; Ye, Z. The enhancement of a self-powered UV photodetector based on vertically aligned Ag-modified ZnO nanowires. RSC Adv. 2015, 5, 66738–66741. [Google Scholar] [CrossRef]
- N’Konou, K.; Chalh, M.; Monnier, V.; Blanchard, N.P.; Chevolot, Y.; Lucas, B.; Vedraine, S.; Torchio, P. Impact of Ag@SiO2 core-shell nanoparticles on the photoelectric current of plasmonic inverted organic solar cells. Synth. Met. 2018, 239, 22–28. [Google Scholar] [CrossRef]
- Zhou, M.J.; Li, J.Z.; Ye, Z.F.; Ma, C.C.; Wang, H.Q.; Huo, P.W.; Shi, W.D.; Yan, Y.S. Transfer Charge and Energy of Ag@CdSe QDs-rGO Core-Shell Plasmonic Photocatalyst for Enhanced Visible Light Photocatalytic Activity. ACS Appl. Mater. Interface 2015, 7, 28231–28243. [Google Scholar] [CrossRef]
- An, X.; Teng, F.; Zhang, Z.; Pan, X.; Zhou, J.; Xie, E. Ultrafast UV switch based on ZnO-Ag heterostructures. Electron. Mater. Lett. 2014, 10, 95–99. [Google Scholar] [CrossRef]
- Dong, H.; Wu, Z.X.; Lu, F.; Gao, Y.C.; El-Shafei, A.; Jiao, B.; Ning, S.Y.; Hou, X. Optics-electrics highways: Plasmonic silver nanowires@TiO2 core-shell nanocomposites for enhanced dye-sensitized solar cells performance. Nano Energy 2014, 10, 181–191. [Google Scholar] [CrossRef]
- Pu, Y.C.; Wang, G.M.; Chang, K.D.; Ling, Y.C.; Lin, Y.K.; Fitzmorris, B.C.; Liu, C.M.; Lu, X.H.; Tong, Y.X.; Zhang, J.Z.; et al. Au Nanostructure-Decorated TiO2 Nanowires Exhibiting Photoactivity Across Entire UV-Visible Region for Photoelectrochemical Water Splitting. Nano Lett 2013, 13, 3817–3823. [Google Scholar] [CrossRef]
- Teng, F.; Zhang, G.Z.; Wang, Y.Q.; Gao, C.T.; Chen, L.L.; Zhang, P.; Zhang, Z.X.; Xie, E.Q. The role of carbon in the photocatalytic reaction of carbon/TiO2 photocatalysts. Appl. Surf. Sci. 2014, 320, 703–709. [Google Scholar] [CrossRef]
- Williams, G.; Kamat, P.V. Graphene-Semiconductor Nanocomposites: Excited-State Interactions between ZnO Nanoparticles and Graphene Oxide. Langmuir 2009, 25, 13869–13873. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Jiang, L.-Y.; Yan, H.-J.; Wang, W.D.; Wang, W.; Song, W.-G.; Guo, Y.-G.; Wan, L.-J. Mono dispersed SnO2 nanoparticles on both sides of single layer graphene sheets as anode materials in Li-ion batteries. J. Mater. Chem. 2010, 20. [Google Scholar] [CrossRef]
- Tian, Y.; Cao, Y.-Y.; Pang, F.; Chen, G.-Q.; Zhang, X. Ag nanoparticles supported on N-doped graphene hybrids for catalytic reduction of 4-nitrophenol. RSC Adv. 2014, 4, 43204–43211. [Google Scholar] [CrossRef]
- Keshvari, A.; Darbari, S.; Taghavi, M. Self-Powered Plasmonic UV Detector, Based on Reduced Graphene Oxide/Ag Nanoparticles. IEEE Electron Device Lett. 2018, 39, 1433–1436. [Google Scholar] [CrossRef]
- Huang, Y.W.; Yu, Q.J.; Wang, J.Z.; Wang, J.A.; Yu, C.L.; Abdalla, J.T.; Zeng, Z.; Jiao, S.J.; Wang, D.B.; Gao, S.Y. Plasmon-Enhanced Self-Powered UV Photodetectors Assembled by Incorporating Ag@SiO2 Core-Shell Nanoparticles into TiO2 Nanocube Photoanodes. ACS Sustain. Chem. Eng. 2018, 6, 438–446. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, F.; Huang, J.X. Langmuir-Blodgett Assembly of Graphite Oxide Single Layers. J. Am. Chem. Soc. 2009, 131, 1043–1049. [Google Scholar] [CrossRef]
- Lim, B.C.; Singu, B.S.; Hong, S.E.; Na, Y.H.; Yoon, K.R. Synthesis and characterization nanocomposite of polyacrylamide-rGO-Ag-PEDOT/PSS hydrogels by photo polymerization method. Polym. Adv. Technol. 2016, 27, 366–373. [Google Scholar] [CrossRef]
- Kar, A.; Sain, S.; Kundu, S.; Bhattacharyya, A.; Pradhan, S.K.; Patra, A. Influence of Size and Shape on the Photocatalytic Properties of SnO2 Nanocrystals. Chemphyschem 2015, 16, 1017–1025. [Google Scholar] [CrossRef]
- Cojocaru, L.; Olivier, C.; Toupance, T.; Sellier, E.; Hirsch, L. Size and shape fine-tuning of SnO2 nanoparticles for highly efficient and stable dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 13789–13799. [Google Scholar] [CrossRef]
- Samantara, A.K.; Mishra, D.K.; Suryawanshi, S.R.; More, M.A.; Thapa, R.; Late, D.J.; Jena, B.K.; Rout, C.S. Facile synthesis of Ag nanowire–rGO composites and their promising field emission performance. RSC Adv. 2015, 5, 41887–41893. [Google Scholar] [CrossRef]
- Lee, S.C.; Some, S.; Kim, S.W.; Kim, S.J.; Seo, J.; Lee, J.; Lee, T.; Ahn, J.H.; Choi, H.J.; Jun, S.C. Efficient Direct Reduction of Graphene Oxide by Silicon Substrate. Sci. Rep. 2015, 5, 12306. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of Graphene’s Properties by Reversible Hydrogenation: Evidence for Graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef]
- Kim, Y.K.; Ok, G.; Choi, S.W.; Jang, H.; Min, D.H. The interfacing structural effect of Ag/graphene oxide nanohybrid films on surface enhanced Raman scattering. Nanoscale 2017, 9, 5872–5878. [Google Scholar] [CrossRef]
- Akbari, E.; Akbari, I.; Ebrahimi, M.R. sp2/sp3 bonding ratio dependence of the band-gap in graphene oxide. Eur. Phys. J. B 2019, 92, 71. [Google Scholar] [CrossRef]
- Xu, W.P.; Zhang, L.C.; Li, J.P.; Lu, Y.; Li, H.H.; Ma, Y.N.; Wang, W.D.; Yu, S.H. Facile synthesis of silver@graphene oxide nanocomposites and their enhanced antibacterial properties. J. Mater. Chem. 2011, 21, 4593–4597. [Google Scholar] [CrossRef]
- Hsu, K.C.; Chen, D.H. Green synthesis and synergistic catalytic effect ofAg/reduced graphene oxide nanocomposite. Nanoscale Res. Lett. 2014, 9, 484. [Google Scholar] [CrossRef]
- Beyene, H.T.; Tichelaar, F.D.; Peeters, P.; Kolev, I.; van de Sanden, M.C.M.; Creatore, M. Hybrid Sputtering-Remote PECVD Deposition of Au Nanoparticles on SiO2 Layers for Surface Plasmon Resonance-Based Colored Coatings. Plasma Process. Polym. 2010, 7, 657–664. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.-Y. Ag/Graphene Heterostructures: Synthesis, Characterization and Optical Properties. Eur. J. Inorg. Chem. 2010, 2010, 1244–1248. [Google Scholar] [CrossRef]
- Khadgi, N.; Li, Y.; Upreti, A.R.; Zhang, C.; Zhang, W.; Wang, Y.; Wang, D. Enhanced Photocatalytic Degradation of 17alpha-Ethinylestradiol Exhibited by Multifunctional ZnFe2O4 -Ag/rGO Nanocomposite Under Visible Light. Photochem. Photobiol. 2016, 92, 238–246. [Google Scholar] [CrossRef]
- Cao, X.; Gu, L.; Lan, X.; Zhao, C.; Yao, D.; Sheng, W. Spinel ZnFe2O4 nanoplates embedded with Ag clusters: Preparation, characterization, and photocatalytic application. Mater. Chem. Phys. 2007, 106, 175–180. [Google Scholar] [CrossRef]
- Xie, W.; Tang, L.; Ying, M.; Liu, J.; Pan, H.; Du, M. Ag–SnO2 nano-heterojunction–reduced graphene oxide by a stepwise photocatalyzed approach and its application in ractopamine determination. RSC Adv. 2017, 7, 54506–54511. [Google Scholar] [CrossRef]
- Lee, J.; Lee, A.; Yu, H.K. Graphene protected Ag nanowires: Blocking of surface migration for thermally stable and wide-range-wavelength transparent flexible electrodes. RSC Adv. 2016, 6, 84985–84989. [Google Scholar] [CrossRef]
- Kim, J.M.; Seo, S.W.; Shin, D.H.; Lee, H.S.; Kim, J.H.; Jang, C.W.; Kim, S.; Choi, S.H. Ag-nanowires-doped graphene/Si Schottky-junction solar cells encapsulated with another graphene layer. Curr. Appl. Phys. 2017, 17, 1136–1141. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Chen, L.L.; Wang, Y.Q.; He, Y.M.; Pan, X.J.; Xie, E.Q. An overview on emerging photoelectrochemical self-powered ultraviolet photodetectors. Nanoscale 2016, 8, 50–73. [Google Scholar] [CrossRef]
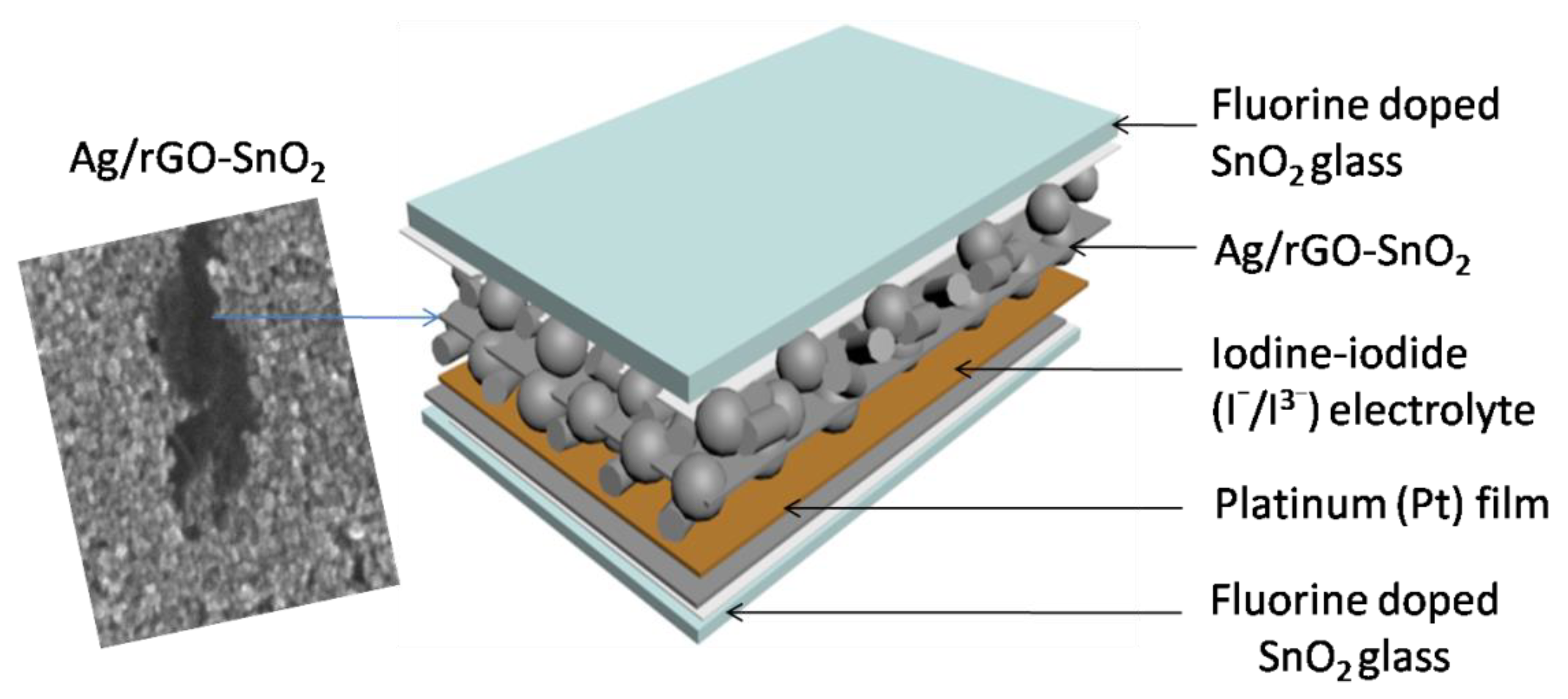

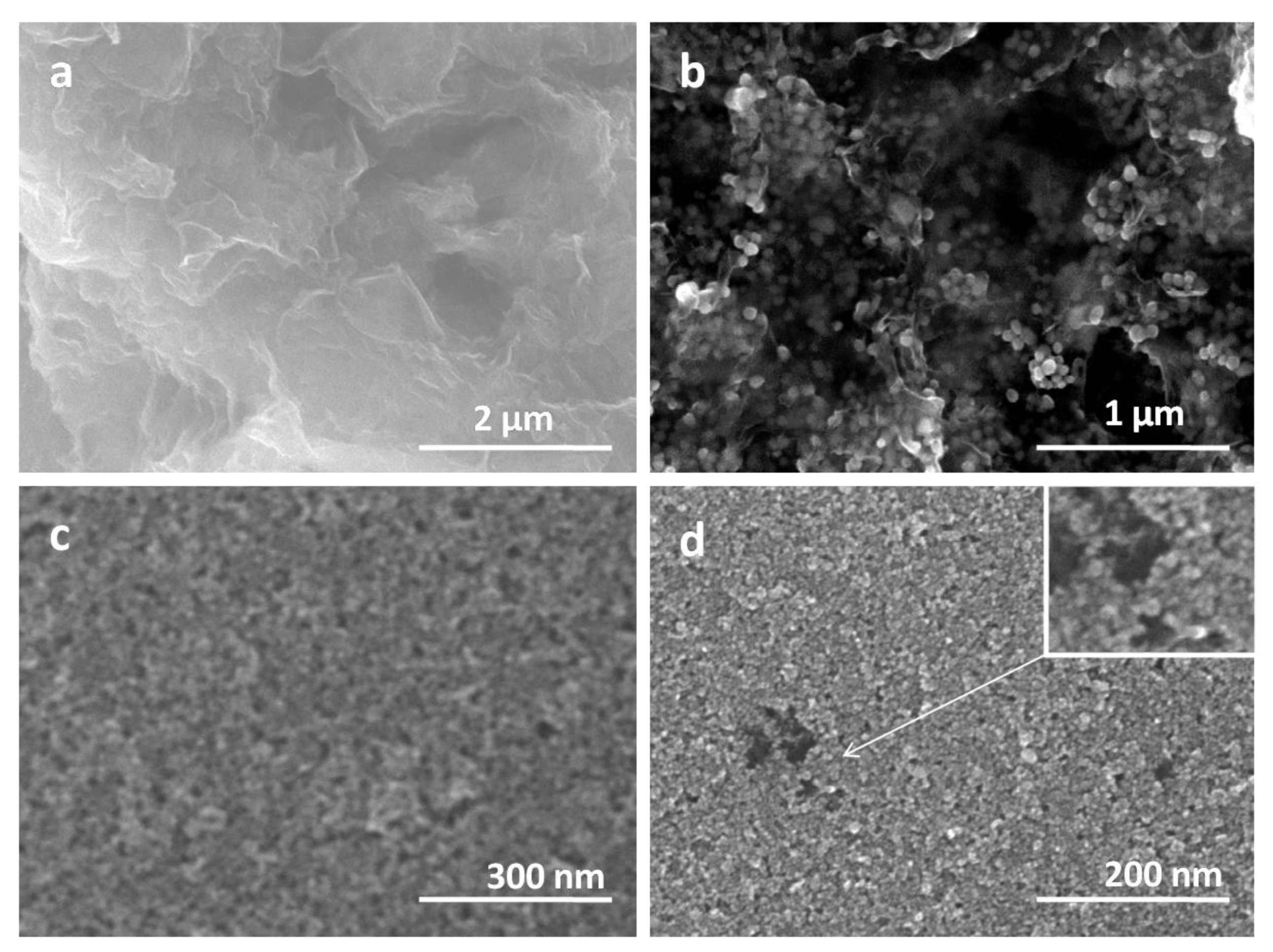
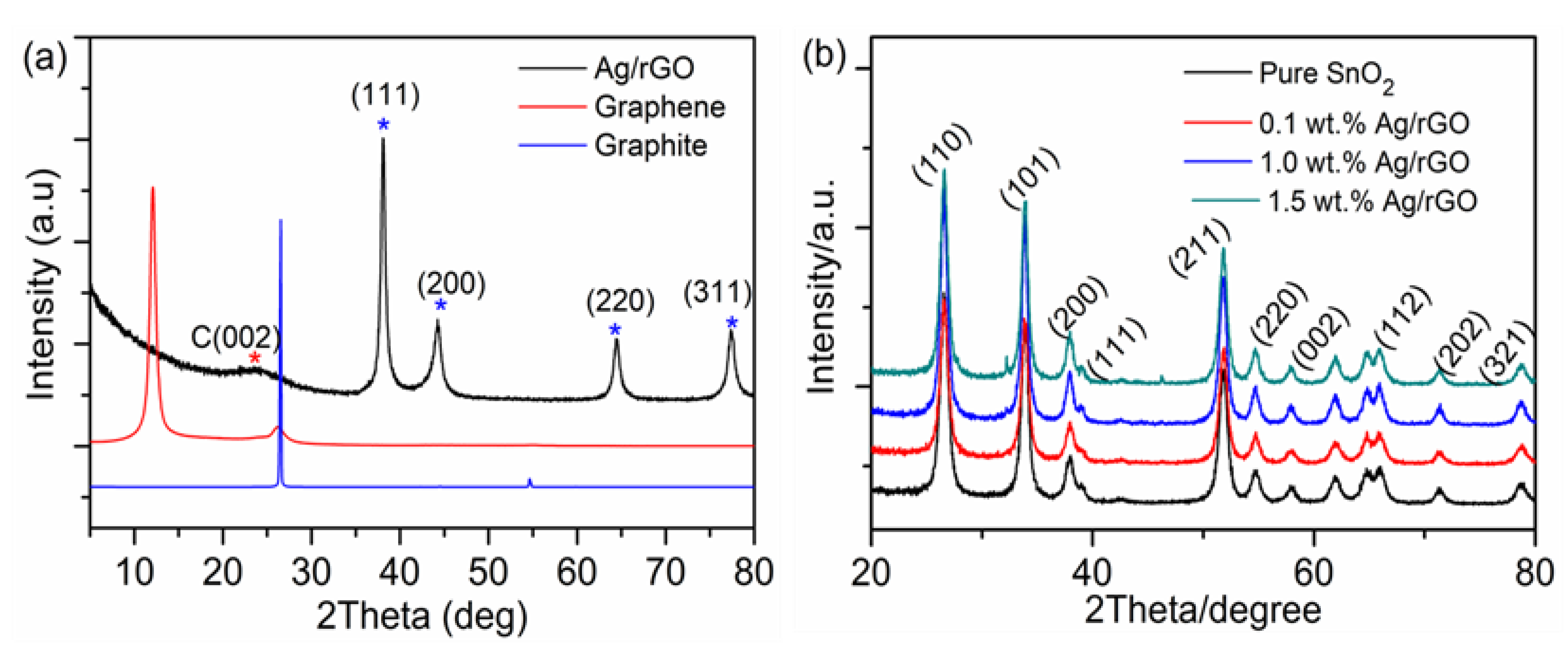
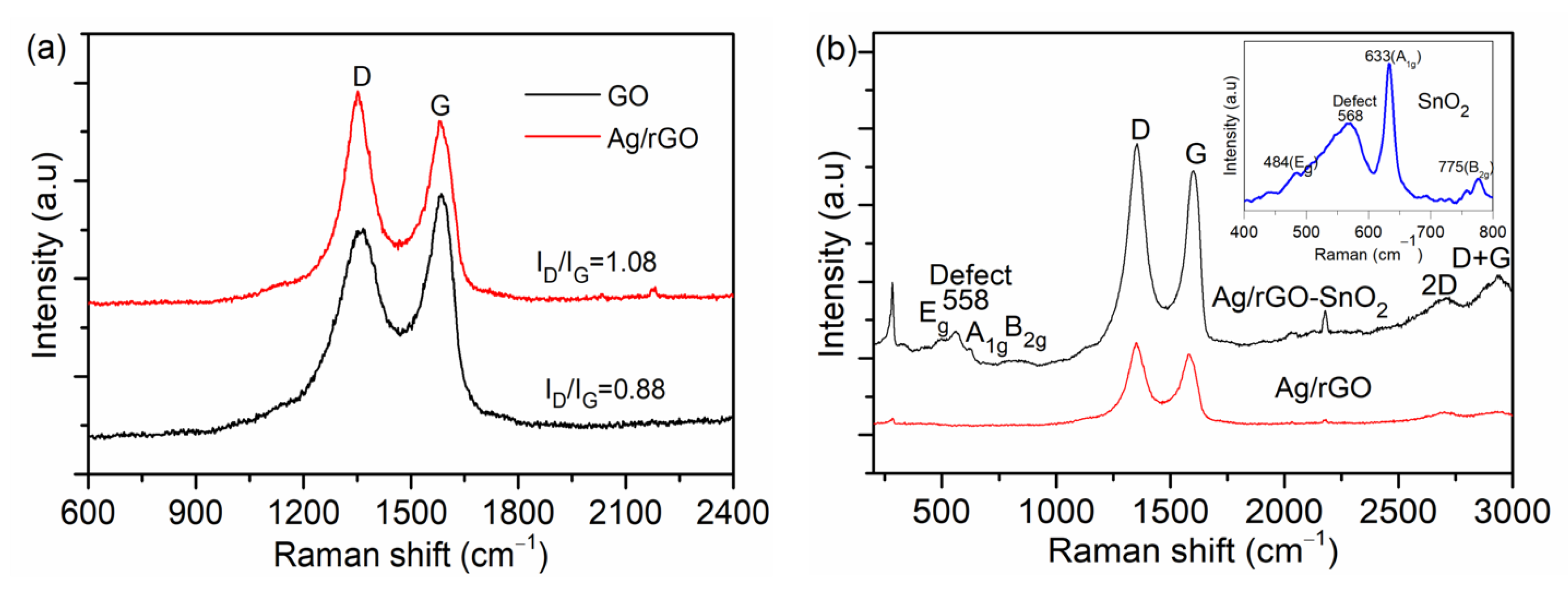
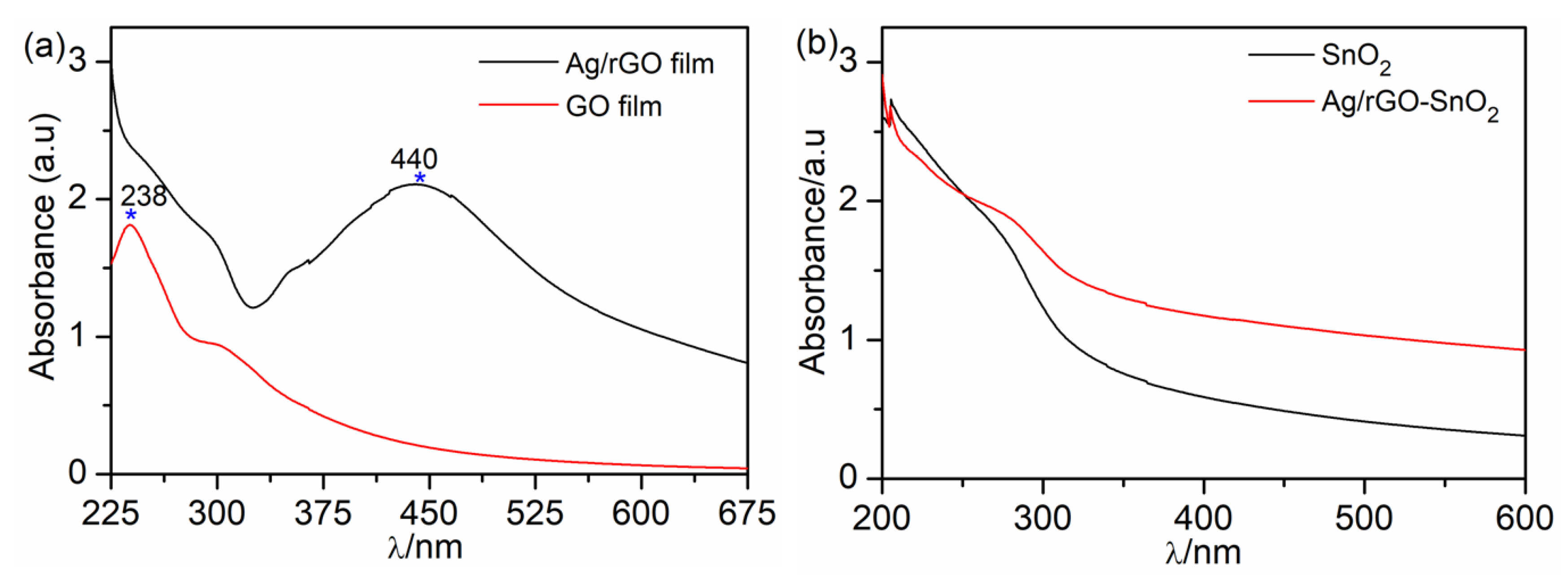
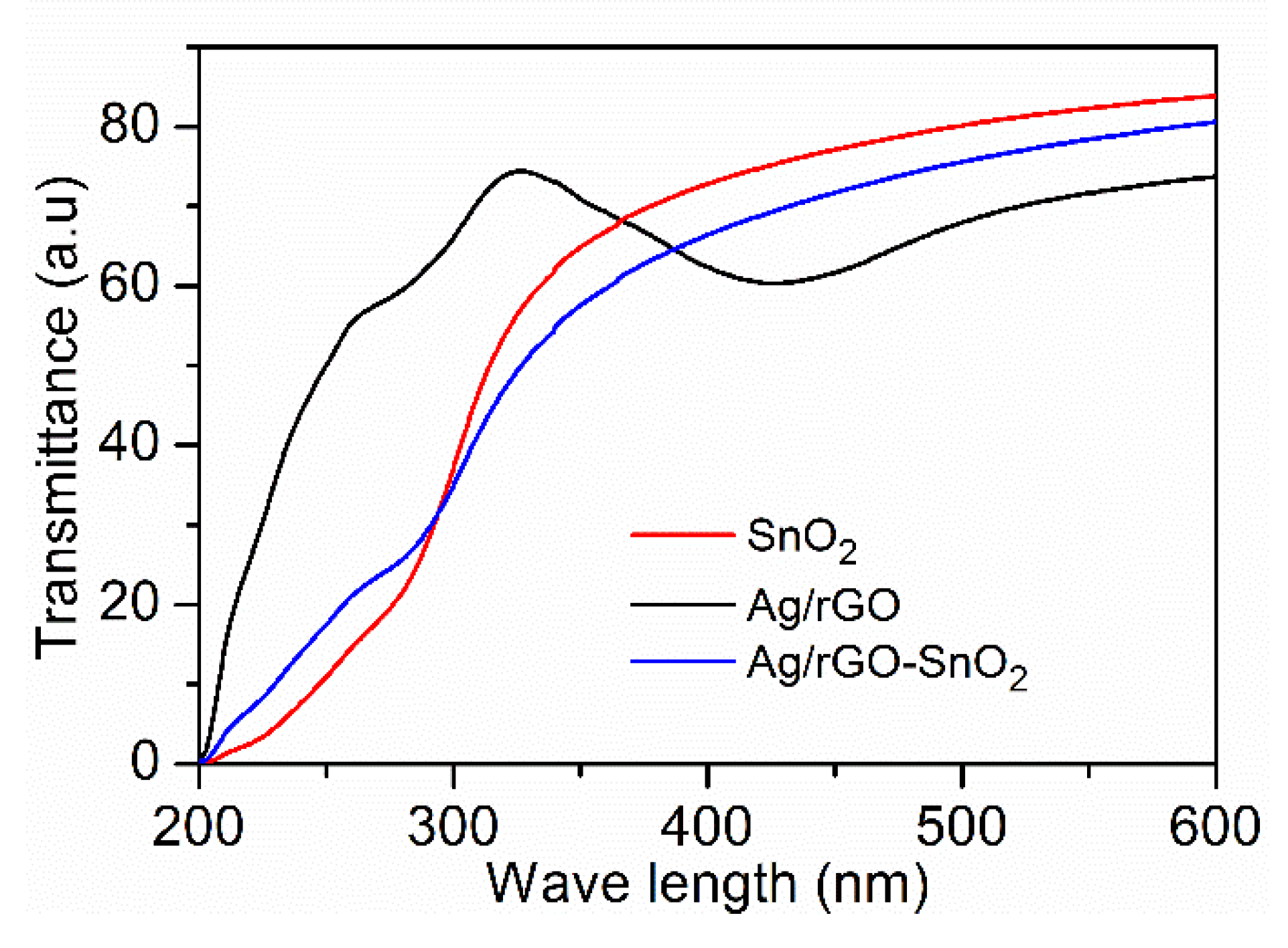
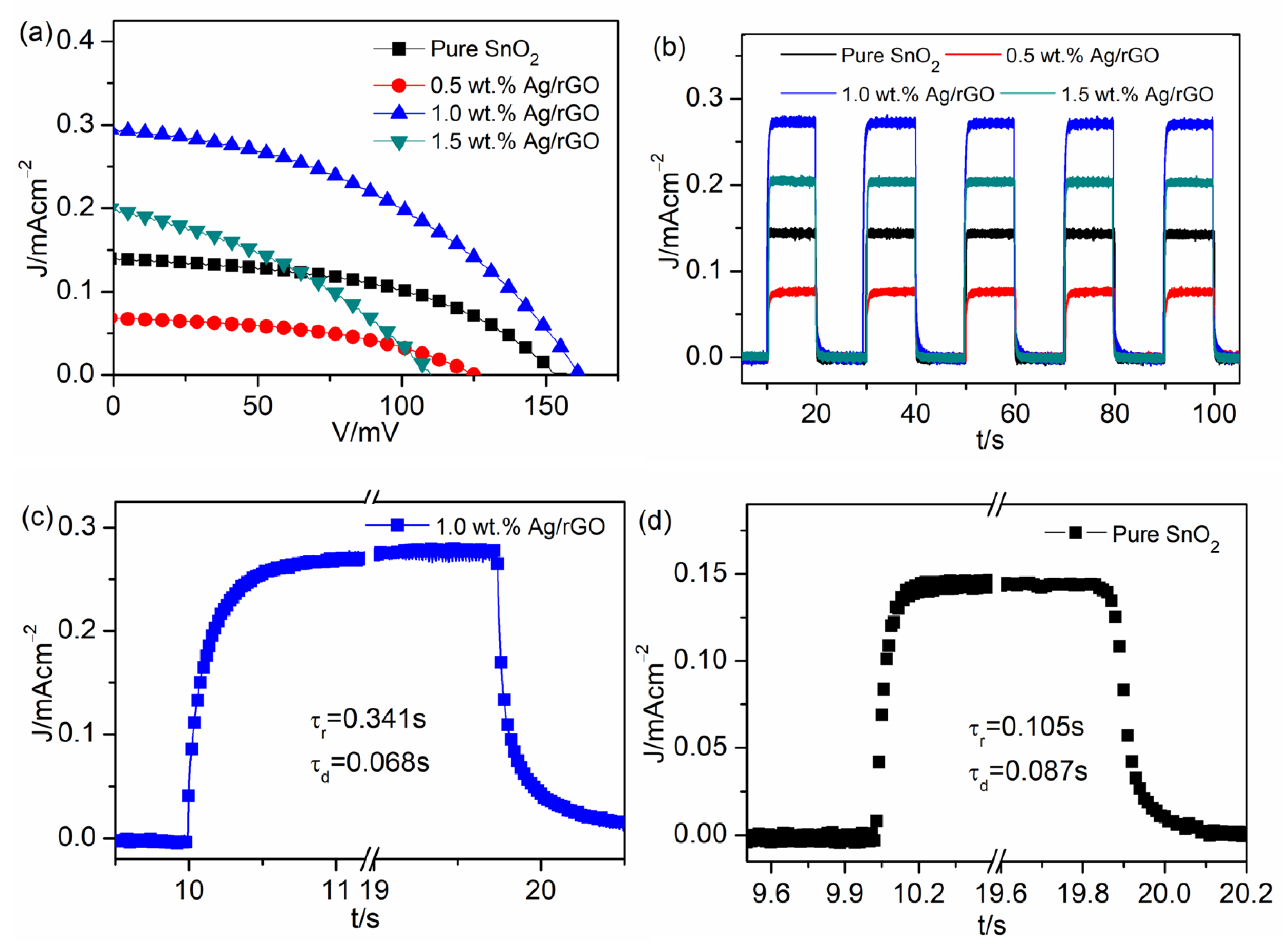
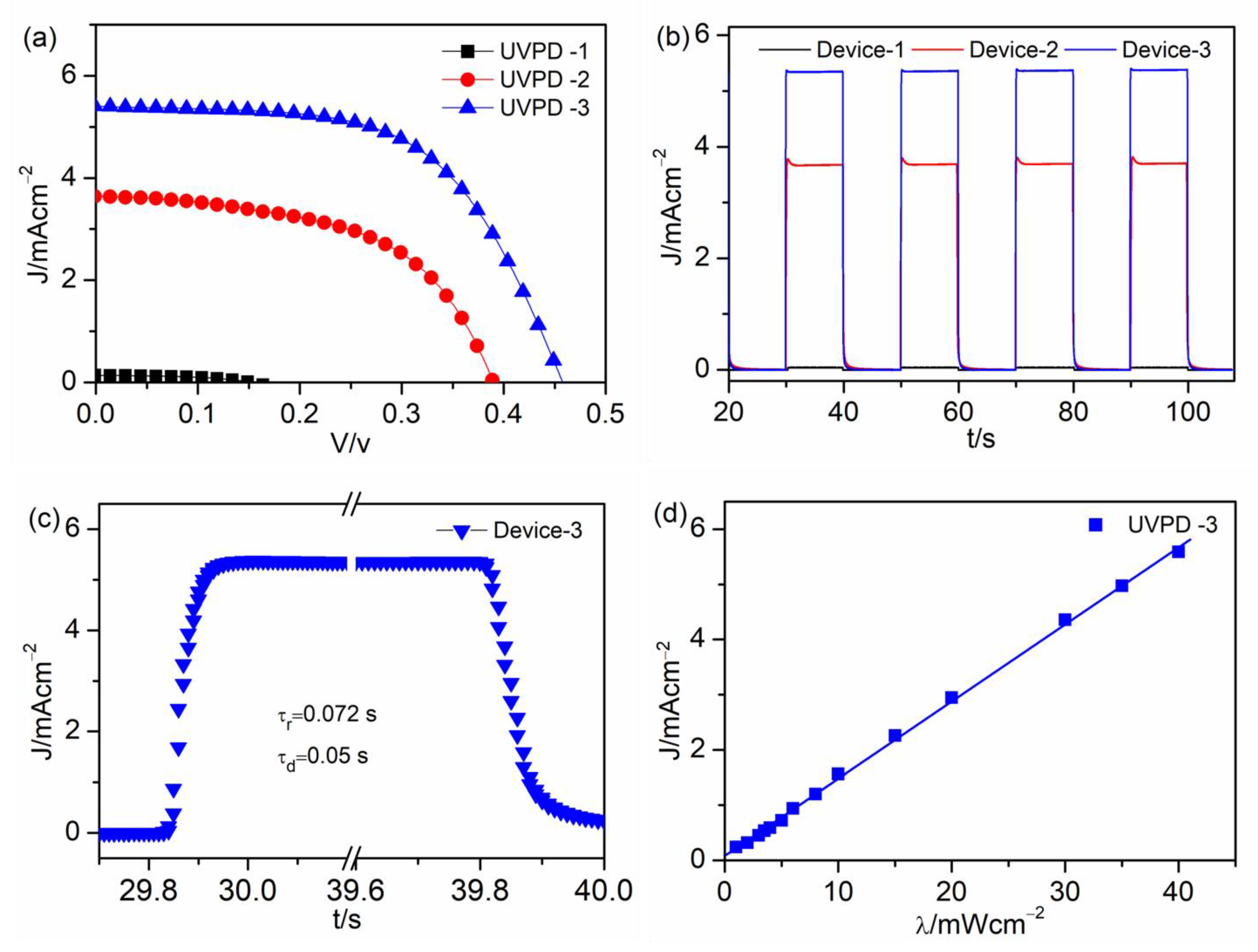
| UVPD | Jsc (mAcm−2) | On:Off Ratio | Rise Time (s) | Decay Time (s) |
|---|---|---|---|---|
| Pure SnO2 | 0.14 | 66 | 0.13 | 0.087 |
| 0.5 wt.% Ag/rGO | 0.07 | 41 | 0.421 | 0.104 |
| 1.0 wt.% Ag/rGO | 0.29 | 195 | 0.341 | 0.068 |
| 1.5 wt.% Ag/rGO | 0.27 | 135 | 0.164 | 0.112 |
| UVPDs | Jsc(mAcm−2) | On:Off Ratio | Rise Time (s) | Decay Time (s) |
|---|---|---|---|---|
| UVPD-1 | 0.14 | 66 | 0.13 | 0.087 |
| UVPD-2 | 3.7 | 544 | 0.14 | 0.05 |
| UVPD-3 | 5.4 | 1441 | 0.072 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, J.T.; Wang, J.; Wang, D. Effect of Ag/rGO on the Optical Properties of Plasmon-Modified SnO2 Composite and Its Application in Self-Powered UV Photodetector. Crystals 2019, 9, 648. https://doi.org/10.3390/cryst9120648
Abdalla JT, Wang J, Wang D. Effect of Ag/rGO on the Optical Properties of Plasmon-Modified SnO2 Composite and Its Application in Self-Powered UV Photodetector. Crystals. 2019; 9(12):648. https://doi.org/10.3390/cryst9120648
Chicago/Turabian StyleAbdalla, James Taban, Jinzhong Wang, and Dongbo Wang. 2019. "Effect of Ag/rGO on the Optical Properties of Plasmon-Modified SnO2 Composite and Its Application in Self-Powered UV Photodetector" Crystals 9, no. 12: 648. https://doi.org/10.3390/cryst9120648
APA StyleAbdalla, J. T., Wang, J., & Wang, D. (2019). Effect of Ag/rGO on the Optical Properties of Plasmon-Modified SnO2 Composite and Its Application in Self-Powered UV Photodetector. Crystals, 9(12), 648. https://doi.org/10.3390/cryst9120648





