Crystallization of CaCO3 in Aqueous Solutions with Extremely High Concentrations of NaCl
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Static Test Experiment
2.3. The Precipitation of CaCO3 by Prompt Addition
2.4. Molecular Dynamics (MD) Simulation
3. Results and Discussion
3.1. Supersaturation Ratio and the Ca2+ Retention Efficiency
3.2. pH Measurement
3.3. Reaction Kinetics of CaCO3 Crystal
3.4. XRD and SEM Characterization for CaCO3 Crystals
3.5. MD Simulation
3.6. The Effect of Inhibitors in NaCl Solutions with Different Concentrations for the Crystallization of CaCO3
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Hasson, D.; Semiat, R.; Shemer, H. Intermediate concentrate demineralization techniques for enhanced brackish water reverse osmosis water recovery—A review. Desalination 2019, 466, 24–35. [Google Scholar] [CrossRef]
- Matin, A.; Rahman, F.; Shafi, H.Z.; Zubair, S.M. Scaling of reverse osmosis membranes used in water desalination: Phenomena, impact, and control; future directions. Desalination 2019, 455, 135–157. [Google Scholar] [CrossRef]
- Oomori, T.; Kaneshima, K.; Kitano, Y. Solubilities of calcite, aragonite and protodolomite in supratidal brines of Minamidaito-Jima Island, Okinawa, Japan. Mar. Chem. 1988, 25, 57–74. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Kamagurov, S.; Tkachenko, S.; Ryabova, A.; Popov, K. Insight into the Mechanisms of Scale Inhibition: A Case Study of a Task-Specific Fluorescent-Tagged Scale Inhibitor Location on Gypsum Crystals. ChemNanoMat 2019, 5, 586–592. [Google Scholar] [CrossRef]
- Ruiz-Garcia, A.; Melian-Martel, N.; Mena, V. Fouling characterization of RO membranes after 11 years of operation in a brackish water desalination plant. Desalination 2018, 430, 180–185. [Google Scholar] [CrossRef]
- Tzotzi, C.; Pahiadaki, T.; Yiantsios, S.G.; Karabelas, A.J.; Andritsos, N. A study of CaCO3 scale formation and inhibition in RO and NF membrane processes. J. Membr. Sci. 2007, 296, 171–184. [Google Scholar] [CrossRef]
- Sim, L.N.; Chong, T.H.; Taheri, A.H.; Sim, S.T.V.; Lai, L.; Krantz, W.B.; Fane, A.G. A review of fouling indices and monitoring techniques for reverse osmosis. Desalination 2018, 434, 169–188. [Google Scholar] [CrossRef]
- Meng, S.; Ye, Y.; Mansouri, J.; Chen, V. Crystallization behavior of salts during membrane distillation with hydrophobic and superhydrophobic capillary membranes. J. Membr. Sci. 2015, 473, 165–176. [Google Scholar] [CrossRef]
- Miyoshi, T.; Hayashi, M.; Shimamura, K.; Matsuyama, H. Important fractions of organic matter causing fouling of seawater reverse osmosis (SWRO) membranes. Desalination 2016, 390, 72–80. [Google Scholar] [CrossRef]
- Rahman, F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination. Desalination 2013, 319, 79–84. [Google Scholar] [CrossRef]
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale formation and control in high pressure membrane water treatment systems: A review. J. Membr. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Bagastyo, A.Y.; Keller, J.; Poussade, Y.; Batstone, D.J. Characterisation and removal of recalcitrants in reverse osmosis concentrates from water reclamation plants. Water Res. 2011, 45, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lu, Y.; Krantz, W.B.; Wang, R.; Fane, A.G. Optimization of operating conditions for a continuous membrane distillation crystallization process with zero salty water discharge. J. Membr. Sci. 2014, 450, 1–11. [Google Scholar] [CrossRef]
- Gal, J.-Y.; Fovet, Y.; Gache, N. Mechanisms of scale formation and carbon dioxide partial pressure influence. Part I. Elaboration of an experimental method and a scaling model. Water Res. 2002, 36, 755–763. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, J.; Yin, Z.; Hou, D.; Luan, Z. Effect of microwave irradiation on typical inorganic salts crystallization in membrane distillation process. J. Membr. Sci. 2014, 455, 24–30. [Google Scholar] [CrossRef]
- Giwa, A.; Dufour, V.; al Marzooqi, F.; al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Naidu, G.; Shim, W.G.; Jeong, S.; Choi, Y.; Ghaffour, N.; Vigneswaran, S. Transport phenomena and fouling in vacuum enhanced direct contact membrane distillation: Experimental and modelling. Sep. Purif. Technol. 2017, 172, 285–295. [Google Scholar] [CrossRef]
- Miao, Y.; Liao, R.; Zhang, X.X.; Liu, B.; Li, Y.; Wu, B.; Li, A. Metagenomic insights into salinity effect on diversity and abundance of denitrifying bacteria and genes in an expanded granular sludge bed reactor treating high-nitrate wastewater. Chem. Eng. J. 2015, 277, 116–123. [Google Scholar] [CrossRef]
- Peyvandi, K.; Haghtalab, A.; Omidkhah, M.R. Using an electrochemical technique to study the effective variables on morphology and deposition of CaCO3 and BaSO4 at the metal surface. J. Cryst. Growth 2012, 354, 109–118. [Google Scholar] [CrossRef]
- Choi, Y.; Naidu, G.; Jeong, S.; Lee, S.; Vigneswaran, S. Effect of chemical and physical factors on the crystallization of calcium sulfate in seawater reverse osmosis brine. Desalination 2018, 426, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Sheikholeslami, R.; Lau, G.T. Effect of bacteria and salinity on calcium sulfate precipitation. Desalination 2012, 287, 301–309. [Google Scholar] [CrossRef]
- Jan, V. IUPAC-NIST Solubility Data Series. 95. Alkaline Earth Carbonates in Aqueous Systems. Part 2. Ca. J. Phys. Chem. Ref. Data 2012, 41, 023105–023137. [Google Scholar]
- Yang, L.; Yang, W.; Xu, B.; Yin, X.; Chen, Y.; Liu, Y.; Ji, Y.; Huan, Y. Synthesis and scale inhibition performance of a novel environmental friendly and hydrophilic terpolymer inhibitor. Desalination 2017, 416, 166–174. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, Y.; Le, J.; Qian, M.; Huan, Y.; Yang, W.; Yin, X.; Liu, Y.; Wang, X.; Chen, Y. Highly effective scale inhibition performance of amino trimethylenephosphonic acid on calcium carbonate. Desalination 2017, 422, 165–173. [Google Scholar] [CrossRef]
- Sousa, M.F.B.; Signorelli, F.; Bertran, C.A. Fast evaluation of inhibitors for calcium carbonate scale based on pH continuous measurements in jar test at high salinity condition. J. Pet. Sci. Eng. 2016, 147, 468–473. [Google Scholar] [CrossRef]
- Shen, C.; Xu, X.; Hou, X.Y.; Wu, D.X.; Yin, J.H. Molecular weight effect on PAA antiscale performance in LT-MED desalination system: Static experiment and MD simulation. Desalination 2018, 445, 1–5. [Google Scholar] [CrossRef]
- Alghamdi, A.O.; Alotaibi, M.B.; Yousef, A.A. Atomistic simulation of calcite interaction with ionic species and oil components in water-flooding. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 529, 760–764. [Google Scholar] [CrossRef]
- Gao, W.; She, F.; Zhang, J.; Dumée, L.F.; He, L.; Hodgson, P.D.; Kong, L. Understanding water and ion transport behaviour and permeability through poly(amide) thin film composite membrane. J. Membr. Sci. 2015, 487, 32–39. [Google Scholar] [CrossRef]
- Shi, W.; Xia, M.; Lei, W.; Wang, F. Molecular dynamics study of polyether polyamino methylene phosphonates as an inhibitor of anhydrite crystal. Desalination 2013, 322, 137–143. [Google Scholar] [CrossRef]
- Kezia, K.; Lee, J.; Zisu, B.; Weeks, M.; Chen, G.; Gras, S.; Kentish, S. Crystallisation of minerals from concentrated saline dairy effluent. Water Res. 2016, 101, 300–308. [Google Scholar] [CrossRef]
- Coto, B.; Martos, C.; Peña, J.L.; Rodríguez, R.; Pastor, G. Effects in the solubility of CaCO3: Experimental study and model description. Fluid Phase Equilib. 2012, 324, 1–7. [Google Scholar] [CrossRef]
- Gal, J.Y.; Bollinger, J.C.; Tolosa, H.; Gache, N. Calcium carbonate solubility: a reappraisal of scale formation and inhibition. Talanta 1996, 43, 1497–1509. [Google Scholar] [CrossRef]
- Qian, H.; Wu, F.; Zhang, F.; Zhou, Z.; Zhang, Z. An accurate calculation model for Na+ concentration in seawater desalination solution. Desalination 2013, 313, 12–17. [Google Scholar] [CrossRef]
- Jaho, S.; Athanasakou, G.D.; Sygouni, V.; Lioliou, M.G.; Koutsoukos, P.G.; Paraskeva, C.A. Experimental Investigation of Calcium Carbonate Precipitation and Crystal Growth in One- and Two-Dimensional Porous Media. Cryst. Growth Des. 2016, 16, 359–370. [Google Scholar] [CrossRef]
- Shin, Y.; Sohn, J. Mechanisms for scale formation in simultaneous membrane distillation crystallization: Effect of flow rate. J. Ind. Eng. Chem. 2016, 35, 318–324. [Google Scholar] [CrossRef]
- Çelikbilek, M.; Ersundu, A.E.; Solak, N.; Aydin, S. Crystallization kinetics of the tungsten–tellurite glasses. J. Non-Cryst. Solids 2011, 357, 88–95. [Google Scholar] [CrossRef]
- Reddy, M.M. Crystallization of calcium carbonate in the presence of trace concentrations of phosphorus-containing anions: I. Inhibition by phosphate and glycerophosphate ions at pH 8.8 and 25 °C. J. Cryst. Growth 1977, 41, 287–295. [Google Scholar] [CrossRef]
- Reddy, M.M.; Hoch, A. Calcium Carbonate Nucleation in an Alkaline Lake Surface Water, Pyramid Lake, Nevada, USA. Aquat. Geochem. 2012, 18, 95–113. [Google Scholar] [CrossRef]
- Reddy, M.M.; Nancollas, G.H. The crystallization of calcium carbonate: IV. The effect of magnesium, strontium and sulfate ions. J. Cryst. Growth 1976, 35, 33–38. [Google Scholar] [CrossRef]
- Al-Hamzah, A.A.; East, C.P.; Doherty, W.O.S.; Fellows, C.M. Inhibition of homogenous formation of calcium carbonate by poly (acrylic acid). The effect of molar mass and end-group functionality. Desalination 2014, 338, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zhang, F.; Cao, Z.; Jing, W.; Chen, Y. Crystallization of CaCO3 in the presence of sulfate and additives: Experimental and molecular dynamics simulation studies. J. Colloid Interface Sci. 2012, 377, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.P.; Subramanian, V.K. Polymorphism in CaCO3—Effect of temperature under the influence of EDTA (di sodium salt). Desalination 2012, 297, 38–47. [Google Scholar] [CrossRef]
- Setta, F.A.; Neville, A. Efficiency assessment of inhibitors on CaCO3 precipitation kinetics in the bulk and deposition on a stainless steel surface (316L). Desalination 2011, 281, 340–347. [Google Scholar] [CrossRef]
- Touati, K.; Alia, E.; Zendah, H.; Elfil, H.; Hannachi, A. Sand filters scaling by calcium carbonate precipitation during groundwater reverse osmosis desalination. Desalination 2018, 430, 24–32. [Google Scholar] [CrossRef]
- Mao, X.; Song, X.; Lu, G.; Sun, Y.; Xu, Y.; Yu, J. Effects of Metal Ions on Crystal Morphology and Size of Calcium Sulfate Whiskers in Aqueous HCl Solutions. Ind. Eng. Chem. Res. 2014, 53, 17625–17635. [Google Scholar] [CrossRef]
- Busenberg, E.; Plummer, L.N. Kinetic and thermodynamic factors controlling the distribution of SO32−and Na+ in calcites and selected aragonites. Geochim. Et Cosmochim. Acta 1985, 49, 713–725. [Google Scholar] [CrossRef]
- Okumura, M.; Kitano, Y. Coprecipitation of alkali metal ions with calcium carbonate. Geochim. Et Cosmochim. Acta 1986, 50, 49–58. [Google Scholar] [CrossRef]
- White, A.F. Sodium and potassium coprecipitation in aragonite. Geochim. Et Cosmochim. Acta 1977, 41, 613–625. [Google Scholar] [CrossRef]
- Kushnir, J. The coprecipitation of strontium, magnesium, sodium, potassium and chloride ions with gypsum. An experimental study. Geochim. Et Cosmochim. Acta 1980, 44, 1471–1482. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Gu, A.; Ding, J.; Shen, Z. Investigation of Calcium Carbonate Scaling Inhibition and Scale Morphology by AFM. J. Colloid Interface Sci. 2001, 240, 608–621. [Google Scholar] [CrossRef]
- Zhang, S.; Qu, H.; Yang, Z.; Fu, C.-E.; Tian, Z.; Yang, W. Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate. Desalination 2017, 419, 152–159. [Google Scholar] [CrossRef]
- Liang, R.; Li, J.; Liu, M.; Huang, Z.Y. Influence of inhibitors on the adhesion of SRB to the stainless steel in circulating cooling water. Colloids Surf. B Biointerfaces 2018, 172, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.H.; Hsieh, L.H.C. A synergistic effect of sodium gluconate and 2-phosphonobutane-1,2,4-tricarboxylic acid on the inhibition of CaCO3 scaling formation. Powder Technol. 2016, 302, 160–167. [Google Scholar] [CrossRef]
- Popov, K.; Rudakova, R.; Larchenko, V.; Tusheva, M.; Afanas’eva, E.; Kombarova, S.; Kamagurov, S.; Kovaleva, N. A comparative performance ranking of some phosphonates and environmentally friendly polymers on CaCO3 scaling inhibition by NACE protocol. Desalin. Water Treat. 2016, 69, 163–172. [Google Scholar] [CrossRef]
- Demadis, K.D.; Chemistry, L.P.J.B. Applications, Chemistry of Organophosphonate Scale Growth lnhibitors: 3. Physicochemical Aspects of 2-Phosphonobutane-1,2,4-Tricarboxylate (PBTC) and its Effect on CaCO3 Crystal Growth. Bioinorg. Chem. Appl. 2005, 3, 135–149. [Google Scholar] [CrossRef] [Green Version]
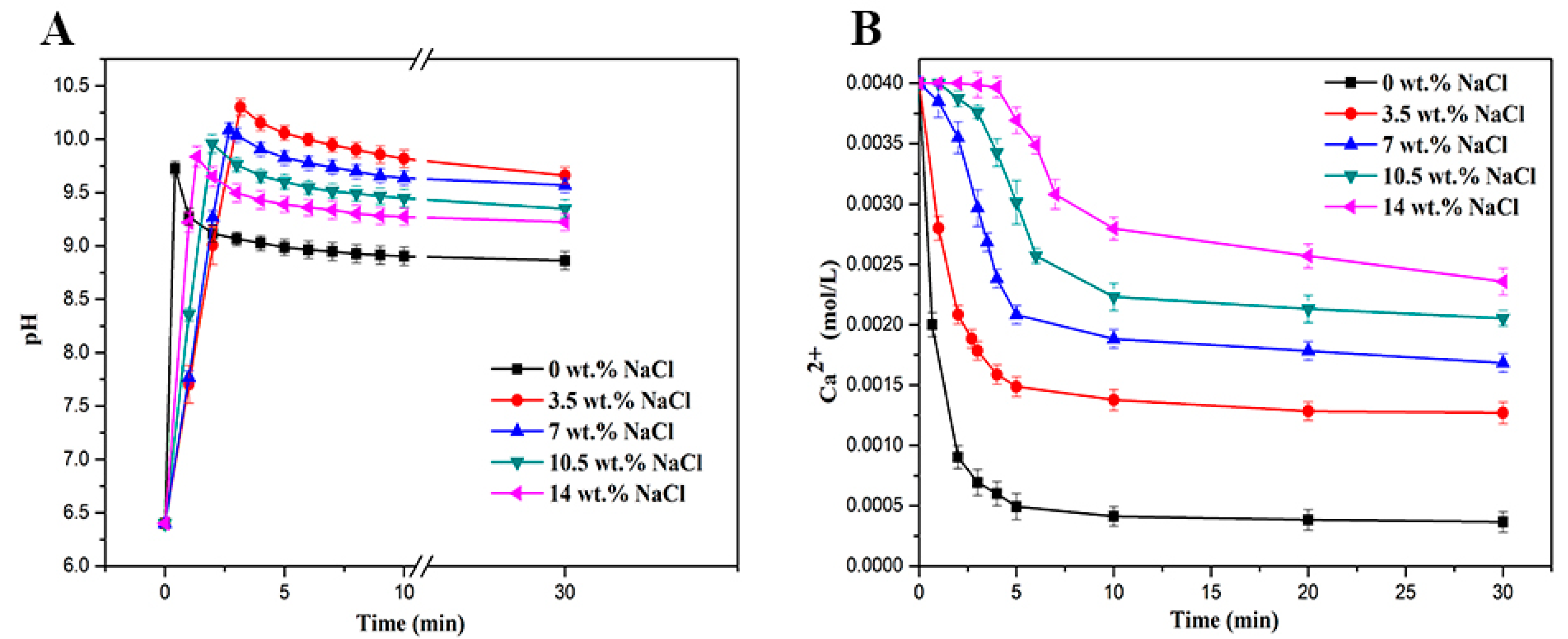
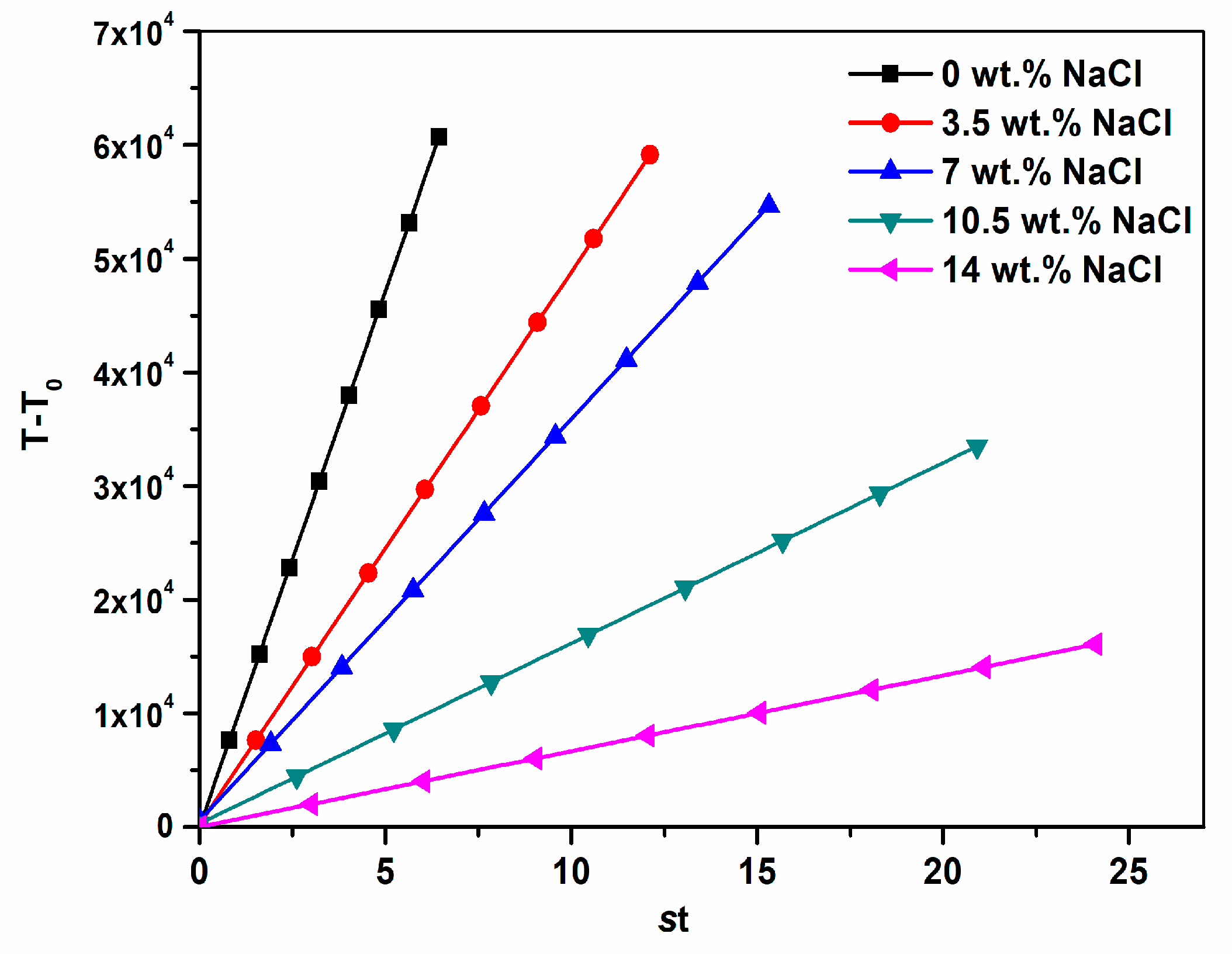
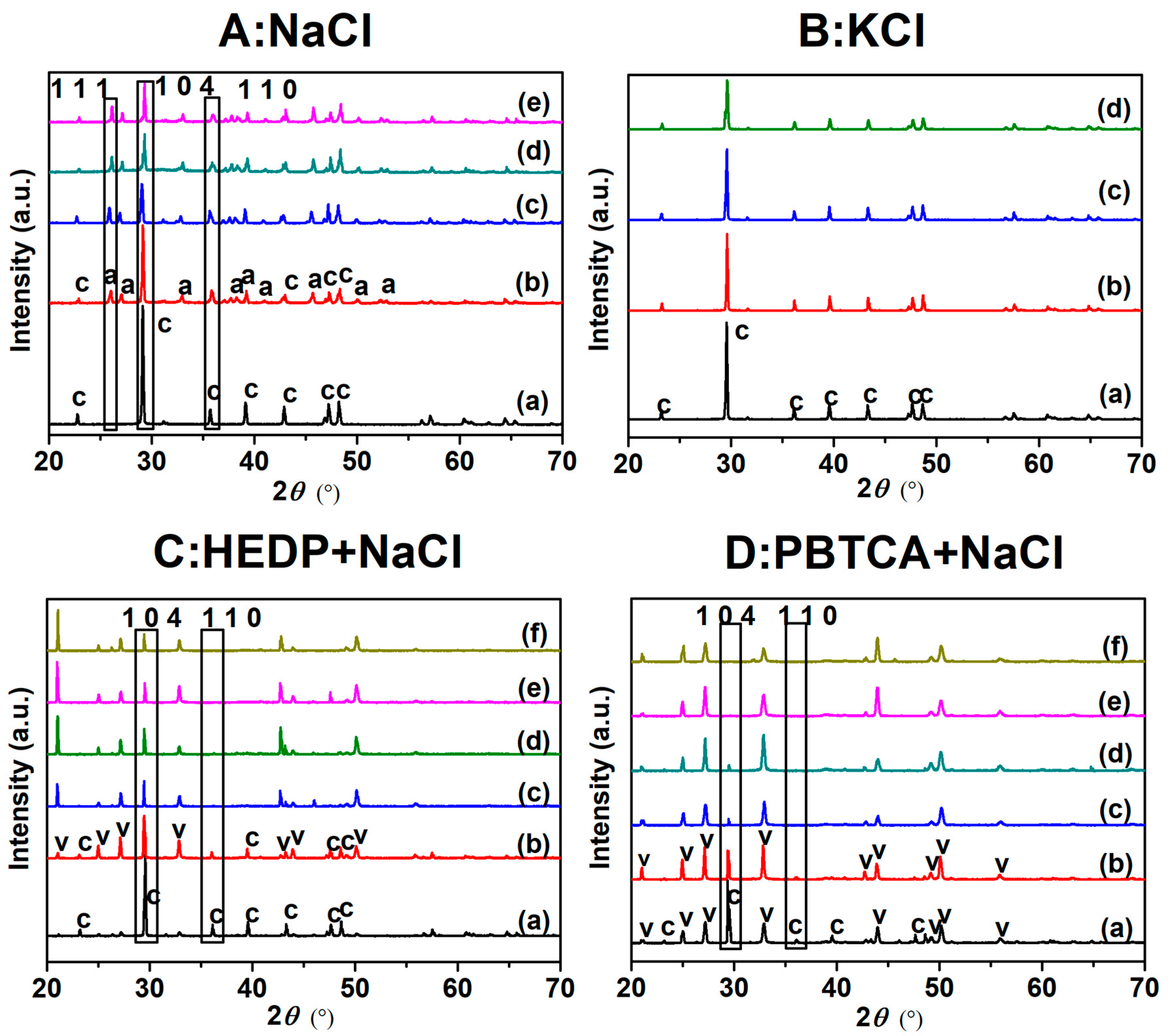
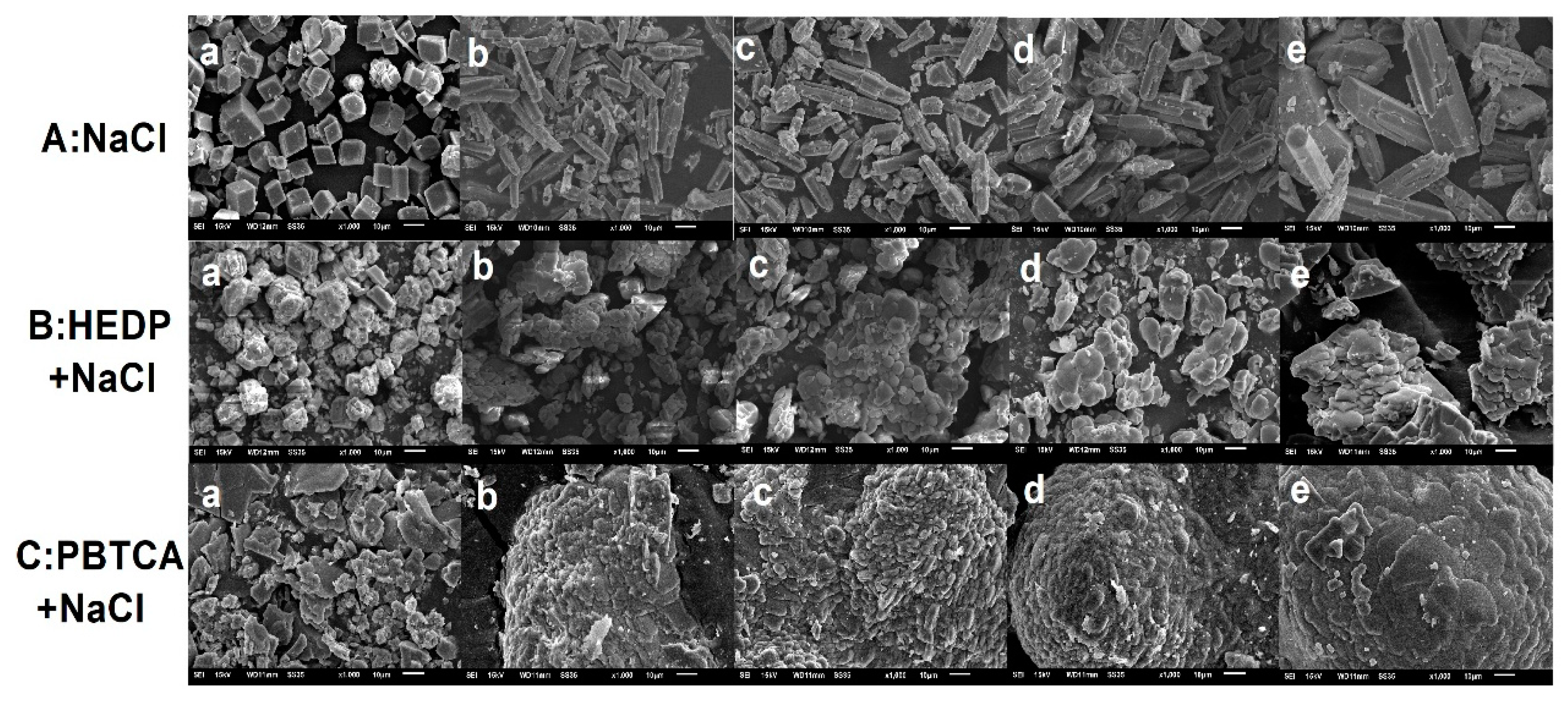

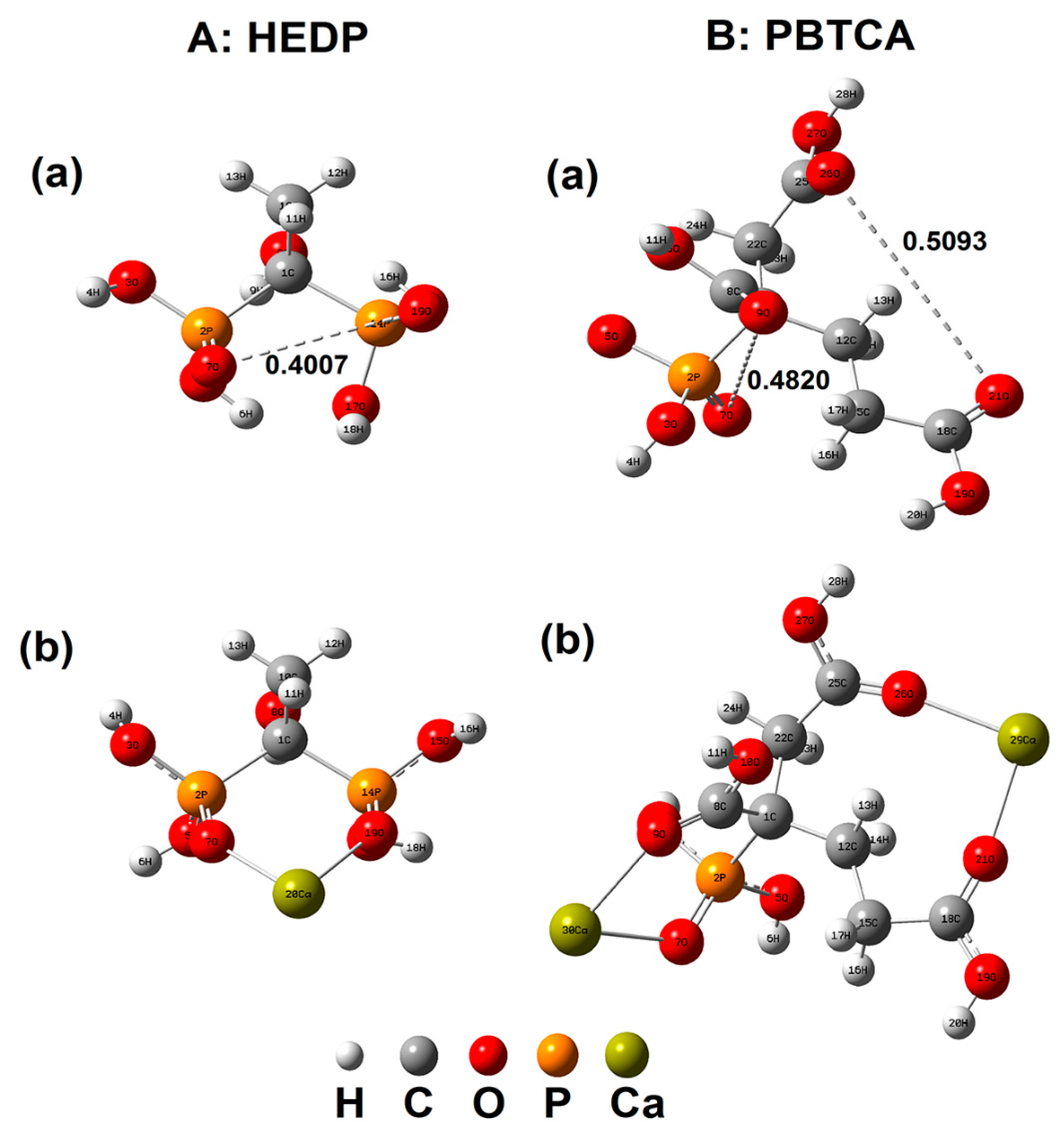
| NaCl (wt.%) | 0 | 3.5 | 7 | 10.5 | 14 |
|---|---|---|---|---|---|
| γCa (×10−2) | 80 ± 3% | 9 ± 1% | 5 ± 3% | 2 ± 6% | 2 ± 7% |
| γCO3 (×10−2) SR | 80 ± 2% 292 ± 9% | 10 ± 3% 20 ± 8% | 8 ± 1% 11 ± 9% | 5 ± 1% 5 ± 4% | 3 ± 8% 4 ± 9% |
| ηNaCl | – | 31 ± 6% | 35 ± 7% | 38 ± 4% | 41 ± 6% |
| η4 μM HEDP | 19 ± 7% | 48 ± 1% | 54 ± 1% | 58 ± 1% | 60 ± 6% |
| η8 μM HEDP | 30 ± 3% | 62 ± 2% | 67 ± 7% | 68 ± 1% | 70 ± 4% |
| η16 μM HEDP | 48 ± 1% | 73 ± 8% | 78 ± 6% | 81 ± 1% | 83 ± 1% |
| η4 μM PBTCA | 27 ± 8% | 56 ± 1% | 61 ± 1% | 65 ± 6% | 70 ± 4% |
| η8 μM PBTCA | 42 ± 5% | 73 ± 8% | 77 ± 9% | 80 ± 6% | 82 ± 3% |
| η16 μM PBTCA | 52 ± 7% | 84 ± 7% | 88 ± 8% | 90 ± 8% | 92 ± 6% |
| NaCl(wt.%) | 0 | 3.5 | 7 | 10.5 | 14 |
|---|---|---|---|---|---|
| pHc | 9 ± 1% | 9 ± 2% | 9 ± 6% | 10 ± 2% | 10 ± 1% |
| pHs Induction time (s) | 8 ± 6% 25 ± 2% | 9 ± 1% 79 ± 8% | 9 ± 5% 118 ± 2% | 9 ± 8% 160 ± 8% | 9 ± 2% 189 ± 6% |
| s (m2/10 g) | 1 ± 6% | 4 ± 4% | 4 ± 6% | 7 ± 2% | 8 ± 5% |
| NaCl (wt%) | 0 | 3.5 | 7 | 10.5 | 14 |
|---|---|---|---|---|---|
| Calcite (%) | 100 | 58 ± 7% | 39 ± 3% | 38 ± 3% | 37 ± 7% |
| Aragonite (%) | 0 | 41 ± 2% | 60 ± 7% | 61 ± 6% | 62 ± 2% |
| Surface | NaCl (wt.%) | Ebinding of HEDP in NaCl | Ebinding of PBTCA in NaCl |
|---|---|---|---|
| (1 0 4) | 0 | 22 ± 3% | 26 ± 1% |
| 3.5 | 48 ± 6% | 64 ± 7% | |
| 7 | 60 ± 3% | 83 ± 9% | |
| 10.5 | 93 ± 1% | 103 ± 2% | |
| 14 | 123 ± 2% | 137 ± 7% | |
| 0 | 64 ± 7% | 74 ± 2% | |
| (1 1 0) | 3.5 | 140 ± 6% | 146 ± 1% |
| 7 | 158 ± 5% | 147 ± 2% | |
| 10.5 | 192 ± 4% | 173 ± 5% | |
| 14 | 216 ± 9% | 184 ± 1% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, M.; Zuo, Y.; Chen, Z.; Yin, X.; Liu, Y.; Yang, W.; Chen, Y. Crystallization of CaCO3 in Aqueous Solutions with Extremely High Concentrations of NaCl. Crystals 2019, 9, 647. https://doi.org/10.3390/cryst9120647
Qian M, Zuo Y, Chen Z, Yin X, Liu Y, Yang W, Chen Y. Crystallization of CaCO3 in Aqueous Solutions with Extremely High Concentrations of NaCl. Crystals. 2019; 9(12):647. https://doi.org/10.3390/cryst9120647
Chicago/Turabian StyleQian, Mengqi, Yuwei Zuo, Zhihao Chen, Xiaoshuang Yin, Ying Liu, Wenzhong Yang, and Yun Chen. 2019. "Crystallization of CaCO3 in Aqueous Solutions with Extremely High Concentrations of NaCl" Crystals 9, no. 12: 647. https://doi.org/10.3390/cryst9120647
APA StyleQian, M., Zuo, Y., Chen, Z., Yin, X., Liu, Y., Yang, W., & Chen, Y. (2019). Crystallization of CaCO3 in Aqueous Solutions with Extremely High Concentrations of NaCl. Crystals, 9(12), 647. https://doi.org/10.3390/cryst9120647





