A Fundamental Reason for the Need of Two Different Semiconductor Technologies for Complementary Thin-Film Transistor Operations
Abstract
:1. Introduction
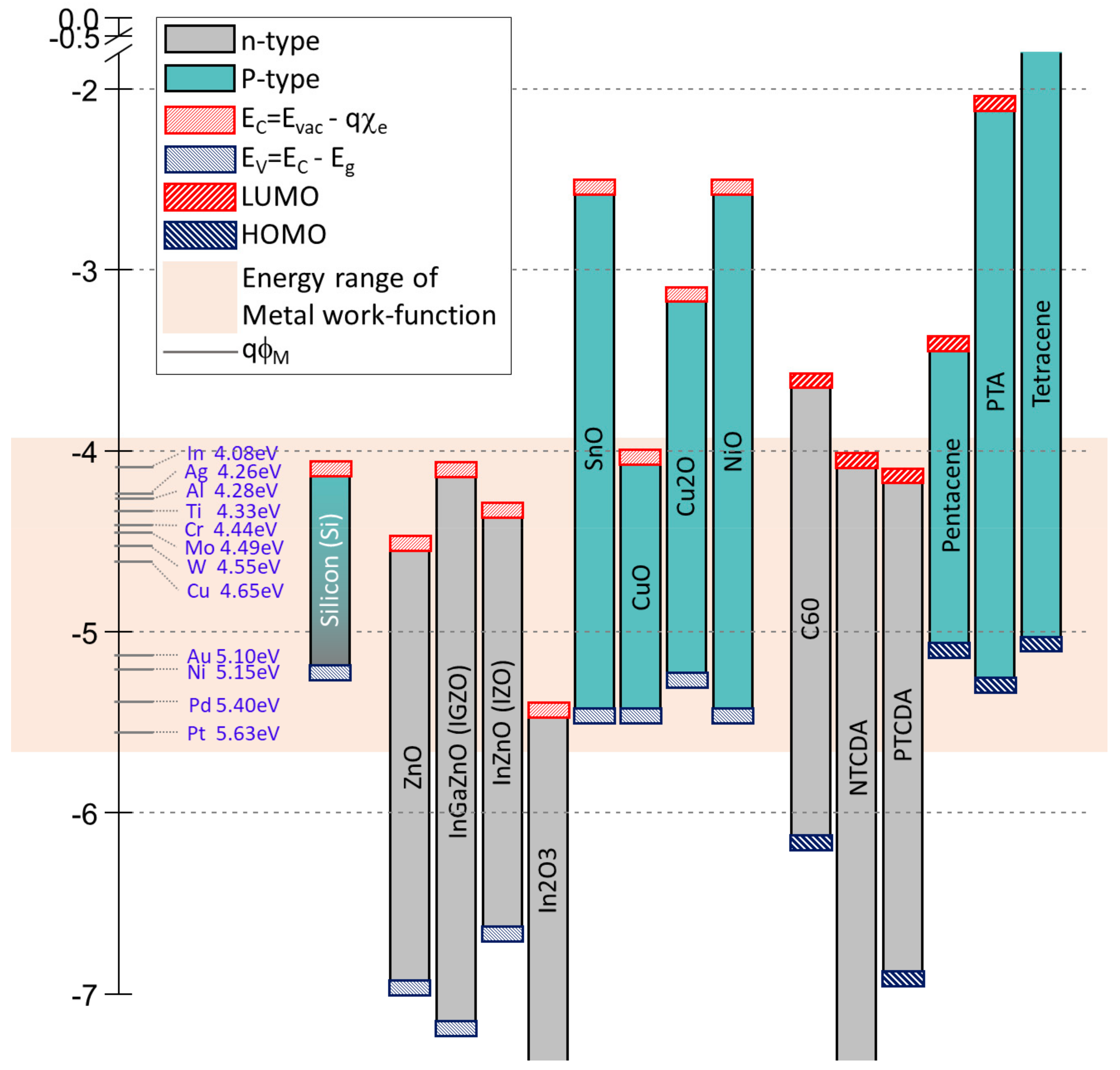
2. Comparative Table of Electronic Material Properties
2.1. n-Type Inorganic Materials
2.2. p-Type Inorganic Materials
2.3. p-Type Organic Materials
2.4. n-Type Organic Materials
2.5. Metals
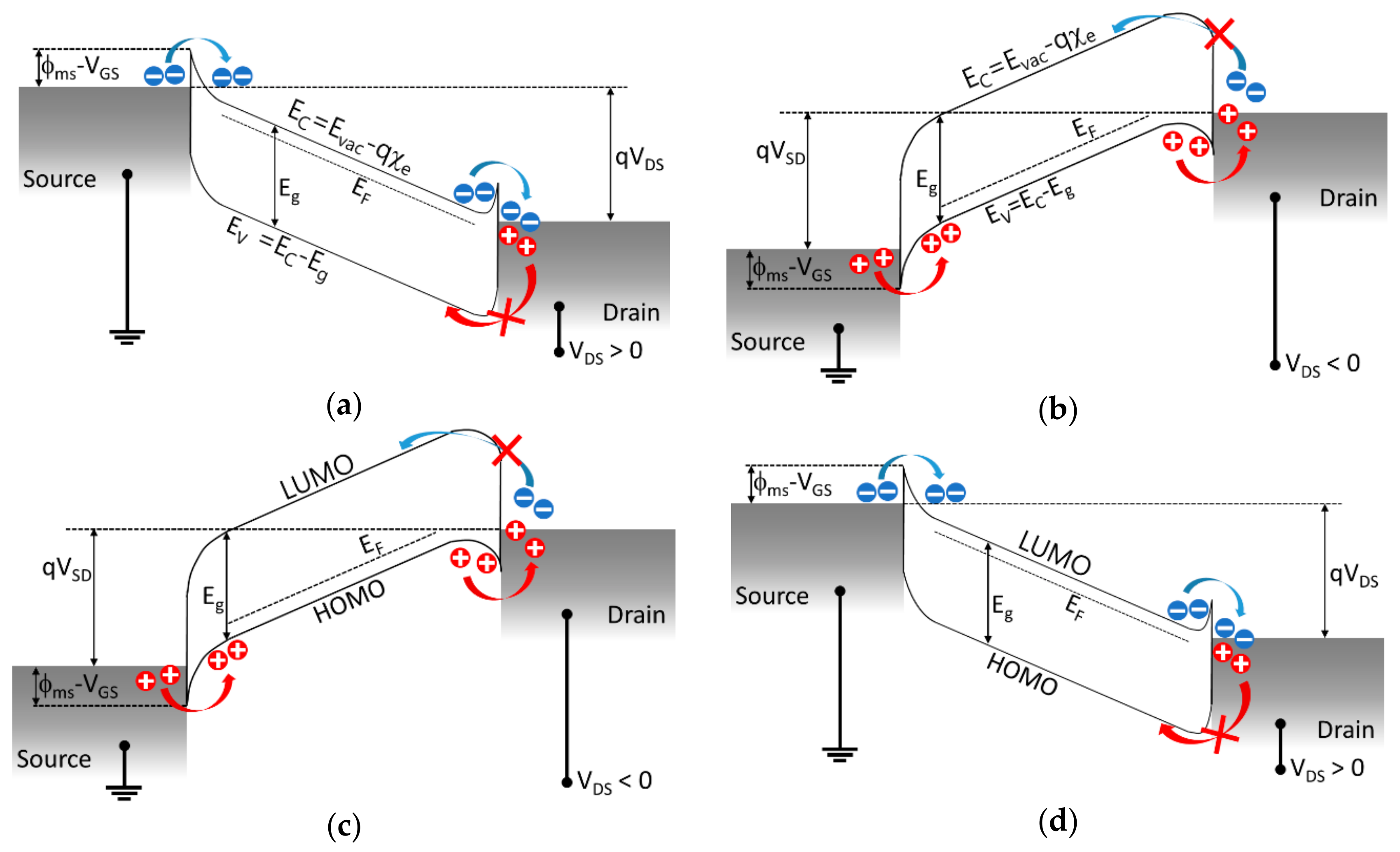
3. Carrier Transport through Semiconductors and Metals in TFTs
3.1. Electron Transport and n-Type Inorganic Materials with Metals
3.2. Hole Transport and p-Type Inorganic Materials with Metals
3.3. Hole Transport and p-Type Organic Materials with Metals
3.4. Electron Transport and n-Type Organic Materials with Metals
4. Summary
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Weste, N.H.; Eshraghian, K. Principles of CMOS VLSI Design: A Systems Perspective; Addison-Wesley Publishing Company: Boston, MA, USA, 1993. [Google Scholar]
- Shur, M.S. Handbook Series on Semiconductor Parameters; World Scientific: Singapore, 1997. [Google Scholar]
- Robertson, J. Band offsets and work function control in field effect transistors. J. Vac. Sci. Technol. B 2009, 27, 277. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, W.; Wu, W.; Liu, B. Research progress on flexible oxide-based thin film transistors. Appl. Sci. 2019, 9, 773. [Google Scholar] [CrossRef]
- Nathan, A.; Ahnood, A.; Cole, M.T.; Lee, S.; Suzuki, Y.; Hiralal, P.; Bonaccorso, F.; Hasan, T.; Garcia-Gancedo, L.; Dyadyusha, A.; et al. Flexible electronics: The next ubiquitous platform. Proc. IEEE 2012, 100, 1486–1517. [Google Scholar] [CrossRef]
- Jang, J.; Kang, Y.; Cha, D.; Bae, J.; Lee, S. Thin-Film Optical Devices Based on Transparent Conducting Oxides: Physical Mechanisms and Applications. Crystals 2019, 9, 192. [Google Scholar] [CrossRef]
- Sun, B.; Sirringhaus, H. Solution-processed zinc oxide field-effect transistors based on self-assembly of colloidal nanorods. Nano Lett. 2005, 5, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Huang, J.; Dong, T.; Xie, J.; Yuan, J.; Luo, D.; Liu, B. Room-temperature fabricated thin-film transistors based on compounds with lanthanum and main family element boron. Molecules 2018, 23, 1373. [Google Scholar] [CrossRef]
- Chen, H.; Cao, Y.; Zhang, J.; Zhou, C. Large-scale complementary macroelectronics using hybrid integration of carbon nanotubes and IGZO thin-film transistors. Nat. Commun. 2014, 5, 4097. [Google Scholar] [CrossRef]
- Weimer, P.K. The TFT a new thin-film transistor. Proc. IRE 1962, 50, 1462–1469. [Google Scholar] [CrossRef]
- Fortunato, E.M.C.; Barquinha, P.M.C.; Pimentel, A.C.M.B.G.; Gonçalves, A.M.F.; Marques, A.J.S.; Martins, R.F.P.; Pereira, L.M. Wide-bandgap high-mobility ZnO thin-film transistors produced at room temperature. Appl. Phys. Lett. 2004, 85, 2541. [Google Scholar] [CrossRef]
- Hussain, B.; Aslam, A.; Khan, T.M.; Creighton, M.; Zohuri, B. Electron affinity and bandgap optimization of zinc oxide for improved performance of ZnO/Si heterojunction solar cell using PC1D simulations. Electronics 2019, 8, 238. [Google Scholar] [CrossRef]
- Kohan, A.F.; Ceder, G.; Morgan, D.; Van De Walle, C.G. First-principles study of native point defects in ZnO. Phys. Rev. B 2000, 61, 15019–15027. [Google Scholar] [CrossRef]
- Afanas’Ev, V.V.; Badylevich, M.; Houssa, M.; Stesmans, A.; Aggrawal, G.; Campbell, S.A. Electron energy band alignment at the NiO/SiO2 interface. Appl. Phys. Lett. 2010, 96, 172105. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Watson, G.W. On the possibility of p-type SnO2. J. Mater. Chem. 2012, 22, 25236–25245. [Google Scholar] [CrossRef]
- Koffyberg, F.P.; Benko, F.A. A photoelectrochemical determination of the position of the conduction and valence band edges ofp-type CuO. J. Appl. Phys. 1982, 53, 1173–1177. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, J.; Gong, H. Transparent p-Type Semiconductors: Copper-Based Oxides and Oxychalcogenides. Coatings 2019, 9, 137. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.J.; Sander, T.; Reindl, C.; Benz, J.; Eickhoff, M.; et al. Binary copper oxide semiconductors: From materials towards devices. Phys. Status Solidi 2012, 249, 1487–1509. [Google Scholar] [CrossRef]
- Dong, H.; Wang, C.; Hu, W. High performance organic semiconductors for field-effect transistors. Chem. Commun. 2010, 46, 5211–5222. [Google Scholar] [CrossRef]
- Djurovich, P.I.; Mayo, E.I.; Forrest, S.R.; Thompson, M.E. Measurement of the lowest unoccupied molecular orbital energies of molecular organic semiconductors. Org. Electron. 2009, 10, 515–520. [Google Scholar] [CrossRef]
- Claves, D. Hole-doping of fullerenes and nanotubes by way of intercalation chemistry. J. Nanosci. Nanotechnol. 2007, 7, 1221–1238. [Google Scholar] [CrossRef]
- Lee, S.; Nathan, A. Conduction threshold in accumulation-mode InGaZnO thin film transistors. Sci. Rep. 2016, 6, 22567. [Google Scholar] [CrossRef]
- Lee, S.; Striakhilev, D.; Jeon, S.; Nathan, A. Unified analytic model for current–voltage behavior in amorphous oxide semiconductor TFTs. EEE Electron Device Lett. 2013, 35, 84–86. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, F.; Lin, Y.; Wang, G.; Pan, F. Correlation of oxygen vacancy variations to band gap changes in epitaxial ZnO thin films. Appl. Phys. Lett. 2013, 102, 181908. [Google Scholar] [CrossRef]
- Chiu, C.; Pei, Z.; Chang, S.; Chang, S.; Chang, S. Effect of oxygen partial pressure on electrical characteristics of amorphous indium gallium zinc oxide thin-film transistors fabricated by thermal annealing. Vacuum 2011, 86, 246–249. [Google Scholar] [CrossRef]
- Isseroff, L.Y.; Carter, E.A. Electronic Structure of Pure and Doped Cuprous Oxide with Copper Vacancies: Suppression of Trap States. Chem. Mater. 2013, 25, 253–265. [Google Scholar] [CrossRef]
- Oehzelt, M.; Koch, N.; Heimel, G. Organic semiconductor density of states controls the energy level alignment at electrode interfaces. Nat. Commun. 2014, 5, 4174. [Google Scholar] [CrossRef]
- Kremer, R.K.; Rabenau, T.; Maser, W.K.; Kaiser, M.; Simon, A.; Haluska, M.; Kuzmany, H. High-temperature conductivity study on single-crystal C 60. Appl. Phys. A 1993, 56, 211–214. [Google Scholar] [CrossRef]
- Berkai, Z.; Daoudi, M.; Mendil, N.; Belghachi, A. Monte Carlo simulation of electric conductivity for pure and doping fullerene (C60). Phys. Lett. A 2019, 383, 2090–2092. [Google Scholar] [CrossRef]
- Gundlach, D.J.; Pernstich, K.P.; Wilckens, G.; Grüter, M.; Haas, S.; Batlogg, B. High mobility n-channel organic thin-film transistors and complementary inverters. J. Appl. Phys. 2005, 98, 064502. [Google Scholar] [CrossRef]
- Chiu, I.-C.; Li, Y.-S.; Tu, M.-S.; Cheng, I.-C. Complementary Oxide–Semiconductor-Based Circuits With n-Channel ZnO and p-Channel SnO Thin-Film Transistors. IEEE Electron. Device Lett. 2014, 35, 1263–1265. [Google Scholar] [CrossRef]
- Oh, M.S.; Hwang, D.K.; Lee, K.; Im, S.; Yi, S. Low voltage complementary thin-film transistor inverters with pentacene-ZnO hybrid channels on AlOx dielectric. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Nomura, K.; Aoki, T.; Nakamura, K.; Kamiya, T.; Nakanishi, T.; Hasegawa, T.; Kimura, M.; Kawase, T.; Hirano, M.; Hosono, H. Three-dimensionally stacked flexible integrated circuit: Amorphous oxide/polymer hybrid complementary inverter using n-type a-In–Ga–Zn–O and p-type poly-(9,9-dioctylfluorene-co-bithiophene) thin-film transistors. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.; Lee, S. A Fundamental Reason for the Need of Two Different Semiconductor Technologies for Complementary Thin-Film Transistor Operations. Crystals 2019, 9, 603. https://doi.org/10.3390/cryst9110603
Jang J, Lee S. A Fundamental Reason for the Need of Two Different Semiconductor Technologies for Complementary Thin-Film Transistor Operations. Crystals. 2019; 9(11):603. https://doi.org/10.3390/cryst9110603
Chicago/Turabian StyleJang, Jiung, and Sungsik Lee. 2019. "A Fundamental Reason for the Need of Two Different Semiconductor Technologies for Complementary Thin-Film Transistor Operations" Crystals 9, no. 11: 603. https://doi.org/10.3390/cryst9110603
APA StyleJang, J., & Lee, S. (2019). A Fundamental Reason for the Need of Two Different Semiconductor Technologies for Complementary Thin-Film Transistor Operations. Crystals, 9(11), 603. https://doi.org/10.3390/cryst9110603




