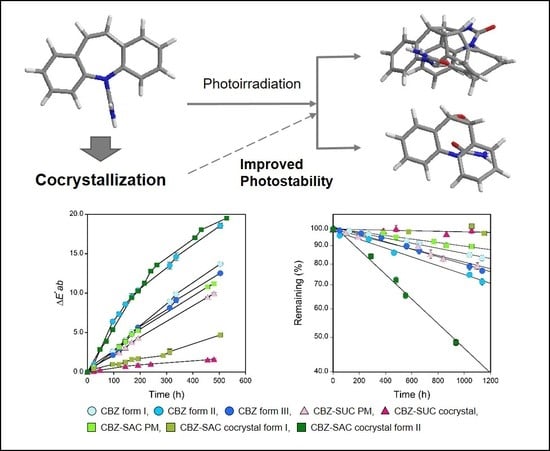
Comparative Evaluation of the Photostability of Carbamazepine Polymorphs and Cocrystals
Abstract
:1. Introduction
2. Materials and Methods
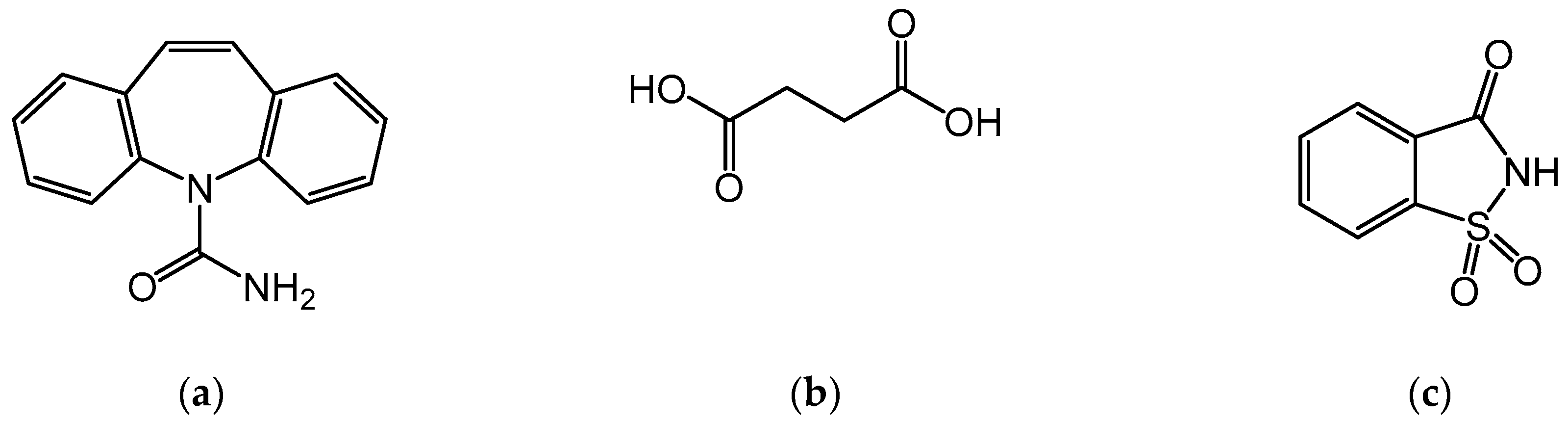
2.1. Materials
2.2. Preparation of CBZ Polymorphs
2.3. Preparation of CBZ Cocrystals
2.4. Powder X-Ray Diffractometry (PXRD)
2.5. Thermal Analysis
2.6. Colorimetric Measurement
2.7. Low-Frequency Raman Spectroscopy
2.8. FT-IR RAS
2.9. SSNMR 1H Spin–Lattice Relaxation Time (T1)
3. Results
3.1. Characterization of CBZ Polymorphs and Cocrystals
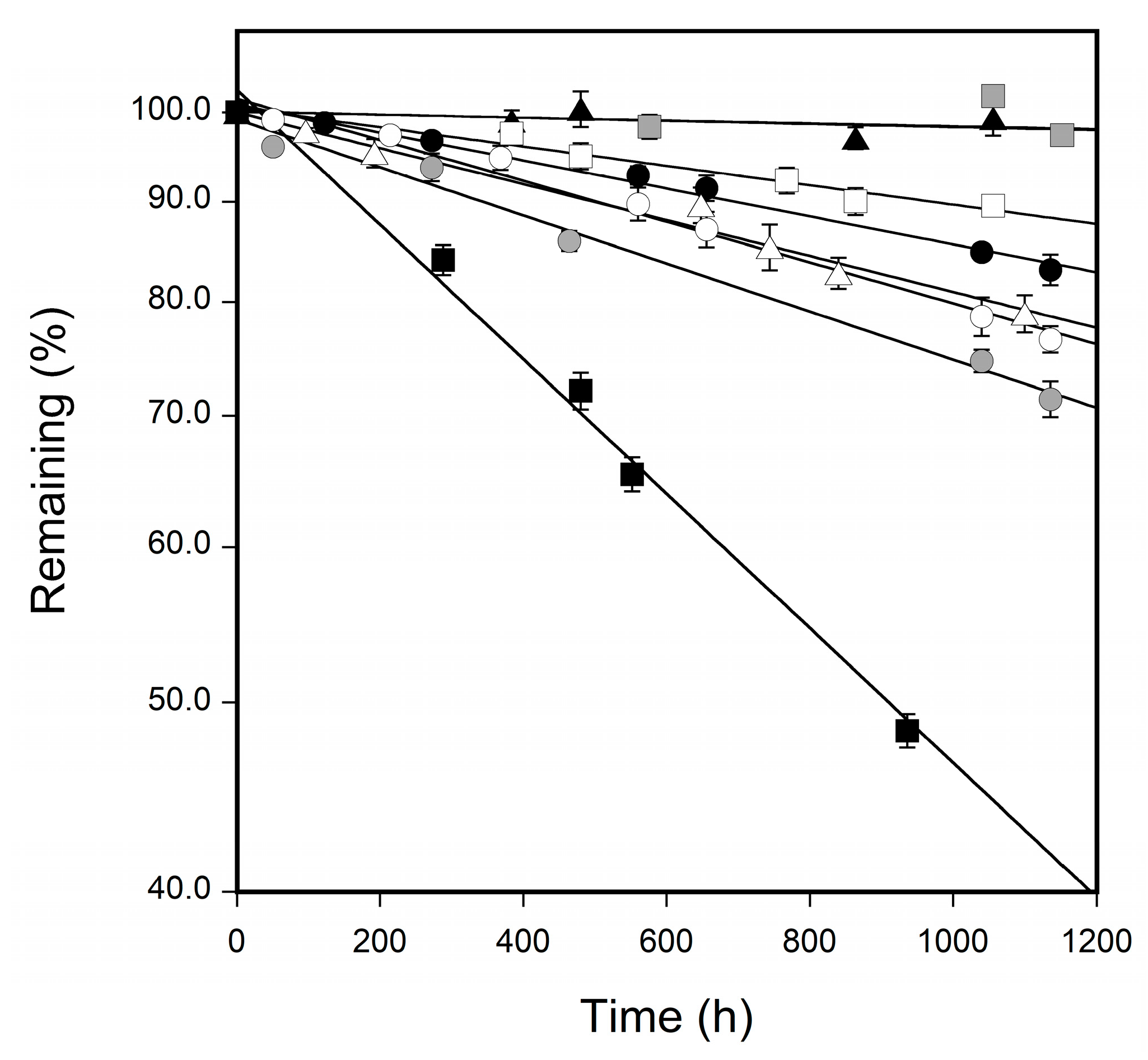
3.2. Changes in the Surface Color of Tablets
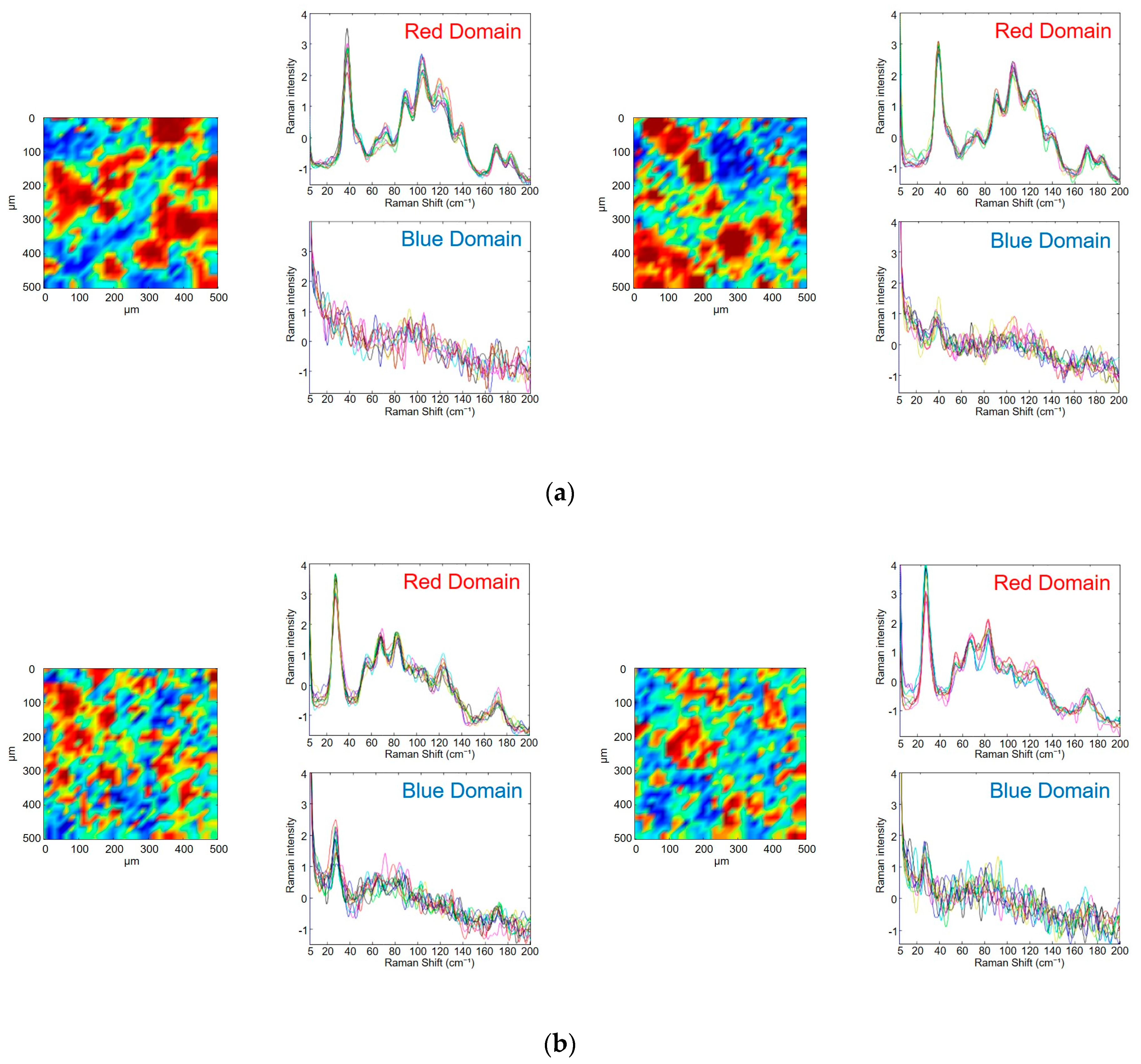
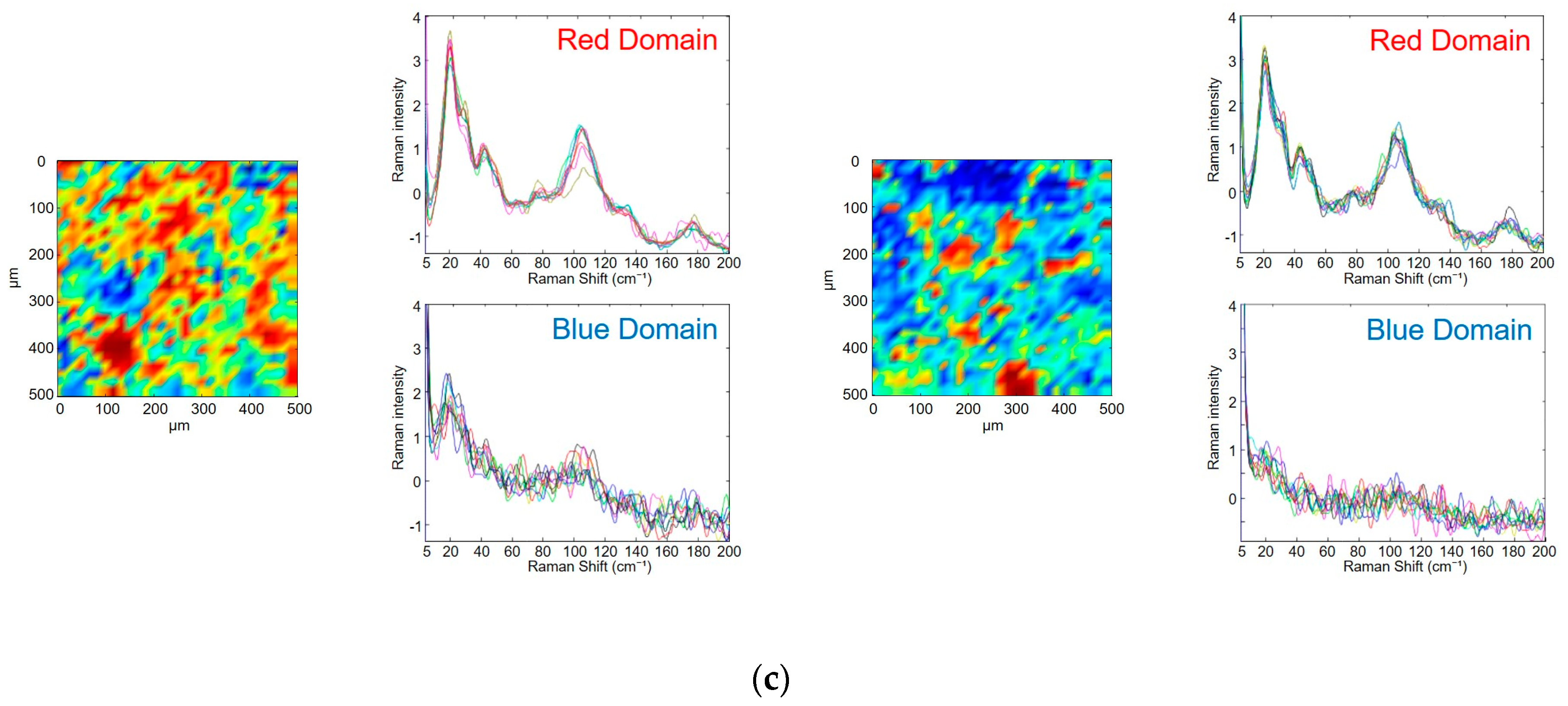
3.3. Evaluation of Photodegradation on the Surface of Tablets Using Low-Frequency Raman Spectroscopy
3.4. Evaluation of Photodegradation on the Surface of Tablets Using FT-IR RAS
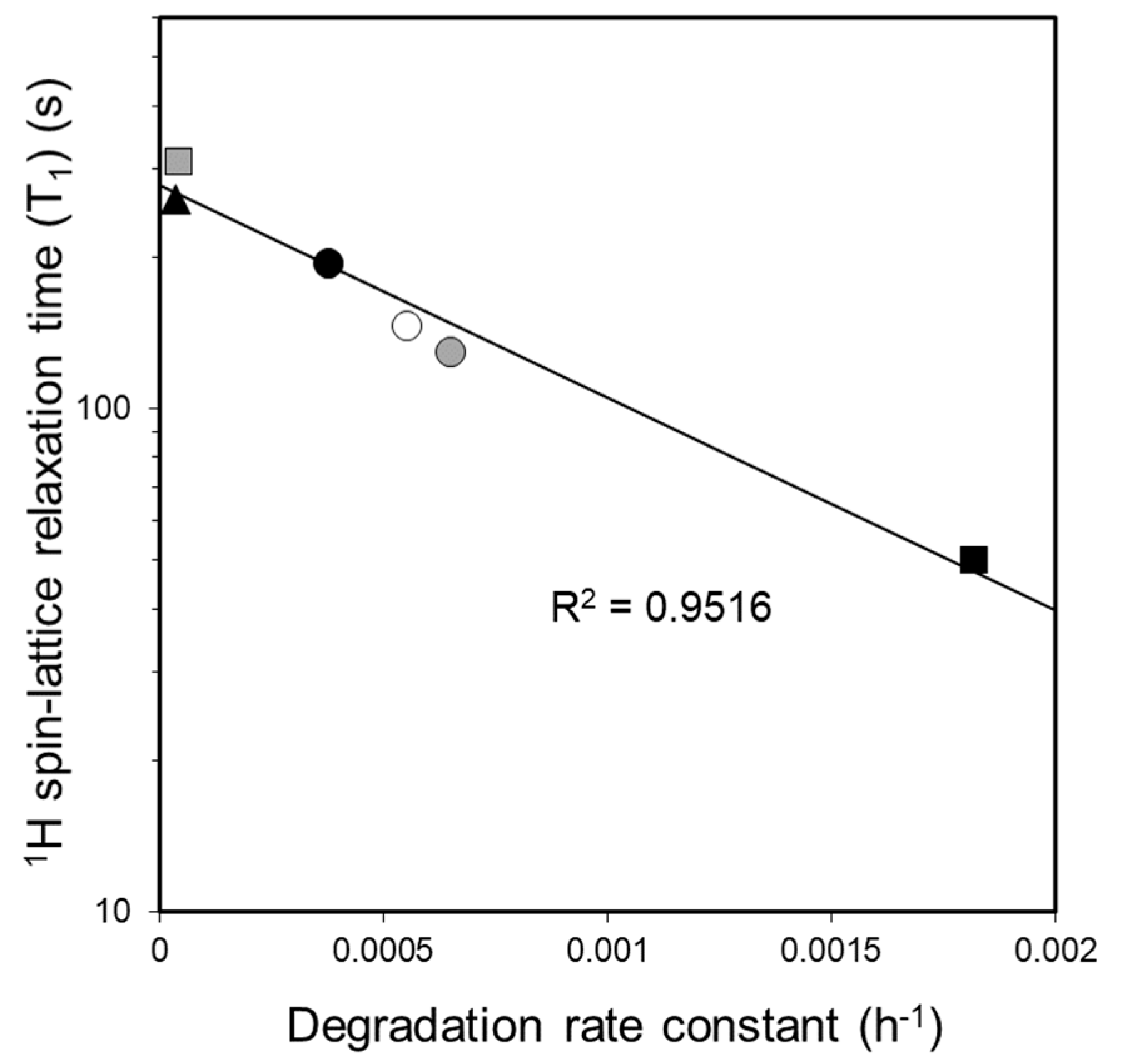
3.5. Investigation of the Relationship between Molecular Mobility and Photostability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coelho, L.; Almeida, I.F.; Sousa Lobo, J.M.; Sousa e Silva, J.P. Photostabilization strategies of photosensitive drugs. Int. J. Pharm. 2018, 541, 19–25. [Google Scholar] [CrossRef]
- Janga, K.Y.; King, T.; Ji, N.; Sarabu, S.; Shadambikar, G.; Sawant, S.; Xu, P.; Repka, M.A.; Murthy, S.N. Photostability issues in pharmaceutical dosage forms and photostabilization. AAPS Pharm. Sci. Tech. 2018, 19, 48–59. [Google Scholar] [CrossRef]
- Tønnesen, H.H. Formulation and stability testing of photolabile drugs. Int. J. Pharm. 2001, 225, 1–14. [Google Scholar] [CrossRef]
- Grzesiak, A.L.; Lang, M.; Kim, K.; Matzger, A.J. Comparison of the four anhydrous polymorphs of carbamazepine and the crystal structure of form I. J. Pharm. Sci. 2003, 92, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.K.; Ghi, P.Y.; Puschmann, H.; Apperley, D.C.; Griesser, U.J.; Hammond, R.B.; Ma, C.; Roberts, K.J.; Pearce, G.J.; Yates, J.R.; et al. Structural studies of the polymorphs of carbamazepine, its dihydrate, and two solvates. Org. Process Res. Dev. 2005, 9, 902–910. [Google Scholar] [CrossRef]
- McMahon, L.E.; Timmins, P.; Williams, A.C.; York, P. Characterization of dihydrates prepared from carbamazepine polymorphs. J. Pharm. Sci. 1996, 85, 1064–1069. [Google Scholar] [CrossRef]
- Lowes, M.M.J.; Caira, M.R.; Lotter, A.P.; Van Watt, J.G.D. Physicochemical properties and x-ray structural studies of the trigonal polymorph of carbamazepine. J. Pharm. Sci. 1987, 76, 744–752. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ito, S.; Itai, S.; Yamamoto, K. Physicochemical properties and bioavailability of carbamazepine polymorphs and dihydrate. Int. J. Pharm. 2000, 193, 137–146. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Salmon, D.J. Building co-crystals with molecular sense and supramolecular sensibility. Cryst. Eng. Comm. 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or salt: Does it really matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef]
- Childs, S.L.; Rodríguez-Hornedo, N.; Reddy, L.S.; Jayasankar, A.; Maheshwari, C.; McCausland, L.; Shipplett, R.; Stahly, B.C. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. Cryst. Eng. Comm. 2008, 10, 856–864. [Google Scholar] [CrossRef]
- Ullah, M.; Hussain, I.; Sun, C.C. The development of carbamazepine-succinic acid cocrystal tablet formulations with improved in vitro and in vivo performance. Drug Dev. Ind. Pharm. 2016, 42, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.B.; Peterson, M.L.; Scoppettuolo, L.A.; Morrisette, S.L.; Vetter, A.; Guzmán, H.; Remenar, J.F.; Zhang, Z.; Tawa, M.D.; Haley, S.; et al. Performance comparison of a co-crystal of carbamazepine with marketed product. Eur. J. Pharm. Biopharm. 2007, 67, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.W., III; Elie, S.C.; Matzger, A.J. Polymorphism in carbamazepine cocrystals. Cryst. Growth Des. 2008, 8, 14–16. [Google Scholar] [CrossRef]
- Wang, J.-R.; Zhou, C.; Yu, X.; Mei, X. Stabilizing vitamin D3 by conformationally selective co-crystallization. Chem. Commun. 2014, 50, 855–858. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, J.-R.; Zhang, Q.; Mei, X. Improving dissolution and photostability of vitamin K3 via cocrystallization with naphthoic acids and sulfamerazine. Cryst. Growth Des. 2016, 16, 483–492. [Google Scholar] [CrossRef]
- Yu, Q.; Yan, Z.; Bao, J.; Wang, J.-R.; Mei, X. Taming photo-induced oxidation degradation of dihydropyridine drugs through cocrystallization. Chem. Commun. 2017, 53, 12266–12269. [Google Scholar] [CrossRef]
- Shinozaki, T.; Ono, M.; Higashi, K.; Moribe, K. A novel drug-drug cocrystal of levofloxacin and metacetamol: Reduced hygroscopicity and improved photostability of levofloxacin. J. Pharm. Sci. 2019, 108, 2383–2390. [Google Scholar] [CrossRef]
- Kaneniwa, N.; Yamaguchi, T.; Watari, N.; Otsuka, M. Hygroscopicity of carbamazepine crystalline powders. Yakugaku Zasshi 1984, 104, 184–190. [Google Scholar] [CrossRef]
- Matsuda, Y.; Teraoka, R.; Sugimoto, I. Comparative evaluation of photostability of solid-state nifedipine under ordinary and intensive light irradiation conditions. Int. J. Pharm. 1989, 54, 211–221. [Google Scholar] [CrossRef]
- Matsuda, Y.; Akazawa, R.; Teraoka, R.; Otsuka, M. Pharmaceutical evaluation of carbamazepine modifications: Comparative study for photostability of carbamazepine polymorphs by using Fourier-transformed reflection-absorption infrared spectroscopy and colorimetric measurement. J. Pharm. Pharmacol. 1994, 46, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Fukami, T.; Hisada, H.; Inoue, M.; Carriere, J.; Heyler, R.; Katori, N.; Okuda, H.; Goda, Y. Identification of pseudopolymorphism of magnesium stearate by using low-frequency Raman spectroscopy. Org. Process. Res. Dev. 2016, 20, 1906–1910. [Google Scholar] [CrossRef]
- Walker, G.; Römann, P.; Poller, B.; Löbmann, K.; Grohganz, H.; Rooney, J.S.; Huff, G.S.; Smith, G.P.S.; Rades, T.; Gordon, K.C.; et al. Probing pharmaceutical mixtures during milling: The potency of low-frequency Raman spectroscopy in identifying disorder. Mol. Pharm. 2017, 14, 4675–4684. [Google Scholar] [CrossRef]
- Teraoka, R.; Otsuka, M.; Matsuda, Y. Evaluation of photostability of solid-state dimethyl 1,4-dihydro-2,6-dimethyl-4-(2-nitro-phenyl)-3, 5-pyridinedicarboxylate by using Fourier-transformed reflection-absorption infrared spectroscopy. Int. J. Pharm. 1999, 184, 35–43. [Google Scholar] [CrossRef]
- Teraoka, R.; Otsuka, M.; Matsuda, Y. Evaluation of photostability of solid-state nicardipine hydrochloride polymorphs by using Fourier-transformed reflection-absorption infrared spectroscopy—Effect of grinding on the photostability of crystal form. Int. J. Pharm. 2004, 286, 1–8. [Google Scholar] [CrossRef]
- Lubach, J.W.; Xu, D.; Segmuller, B.E.; Munson, E.J. Investigation of the effects of pharmaceutical processing upon solid-state NMR relaxation times and implications to solid-state formulation stability. J. Pharm. Sci. 2007, 96, 777–787. [Google Scholar] [CrossRef]
- Hudson, S.P.; Owens, E.; Hughes, H.; McLoughlin, P. Enhancement and restriction of chain motion in polymer networks. Int. J. Pharm. 2012, 430, 34–41. [Google Scholar] [CrossRef]
- Robson, J.K.; Sharples, D. Photoirradiation products of cyproheptadine and carbamazepine. J. Pharm. Pharmacol. 1984, 36, 843–844. [Google Scholar] [CrossRef]
- Shenmin, X.; Dang, L.; Wei, H. Solid-liquid phase equilibrium and phase diagram for the ternary carbamazepine-succinic acid-ethanol or acetone system at (298.15 and 308.15) K. J. Chem. Eng. Data 2011, 56, 2746–2750. [Google Scholar] [CrossRef]
- Pagire, S.K.; Jadav, N.; Vangala, V.R.; Whiteside, B.; Paradkar, A. Thermodynamic investigation of carbamazepine-saccharin co-crystal polymorphs. J. Pharm. Sci. 2017, 106, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
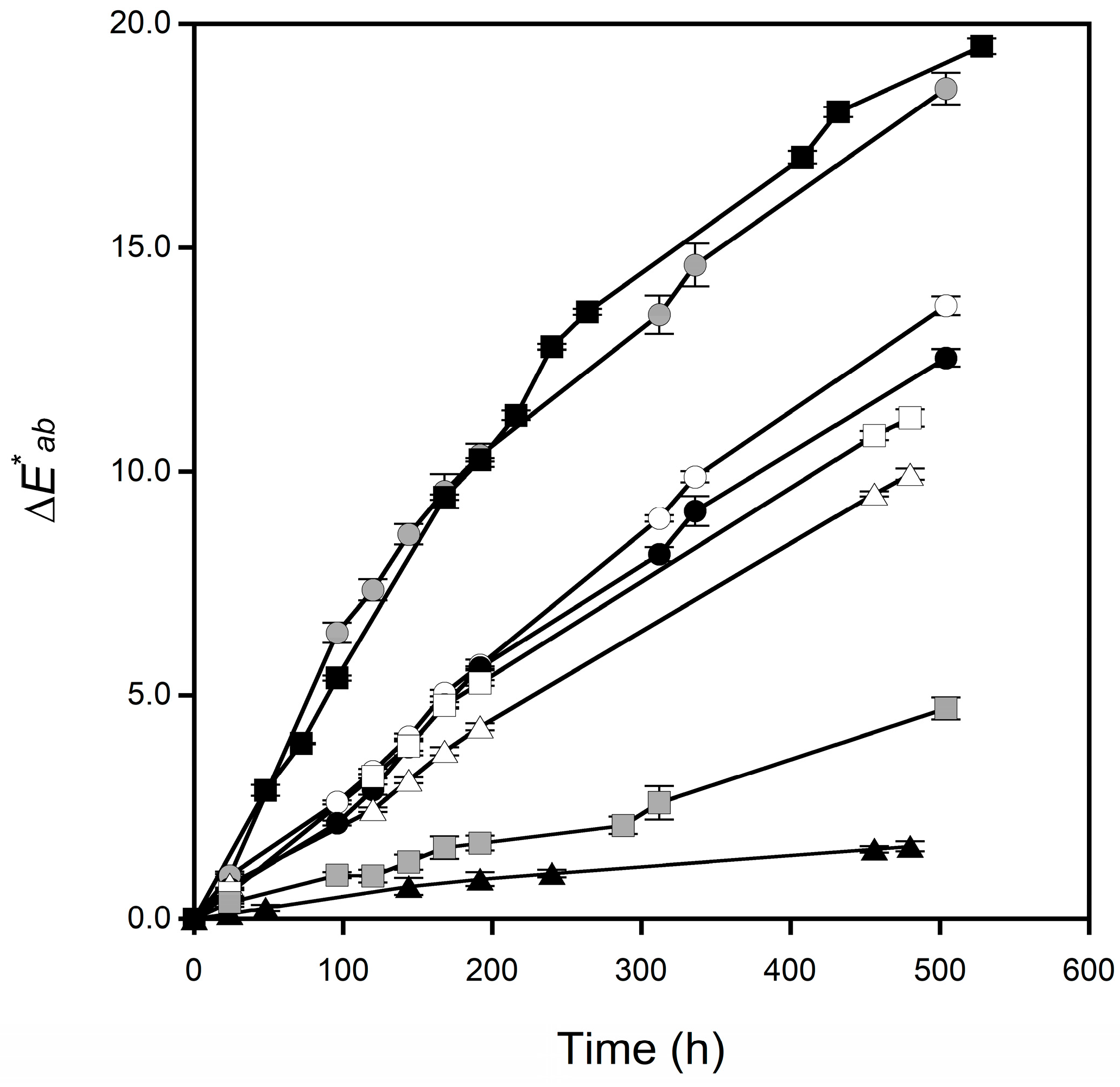
| Sample | Apparent Discoloration Rate Constant (h−1) |
|---|---|
| CBZ form I | 0.032 |
| CBZ form II | 0.054 |
| CBZ form III | 0.026 |
| CBZ–SUC PM | 0.024 |
| CBZ–SUC | 0.004 |
| CBZ–SAC PM | 0.026 |
| CBZ–SAC form I | 0.008 |
| CBZ–SAC form II | 0.064 |
| Sample | Degradation Rate Constant (h−1) |
|---|---|
| CBZ form I | 5.53 × 10−4 |
| CBZ form II | 6.51 × 10−4 |
| CBZ form III | 3.78 × 10−4 |
| CBZ–SUC PM | 4.86 × 10−4 |
| CBZ–SUC | 3.79 × 10−5 |
| CBZ–SAC PM | 2.63 × 10−4 |
| CBZ–SAC form I | 4.23 × 10−5 |
| CBZ–SAC form II | 1.82 × 10−3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yutani, R.; Haku, R.; Teraoka, R.; Tode, C.; Koide, T.; Kitagawa, S.; Sakane, T.; Fukami, T. Comparative Evaluation of the Photostability of Carbamazepine Polymorphs and Cocrystals. Crystals 2019, 9, 553. https://doi.org/10.3390/cryst9110553
Yutani R, Haku R, Teraoka R, Tode C, Koide T, Kitagawa S, Sakane T, Fukami T. Comparative Evaluation of the Photostability of Carbamazepine Polymorphs and Cocrystals. Crystals. 2019; 9(11):553. https://doi.org/10.3390/cryst9110553
Chicago/Turabian StyleYutani, Reiko, Ryotaro Haku, Reiko Teraoka, Chisato Tode, Tatsuo Koide, Shuji Kitagawa, Toshiyasu Sakane, and Toshiro Fukami. 2019. "Comparative Evaluation of the Photostability of Carbamazepine Polymorphs and Cocrystals" Crystals 9, no. 11: 553. https://doi.org/10.3390/cryst9110553
APA StyleYutani, R., Haku, R., Teraoka, R., Tode, C., Koide, T., Kitagawa, S., Sakane, T., & Fukami, T. (2019). Comparative Evaluation of the Photostability of Carbamazepine Polymorphs and Cocrystals. Crystals, 9(11), 553. https://doi.org/10.3390/cryst9110553