Crystal Structures of Putative Flavin Dependent Monooxygenase from Alicyclobacillus Acidocaldarius
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning and Protein Preparation
2.2. Crystallization, Data Collection, and Structure Determination
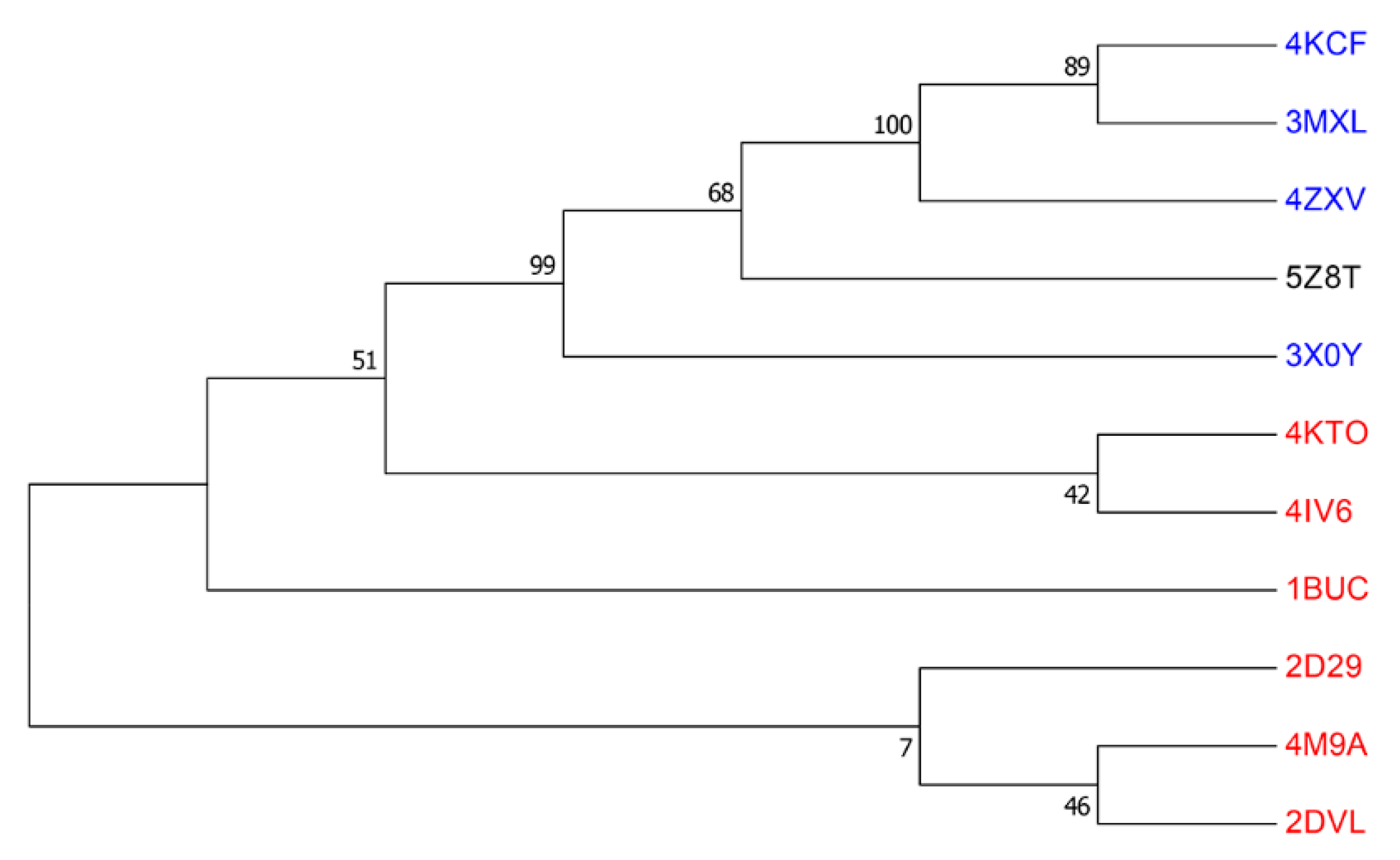
2.3. Phylogenetic Analysis
3. Results and Discussion
3.1. Overall Structure
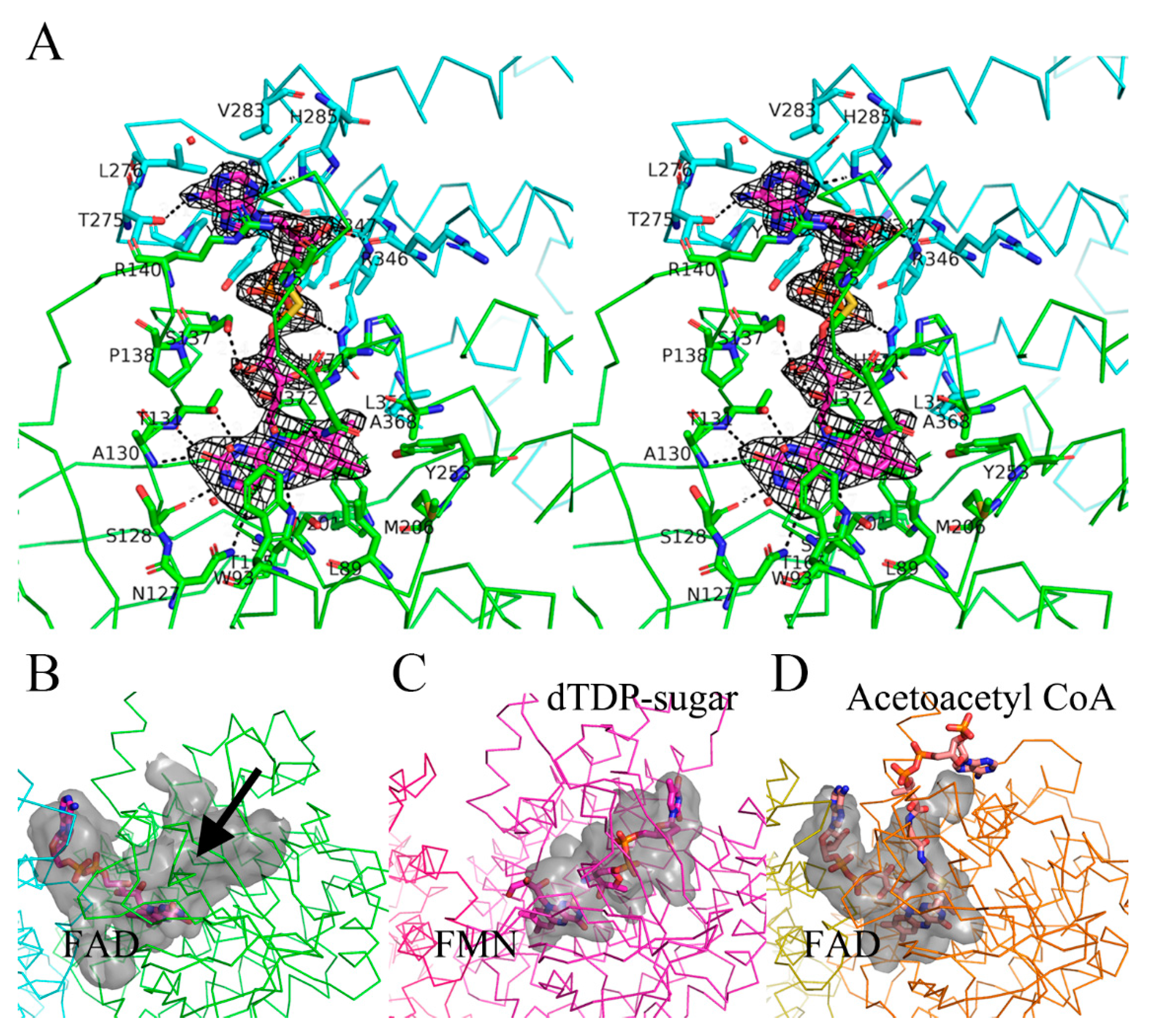
3.2. FAD and Potential Substrate Binding Site
3.3. Features of Group D Flavin Dependent Monooxygenase
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ballou, D.P.; Entsch, B.; Cole, L.J. Dynamics involved in catalysis by single-component and two-component flavin-dependent aromatic hydroxylases. Biochem. Biophys. Res. Commun. 2005, 338, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, M.M.; Montersino, S.; Westphal, A.H.; Tischler, D.; van Berkel, W.J. Flavin dependent monooxygenases. Arch. Biochem. Biophys. 2014, 544, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Torres Pazmino, D.E.; Winkler, M.; Glieder, A.; Fraaije, M.W. Monooxygenases as biocatalysts: Classification, mechanistic aspects and biotechnological applications. J. Biotechnol. 2010, 146, 9–24. [Google Scholar] [CrossRef] [PubMed]
- van Berkel, W.J.; Kamerbeek, N.M.; Fraaije, M.W. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J. Biotechnol. 2006, 124, 670–689. [Google Scholar] [CrossRef]
- Massey, V. Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem. 1994, 269, 22459–22462. [Google Scholar]
- Alfieri, A.; Fersini, F.; Ruangchan, N.; Prongjit, M.; Chaiyen, P.; Mattevi, A. Structure of the monooxygenase component of a two-component flavoprotein monooxygenase. Proc. Natl. Acad. Sci. USA 2007, 104, 1177–1182. [Google Scholar] [CrossRef]
- Guan, L.J.; Lee, W.C.; Wang, S.; Ohshiro, T.; Izumi, Y.; Ohtsuka, J.; Tanokura, M. Crystal structures of apo-DszC and FMN-bound DszC from Rhodococcus erythropolis D-1. FEBS J. 2015, 282, 3126–3135. [Google Scholar] [CrossRef]
- Parry, R.J.; Li, W. Purification and characterization of isobutylamine N-hydroxylase from the valanimycin producer Streptomyces viridifaciens MG456-hF10. Arch. Biochem. Biophys. 1997, 339, 47–54. [Google Scholar] [CrossRef]
- Vey, J.L.; Al-Mestarihi, A.; Hu, Y.; Funk, M.A.; Bachmann, B.O.; Iverson, T.M. Structure and mechanism of ORF36, an amino sugar oxidizing enzyme in everninomicin biosynthesis. Biochemistry 2010, 49, 9306–9317. [Google Scholar] [CrossRef]
- Bruender, N.A.; Thoden, J.B.; Holden, H.M. X-Ray structure of kijd3, a key enzyme involved in the biosynthesis of D-kijanose. Biochemistry 2010, 49, 3517–3524. [Google Scholar] [CrossRef]
- Sartor, L.; Ibarra, C.; Al-Mestarihi, A.; Bachmann, B.O.; Vey, J.L. Structure of DnmZ, a nitrososynthase in the Streptomyces peucetius anthracycline biosynthetic pathway. Acta Cryst. F Struct. Biol. Commun. 2015, 71, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Otwinowski, Z.; Minor, W. Processing of X-Ray diffraction data collected in oscillation mode. Methods Enzym. 1997, 276, 307–326. [Google Scholar]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Cryst. D Biol. Cryst. 2011, 67, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Vagin, A.A.; Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Cryst. D Biol. Cryst. 1997, 53, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Cryst. D Biol. Cryst. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall, W.B., 3rd; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-Atom structure validation for macromolecular crystallography. Acta Cryst. D Biol. Cryst. 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Oliveira, S.H.; Ferraz, F.A.; Honorato, R.V.; Xavier-Neto, J.; Sobreira, T.J.; de Oliveira, P.S. KVFinder: Steered identification of protein cavities as a PyMOL plugin. BMC Bioinform. 2014, 15, 197. [Google Scholar] [CrossRef]
- Kim, S.H.; Hisano, T.; Takeda, K.; Iwasaki, W.; Ebihara, A.; Miki, K. Crystal structure of the oxygenase component (HpaB) of the 4-hydroxyphenylacetate 3-monooxygenase from Thermus thermophilus HB8. J. Biol. Chem. 2007, 282, 33107–33117. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.; Rosenstrom, P. Dali server: Conservation mapping in 3D. Nucleic Acids Res. 2010, 38, W545–W549. [Google Scholar] [CrossRef] [PubMed]
- Battaile, K.P.; Molin-Case, J.; Paschke, R.; Wang, M.; Bennett, D.; Vockley, J.; Kim, J.J. Crystal structure of rat short chain acyl-CoA dehydrogenase complexed with acetoacetyl-CoA: Comparison with other acyl-CoA dehydrogenases. J. Biol. Chem. 2002, 277, 12200–12207. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Wang, M.; Paschke, R. Crystal structures of medium-chain acyl-CoA dehydrogenase from pig liver mitochondria with and without substrate. Proc. Natl. Acad. Sci. USA 1993, 90, 7523–7527. [Google Scholar] [CrossRef] [PubMed]
- Swigonova, Z.; Mohsen, A.W.; Vockley, J. Acyl-CoA dehydrogenases: Dynamic history of protein family evolution. J. Mol. Evol. 2009, 69, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]

| Apo (5GJ7) | FAD Bound (5Z8T) | |
|---|---|---|
| Data Collection | ||
| Space group | P21212 | I4122 |
| Unit Cell Dimensions (Å) | a = 155.7, b = 69.4, c = 83.3 | a = b = 177.3, c = 285.3 |
| Resolution (Å) a | 20-1.95 (1.98-1.95) | 20-2.35 (2.39-2.35) |
| Observed reflections | 1,136,579 | 4,407,376 |
| Unique reflections | 65,742 | 95,626 |
| Completeness (%) | 99.6 (100) | 100 (100) |
| Rsym b | 0.10 (0.65) | 0.14 (0.66) |
| I/σ(I) c | 19.1 (4.7) | 19.6 (4.0) |
| Refinement | ||
| Rcryst (%)/Rfree (%) d | 19.8/22.3 | 22.4/25.6 |
| No. of Residues | 776 | 1182 |
| No. of FAD | - | 3 |
| No. of Water molecules | 271 | 610 |
| R.m.s deviations | ||
| Bond lengths (Å) | 0.016 | 0.017 |
| Bond angles (°) | 1.667 | 1.605 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, H.; Shin, S.; Choe, J. Crystal Structures of Putative Flavin Dependent Monooxygenase from Alicyclobacillus Acidocaldarius. Crystals 2019, 9, 548. https://doi.org/10.3390/cryst9110548
Moon H, Shin S, Choe J. Crystal Structures of Putative Flavin Dependent Monooxygenase from Alicyclobacillus Acidocaldarius. Crystals. 2019; 9(11):548. https://doi.org/10.3390/cryst9110548
Chicago/Turabian StyleMoon, Hyunjin, Sungwook Shin, and Jungwoo Choe. 2019. "Crystal Structures of Putative Flavin Dependent Monooxygenase from Alicyclobacillus Acidocaldarius" Crystals 9, no. 11: 548. https://doi.org/10.3390/cryst9110548
APA StyleMoon, H., Shin, S., & Choe, J. (2019). Crystal Structures of Putative Flavin Dependent Monooxygenase from Alicyclobacillus Acidocaldarius. Crystals, 9(11), 548. https://doi.org/10.3390/cryst9110548




