Se–Cl Interactions in Selenite Chlorides: A Theoretical Study
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Zn2(SeO3)Cl2 Polymorphs
3.2. Cu5O2(SeO3)2Cl2 Polymorphs
- (1)
- the Cu–O, Cu–Cl and Se–O interactions with ∇2ρ(rc) > 0 and H(rc) < 0 (intermediate bonding interactions);
- (2)
- the Cu–O and Cu–Cl interactions with ∇2ρ(rc) > 0 and H(rc) > 0 (closed-shell interactions); the Cu3–Cl interaction (2.739 Å) deserves special attention as this interaction lies exactly on the border between intermediate and closed-shell interactions (H(rc) = 0);
- (3)
- the closed-shell Se–Cl, Cl–Cl, and Cl–O interactions with ∇2ρ(rc) > 0 and H(rc) > 0.
3.3. Burnsite, KCdCu7O2(SeO3)2Cl9
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hellenbrandt, M. The Inorganic Crystal Structure Database (ICSD)—Present and future. Crystallogr. Rev. 2004, 10, 17–22. [Google Scholar] [CrossRef]
- Millet, P.; Bastide, B.; Pashchenko, V.; Gnatchenko, S.; Gapon, V.; Ksarid, Y.; Stepanov, A. Syntheses, crystal structures and magnetic properties of francisite compounds Cu3Bi(SeO3)2O2X (X = Cl, Br and I). J. Mater. Chem. 2001, 11, 1152–1157. [Google Scholar] [CrossRef]
- Shen, Y.-L.; Mao, J.-G.; Jiang, H.-L. Synthesis, crystal structure and magnetic property of a new nickel selenite chloride: Ni5(SeO3)4Cl2. J. Solid State Chem. 2005, 178, 2942–2946. [Google Scholar] [CrossRef]
- Becker, R.; Prester, M.; Berger, H.; Lin, P.H.; Johnsson, M.; Drobac, D.; Zivkovic, I. Crystal structure and magnetic properties of two new cobalt selenite halides: Co5(SeO3)4X2 (X = Cl, Br). J. Solid State Chem. 2007, 180, 1051–1059. [Google Scholar] [CrossRef]
- Zhang, D.; Berger, H.; Kremer, R.K.; Wulferding, D.; Lemmens, P.; Johnsson, M. Synthesis, crystal structure, and magnetic properties of the copper selenite chloride Cu5(SeO3)4Cl2. Inorg. Chem. 2010, 49, 9683–9688. [Google Scholar] [CrossRef] [PubMed]
- Berdonosov, P.S.; Janson, O.; Olenev, A.V.; Krivovichev, S.V.; Rosner, H.; Dolgikh, V.A.; Tsirlin, A.A. Crystal structures and variable magnetism of PbCu2(XO3)2Cl2 with X = Se, Te. Dalton Trans. 2013, 42, 9547–9554. [Google Scholar] [CrossRef] [PubMed]
- Berdonosov, P.S.; Kuznetsova, E.S.; Dolgikh, V.A.; Sobolev, A.V.; Presniakov, I.A.; Olenev, A.V.; Rahaman, B.; Saha-Dasgupta, T.; Zakharov, K.V.; Zvereva, E.A.; et al. Crystal structure, physical properties, and electronic and magnetic structure of the spin S = 5/2 zigzag chain compound Bi2Fe(SeO3)2OCl3. Inorg. Chem. 2014, 53, 5830–5838. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, K.V.; Zvereva, E.A.; Berdonosov, P.S.; Kuznetsova, E.S.; Dolgikh, V.A.; Clark, L.; Black, C.; Lightfoot, P.; Kockelmann, W.; Pchelkina, Z.V.; et al. Thermodynamic properties, electron spin resonance, and underlying spin model in Cu3Y(SeO3)2O2Cl. Phys. Rev. B 2014, 90, 214417. [Google Scholar] [CrossRef]
- Markina, M.M.; Zakharov, K.V.; Zvereva, E.A.; Denisov, R.S.; Berdonosov, P.S.; Dolgikh, V.A.; Kuznetsova, E.S.; Olenev, A.V.; Vasiliev, A.N. Static and dynamic magnetic properties of two synthetic francisites Cu3La(SeO3)2O2X (X = Br and Cl). Phys. Chem. Miner. 2017, 44, 277–285. [Google Scholar] [CrossRef]
- Badrtdinov, D.I.; Kuznetsova, E.S.; Verchenko, V.Y.; Berdonosov, P.S.; Dolgikh, V.A.; Mazurenko, V.V.; Tsirlin, A.A. Magnetism of coupled spin tetrahedra in ilinskite-type KCu5O2(SeO3)2Cl3. Sci. Rep. 2018, 8, 2379. [Google Scholar] [CrossRef] [PubMed]
- Berdonosov, P.S.; Kuznetsova, E.S.; Dolgikh, V.A. Transition metal selenite halides: A fascinating family of magnetic compounds. Crystals 2018, 8, 159. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Filatov, S.K.; Semenova, T.F.; Filosofova, T.M. Sofiite Zn2(SeO3)Cl2—A new mineral from volcanic sublimates. Zap. Vses. Mineral. Obshch. 1989, 118, 65–69. (In Russian) [Google Scholar]
- Pring, A.; Gatehouse, B.M.; Birch, W.D. Francisite, Cu3Bi(SeO3)2O2Cl, new mineral from Iron Monarch, South Australia: Description and crystal structure. Am. Mineral. 1990, 75, 1421–1425. [Google Scholar]
- Vergasova, L.P.; Semenova, T.F.; Shuvalov, R.R.; Filatov, S.K.; Anan’yev, V.V. Ilinskite NaCu5O2(SeO3)2Cl3—A new mineral of volcanic exhalations. Dokl. Akad. Nauk 1997, 353, 641–644. (In Russian) [Google Scholar]
- Vergasova, L.; Krivovichev, S.; Semenova, T.; Filatov, S.; Ananiev, V. Chloromenite, Cu9O2(SeO3)4Cl6, a new mineral from the Tolbachik volcano, Kamchatka, Russia. Eur. J. Mineral. 1999, 11, 119–123. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Semenova, T.F.; Filatov, S.K.; Krivovichev, S.V.; Shuvalov, R.R.; Ananiev, V.V. Georgbokiite Cu5O2(SeO3)2Cl2—A new mineral from volcanic sublimates. Dokl. Akad. Nauk 1999, 364, 527–531. (In Russian) [Google Scholar]
- Campostrini, I.; Gramaccioli, C.M.; Demartin, F. Orlandiite, Pb3Cl4(SeO3)·H2O, a new mineral species, and an associated lead-copper selenite chloride from the Baccu Locci mine, Sardinia, Italy. Can. Mineral. 1999, 37, 1493–1498. [Google Scholar]
- Krivovichev, S.V.; Vergasova, L.P.; Starova, G.L.; Filatov, S.K.; Britvin, S.N.; Roberts, A.C.; Steele, I.M. Burnsite, KCdCu7O2(SeO3)2Cl9, a new mineral species from the Tolbachik Volcano, Kamchatka Peninsula, Russia. Can. Mineral. 2002, 40, 1171–1175. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Krivovichev, S.V.; Britvin, S.N.; Filatov, S.K.; Burns, P.C.; Ananyev, V.V. Allochalcoselite, Cu+Cu2+5PbO2(SeO3)2Cl5—A new mineral from volcanic exhalations (Kamchatka, Russia). Zap. Ross. Mineral. Obshch. 2005, 134, 70–74. (In Russian) [Google Scholar]
- Vergasova, L.P.; Krivovichev, S.V.; Filatov, S.K.; Britvin, S.N.; Burns, P.C.; Ananyev, V.V. Parageorgbokiite, β-Cu5O2(SeO3)2Cl2—A new mineral from volcanic exhalations (Kamchatka peninsula, Russia). Zap. Ross. Mineral. Obshch. 2006, 135, 24–28. [Google Scholar] [CrossRef]
- Gemmi, M.; Campostrini, I.; Demartin, F.; Gorelik, T.E.; Gramaccioli, C.M. Structure of the new mineral sarrabusite, Pb5CuCl4(SeO3)4, solved by manual electron-diffraction tomography. Acta Crystallogr. 2012, B68, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Shuvalov, R.R.; Vegasova, L.P.; Semenova, T.F.; Filatov, S.K.; Krivovichev, S.V.; Siidra, O.I.; Rudashevsky, N.S. Prewittite, KPb1.5Cu6Zn(SeO3)2O2Cl10, a new mineral from Tolbachik fumaroles, Kamchatka peninsula, Russia: Description and crystal structure. Am. Mineral. 2013, 98, 463–469. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Semenova, T.F.; Krivovichev, S.V.; Filatov, S.K.; Zolotarev, A.A., Jr.; Ananiev, V.V. Nicksobolevite, Cu7(SeO3)2O2Cl6, a new complex copper oxoselenite chloride from Tolbachik fumaroles, Kamchatka peninsula, Russia. Eur. J. Mineral. 2014, 26, 439–449. [Google Scholar] [CrossRef]
- Johnsson, M.; Tornroos, K.W.; Lemmens, P.; Millet, P. Crystal structure and magnetic properties of a new two-dimensional S = 1 quantum system Ni5(TeO3)3X2 (X = Cl, Br). Chem. Mater. 2003, 15, 68–73. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules; Oxford Science Publications: Oxford, UK, 1990; ISBN 9780198558651. [Google Scholar]
- Gatti, C. Chemical bonding in crystals: New directions. Z. Kristallogr. 2005, 220, 399–457. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H…F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Pendás, A.M.; Costales, A.; Luaña, V. Ions in crystals: The topology of the electron density in ionic materials. I. Fundamentals. Phys. Rev. B 1997, 55, 4275. [Google Scholar] [CrossRef]
- Vegas, A.; Santamaria-Perez, D.; Marques, M.; Florez, M.; Garcia-Baonza, V.; Recio, J.M. Anions in metallic matrices model: Application to the aluminium crystal chemistry. Acta Crystallogr. 2006, B62, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Dunitz, J.D. Intermolecular atom-atom bonds in crystals? IUCrJ 2015, 2, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, C.; Espinosa, E.; Matta, C.F. On atom-atom ‘short contact’ bonding interactions in crystals. IUCrJ 2015, 2, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Nelyubina, Y.V.; Antipin, M.Y.; Lyssenko, K.A. Anion–anion interactions: Their nature, energy and role in crystal formation. Russ. Chem. Rev. 2010, 79, 167–187. [Google Scholar] [CrossRef]
- Gibbs, G.V.; Downs, R.T.; Cox, D.F.; Ross, N.L.; Boisen, M.B., Jr.; Rosso, K.M. Shared and closed-shell O–O interactions in silicates. J. Phys. Chem. A 2008, 112, 3693–3699. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, G.V.; Wallace, A.F.; Cox, D.F.; Dove, P.M.; Downs, R.T.; Ross, N.L.; Rosso, K.M. Role of directed van der Waals bonded interactions in the determination of the structures of molecular arsenate solids. J. Phys. Chem. A 2009, 113, 736–749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gibbs, G.V.; Wallace, A.F.; Zallen, R.; Downs, R.T.; Ross, N.L.; Cox, D.F.; Rosso, K.M. Bond paths and van der Waals interactions in orpiment, As2S3. J. Phys. Chem. A 2010, 114, 6550–6557. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, G.V.; Wallace, A.F.; Downs, R.T.; Ross, N.L.; Cox, D.F.; Rosso, K.M. Thioarsenides: A case for long-range Lewis acid–base-directed van der Waals interactions. Phys. Chem. Miner. 2011, 38, 267–291. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C. Crystal chemistry of lead oxide chlorides. I. Crystal structures of synthetic mendipite, Pb3O2Cl2, and synthetic damaraite, Pb3O2(OH)Cl. Eur. J. Mineral. 2001, 13, 801–809. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Armbruster, T.; Depmeier, W. Crystal structures of Pb8O5(AsO4)2 and Pb5O4(CrO4), and review of PbO-related structural units in inorganic compounds. J. Solid State Chem. 2004, 177, 1321–1332. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Siidra, O.I.; Nazarchuk, E.V.; Burns, P.C.; Depmeier, W. Particular topological complexity of lead oxide blocks in Pb31O22X18 (X = Br, Cl). Inorg. Chem. 2006, 45, 3846–3848. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V.; Armbruster, T.; Depmeier, W. One-dimensional lone electron pair micelles in the crystal structure of Pb5(SiO4)(VO4)2. Mater. Res. Bull. 2004, 39, 1717–1722. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C. Crystal chemistry of uranyl molybdates. VIII. Crystal structures of Na3Tl3[(UO2)(MoO4)4], Na13Tl3[(UO2)(MoO4)3]4(H2O)5, Na3Tl5[(UO2)(MoO4)3]2(H2O)3 and Na2[(UO2)(MoO4)2](H2O)4. Can. Mineral. 2003, 41, 707–719. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K.; Burns, P.C.; Vergasova, L.P. The crystal structure of allochalcoselite, Cu+Cu2+5PbO2(SeO3)2Cl5, a mineral with well-defined Cu+ and Cu2+ positions. Can. Miner. 2006, 44, 507–514. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K.; Vergasova, L.P. The crystal structure of ilinskite, NaCu5O2(SeO3)2Cl3, and review of mixed-ligand CuOmCln coordination geometries in minerals and inorganic compounds. Miner. Petrol. 2013, 107, 235–242. [Google Scholar] [CrossRef]
- Semenova, T.F.; Rozhdestvenskaya, I.V.; Filatov, S.K.; Vergasova, L.P. Crystal structure and physical properties of sophiite, Zn2(SeO3)Cl2, a new mineral. Mineral. Mag. 1992, 56, 241–245. [Google Scholar] [CrossRef]
- Johnsson, M.; Tornroos, K.W. Zinc selenium oxochloride, β-Zn2(SeO3)Cl2, a synthetic polymorph of the mineral sophiite. Acta Crystallogr. C 2007, 63, i34–i36. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V.; Shuvalov, R.R.; Semenova, T.F.; Filatov, S.K. Crystal chemistry of inorganic compounds based on chains of oxocentered tetrahedra. III. Crystal structure of georgbokiite, Cu5O2(SeO3)2Cl2. Z. Kristallogr. 1999, 214, 135–138. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K.; Burns, P.C.; Vergasova, L.P. The crystal structure of parageorgbokiite, β-Cu5O2(SeO3)2Cl2. Can. Mineral. 2007, 45, 929–934. [Google Scholar] [CrossRef]
- Burns, P.C.; Krivovichev, S.V.; Filatov, S.K. New Cu2+ coordination polyhedra in the crystal structure of burnsite, KCdCu7O2(SeO3)2Cl9. Can. Mineral. 2002, 40, 1587–1595. [Google Scholar] [CrossRef]
- Dovesi, R.; Orlando, R.; Erba, A.; Zicovich-Wilson, C.M.; Civalleri, B.; Casassa, S.; Maschio, L.; Ferrabone, M.; De La Pierre, M.; D’Arco, P.; et al. CRYSTAL14: A program for ab initio investigation of crystalline solids. Int. J. Quantum Chem. 2014, 114, 1287–1317. [Google Scholar] [CrossRef]
- Peintinger, M.F.; Oliveira, D.V.; Bredow, T. Consistent Gaussian basis sets of triple-zeta valence with polarization quality for solid-state calculations. J. Comput. Chem. 2013, 34, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Egdell, R.G.; Law, D.S.L.; Harrison, N.M.; Searle, B.G. An experimental and theoretical investigation of the electronic structure of CdO. J. Phys. Condens. Matter 1998, 10, 8447–8458. [Google Scholar] [CrossRef][Green Version]
- Gatti, C.; Casassa, S. TOPOND14. User's Manual; CNR-ISTM of Milano: Milano, Italy, 2013. [Google Scholar]
- Gatti, C.; Saunders, V.R.; Roetti, C. Crystal field effects on the topological properties of the electron density in molecular crystals. The case of urea. J. Chem. Phys. 1994, 101, 10686–10696. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Siidra, O.I.; Colmont, M.; Mentré, O.; Krivovichev, S.V. Emulating exhalative chemistry: Synthesis and structural characterization of ilinskite, Na[Cu5O2](SeO3)2Cl3, and its K-analogue. Miner. Petrol. 2015, 109, 421–430. [Google Scholar] [CrossRef]
- Galy, J.; Bonnet, J.J.; Andersson, S. The crystal structure of a new oxide chloride of copper(II) and selenium(IV) Cu5Se2O8Cl2. Acta Chem. Scand. 1979, A33, 383–389. [Google Scholar] [CrossRef][Green Version]
- Bergerhoff, G.; Paeslack, J. Sauerstoff als Koordinationszentrum in Kristallstrukturen. Z. Kristallogr. 1968, 126, 112–123. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Mentré, O.; Siidra, O.I.; Colmont, M.; Filatov, S.K. Anion-centered tetrahedra in inorganic compounds. Chem. Rev. 2013, 113, 6459–6535. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V. Structure description, interpretation and classification in mineralogical crystallography. Crystallogr. Rev. 2017, 23, 2–71. [Google Scholar] [CrossRef]
- Haas, H.; Jansen, M. Synthese und Charakterisierung von Na5AsO5. Z. Anorg. Allg. Chem. 2001, 627, 1013–1016. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Minerals with antiperovskite structure: A review. Z. Kristallogr. 2008, 223, 109–113. [Google Scholar] [CrossRef]
- Reckeweg, O.; Blaschkowski, B.; Schleid, T. Li5OCl3 and Li3OCl: Two remarkably different lithium oxide chlorides. Z. Anorg. Allg. Chem. 2012, 638, 2081–2086. [Google Scholar] [CrossRef]
- Nuss, J.; Muehle, C.; Hayama, K.; Abdolazimi, V.; Takagi, H. Tilting structures in inverse perovskites, M3TtO (M = Ca, Sr, Ba, Eu; Tt = Si, Ge, Sn, Pb). Acta Crystallogr. 2015, B71, 300–312. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gfeller, F.; Armbruster, T.; Galuskina, I.O.; Vapnik, Y.; Murashko, M.; Włodyka, R.; Dzierżanowski, P. New minerals with a modular structure derived from hatrurite from the pyrometamorphic Hatrurim Complex. Part I. Nabimusaite, KCa12(SiO4)4(SO4)2O2F, from larnite rocks of Jabel Harmun, Palestinian Autonomy, Israel. Mineral. Mag. 2015, 79, 1061–1072. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gfeller, F.; Galuskina, I.O.; Pakhomova, A.; Armbruster, T.; Vapnik, Y.; Włodyka, R.; Dzierżanowski, P.; Murashko, M. New minerals with a modular structure derived from hatrurite from the pyrometamorphic Hatrurim Complex. Part II. Zadovite, BaCa6[(SiO4)(PO4)](PO4)2F and aradite, BaCa6[(SiO4)(VO4)](VO4)2F, from paralavas of the Hatrurim Basin, Negev Desert, Israel. Mineral. Mag. 2015, 79, 1073–1087. [Google Scholar] [CrossRef]
- Oudah, M.; Ikeda, A.; Hausmann, J.N.; Yonezawa, S.; Fukumoto, T.; Kobayashi, S.; Sato, M.; Maeno, Y. Superconductivity in the antiperovskite Dirac-metal oxide Sr3-xSnO. Nat. Commun. 2016, 12, 13617. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.T.; Antonyshyn, I.; Prots, Y.; Valldor, M. Anti-perovskite Li-battery cathode materials. J. Am. Chem. Soc. 2017, 139, 9645–9649. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V.; Starova, G.L.; Filatov, S.K. “Face-to-face” relationships between oxocentered tetrahedra and cation-centered tetrahedral oxyanions in crystal structures of minerals and inorganic compounds. Mineral. Mag. 1999, 63, 263–266. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K. Structural principles for minerals and inorganic compounds containing anion-centered tetrahedra. Am. Mineral. 1999, 84, 1099–1106. [Google Scholar] [CrossRef]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states. I. Orbital degeneracy. Proc. R. Soc. A 1937, A161, 220–235. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry. The Bond Valence Model, 2nd ed.; Oxford University Press: Oxford, UK, 2016; ISBN 9780198742951. [Google Scholar]
- Semenova, T.F.; Pankratova, O.Y.; Habanova, A.A.; Shuvalov, R.R. Synthesis of exhalation copper selenites analogues by chemical gas transport reactions method. Vestnik Sankt-Peterb. Univ. Ser. 4 Fiz. Khim. 2005, 2, 75–81. (In Russian) [Google Scholar]
- Kovrugin, V.M.; Colmont, M.; Mentré, O.; Siidra, O.I.; Krivovichev, S.V. Dimers of oxocentred [OCu4]6+ tetrahedra in two novel copper selenite chlorides, K[Cu3O](SeO3)2Cl and Na2[Cu7O2](SeO3)4Cl4, and related minerals and inorganic compounds. Mineral Mag. 2016, 80, 227–238. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, W.; Zhang, S.; Xiang, H.; Cui, M.; He, Z. Na2Cu7(SeO3)4O2Cl4: A selenite chloride compound with Cu7 units showing spin-frustration and a magnetization plateau. Dalton Trans. 2016, 45, 8324–8326. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Benz, S.; Macchione, M.; Verolet, Q.; Mareda, J.; Sakai, N.; Matile, S. Anion transport with chalcogen bonds. J. Am. Chem. Soc. 2016, 138, 9093–9096. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sanz, G.; Trujillo, C. Improvement of anion transport systems by modulation of chalcogen interactions: The influence of solvent. J. Phys. Chem. A 2018, 122, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Batsanov, S.S. Van der Waals radii of elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Rozas, I.; Elguero, J.; Molins, E. About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem. Phys. Lett. 2001, 336, 457–461. [Google Scholar] [CrossRef]
- Desiraju, G.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 2001; ISBN 9780198509707. [Google Scholar]
- Nelyubina, Y.V.; Antipin, M.Y.; Lyssenko, K.A. Extremely short halogen bond: The nature and energy of iodine–oxygen interactions in crystalline iodic acid. Mendeleev Commun. 2011, 21, 250–252. [Google Scholar] [CrossRef]
- Mata, I.; Alkorta, I.; Molins, E.; Espinosa, E. Electrostatics at the origin of the stability of phosphate-phosphate complexes locked by hydrogen bonds. ChemPhysChem 2012, 13, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, F.; Klein, R.A. Anti-electrostatic hydrogen bonds. Angew. Chem. Int. Ed. 2014, 53, 11214–11217. [Google Scholar] [CrossRef] [PubMed]

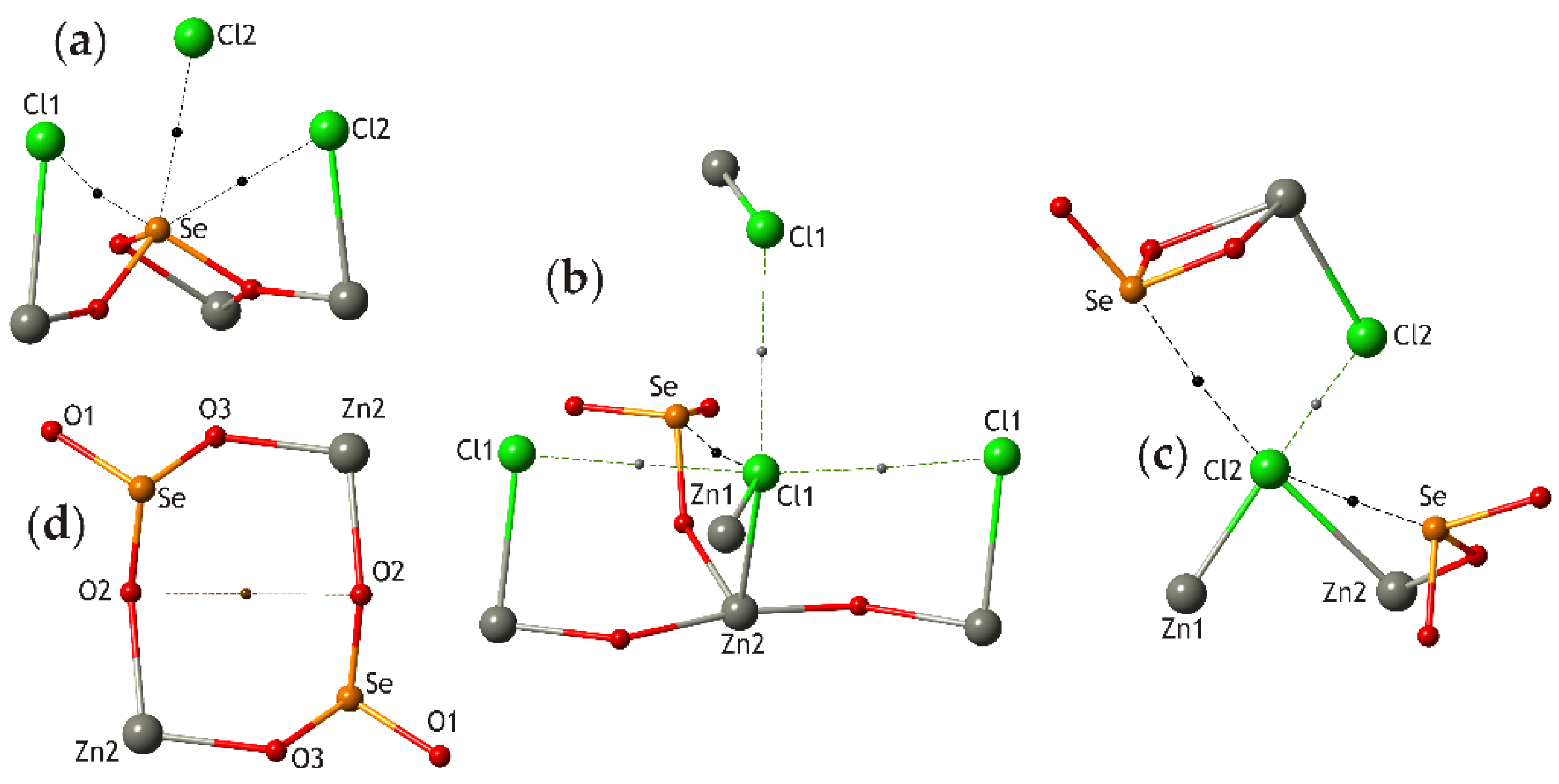
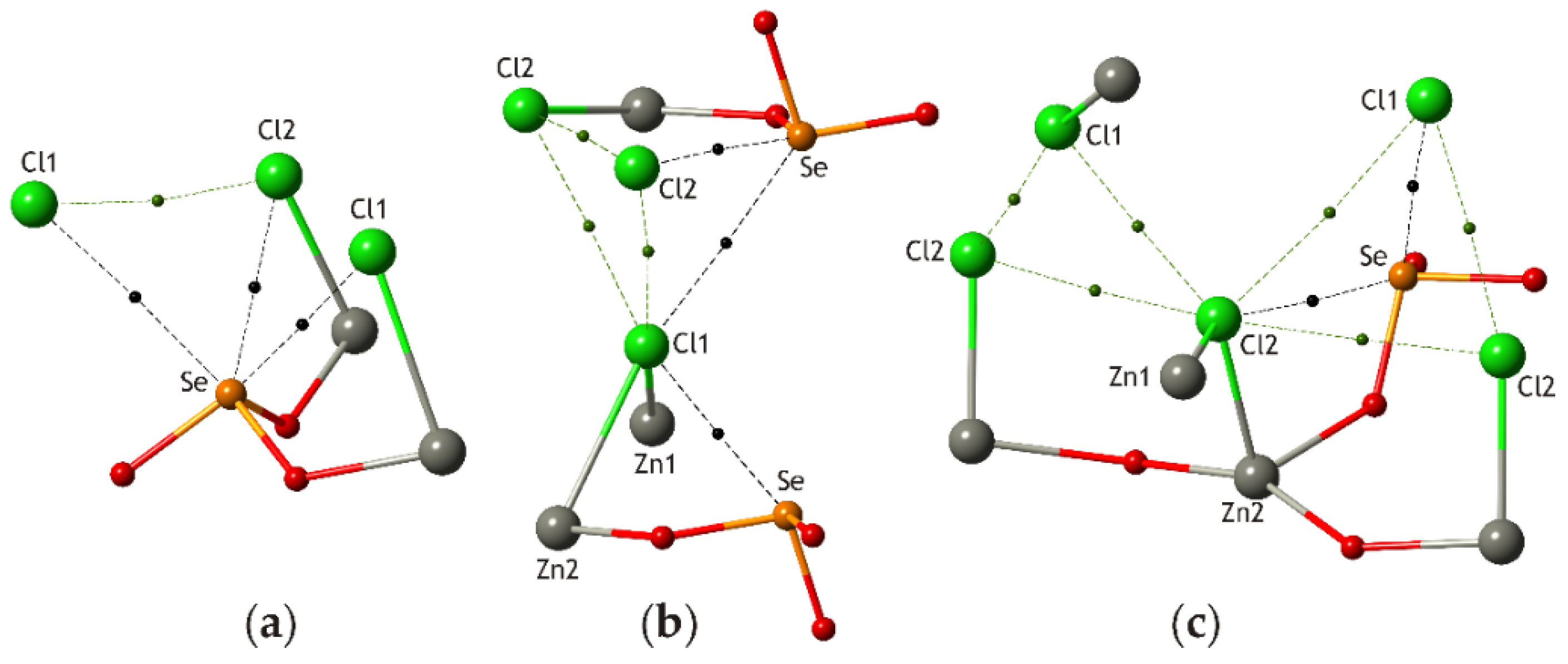
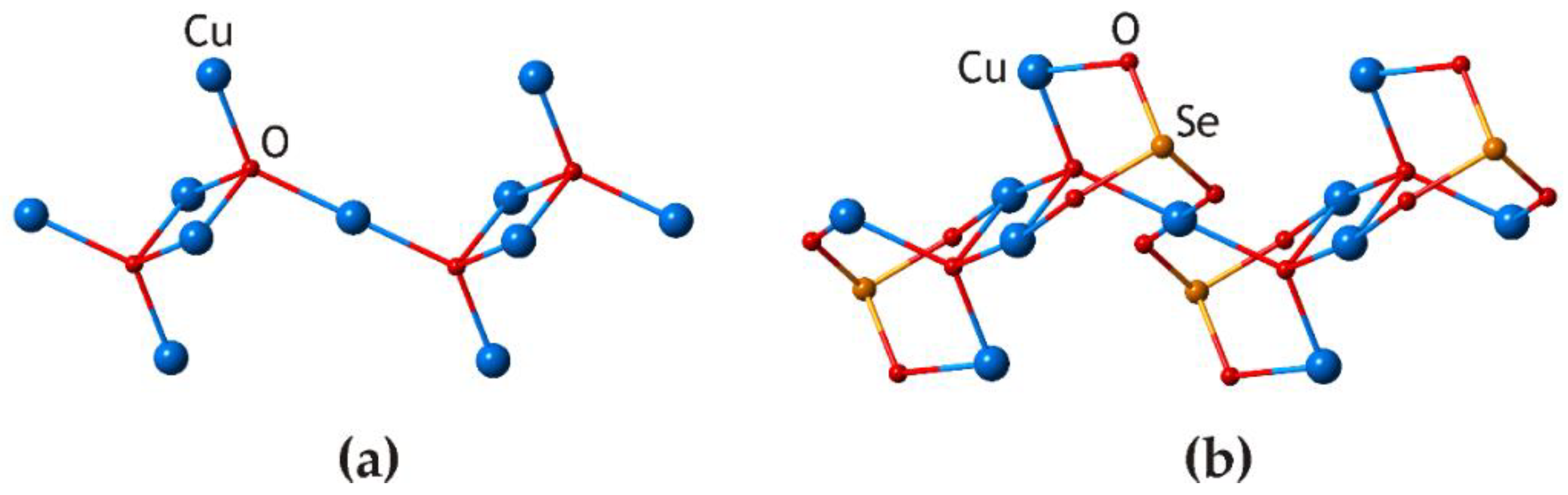
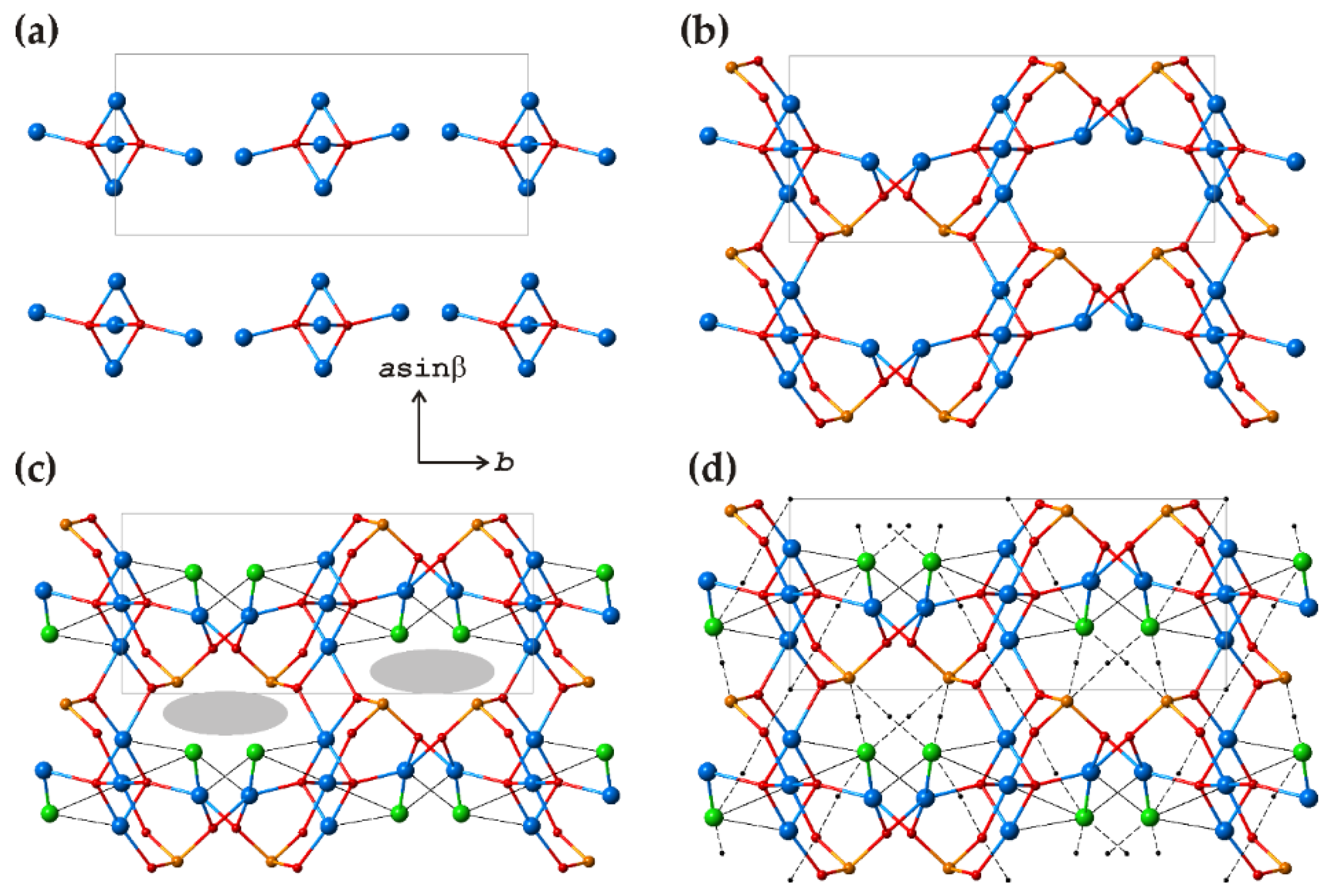
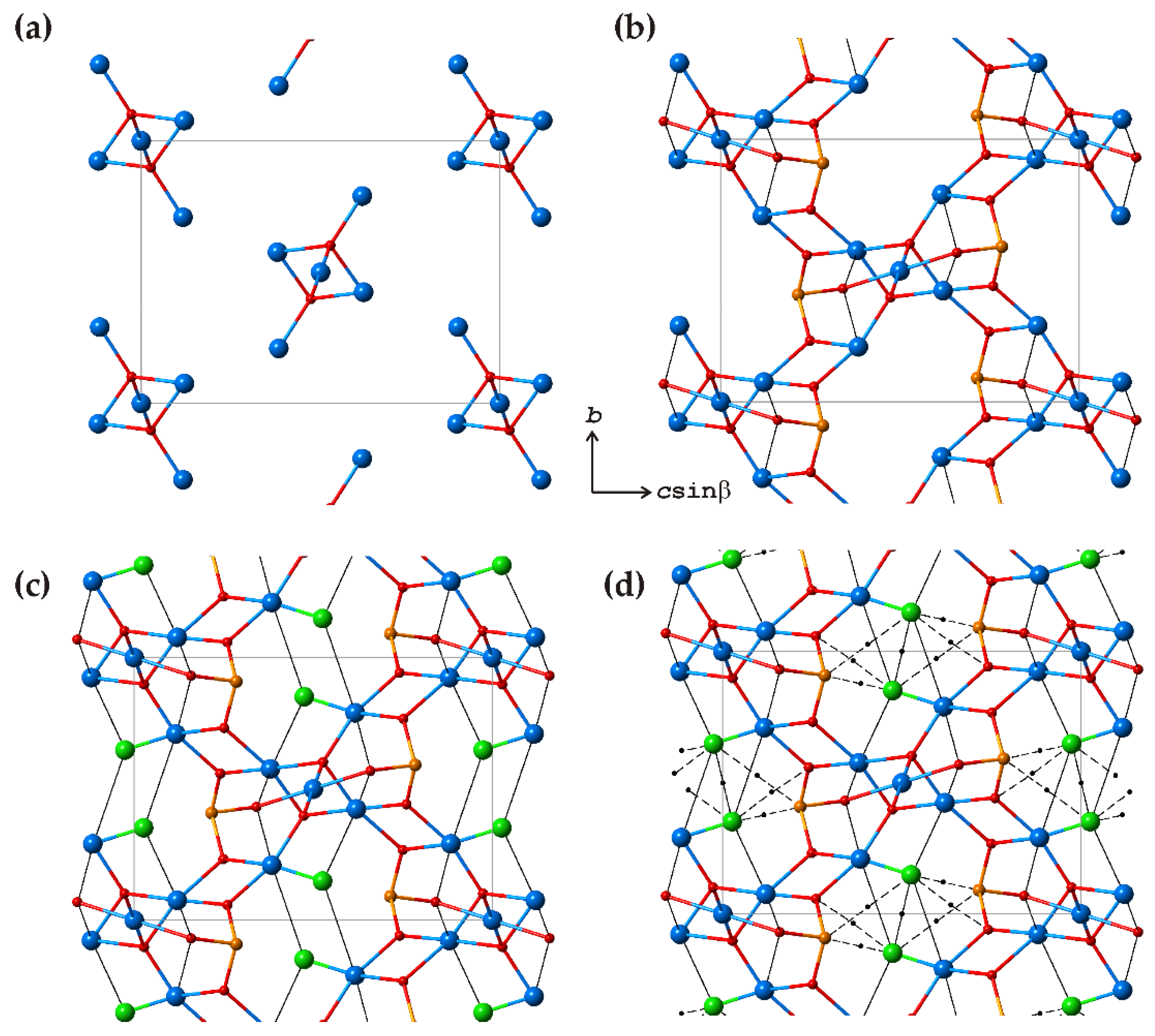
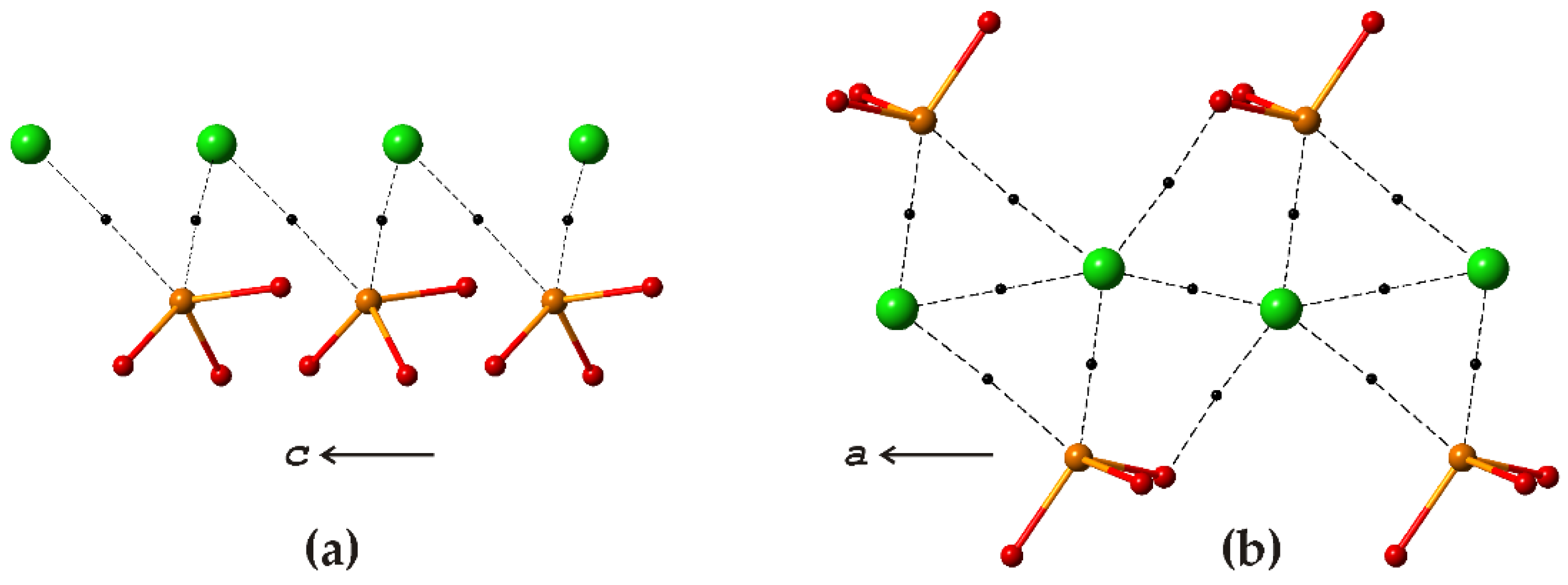
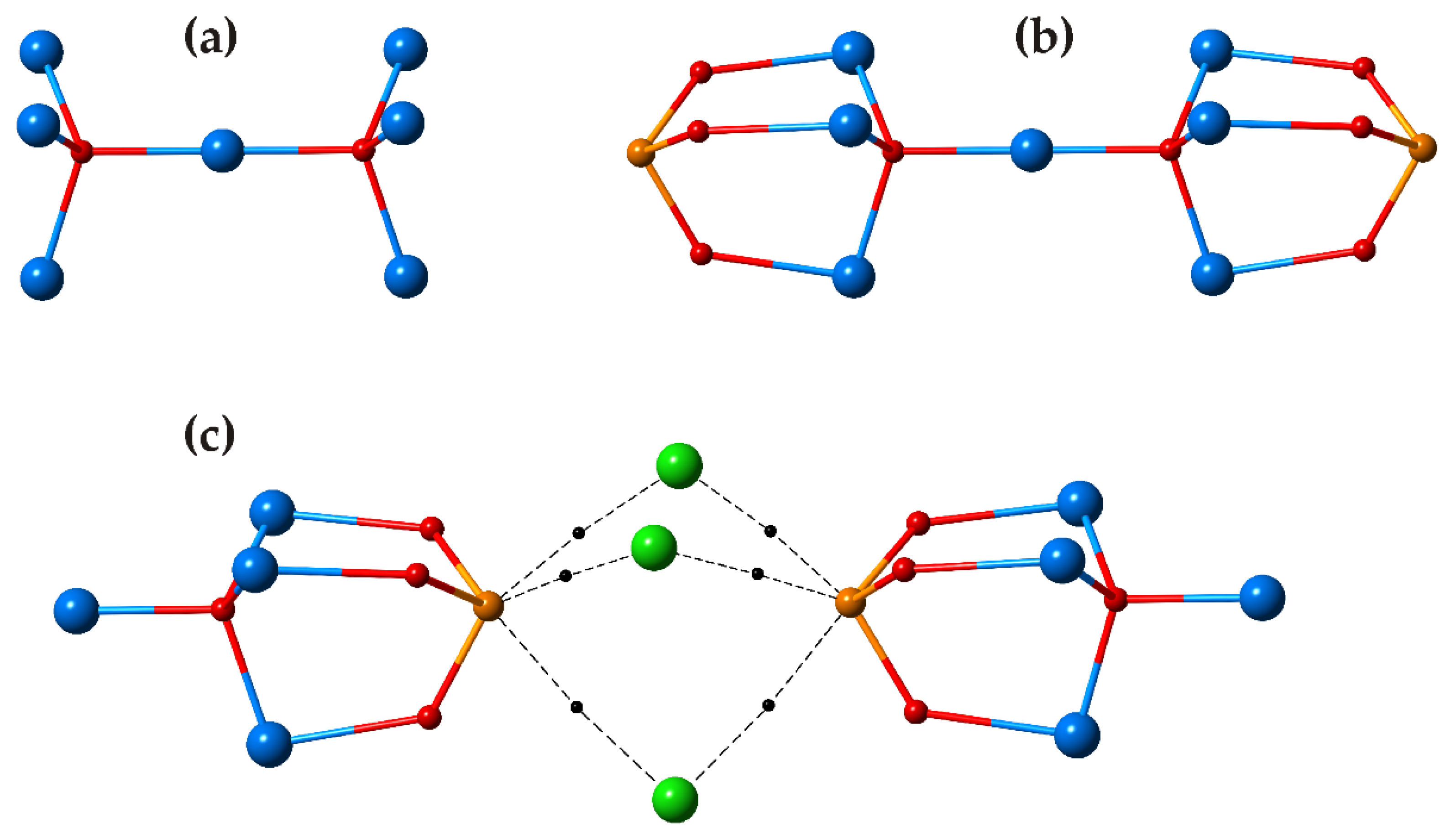
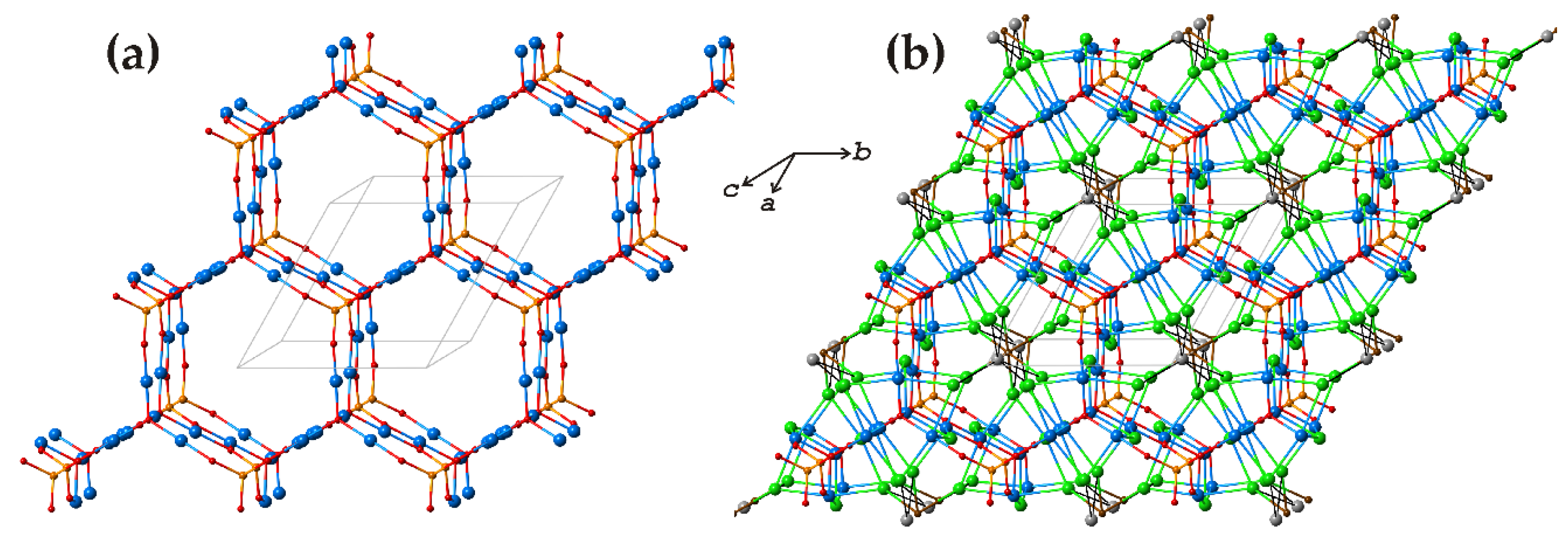
| # | Chemical Formula | Mineral Name | Space Group | a, Å/α, Deg. | b, Å/β, Deg. | c, Å/γ, Deg. | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | α-Zn2(SeO3)Cl2 | sofiite | Pccn | 10.251/90 | 15.223/90 | 7.666/90 | [44] |
| 2 | β-Zn2(SeO3)Cl2 | - | P21/c | 7.670/90 | 10.261/100.0 | 7.657/90 | [45] |
| 3 | α-Cu5O2(SeO3)2Cl2 | georgbokiite | P21/c | 6.030/90 | 13.744/95.8 | 5.562/90 | [46] |
| 4 | β-Cu5O2(SeO3)2Cl2 | parageorgbokiite | P21/c | 5.398/90 | 8.054/99.3 | 11.128/90 | [47] |
| 5 | KCdCu7O2(SeO3)2Cl9 | burnsite | P63/mmc | 8.781/90 | 8.781/90 | 15.521/120 | [48] |
| A1 | A2 | d [Å] | ρ(rc) | ∇2ρ(rc) | G(rc) | V(rc) | H(rc) | BD |
|---|---|---|---|---|---|---|---|---|
| Se–O and Zn–X bonds (X = O, Cl) | ||||||||
| Se | O1 | 1.688 | 0.1940 | 0.4591 | 0.2357 | −0.3566 | −0.1209 | −0.624 |
| Se | O3 | 1.697 | 0.1922 | 0.4144 | 0.2247 | −0.3458 | −0.1211 | −0.630 |
| Se | O2 | 1.701 | 0.1883 | 0.4108 | 0.2196 | −0.3364 | −0.1168 | −0.620 |
| Zn1 | O1 | 1.993 | 0.0758 | 0.3908 | 0.1109 | −0.1242 | −0.0133 | −0.175 |
| Zn1 | O2 | 2.057 | 0.0667 | 0.3232 | 0.0908 | −0.1007 | −0.0099 | −0.148 |
| Zn1 | Cl2 | 2.205 | 0.0784 | 0.2664 | 0.0849 | −0.1033 | −0.0184 | −0.235 |
| Zn1 | Cl1 | 2.244 | 0.0732 | 0.2346 | 0.0746 | −0.0905 | −0.0159 | −0.217 |
| Zn2 | O1 | 1.993 | 0.0781 | 0.3984 | 0.1144 | −0.1292 | −0.0148 | −0.190 |
| Zn2 | O2 | 2.115 | 0.0558 | 0.2667 | 0.0717 | −0.0766 | −0.0049 | −0.088 |
| Zn2 | O3 | 1.955 | 0.0837 | 0.4391 | 0.1267 | −0.1435 | −0.0168 | −0.201 |
| Zn2 | O3 | 2.147 | 0.0551 | 0.2511 | 0.0687 | −0.0747 | −0.0060 | −0.109 |
| Zn2 | Cl2 | 2.685 | 0.0298 | 0.0783 | 0.0220 | −0.0245 | −0.0025 | −0.084 |
| Zn2 | Cl1 | 2.753 | 0.0256 | 0.0652 | 0.0180 | −0.0197 | −0.0017 | −0.066 |
| Se–Cl, Cl–Cl, and O–O interactions | ||||||||
| Se | Cl2 | 3.317 | 0.0121 | 0.0349 | 0.0078 | −0.0069 | 0.0009 | 0.074 |
| Se | Cl2 | 3.444 | 0.0103 | 0.0312 | 0.0069 | −0.0060 | 0.0009 | 0.087 |
| Se | Cl1 | 3.533 | 0.0080 | 0.0268 | 0.0057 | −0.0046 | 0.0011 | 0.138 |
| Cl1 | Cl1 | 3.898 | 0.0039 | 0.0143 | 0.0028 | −0.0021 | 0.0007 | 0.179 |
| Cl1 | Cl1 | 3.955 | 0.0029 | 0.0115 | 0.0022 | −0.0015 | 0.0007 | 0.241 |
| Cl2 | Cl2 | 3.567 | 0.0071 | 0.0208 | 0.0046 | −0.0041 | 0.0005 | 0.070 |
| O2 | O2 | 3.362 | 0.0055 | 0.0181 | 0.0038 | 0.0030 | 0.0008 | 0.145 |
| A1 | A2 | d [Å] | ρ(rc) | ∇2ρ(rc) | G(rc) | V(rc) | H(rc) | BD |
|---|---|---|---|---|---|---|---|---|
| Se–O and Zn–X bonds (X = O, Cl) | ||||||||
| Se1 | O3 | 1.703 | 0.1901 | 0.3856 | 0.2163 | −0.3361 | −0.1198 | −0.630 |
| Se1 | O1 | 1.707 | 0.1879 | 0.3797 | 0.2123 | −0.3297 | −0.1174 | −0.625 |
| Se1 | O2 | 1.715 | 0.1836 | 0.3736 | 0.2065 | −0.3196 | −0.1131 | −0.616 |
| Zn2 | O1 | 2.126 | 0.0574 | 0.2672 | 0.0733 | −0.0798 | −0.0065 | −0.113 |
| Zn1 | O1 | 1.983 | 0.0779 | 0.4009 | 0.1147 | −0.1291 | −0.0144 | −0.185 |
| Zn1 | O2 | 2.031 | 0.0702 | 0.3492 | 0.0984 | −0.1096 | −0.0112 | −0.160 |
| Zn1 | Cl1 | 2.222 | 0.0761 | 0.2519 | 0.0801 | −0.0972 | −0.0171 | −0.225 |
| Zn1 | Cl2 | 2.226 | 0.0751 | 0.2519 | 0.0795 | −0.0961 | −0.0166 | −0.221 |
| Zn2 | O3 | 2.008 | 0.0737 | 0.3733 | 0.1056 | −0.1178 | −0.0122 | −0.166 |
| Zn2 | O2 | 2.049 | 0.0663 | 0.3304 | 0.0914 | −0.1002 | −0.0088 | −0.133 |
| Zn2 | O3 | 2.150 | 0.0546 | 0.2470 | 0.0676 | −0.0735 | −0.0059 | −0.108 |
| Zn2 | Cl2 | 2.430 | 0.0487 | 0.1555 | 0.0445 | −0.0501 | −0.0056 | −0.115 |
| Zn2 | Cl1 | 2.765 | 0.0250 | 0.0649 | 0.0179 | −0.0196 | −0.0017 | −0.068 |
| Se–Cl, Cl–Cl, and O–O interactions | ||||||||
| Se1 | Cl2 | 3.382 | 0.0106 | 0.0343 | 0.0075 | −0.0064 | 0.0009 | 0.085 |
| Se1 | Cl1 | 3.482 | 0.0096 | 0.0297 | 0.0065 | −0.0055 | 0.0010 | 0.104 |
| Se1 | Cl1 | 3.514 | 0.0085 | 0.0252 | 0.0053 | −0.0044 | 0.0009 | 0.106 |
| Cl1 | Cl2 | 3.534 | 0.0079 | 0.0225 | 0.0051 | −0.0046 | 0.0005 | 0.063 |
| Cl1 | Cl2 | 3.956 | 0.0029 | 0.0113 | 0.0021 | −0.0014 | 0.0007 | 0.241 |
| Cl2 | Cl2 | 3.869 | 0.0041 | 0.0151 | 0.0030 | −0.0022 | 0.0008 | 0.195 |
| O2 | O2 | 3.470 | 0.0048 | 0.0155 | 0.0032 | −0.0026 | 0.0006 | 0.125 |
| A1 | A2 | d [Å] | ρ(rc) | ∇2ρ(rc) | G(rc) | V(rc) | H(rc) | BD |
|---|---|---|---|---|---|---|---|---|
| Cu1 | O1 | 1.923 | 0.0979 | 0.3812 | 0.1339 | −0.1725 | −0.0386 | −0.394 |
| Cu1 | O4 | 1.962 | 0.0855 | 0.3506 | 0.1189 | −0.1501 | −0.0312 | −0.365 |
| Cu1 | O4 | 2.046 | 0.0694 | 0.2766 | 0.0922 | −0.1153 | −0.0231 | −0.333 |
| Cu1 | Cl | 2.282 | 0.0682 | 0.1701 | 0.0658 | −0.0890 | −0.0232 | −0.340 |
| Cu1 | Cl | 2.570 | 0.0371 | 0.1248 | 0.0352 | −0.0392 | −0.0040 | −0.108 |
| Cu2 | O2 | 1.944 | 0.0877 | 0.3727 | 0.1248 | −0.1564 | −0.0316 | −0.360 |
| Cu2 | O1 | 1.971 | 0.0857 | 0.3284 | 0.1144 | −0.1467 | −0.0323 | −0.377 |
| Cu2 | Cl | 2.954 | 0.0164 | 0.0608 | 0.0138 | −0.0123 | 0.0015 | 0.091 |
| Cu3 | O2 | 1.989 | 0.0786 | 0.3254 | 0.1089 | −0.1364 | −0.0275 | −0.350 |
| Cu3 | O1 | 1.956 | 0.0911 | 0.3433 | 0.1214 | −0.1570 | −0.0356 | −0.391 |
| Cu3 | O1 | 1.952 | 0.0935 | 0.3464 | 0.1238 | −0.1611 | −0.0373 | −0.399 |
| Cu3 | O3 | 2.024 | 0.0723 | 0.2917 | 0.0973 | −0.1217 | −0.0244 | −0.337 |
| Cu3 | O2 | 2.430 | 0.0283 | 0.1314 | 0.0327 | −0.0326 | 0.0001 | 0.004 |
| Cu3 | Cl | 2.739 | 0.0254 | 0.0915 | 0.0229 | −0.0229 | 0.0000 | 0.000 |
| Se | O2 | 1.674 | 0.1998 | 0.4741 | 0.2453 | −0.3721 | −0.1268 | −0.635 |
| Se | O3 | 1.709 | 0.1870 | 0.3738 | 0.2105 | −0.3275 | −0.1170 | −0.626 |
| Se | O4 | 1.724 | 0.1820 | 0.3276 | 0.1952 | −0.3084 | −0.1132 | −0.622 |
| Se | Cl | 3.310 | 0.0129 | 0.0378 | 0.0083 | −0.0072 | 0.0011 | 0.085 |
| Se | Cl | 3.544 | 0.0086 | 0.0244 | 0.0053 | −0.0045 | 0.0008 | 0.093 |
| Cl | O2 | 3.216 | 0.0113 | 0.0358 | 0.0082 | −0.0074 | 0.0008 | 0.071 |
| O2 | O2 | 3.972 | 0.0083 | 0.0297 | 0.0062 | −0.0050 | 0.0012 | 0.145 |
| A1 | A2 | d [Å] | ρ(rc) | ∇2ρ(rc) | G(rc) | V(rc) | H(rc) | BD |
|---|---|---|---|---|---|---|---|---|
| Cu1 | O1 | 1.919 | 0.0976 | 0.3890 | 0.1350 | −0.1727 | −0.0377 | −0.386 |
| Cu1 | O2 | 1.966 | 0.0824 | 0.3500 | 0.1164 | −0.1453 | −0.0289 | −0.351 |
| Cu1 | O3 | 1.969 | 0.0834 | 0.3406 | 0.1153 | −0.1455 | −0.0302 | −0.362 |
| Cu1 | Cl | 2.268 | 0.0706 | 0.1716 | 0.0676 | −0.0923 | −0.0247 | −0.350 |
| Cu1 | O4 | 2.944 | 0.0096 | 0.0425 | 0.0081 | −0.0056 | 0.0025 | 0.260 |
| Cu1 | Cl | 3.185 | 0.0096 | 0.0392 | 0.0079 | −0.0060 | 0.0019 | 0.198 |
| Cu2 | O1 | 1.929 | 0.0944 | 0.3815 | 0.1314 | −0.1675 | −0.0361 | −0.382 |
| Cu2 | O4 | 1.974 | 0.0823 | 0.3329 | 0.1128 | −0.1425 | −0.0297 | −0.361 |
| Cu3 | O1 | 1.945 | 0.0934 | 0.3585 | 0.1260 | −0.1625 | −0.0365 | −0.391 |
| Cu3 | O2 | 1.961 | 0.0832 | 0.3576 | 0.1187 | −0.1480 | −0.0293 | −0.352 |
| Cu3 | O1 | 1.975 | 0.0885 | 0.3237 | 0.1158 | −0.1506 | −0.0348 | −0.393 |
| Cu3 | O3 | 2.057 | 0.0672 | 0.2650 | 0.0881 | −0.1099 | −0.0218 | −0.324 |
| Cu3 | O4 | 2.399 | 0.0311 | 0.1409 | 0.0363 | −0.0374 | −0.0011 | −0.035 |
| Cu3 | Cl | 2.705 | 0.0283 | 0.0977 | 0.0254 | −0.0264 | −0.0010 | −0.035 |
| Se | O4 | 1.675 | 0.2000 | 0.4610 | 0.2428 | −0.3704 | −0.1276 | −0.638 |
| Se | O3 | 1.713 | 0.1850 | 0.3654 | 0.2061 | −0.3208 | −0.1147 | −0.620 |
| Se | O2 | 1.724 | 0.1813 | 0.3406 | 0.1971 | −0.3091 | −0.1120 | −0.618 |
| Se | Cl | 3.300 | 0.0133 | 0.0408 | 0.0093 | −0.0083 | 0.0010 | 0.075 |
| Se | Cl | 3.327 | 0.0123 | 0.0357 | 0.0078 | −0.0067 | 0.0011 | 0.089 |
| Cl | O2 | 3.321 | 0.0097 | 0.0278 | 0.0065 | −0.0060 | 0.0005 | 0.052 |
| Cl | Cl | 3.501 | 0.0104 | 0.0284 | 0.0068 | −0.0065 | 0.0003 | 0.029 |
| Cl | Cl | 3.810 | 0.0046 | 0.0158 | 0.0032 | −0.0025 | 0.0007 | 0.152 |
| A1 | A2 | d [Å] | ρ(rc) | ∇2ρ(rc) | G(rc) | V(rc) | H(rc) | BD |
|---|---|---|---|---|---|---|---|---|
| Cd | Cl1 | 2.614 | 0.0426 | 0.1420 | 0.0395 | −0.0436 | −0.0041 | −0.096 |
| Cu1 | O1 | 1.899 | 0.1016 | 0.4263 | 0.1447 | −0.1828 | −0.0381 | −0.375 |
| Cu1 | O2 | 1.916 | 0.0906 | 0.4215 | 0.1362 | −0.1670 | −0.0308 | −0.340 |
| Cu1 | O2 | 2.127 | 0.0580 | 0.2304 | 0.0736 | −0.0896 | −0.0160 | −0.276 |
| Cu1 | Cl2 | 2.555 | 0.0369 | 0.1152 | 0.0331 | −0.0374 | −0.0043 | −0.117 |
| Cu1 | Cl1 | 2.612 | 0.0331 | 0.1101 | 0.0300 | −0.0324 | −0.0024 | −0.073 |
| Cu2 | O1 | 1.914 | 0.0980 | 0.4169 | 0.1409 | −0.1776 | −0.0367 | −0.374 |
| Cu2 | Cl2 | 2.451 | 0.0484 | 0.1460 | 0.0464 | −0.0562 | −0.0098 | −0.202 |
| Se | O2 | 1.687 | 0.1936 | 0.4591 | 0.2350 | −0.3553 | −0.1203 | −0.621 |
| Se | Cl2 | 3.532 | 0.0085 | 0.0270 | 0.0058 | −0.0048 | 0.0010 | 0.118 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivovichev, S.V.; Gorelova, L.A. Se–Cl Interactions in Selenite Chlorides: A Theoretical Study. Crystals 2018, 8, 193. https://doi.org/10.3390/cryst8050193
Krivovichev SV, Gorelova LA. Se–Cl Interactions in Selenite Chlorides: A Theoretical Study. Crystals. 2018; 8(5):193. https://doi.org/10.3390/cryst8050193
Chicago/Turabian StyleKrivovichev, Sergey V., and Liudmila A. Gorelova. 2018. "Se–Cl Interactions in Selenite Chlorides: A Theoretical Study" Crystals 8, no. 5: 193. https://doi.org/10.3390/cryst8050193
APA StyleKrivovichev, S. V., & Gorelova, L. A. (2018). Se–Cl Interactions in Selenite Chlorides: A Theoretical Study. Crystals, 8(5), 193. https://doi.org/10.3390/cryst8050193






