Self-Assembly of a Carboxyl-Functionalized BODIPY Dye via Hydrogen Bonding
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis
2.2.1. Synthesis of Compound 2
2.2.2. Synthesis of Compound 1
2.3. Crystal Structure Determination of Compound 1
3. Results and Discussion
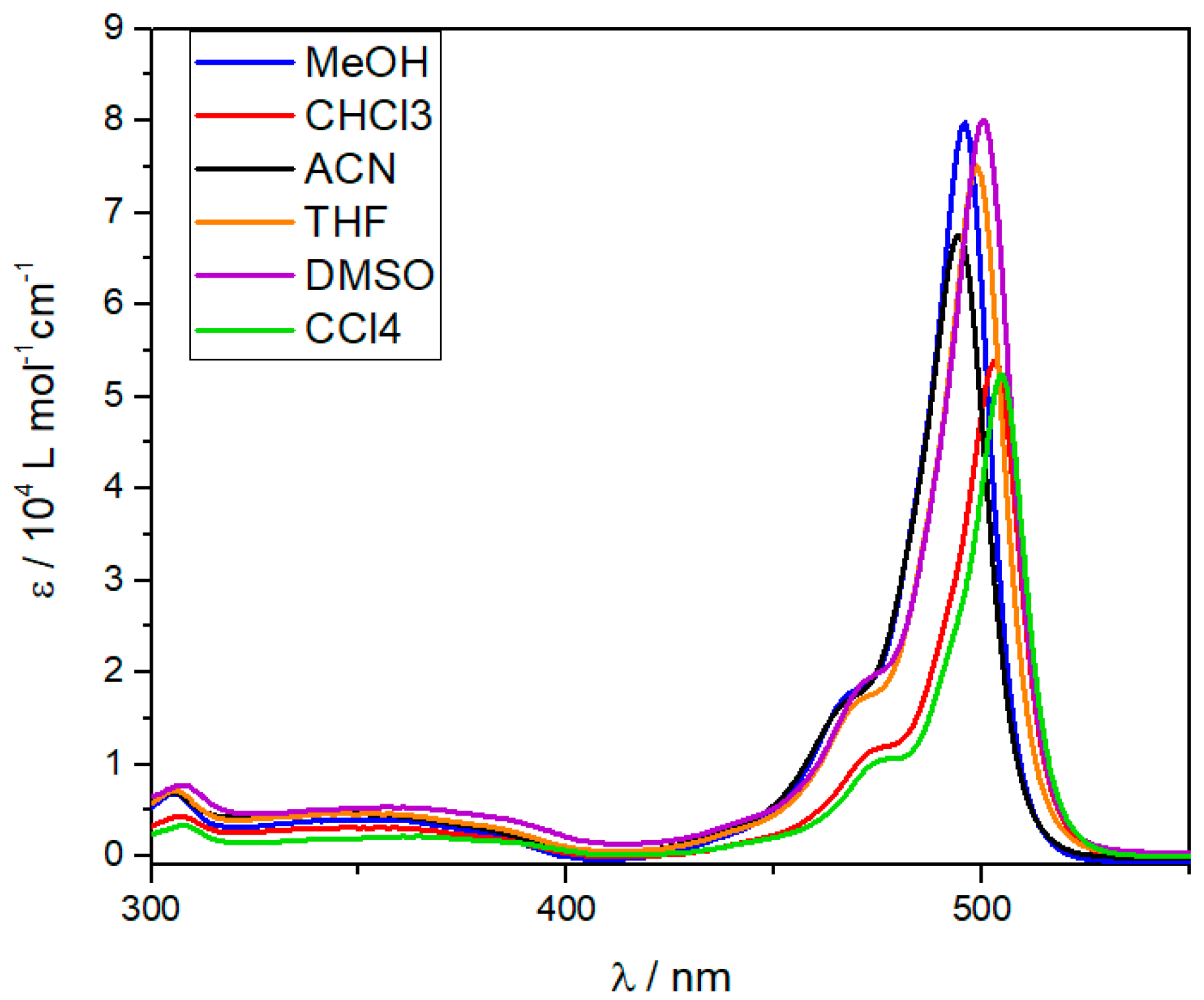
3.1. UV/Vis Experiments
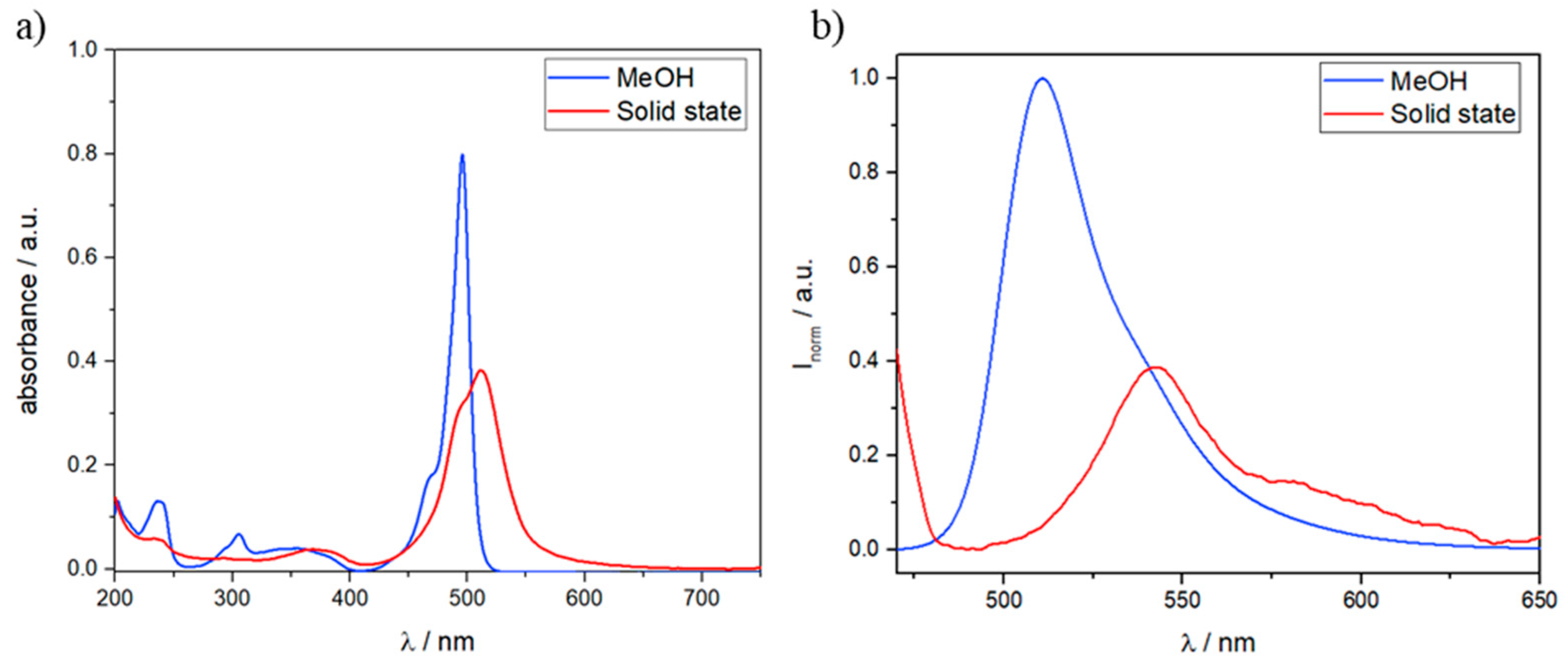
3.2. Solid State UV/Vis and Fluorescence Spectra
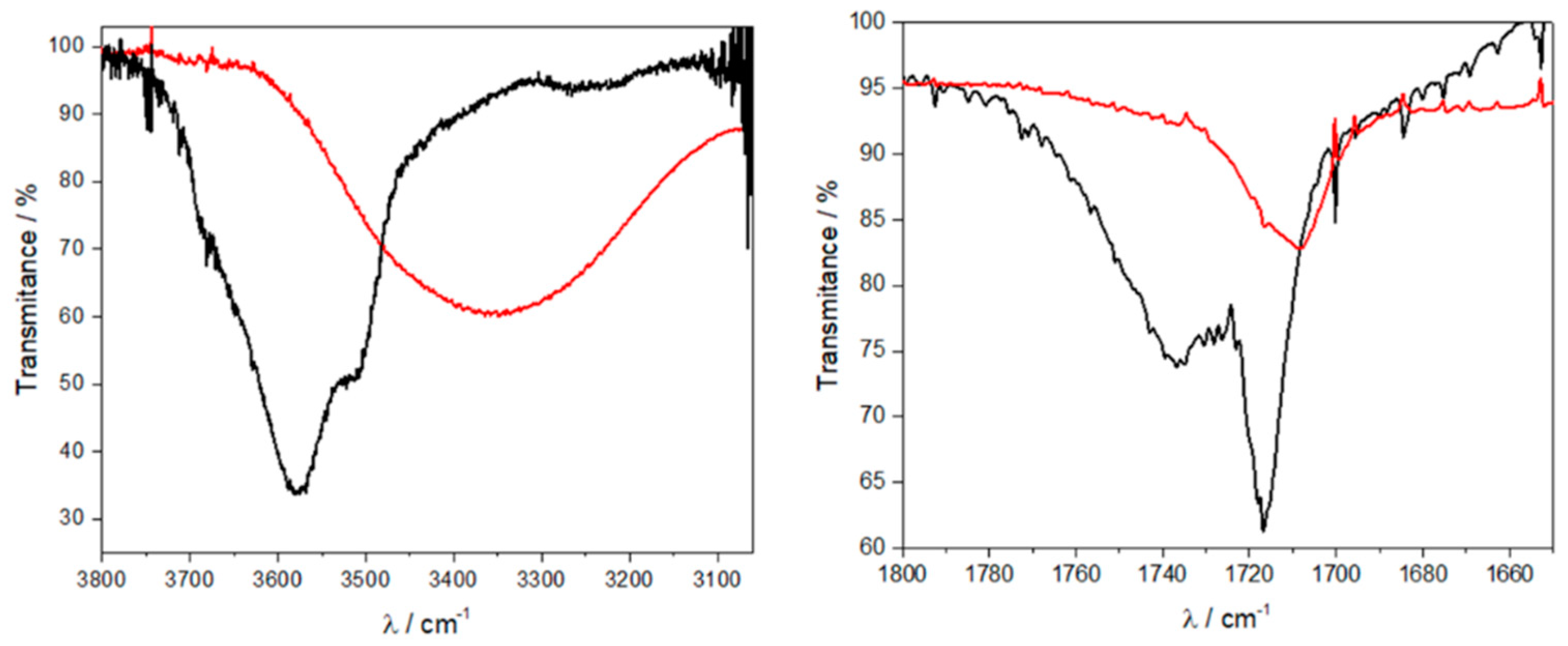
3.3. FTIR Spectra
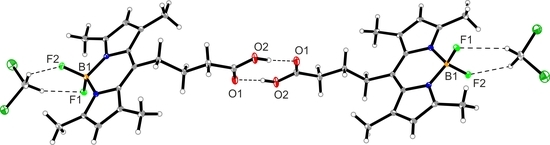
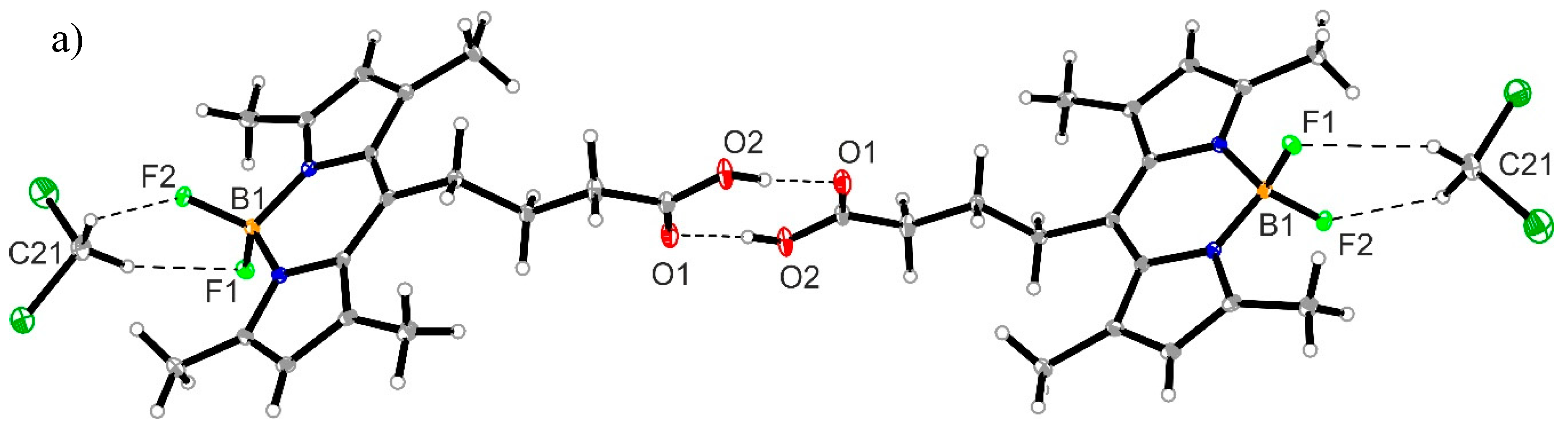
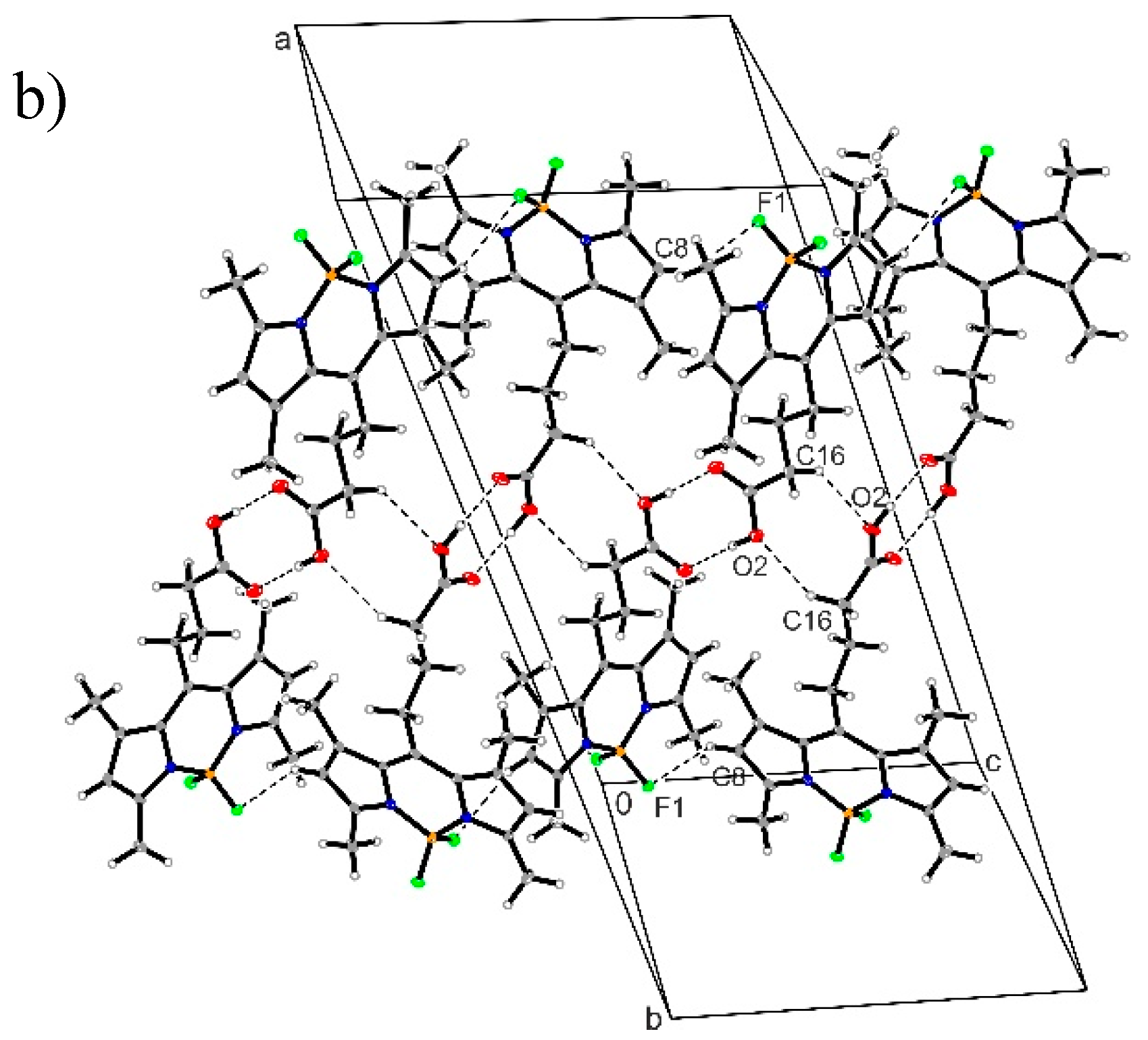
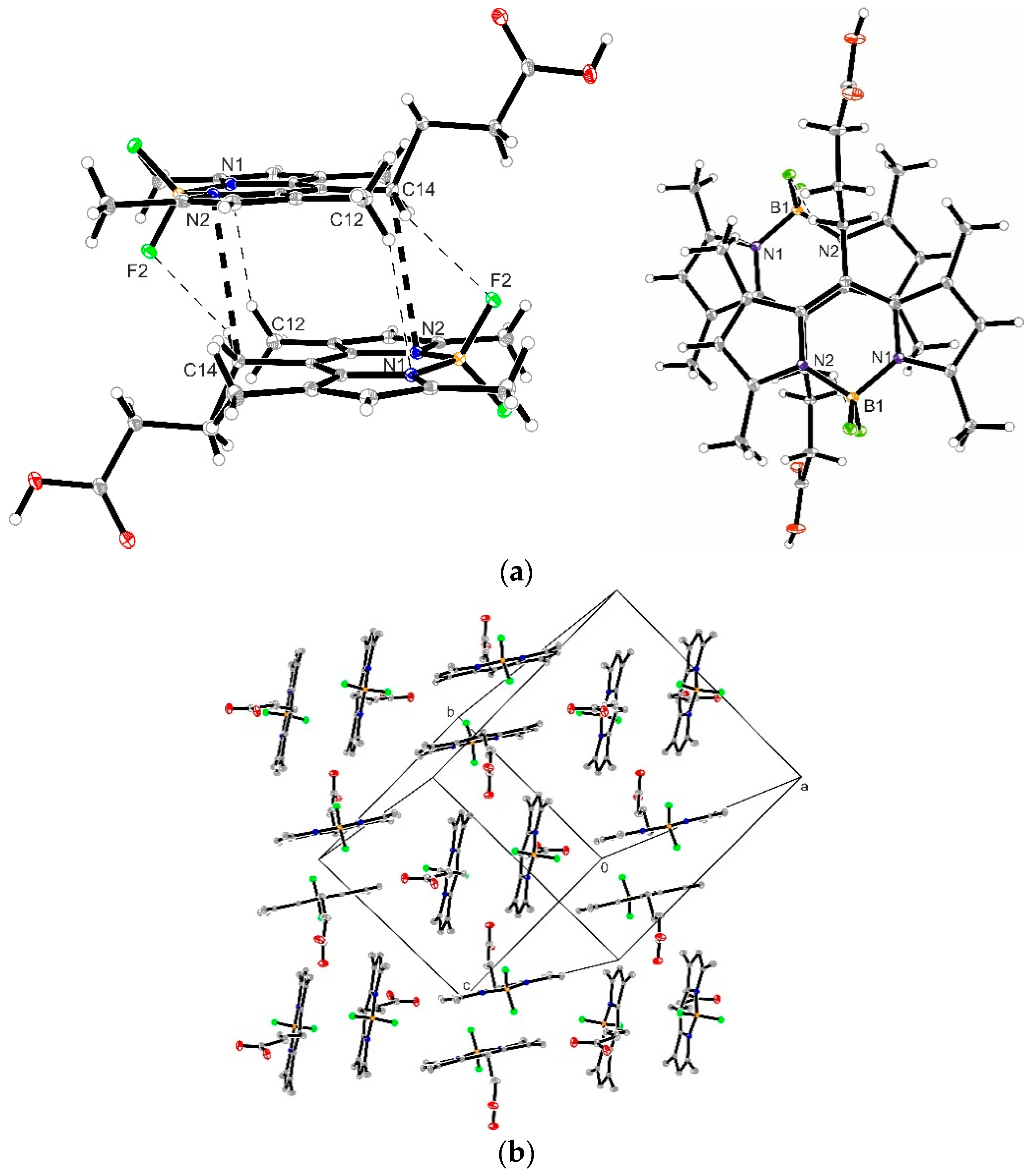
3.4. Crystal Structure of Compound 1
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Fan, G.; Yang, L.; Chen, Z. Water-soluble BODIPY and aza-BODIPY dyes: Synthetic progress and applications. Chem. Sci Eng. 2014, 8, 405–417. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Cherumukkil, S.; Vedhanarayanan, B.; Das, G.; Praveen, V.K.; Ajayaghosh, A. Self-assembly of bodipy-derived extended π-systems. Bull. Chem. Soc. Jpn. 2018, 91, 100–120. [Google Scholar] [CrossRef]
- Olivier, J.H.; Barber, J.; Bahaidarah, E.; Harriman, A.; Ziessel, R. Self-assembly of charged bodipy dyes to form cassettes that display intracomplex electronic energy transfer and accrete into liquid crystals. J. Am. Chem. Soc. 2012, 134, 6100–6103. [Google Scholar] [CrossRef] [PubMed]
- Frein, S.; Camerel, F.; Ziessel, R.; Barber, J.; Deschenaux, R. Highly fluorescent liquid-crystalline dendrimers based on borondipyrromethene dyes. Chem. Mater. 2009, 21, 3950–3959. [Google Scholar] [CrossRef]
- Fan, G.; Lin, Y.-X.; Yang, L.; Gao, F.-P.; Zhao, Y.-X.; Qiao, Z.-Y.; Zhao, Q.; Fan, Y.-S.; Chen, Z.; Wang, H. Co-self-assembled nanoaggregates of BODIPY amphiphiles for dual colour imaging of live cells. Chem. Commun. 2015, 51, 12447–12450. [Google Scholar] [CrossRef] [PubMed]
- Tokoro, Y.; Nagai, A.; Chujo, Y. Nanoparticles via H-aggregation of amphiphilic BODIPY dyes. Tetrahedron Lett. 2010, 51, 3451–3454. [Google Scholar] [CrossRef]
- Yang, L.; Fan, G.; Ren, X.; Zhao, L.; Wang, J.; Chen, Z. Aqueous self-assembly of a charged BODIPY amphiphile via nucleation–growth mechanism. Phys. Chem. Chem. Phys. 2015, 17, 9167–9172. [Google Scholar] [CrossRef] [PubMed]
- Cherumukkil, S.; Gosh, S.; Praveen, V.K.; Ajayaghosh, A. An unprecedented amplification of near-infrared emission in a Bodipy derived p-system by stress or gelation. Chem. Sci. 2017, 8, 5644–5649. [Google Scholar] [CrossRef] [PubMed]
- Florian, A.; Mayoral, M.J.; Stepanenko, V.; Fernández, G. Alternated stacks of nonpolar oligo(p-phenyleneethynylene)-BODIPY systems. Chem. Eur. J. 2012, 18, 14957–14961. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lui, Y.; Wagner, W.; Stepanenko, V.; Ren, X.; Ogi, S.; Würthner, F. Near-IR absorbing j-aggregate of an amphiphilic BF2-azadipyrromethene dye by kinetic cooperative self-assembly. Angew. Chem. Int. Ed. 2017, 56, 5729–5733. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Fennel, F.; Wagner, W.; Würthner, F.; Chen, Y.; Chen, Z. Coupled cooperative supramolecular polymerization: A new model applied to the competing aggregation pathways of an amphiphilic aza-BODIPY dye into spherical and rod-like aggregates. Chem. Eur. J. 2018, 24, 16388–16394. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, D.; Schenning, A.P.H.J. Hydrogen-bonded supramolecular π-functional materials. Chem. Mater. 2011, 23, 310–325. [Google Scholar] [CrossRef]
- Yagai, S. Supramolecularly engineered functional π-assemblies based on complementary hydrogen bonding interactions. Bull. Chem. Soc. Jpn. 2015, 88, 28–58. [Google Scholar] [CrossRef]
- Rödle, A.; Ritschel, B.; Mück-Lichtenfeld, C.; Stepanenko, V.; Fernández, G. Influence of ester versus amide linkers on the supramolecular polymerization mechanisms of planar bodipy dyes. Chem. Eur. J. 2016, 22, 15772–15777. [Google Scholar] [CrossRef] [PubMed]
- Rödle, A.; Lambov, M.; Mück-Lichtenfeld, C.; Stepanenko, V.; Fernández, G. Cooperative nanoparticle H-type self-assembly of a bolaamphiphilic BODIPY derivative in aqueous medium. Polymer 2017, 128, 317–324. [Google Scholar] [CrossRef]
- Sampedro, A.; Ramos-Torres, A.; Schwöppe, C.; Mück-Lichtenfeld, M.; Helmers, I.; Bort, A.; Díaz-Laviada, I.; Fernández, G. Hierarchical self-assembly of BODIPY dyes as a tool to improve the antitumor activity of capsaicin in prostate cancer. Angew. Chem. Int. Ed. 2018. [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. Available online: http://journals.iucr.org/c/issues/2015/01/00/fa3356/index.html (accessed on 20 November 2018).
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112. Available online: http:// http://journals.iucr.org/a/issues/2008/01/00/sc5010/index.html (accessed on 20 November 2018). [CrossRef] [PubMed]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Yagai, S.; Tamiaki, H. Synthesis and self-aggregation of zinc chlorophylls possessing an -hydroxyalkyl group: Effect of distance between interactive hydroxy group and chlorin moiety on aggregation. J. Chem. Soc. Perkin Trans. 2001, 3135–3144. [Google Scholar] [CrossRef]
- Takasuka, M.; Matsumura, K.; Ishizuka, N. Study of substituent effects on rotational isomers and monomer±dimer equilibria of the 3-carboxy group in 4-substituted (R)-2-(3,4-methylenedioxyphenyl)-6-isopropyloxy-2H-chromen3-carboxylic acids in dilute CCl4 and CHCl3 solutions by FTIR spectroscopy. Vib. Spectrosc. 2001, 25, 63–79. [Google Scholar] [CrossRef]
- Takasuka, M.; Yamakawa, M.; Watanabe, F. F.t.i.r. spectral study of intramolecular hydrogen bonding in thromboxane A2 receptor antagonist s-l45t and related compounds. Part 1. J. Chem. Soc. Perkin Trans. 1989, 1173–1179. [Google Scholar] [CrossRef]
- Choi, S.; Bouffard, J.; Kim, Y. Aggregation-induced emission enhancement of a meso-trifluoromethyl BODIPY via J-aggregation. Chem. Sci. 2014, 5, 751–755. [Google Scholar] [CrossRef]
- Kim, S.; Bouffard, J.; Kim, Y. Tailoring the Solid-State Fluorescence Emission of BODIPY Dyes by meso Substitution. Chem. Eur. J. 2015, 21, 17459–17465. [Google Scholar] [CrossRef] [PubMed]

| Empirical Formula | C17H21BF2N2O·CH2Cl2 |
|---|---|
| Formula weight | 419.09 |
| Temperature/K | 100(2) |
| Wavelength/Å | 1.54178 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| Unit cell dimensions | |
| a/Å | 27.4033(11) |
| b/Å | 12.5495(5) |
| c/Å | 12.3648(5) |
| α/° | 90 |
| β/° | 110.442(2) |
| γ/° | 90 |
| V/Å3 | 3984.4(3) |
| Z | 8 |
| Dc/gcm−3 | 1.397 |
| μ/mm−1 | 3.234 |
| F (000) | 1.744 |
| Crystal size/mm | 0.020 × 0.160 × 0.200 |
| θ range/° | 3.92–66.74 |
| Index ranges | −32 ≤ h ≤ 32 |
| −14 ≤ k ≤ 14 | |
| −14 ≤ l ≤ 14 | |
| Reflections collected | 35,692 |
| Independent reflections | 3520 |
| R int | 0.0373 |
| Data/Restraints/Parameters | 3520/0/252 |
| GOF | 1.035 |
| Final R indexes [I > 2σ(I)] | R1 = 0.0324 |
| ωR2 = 0.0816 | |
| Final R indexes (all data) | R1 = 0.0348 |
| ωR2 = 0.0830 | |
| Largest diff. peak/hole/eÅ−3 | 0.375/−0.413 |
| Solvent | λabs/nm S0 → S1 | ε/104 M−1·cm−1 | λem/nm | ΦF |
|---|---|---|---|---|
| MeOH | 496 | 7.98 | 506 | 12% |
| DMSO | 500 | 8.01 | 511 | 79% |
| THF | 499 | 7.50 | 510 | 17% |
| MeCN | 494 | 6.75 | 505 | 55% |
| CHCl3 | 503 | 5.38 | 513 | 8% |
| CCl4 | 505 | 5.24 | 513 | 14% |
| D–H⋯A | d(D–H) | d(H–A) | d(D⋯A) | ∠(DHA) |
|---|---|---|---|---|
| O2–H1⋯O1#1 | 0.87(3) | 1.76(3) | 2.629(2) | 175.0(3) |
| C8–H8⋯F1#2 | 0.95 | 2.55 | 3.362(2) | 142.9 |
| C21–H21A⋯F2 | 0.99 | 2.30 | 3.129(2) | 141.0 |
| C21–H21B⋯F1 | 0.99 | 2.51 | 3.198(2) | 126.0 |
| C12–H21B⋯N1#3 | 0.98 | 2.73 | 3.662(3) | 157.7 |
| C14–H14A⋯F2#3 | 0.99 | 2.56 | 3.383(3) | 140.8 |
| C16–H16B⋯O2#4 | 0.99 | 2.63 | 3.430(2) | 138.2 |
| Cg1⋯Cg1#3, a | 3.523 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matarranz, B.; Sampedro, A.; G. Daniliuc, C.; Fernández, G. Self-Assembly of a Carboxyl-Functionalized BODIPY Dye via Hydrogen Bonding. Crystals 2018, 8, 436. https://doi.org/10.3390/cryst8110436
Matarranz B, Sampedro A, G. Daniliuc C, Fernández G. Self-Assembly of a Carboxyl-Functionalized BODIPY Dye via Hydrogen Bonding. Crystals. 2018; 8(11):436. https://doi.org/10.3390/cryst8110436
Chicago/Turabian StyleMatarranz, Beatriz, Angel Sampedro, Constantin G. Daniliuc, and Gustavo Fernández. 2018. "Self-Assembly of a Carboxyl-Functionalized BODIPY Dye via Hydrogen Bonding" Crystals 8, no. 11: 436. https://doi.org/10.3390/cryst8110436
APA StyleMatarranz, B., Sampedro, A., G. Daniliuc, C., & Fernández, G. (2018). Self-Assembly of a Carboxyl-Functionalized BODIPY Dye via Hydrogen Bonding. Crystals, 8(11), 436. https://doi.org/10.3390/cryst8110436