Aging Behavior and Precipitation Analysis of Cu-Ni-Co-Si Alloy
Abstract
1. Introduction
2. Experimental
3. Results and Discussions
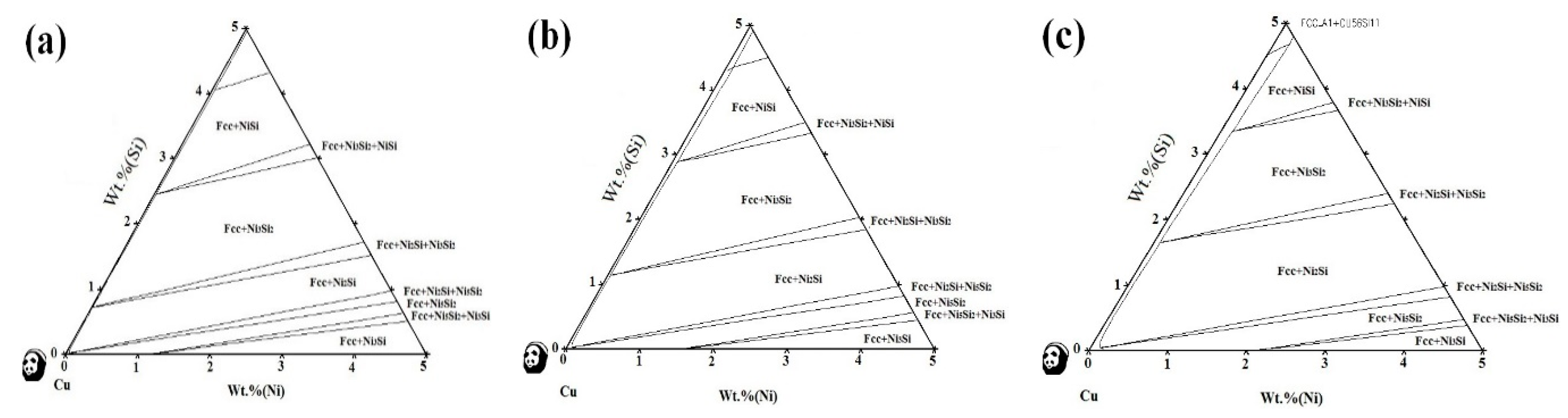
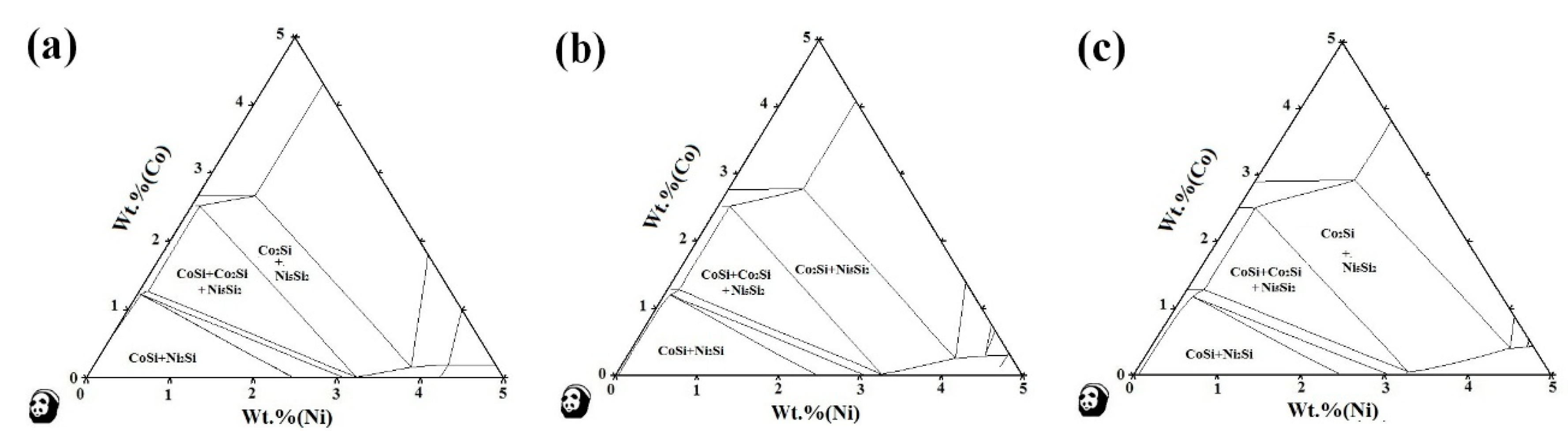
3.1. Phase Diagram Calculation
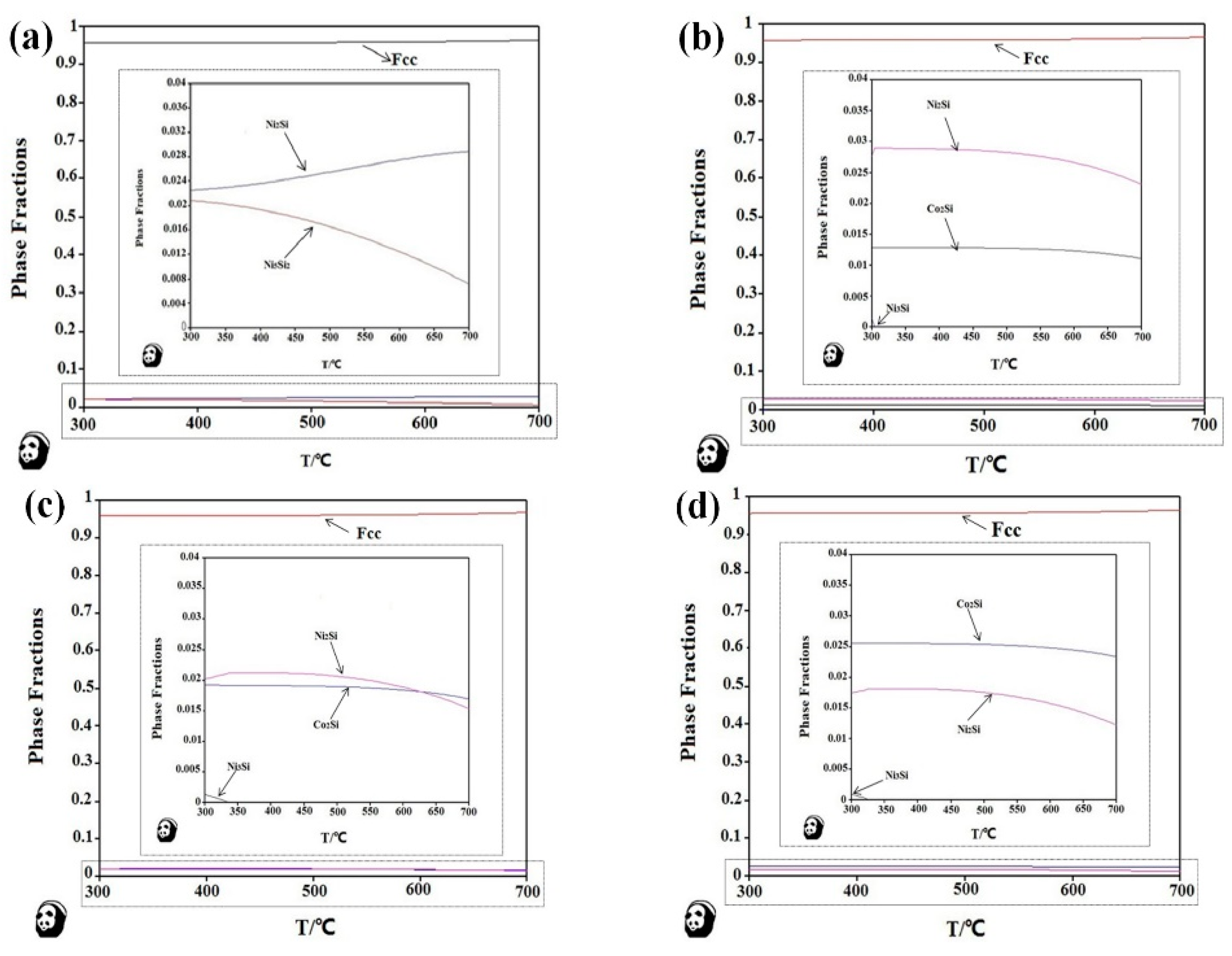
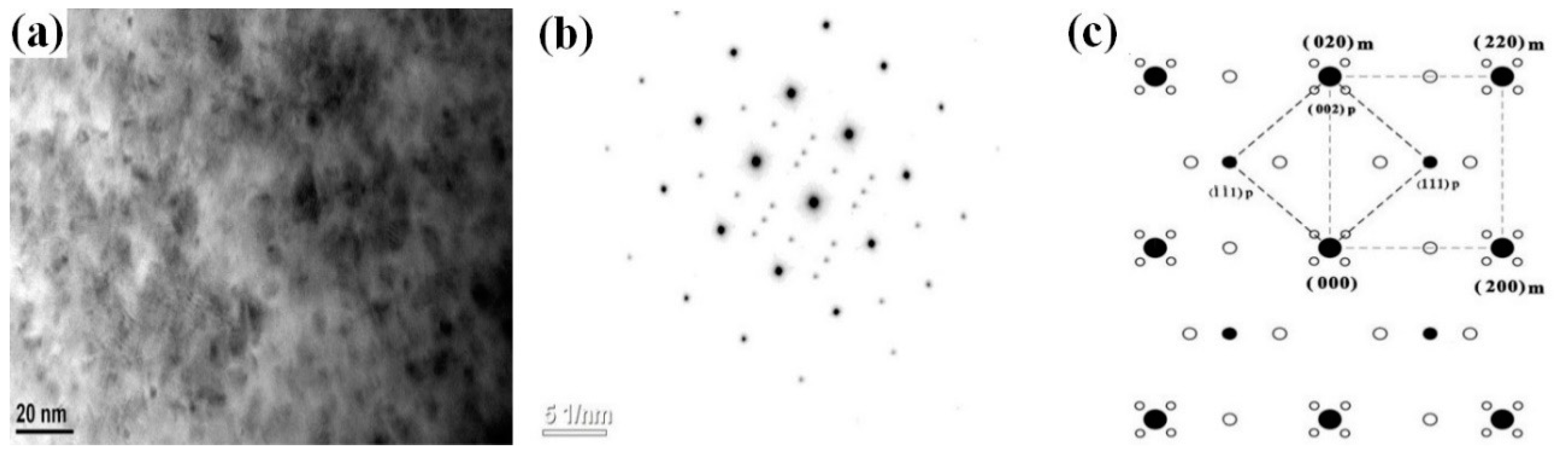
3.2. Aging Precipitation Evolution
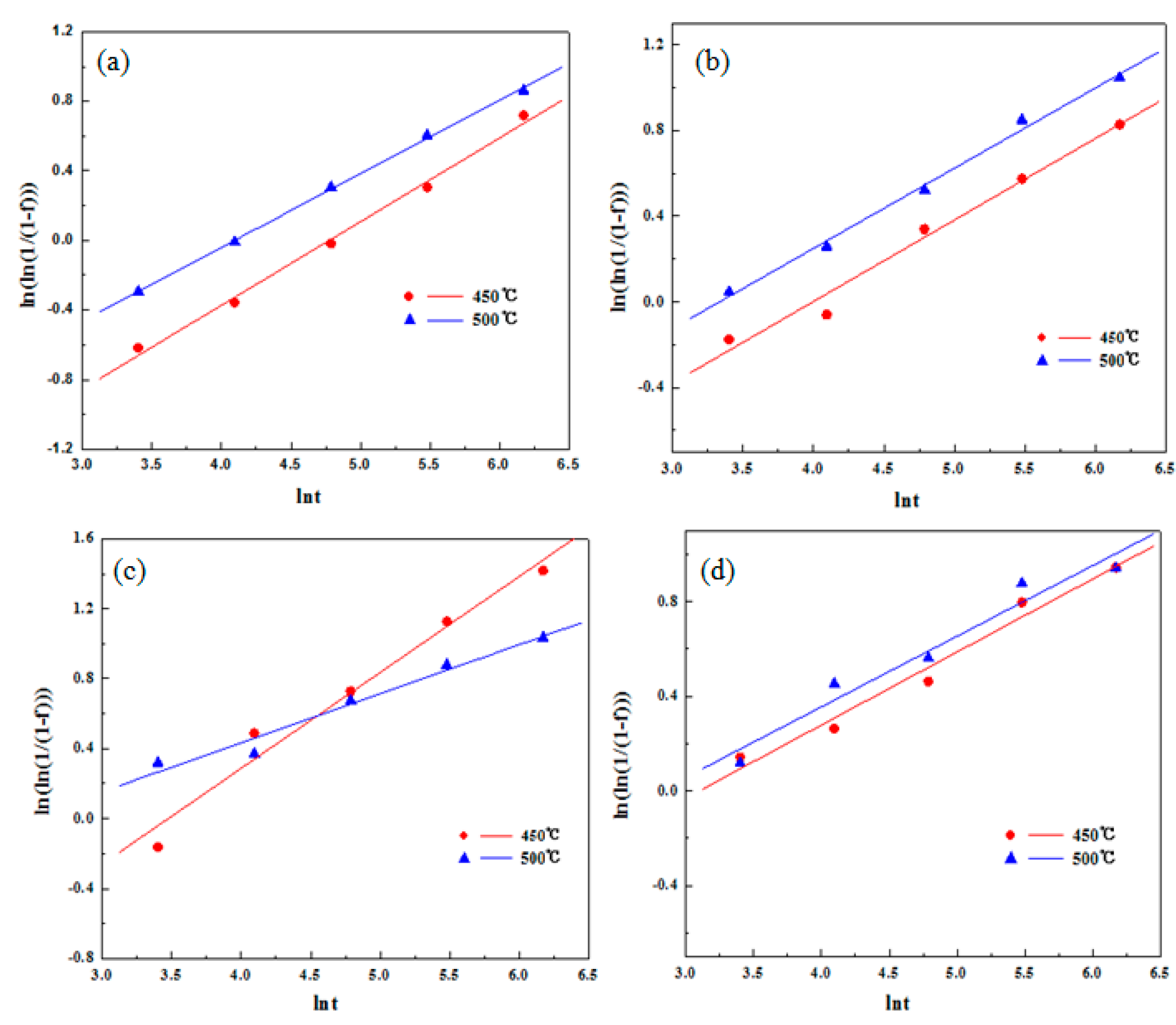
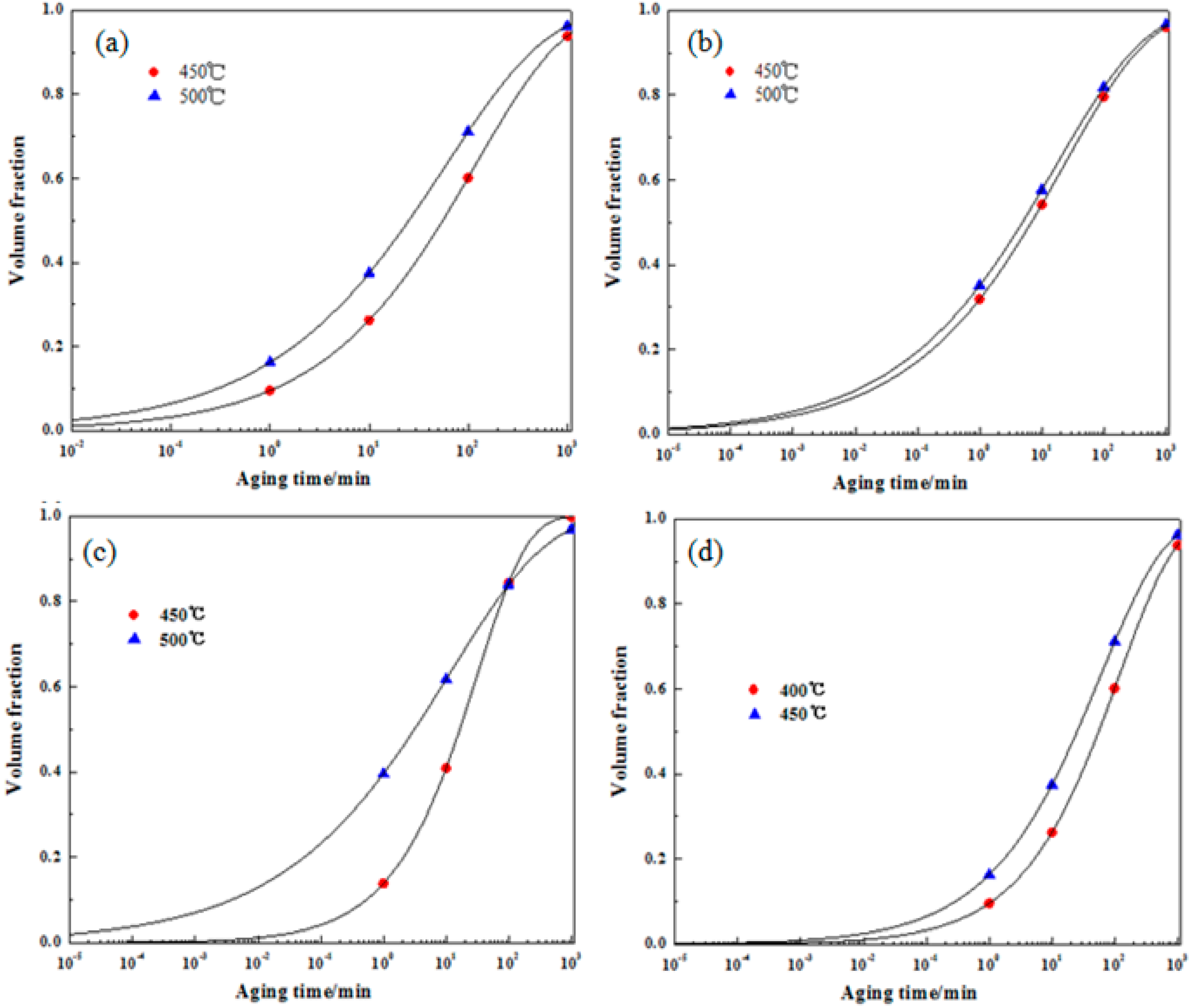
3.3. Phase Transformation Kinetics Equation
4. Conclusions
- (1)
- There are various combinations of NixSiy phases in the Cu-rich isothermal section of the Cu-Ni-Si ternary alloy. With the increase of temperature, the Ni2Si two-phase region is widened. After the addition of the Co element, a part of the Ni atom is replaced to form a Co2Si phase, which increases as the content of the Co element increases.
- (2)
- The addition of the Co element will delay the formation of spinodal decomposition during the aging of the Cu-Ni-Si alloy. After adding the Co element to the Cu-Ni-Si alloy to form the δ-Ni2Si phase, which has the , orientation relationship with the matrix and will precipitate the (Ni,Co)2Si phase, the relationship between the matrix and (Ni,Co)2Si can be expressed as , . In addition, with the aging time prolonged, the precipitates of the Cu-Ni-Si alloy with a high content of the Co element is more likely to grow.
- (3)
- The precipitation kinetics of the Cu-Ni-Si alloy at 450 °C and 500 °C was calculated. The kinetic equation agrees with the experimental values and it can be seen from the precipitation kinetic curves that the addition of Co can promote the precipitation rate of the precipitated phase.
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Hatano, T. Cu-Ni-Si System Alloy. U.S. Patent 20100000637A1, 21 September 2010. [Google Scholar]
- Zhao, D.M.; Dong, Q.M.; Liu, P.; Kang, B.X.; Huang, J.L. Transformation and strengthening of early stage of aging in Cu-3.2Ni-0.75Si alloy. Chin. J. Nonferr. Met. 2002, 12, 1167–1171. [Google Scholar]
- Monzen, R.; Watanabe, C. Microstructure and mechanical properties of Cu-Ni-Si alloys. Mate. Sci. Eng. A 2008, 483–484, 117–119. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Xiao, T.; Pang, Y.; Xiang, Z.Q.; Qiu, W.T.; Xiao, Z. A new ultrahigh strength Cu-Ni-Si alloy. Intermetallics 2013, 42, 77–84. [Google Scholar] [CrossRef]
- Liu, H.Y.; Liu, P.; Tian, B.H.; Jia, S.G.; Ren, F.Z.; Zhang, Y. Development of Cu-Ni-Si alloy for lead frame. Mater. Res. Appl. 2007, 1, 260–264. [Google Scholar]
- Li, D.M.; Wang, Q.; Jiang, B.B.; Li, X.; Zhou, W.; Dong, C.; Wang, H.; Chen, Q. Minor-alloyed Cu-Ni-Si alloys with high hardness and electric conductivity designed by a cluster formula approach. Prog. Nat. Sci. Mater. Int. 2017, 27, 467–473. [Google Scholar] [CrossRef]
- Chen, Z.P.; Xiang, C.J.; Li, H.Q. The Oxide Defect of C70250 Alloy. Adv. Mater. Res. 2014, 936, 1168–1172. [Google Scholar] [CrossRef]
- Kuhn, A.K.; Käufler, A.; Ringhand, D.; Theobald, S. A new high performance copper based alloy for electro-mechanical connectors. Materialwiss. Werkstofftech. 2007, 38, 624–634. [Google Scholar] [CrossRef]
- Xiao, X.P.; Yi, Z.Y.; Chen, T.T.; Liu, R.; Wang, H. Suppressing spinodal decomposition by adding Co into Cu-Ni-Si alloy. J. Alloys Compd. 2016, 660, 178–183. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Arpshofen, I.; Seifert, H.J.; Sommer, F.; Aldinger, F. Enthalpy of mixing of liquid and undercooled liquid ternary and quaternary Cu-Ni-Si-Zr alloys. J. Alloys Compd. 2002, 337, 155–167. [Google Scholar] [CrossRef]
- Zhao, D.M.; Dong, Q.M.; Liu, P.; Kang, B.X.; Huang, J.L.; Jin, Z.H. Structure and strength of the age hardened Cu-Ni-Si alloy. Mater. Chem. Phys. 2003, 79, 81–86. [Google Scholar] [CrossRef]
- Qian, L.; Zhou, L.; Yang, G.; Peng, X.; Derby, B. Microstructure and mechanical properties of a high strength Cu-Ni-Si alloy treated by combined aging processes. J. Alloys Compd. 2017, 695, 2413–2423. [Google Scholar]
- Semboshi, S.; Sato, S.; Iwase, A.; Takasugi, T. Discontinuous precipitates in age-hardening Cu-Ni-Si alloys. Mater. Charact. 2016, 115, 39–45. [Google Scholar] [CrossRef]
- Semboshi, S.; Ishikuro, M.; Iwase, A.; Takasugi, T. Microstructural Subsequence and Phase Equilibria in an Age-Hardenable Cu-Ni-Si Alloy. Mater. Trans. 2017, 59, 182–187. [Google Scholar] [CrossRef]
- Chalon, J.; Guérin, J.D.; Dubar, L.; Dubois, A.; Puchi-Cabrera, E.S. Characterization of the hot-working behavior of a Cu-Ni-Si alloy. Mater. Sci. Eng. A 2016, 667, 77–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Volinsky, A.A.; Xu, Q.Q.; Chai, Z.; Tian, B.; Liu, P.; Tran, H.T. Deformation Behavior and Microstructure Evolution of the Cu-2Ni-0.5Si-0.15Ag Alloy During Hot Compression. Met. Mater. Trans. A 2015, 46, 5871–5876. [Google Scholar] [CrossRef]
- Watanabe, C.; Takeshita, S.; Monzen, R. Effects of Small Addition of Ti on Strength and Microstructure of a Cu-Ni-Si Alloy. Met. Mater. Trans. A 2015, 46, 2469–2475. [Google Scholar] [CrossRef]
- Li, S.H.; Li, Z.; Lei, Q.; Wang, M.P.; Sheng, L.N.; Pan, Z.Y.; Liu, H.Q. Microstructure and Properties of Superhigh Strength Cu-Ni-(Al)-Si Alloy. Rare Met. Mater. Eng. 2015, 44, 1427–1431. [Google Scholar]
- Wang, H.S.; Chen, H.G.; Gu, J.W.; Hsu, C.E.; Wu, C.Y. Improvement in strength and thermal conductivity of powder metallurgy produced Cu-Ni-Si-Cr alloy by adjusting Ni/Si weight ratio and hot forging. J. Alloys Compd. 2015, 633, 59–64. [Google Scholar] [CrossRef]
- Lei, J.-G.; Liu, P. The effects of aging precipitation on the recrystallization of CuNiSi alloy. Wuhan Univ. Technol. Mater. Sci. Ed. 2005, 20, 21–25. [Google Scholar]
- Cheng, J.Y.; Tang, B.B.; Yu, F.X.; Shen, B. Evaluation of nanoscaled precipitates in a Cu-Ni-Si-Cr alloy during aging. J. Alloys Compd. 2014, 614, 189–195. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.S.; Fu, X.Q.; Xue, F.; Chen, X. Microstructure and properties of Cu-Ni-Si based alloys for lead frame. J. Southeast Univ. 2005, 35, 729–732. [Google Scholar]
- Kim, Y.G.; Seong, T.Y.; Han, J.H.; Ardell, A.J. Effect of heat treatment on precipitation behaviour in a Cu-Ni-Si-P alloy. J. Mater. Sci. 1986, 21, 1357–1362. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Biancaniello, F.S.; Williams, M.E. Evolution of the two-phase microstructure L12+DO22 in near-Eutectoid Ni3(Al,V) alloy. J. Mater. Res. 1994, 9, 3068–3082. [Google Scholar] [CrossRef]
- Guy, A.G.; Hren, J.J. Elements of Physical Metallurgy; Addison-Wesley Publishing Company: Boston, MA, USA, 1956. [Google Scholar]
- Nagarjuna, S.; Balasubramanian, K.; Sarma, D.S. Effect of prior cold work on mechanical properties, electrical conductivity and microstructure of aged Cu-Ti alloys. J. Mater. Sci. 1999, 34, 2929–2942. [Google Scholar] [CrossRef]
- Fernee, H.; Nairn, J.; Atrens, A. Precipitation hardening of Cu-Fe-Cr alloys part I Mechanical and electrical properties. J. Mater. Sci. 2001, 36, 2711–2719. [Google Scholar] [CrossRef]

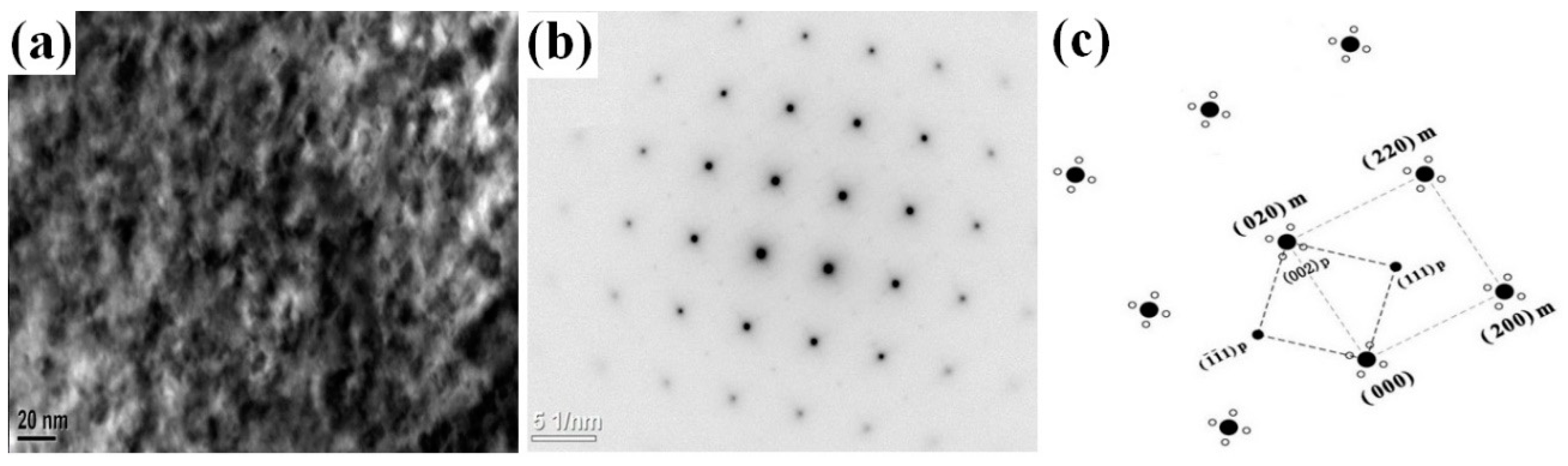
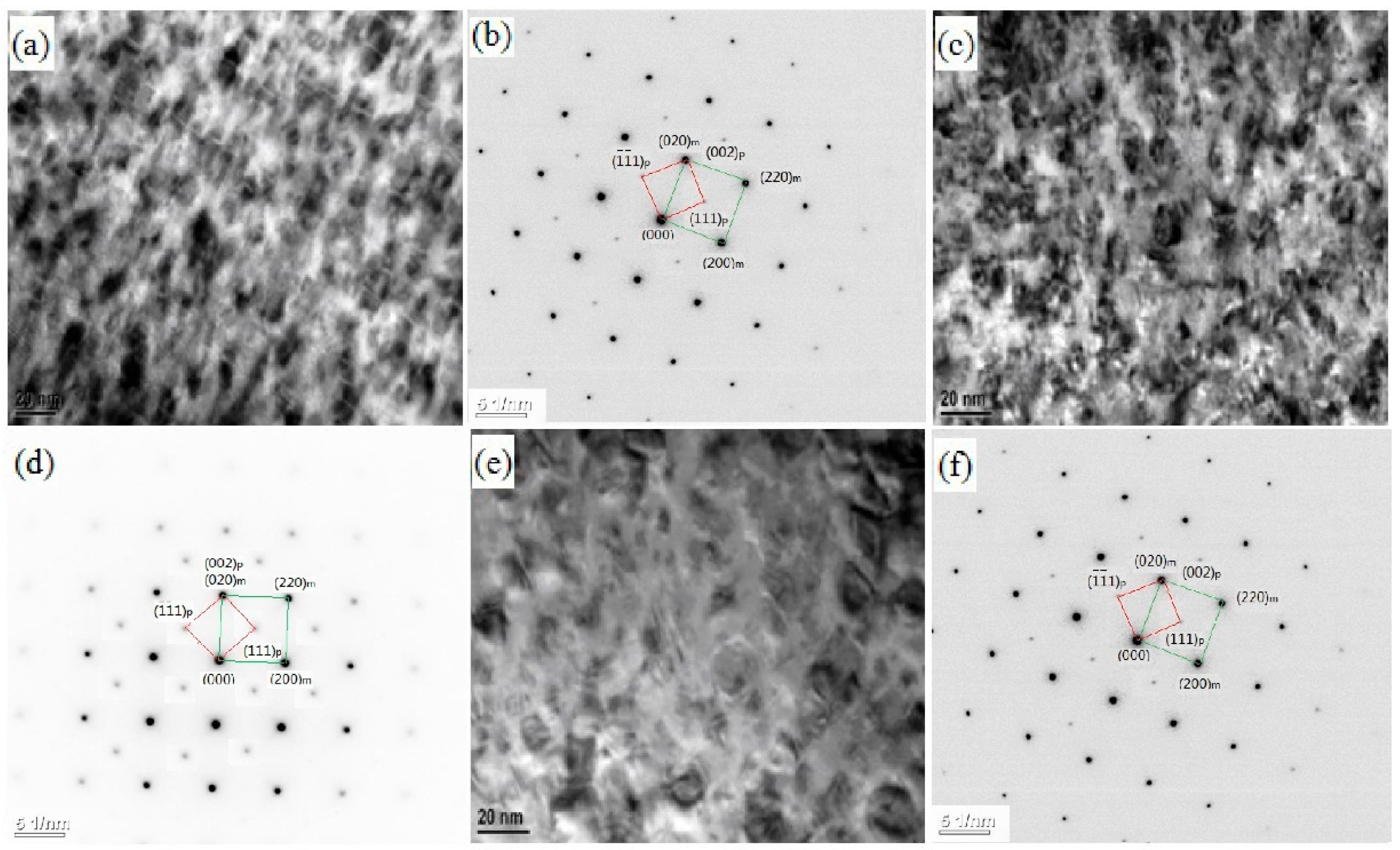
| Sample | Cu | Ni | Co | Si | Ni/Co |
|---|---|---|---|---|---|
| S1 | Bal. | 2.8 | 0.0 | 0.6 | 0.00 |
| S2 | Bal. | 1.8 | 0.8 | 0.6 | 3.00 |
| S3 | Bal. | 1.4 | 1.2 | 0.6 | 1.17 |
| S4 | Bal. | 1.2 | 1.4 | 0.6 | 0.86 |
| t/min | S1 | S2 | S3 | S4 | ||||
|---|---|---|---|---|---|---|---|---|
| σ/IACS% | f/% | σ/IACS% | f/% | σ/IACS% | f/% | σ/IACS% | f/% | |
| 0 | 17.6 | 0.00 | 15.2 | 0.00 | 14.5 | 0.00 | 19.6 | 0.00 |
| 30 | 24 | 41.67 | 28.6 | 56.78 | 35.8 | 68.39 | 44.7 | 68.39 |
| 60 | 27.4 | 50.38 | 29.6 | 61.02 | 44.4 | 72.75 | 46.3 | 72.75 |
| 120 | 29.6 | 62.50 | 33 | 75.42 | 47 | 79.56 | 48.8 | 79.56 |
| 240 | 31.6 | 74.24 | 34.8 | 83.05 | 50 | 89.10 | 52.3 | 89.10 |
| 480 | 34.3 | 87.12 | 36.4 | 89.83 | 51.1 | 92.37 | 53.5 | 92.37 |
| 960 | 35.6 | 100.00 | 38.8 | 100.00 | 51.7 | 100.00 | 56.3 | 100.00 |
| t/min | S1 | S2 | S3 | S4 | ||||
|---|---|---|---|---|---|---|---|---|
| σ/IACS% | f/% | σ/IACS% | f/% | σ/IACS% | f/% | σ/IACS% | f/% | |
| 0 | 17.6 | 0.00 | 15.2 | 0.00 | 14.5 | 0.00 | 19.6 | 0.00 |
| 30 | 33.3 | 52.51 | 32 | 64.86 | 44.3 | 74.69 | 45 | 67.55 |
| 60 | 36.4 | 62.88 | 34 | 72.59 | 45 | 76.44 | 49.4 | 79.26 |
| 120 | 39.8 | 74.25 | 36.3 | 81.47 | 48.8 | 85.96 | 50.7 | 82.71 |
| 240 | 42.7 | 83.95 | 38.6 | 90.35 | 50.8 | 90.98 | 53.8 | 90.96 |
| 480 | 44.7 | 90.64 | 39.6 | 94.21 | 52 | 93.98 | 54.3 | 92.29 |
| 960 | 47.5 | 100.00 | 41.1 | 100.00 | 54.4 | 100.00 | 57.2 | 100.00 |
| Alloy | 450 °C | 500 °C |
|---|---|---|
| S1 | f = 1 − exp (−0.1007t0.4807) | f = 1 − exp (−0.1776t0.4223) |
| S2 | f = 1 − exp (−0.2184t0.3806) | f = 1 − exp (−0.2870t0.3744) |
| S3 | f = 1 − exp (−0.1488t0.5481) | f = 1 − exp (−0.5035t0.28) |
| S4 | f = 1 − exp (−0.3839t0.3089) | f = 1 − exp (−0.4322t0.2985) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Huang, J.; Chen, J.; Xu, H.; Li, Z.; Zhang, J. Aging Behavior and Precipitation Analysis of Cu-Ni-Co-Si Alloy. Crystals 2018, 8, 435. https://doi.org/10.3390/cryst8110435
Xiao X, Huang J, Chen J, Xu H, Li Z, Zhang J. Aging Behavior and Precipitation Analysis of Cu-Ni-Co-Si Alloy. Crystals. 2018; 8(11):435. https://doi.org/10.3390/cryst8110435
Chicago/Turabian StyleXiao, Xiangpeng, Jian Huang, Jinshui Chen, Hai Xu, Zhao Li, and Jianbo Zhang. 2018. "Aging Behavior and Precipitation Analysis of Cu-Ni-Co-Si Alloy" Crystals 8, no. 11: 435. https://doi.org/10.3390/cryst8110435
APA StyleXiao, X., Huang, J., Chen, J., Xu, H., Li, Z., & Zhang, J. (2018). Aging Behavior and Precipitation Analysis of Cu-Ni-Co-Si Alloy. Crystals, 8(11), 435. https://doi.org/10.3390/cryst8110435





