Structural Variation in Polyoxomolybdate Hybrid Crystals Comprising Ionic-Liquid Surfactants
Abstract
:1. Introduction
2. Results and Discussion
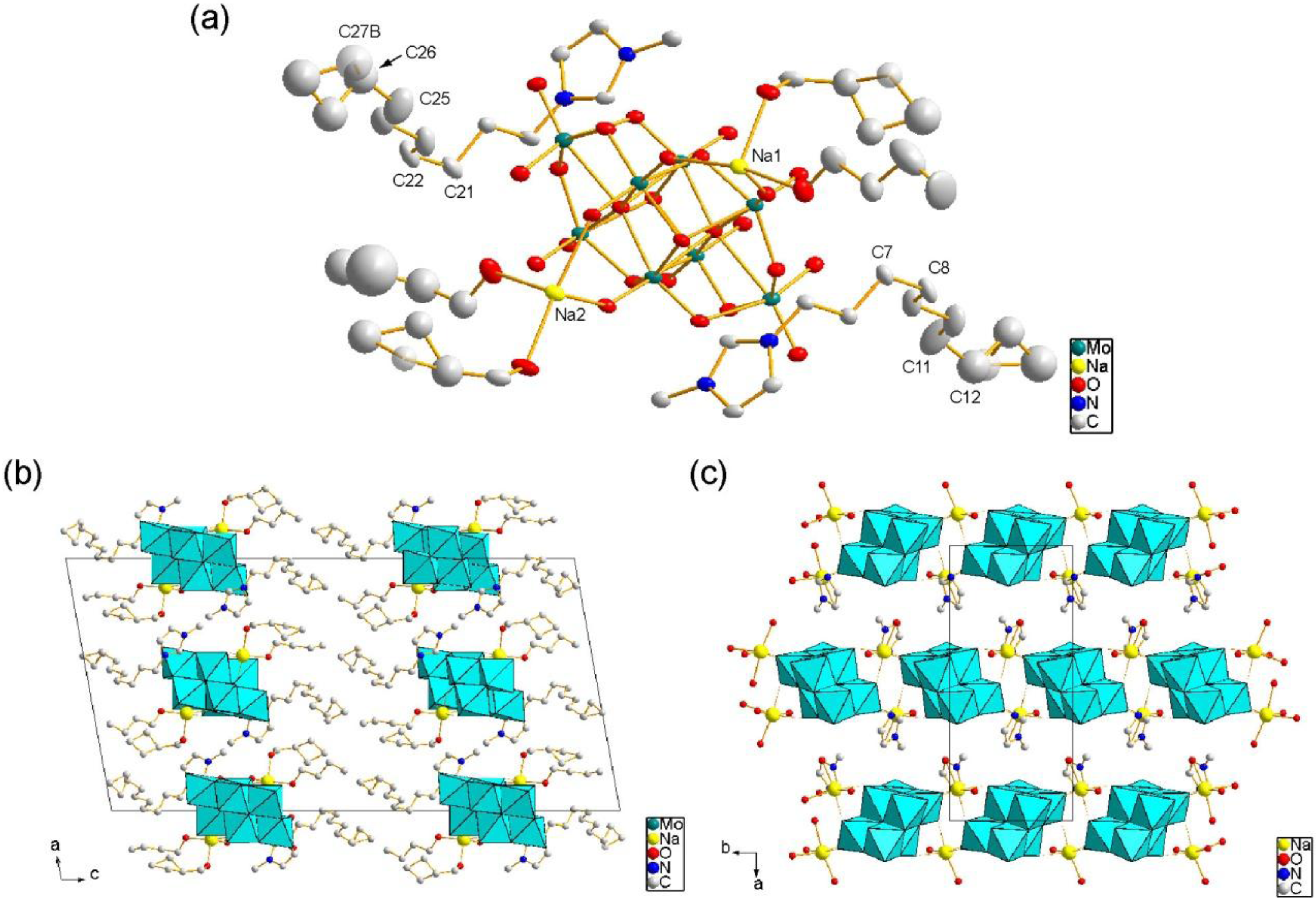
2.1. Crystal Structure of C10im-Na-Mo8
| Compound | C10im-Na-Mo8 | C12im-Na-Mo8 |
|---|---|---|
| Chemical formula | C44H55N4Na2Mo8O30 | C44H94N4Na2Mo8O30 |
| Formula weight | 1933.43 | 1972.73 |
| Crystal system | monoclinic | triclinic |
| Space group | P21/n (No. 14) | P1 (No. 2) |
| a (Å) | 20.089 (4) | 9.5496 (6) |
| b (Å) | 8.8791 (18) | 11.3505 (8) |
| c (Å) | 39.931 (9) | 16.8466 (12) |
| α (°) | – | 102.283 (8) |
| β (°) | 100.301 (3) | 90.927 (7) |
| γ (°) | – | 104.802 (8) |
| V (Å3) | 7008 (3) | 1720.2 (3) |
| Z | 4 | 1 |
| ρcalcd (g·cm−3) | 1.832 | 1.904 |
| T (K) | 93 | 93 |
| µ (Mo Kα) (mm−1) | 1.472 | 1.500 |
| No. of reflections measured | 70784 | 21352 |
| No. of independent reflections | 15939 | 7869 |
| Rint | 0.0874 | 0.0536 |
| No. of parameters | 730 | 381 |
| R1 (I > 2σ(I)) | 0.0933 | 0.0494 |
| wR2 (all data) | 0.2612 | 0.1276 |
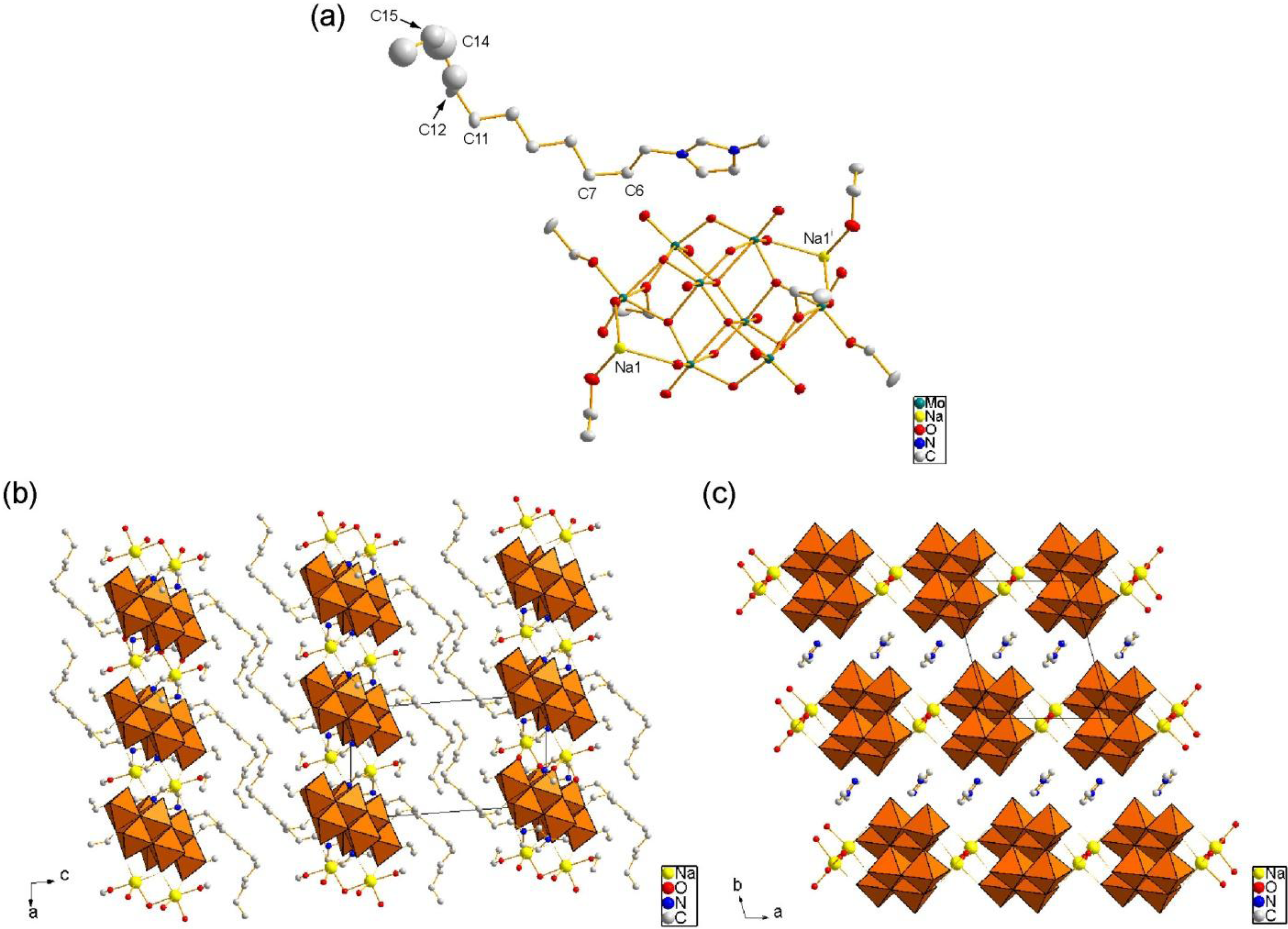
2.2. Crystal Structure of C12im-Na-Mo8


3. Experimental Section
3.1. Syntheses and Methods
3.2. X-Ray Crystallography
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar]
- Wasserscheid, P.; Keim, W. Ionic liquids-new “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Haumann, M.; Riisager, A. Hydroformylation in room temperature ionic liquids (RTILs): Catalyst and process developments. Chem. Rev. 2008, 108, 1474–1497. [Google Scholar]
- Honma, I.; Yamada, M. Bio-inspired membranes for advanced polymer electrolyte fuel cells. Anhydrous proton-conducting membrane via molecular self-assembly. Bull. Chem. Soc. Jpn. 2007, 80, 2110–2123. [Google Scholar] [CrossRef]
- Coronado, E.; Gómez-García, C.J. Polyoxometalate-based molecular materials. Chem. Rev. 1998, 98, 273–296. [Google Scholar] [CrossRef]
- Coronado, E.; Giménez-Saiz, C.; Gómez-García, C.J. Recent advances in polyoxometalate-containing molecular conductors. Coord. Chem. Rev. 2005, 249, 1776–1796. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin, Germany, 1983. [Google Scholar]
- Hill, C.L. Introduction: Polyoxometalates multicomponent molecular vehicles to probe fundamental issues and practical problems. Chem. Rev. 1998, 98, 1–2. [Google Scholar] [CrossRef]
- Long, D.-L.; Burkholder, E.; Cronin, L. Polyoxometalate clusters, nanostructures and materials: From self assembly to designer materials and devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalytic chemistry of heteropoly compounds. Adv. Catal. 1996, 41, 113–252. [Google Scholar] [CrossRef]
- Sadakane, M.; Steckhan, E. Electrochemical properties of polyoxometalates as electrocatalysts. Chem. Rev. 1998, 98, 219–237. [Google Scholar] [CrossRef]
- Song, Y.-F.; Long, D.-L.; Ritchie, C.; Cronin, L. Nanoscale polyoxometalate-based inorganic/organic hybrids. Chem. Rec. 2011, 11, 158–171. [Google Scholar] [CrossRef]
- Qi, W.; Wu, L. Polyoxometalate/polymer hybrid materials: Fabrication and properties. Polym. Int. 2009, 58, 1217–1225. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Soriano-Portillo, A.; Mingotaud, C.; Dominguez-Vera, J.M. Langmuir–Blodgett films based on inorganic molecular complexes with magnetic or optical properties. Adv. Colloid Interface Sci. 2005, 116, 193–203. [Google Scholar] [CrossRef]
- Stein, A.; Fendorf, M.; Jarvie, T.P.; Mueller, K.T.; Benesi, A.J.; Mallouk, T.E. Salt-gel synthesis of porous transition-metal oxides. Chem. Mater. 1995, 7, 304–313. [Google Scholar] [CrossRef]
- Janauer, G.G.; Dobley, A.; Guo, J.; Zavalij, P.; Whittingham, M.S. Novel tungsten, molybdenum, and vanadium oxides containing surfactant ions. Chem. Mater. 1996, 8, 2096–2101. [Google Scholar] [CrossRef]
- Taguchi, A.; Abe, T.; Iwamoto, M. Non-silica-based mesostructured materials: Hexagonally mesostructured array of surfactant micelles and 11-tungstophosphoric heteropoly anions. Adv. Mater. 1998, 10, 667–669. [Google Scholar]
- Landsmann, S.; Lizandara-Pueyo, C.; Polarz, S. A new class of surfactants with multinuclear, inorganic head groups. J. Am. Chem. Soc. 2010, 132, 5315–5321. [Google Scholar] [CrossRef]
- Zhang, G.; Ke, H.; He, T.; Xiao, D.; Chen, Z.; Yang, W.; Yao, J. Synthesis and characterization of new layered polyoxometallates-1,10-decanediamine intercalative nanocomposites. J. Mater. Res. 2004, 19, 496–500. [Google Scholar] [CrossRef]
- Janauer, G.G.; Dobley, A.D.; Zavalij, P.Y.; Whittingham, M.S. Evidence for decavanadate clusters in the lamellar surfactant ion phase. Chem. Mater. 1997, 9, 647–649. [Google Scholar] [CrossRef]
- Spahr, M.E.; Nesper, R. Anhydrous octamolybdate with trimethyl hexadecyl ammonium cations. Z. Anorg. Allg. Chem. 2001, 627, 2133–2138. [Google Scholar] [CrossRef]
- Nyman, M.; Ingersoll, D.; Singh, S.; Bonhomme, F.; Alam, T.M.; Brinker, C.J.; Rodriguez, M.A. Comparative study of inorganic cluster-surfactant arrays. Chem. Mater. 2005, 17, 2885–2895. [Google Scholar]
- Nyman, M.; Rodriguez, M.A.; Anderson, T.M.; Ingersoll, D. Two structures toward understanding evolution from surfactant-polyoxometalate lamellae to surfactant-encapsulated polyoxometalates. Cryst. Growth Des. 2009, 9, 3590–3597. [Google Scholar] [CrossRef]
- Yin, P.; Wu, P.; Xiao, Z.; Li, D.; Bitterlich, E.; Zhang, J.; Cheng, P.; Vezenov, D.V.; Liu, T.; Wei, Y. A double-tailed fluorescent surfactant with a hexavanadate cluster as the head group. Angew. Chem. Int. Ed. 2011, 50, 2521–2525. [Google Scholar] [CrossRef]
- Ito, T.; Sawada, K.; Yamase, T. Crystal structure of bis(dimethyldioctadecylammonium) hexamolybdate: A molecular model of Langmuir-Blodgett films. Chem. Lett. 2003, 32, 938–939. [Google Scholar] [CrossRef]
- Ito, T.; Mikurube, K.; Abe, Y.; Koroki, T.; Saito, M.; Iijima, J.; Naruke, H.; Ozeki, T. Hybrid inorganic-organic crystals composed of octamolybdate isomers and pyridinium surfactant. Chem. Lett. 2010, 39, 1323–1325. [Google Scholar] [CrossRef]
- Ito, T.; Mikurube, K.; Hasegawa, K.; Kurasawa, M.; Naruke, H.; Ozeki, T. Polyoxomolybdate-surfactant hybrid layered crystal with unusually long periodicity. Chem. Lett. 2011, 40, 626–628. [Google Scholar] [CrossRef]
- Ito, T.; Ide, R.; Kosaka, K.; Hasegawa, S.; Mikurube, K.; Taira, M.; Naruke, H.; Koguchi, S. Polyoxomolybdate-surfactant layered crystals derived from long-tailed alkylamine and ionic-liquid. Chem. Lett. 2013, 42, 1400–1402. [Google Scholar] [CrossRef]
- Ito, T. Polyoxometalate-surfactant hybrids as building strategy for two-dimensional molecular arrays. Polyoxometalate Chem. 2012, 1, 6–14. [Google Scholar]
- Bourlinos, A.B.; Raman, K.; Herrera, R.; Zhang, Q.; Archer, L.A.; Giannelis, E.P. A liquid derivative of 12-tungstophosphoric acid with unusually high conductivity. J. Am. Chem. Soc. 2004, 126, 15358–15359. [Google Scholar] [CrossRef]
- Leng, Y.; Wang, J.; Zhu, D.; Ren, X.; Ge, H.; Shen, L. Heteropolyanion-based ionic liquids: Reaction-induced self-separation catalysts for esterification. Angew. Chem. Int. Ed. 2009, 48, 168–171. [Google Scholar] [CrossRef]
- Rafiee, E.; Evani, S. Polyoxometalate-based acid salts with tunable separation properties as recyclable Brönsted acid catalysts for the synthesis of β-keto enol ethers. Catal. Commun. 2012, 25, 64–68. [Google Scholar] [CrossRef]
- Chen, X.; Souvanhthong, B.; Wang, H.; Zheng, H.; Wang, X.; Huo, M. Polyoxometalate-based ionic liquid as thermoregulated and environmentally friendly catalyst for starch oxidation. Appl. Catal. B 2013, 138–139, 161–166. [Google Scholar]
- Jiang, Y.; Liu, S.; Li, S.; Miao, J.; Zhang, J.; Wu, L. Anisotropic ionic liquids built from nonmesogenic cation surfactants and Keggin-type polyoxoanions. Chem. Commun. 2011, 47, 10287–10289. [Google Scholar]
- Rickert, P.G.; Antonio, M.R.; Firestone, M.A.; Kubatko, K.-A.; Szreder, T.; Wishart, J.F.; Dietz, M.L. Tetraalkylphosphonium polyoxometalate ionic liquids: Novel, organic-inorganic hybrid materials. J. Phys. Chem. B 2007, 111, 4685–4692. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: New York, NY, USA, 1999; pp. 12–16. [Google Scholar]
- McCarron, E.M., III; Harlow, R.L. Synthesis and structure of Na4[Mo8O24(OCH3)4]·8MeOH: A novel isopolymolybdate that decomposes with the loss of formaldehyde. J. Am. Chem. Soc. 1983, 105, 6179–6181. [Google Scholar] [CrossRef]
- Niven, M.L.; Cruywagen, J.J.; Heyns, J.B.B. The first observation of γ-type octamolybdates: Synthesis, crystal and molecular structure of [Me3N(CH2)6NMe3]2[Mo8O26]·2H2O. J. Chem. Soc. Dalton Trans. 1991, 20, 2007–2011. [Google Scholar]
- Inoue, M.; Yamase, T. Synthesis and crystal structures of γ-type octamolybdates coordinated by chiral lysines. Bull. Chem. Soc. Jpn. 1995, 68, 3055–3063. [Google Scholar] [CrossRef]
- Khan, M.I.; Zubieta, J. Oxovanadium and oxomolybdenum clusters and solids incorporating oxygen-donor ligands. Prog. Inorg. Chem. 1995, 43, 1–149. [Google Scholar] [CrossRef]
- Aupoix, A.; Pégot, B.; Vo-Thanh, G. Synthesis of imidazolium and pyridinium-based ionic liquids and application of 1-alkyl-3-methylimidazolium salts as pre-catalysts for the benzoin condensation using solvent-free and microwave activation. Tetrahedron 2010, 66, 1352–1356. [Google Scholar] [CrossRef]
- CrystalClear; Rigaku Corporation: Tokyo, Japan, 1999.
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Cryst. 1994, 27, 435–436. [Google Scholar]
- Beurskens, P.T.; Admiraal, G.; Beurskens, G.; Bosman, W.P.; Garcia-Granda, S.; Gould, R.O.; Smits, J.M.M.; Smykalla, C. The DIRDIF Program System; University of Nijmegen: Nijmegen, The Netherlands, 1992. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect. A 2007, 64, 112–122. [Google Scholar] [CrossRef]
- Crystal Structure 4.0; Rigaku Corporation: Tokyo, Japan, 2010.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ito, T.; Mikurube, K.; Hasegawa, K.; Matsumoto, T.; Kosaka, K.; Naruke, H.; Koguchi, S. Structural Variation in Polyoxomolybdate Hybrid Crystals Comprising Ionic-Liquid Surfactants. Crystals 2014, 4, 42-52. https://doi.org/10.3390/cryst4010042
Ito T, Mikurube K, Hasegawa K, Matsumoto T, Kosaka K, Naruke H, Koguchi S. Structural Variation in Polyoxomolybdate Hybrid Crystals Comprising Ionic-Liquid Surfactants. Crystals. 2014; 4(1):42-52. https://doi.org/10.3390/cryst4010042
Chicago/Turabian StyleIto, Takeru, Keisuke Mikurube, Kimiko Hasegawa, Takashi Matsumoto, Kurato Kosaka, Haruo Naruke, and Shinichi Koguchi. 2014. "Structural Variation in Polyoxomolybdate Hybrid Crystals Comprising Ionic-Liquid Surfactants" Crystals 4, no. 1: 42-52. https://doi.org/10.3390/cryst4010042
APA StyleIto, T., Mikurube, K., Hasegawa, K., Matsumoto, T., Kosaka, K., Naruke, H., & Koguchi, S. (2014). Structural Variation in Polyoxomolybdate Hybrid Crystals Comprising Ionic-Liquid Surfactants. Crystals, 4(1), 42-52. https://doi.org/10.3390/cryst4010042






