Research on the Production of Methyltrioxorhenium and Heterogenous Catalysts from Waste Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Techniques
2.3. Experiment Procedures
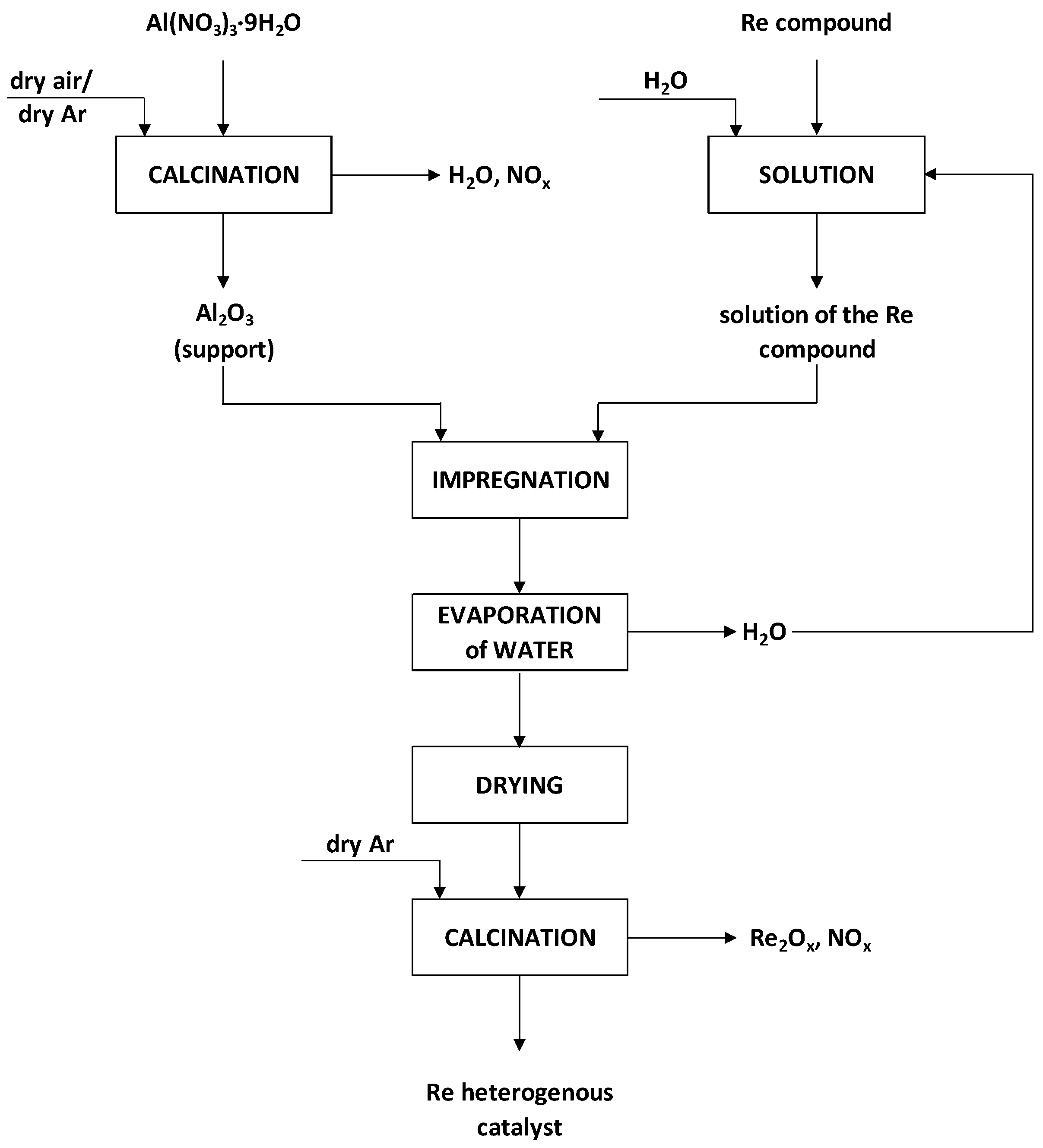
2.3.1. Preparation of Support for Heterogeneous Catalysts
2.3.2. Synthesis of Heterogeneous Catalysts of Re2O7/Al2O3 and M-Re2O7/Al2O3 Type
2.3.3. Synthesis of MTO According to Modified Recipe from AgReO4 [17]
2.3.4. Synthesis of MTO According to Modified Recipe from M(ReO4)2 (Where M = Ni, Zn or Co) [17]
2.3.5. The Procedure for Carrying out the Epoxidation Reaction of Cyclohexene to Cyclohexene Oxide Using MTO
2.3.6. The Procedure for Carrying out the Homometathesis Reaction of Hex-1-ene to Dec-5-ene
2.4. Calculations
- —yield in relation to the amount of rhenium.
- —rhenium content in metal perrhenate.
- —mass of metal perrhenate used in the reaction to obtain MTO.
- —rhenium content in MTO (a constant value was assumed—74.71%).
- —mass of obtained MTO.
- —yield in relation to the amount of rhenium.
- —rhenium content in rhenium catalyst precursor.
- rhenium content in the obtained rhenium catalyst after the calcination process.
- —degree of conversion of the substrate to product(s).
- —the amount of substrate was determined based on the interpretation of spectrum 1H NMR.
- —the amount of product(s) was determined based on the interpretation of spectrum 1H NMR.
3. Results
3.1. Synthesis and Characterization of Catalysts
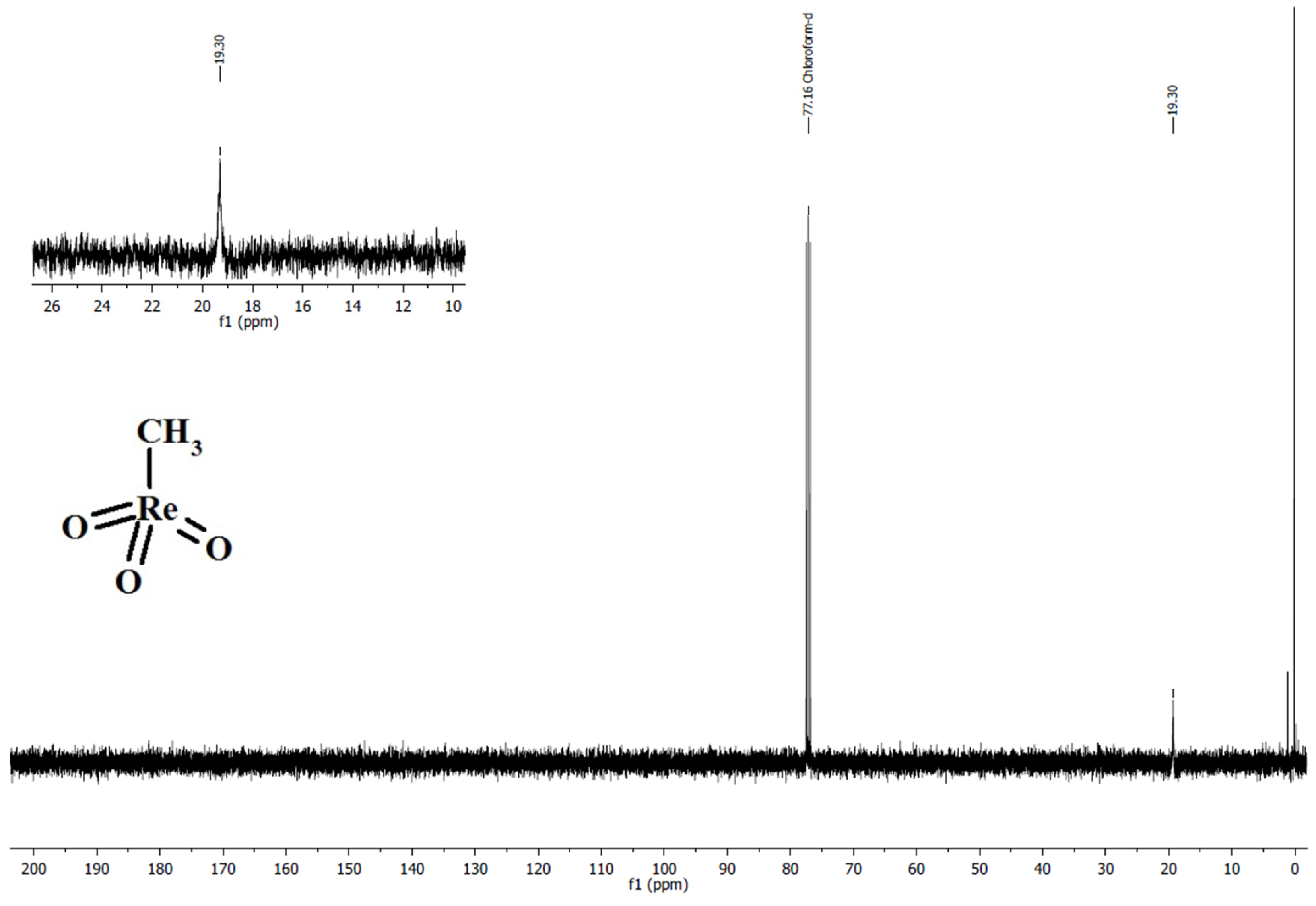
3.1.1. Synthesis and Characterization of Methyltrioxorhenium
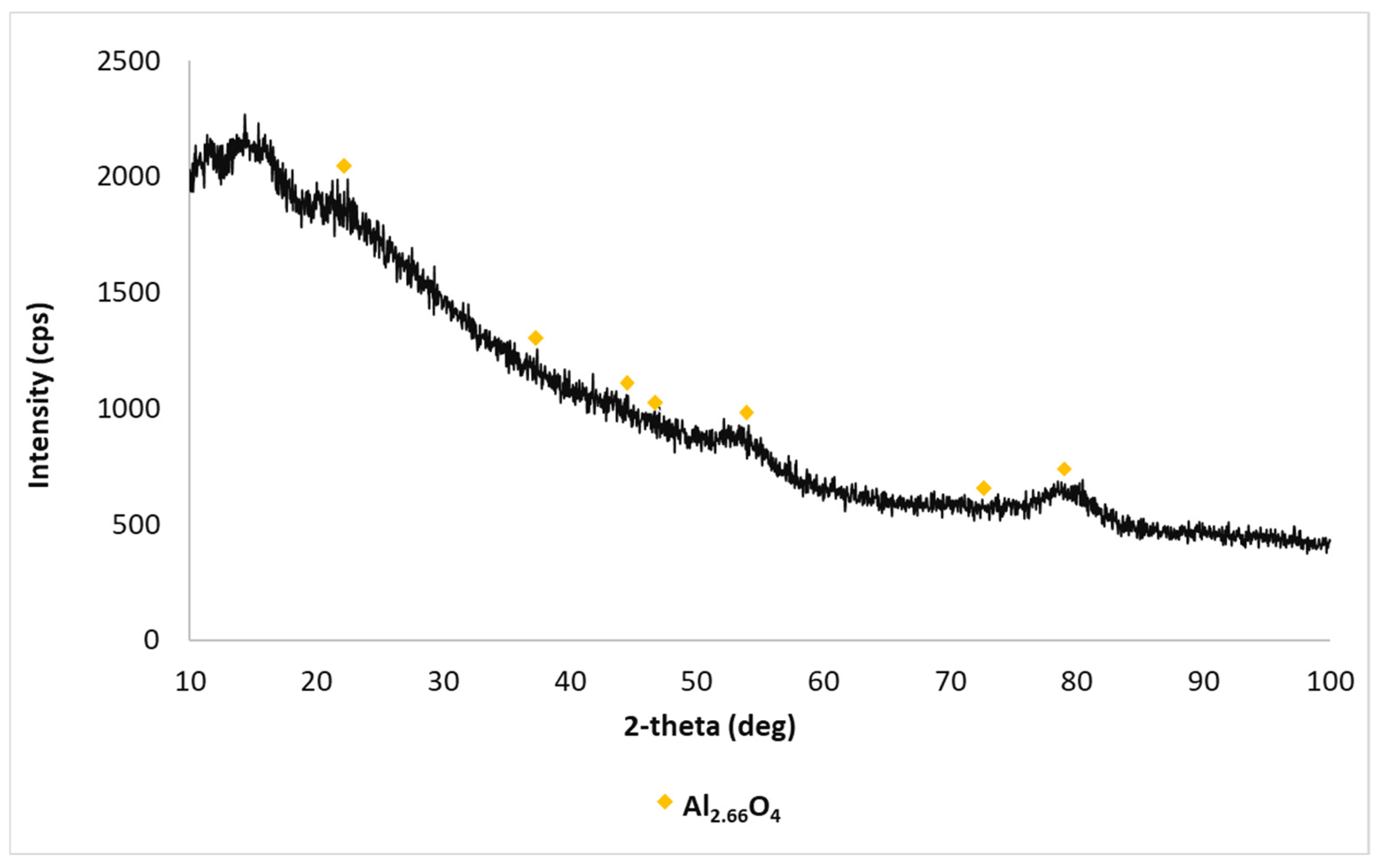
3.1.2. Synthesis and Characterization of Re2O7/Al2O3 and M-Re2O7/Al2O3 Type
3.2. Catalytic Test
3.2.1. Methyltrioxorhenium
3.2.2. Re2O7/Al2O3 and M-Re2O7/Al2O3 Type
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verma, S.; Joshi, A.; De, S.R.; Jat, J.L. Methyltrioxorhenium (MTO) catalysis in the epoxidation of alkenes: A synthetic overview. New J. Chem. 2022, 46, 2005–2027. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Zoller, J.P.; Fischer, R.W. The selective catalytic oxidation of terminal alcohols: A novel four-component system with MTO as catalyst. J. Organomet. Chem. 1999, 579, 404–407. [Google Scholar] [CrossRef]
- Matassini, C.; Bonanni, M.; Cardona, F.; Goti, A. Nitrone or Oxaziridine? Further Insights into the Selectivity of Imine Oxidation Catalyzed by Methyltrioxorhenium. Catalysts 2025, 15, 344. [Google Scholar] [CrossRef]
- Adam, W.; Mitchell, C.M.; Saha-Moller, C.R. Regio- and Diastereoselective Catalytic Epoxidation of Acyclic Allylic Alcohols with Methyltrioxorhenium: A Mechanistic Comparison with Metal (Peroxy and Peroxo Complexes) and Nonmetal (Peracids and Dioxirane) Oxidants. J. Org. Chem. 1999, 64, 3699–3707. [Google Scholar] [CrossRef]
- Al-ajlouni, A.M.; Espenson, J.H. Kinetics and Mechanism of the Epoxidation of Alkyl-Substituted Alkenes by Hydrogen Peroxide, Catalyzed by Methylrhenium Trioxide. J. Org. Chem. 1996, 61, 3969–3976. [Google Scholar] [CrossRef]
- Adam, W.; Mitchell, C.M. Methyltrioxorhenium(VII)-Catalyzed Epoxidation of Alkenes with the Urea/Hydrogen Peroxide Adduct. Angew. Chem. Int. Ed. Engl. 1996, 35, 533–535. [Google Scholar] [CrossRef]
- Yamazaki, S. Methyltrioxorhenium-Catalyzed Epoxidation of Homoallylic Alcohols with Hydrogen Peroxide. J. Org. Chem. 2012, 77, 9884–9888. [Google Scholar] [CrossRef]
- Adam, W.; Mitchell, C.M.; Saha-Möller, C.R. Chemoselective methyltrioxorhenium(VII)-catalyzed sulfoxidations with hydrogen peroxide. Tetrahedron 1994, 50, 13121–13124. [Google Scholar] [CrossRef]
- Goti, A.; Nanneili, L. Synthesis of nitrones by methyltrioxorhenium catalyzed direct oxidation of secondary amines. Tetrahedron Lett. 1996, 37, 6025–6028. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, R.-L.; Lin, H.; Sun, X.W. Methyltrioxorhenium/urea hydrogen peroxide catalyzed oxidation of N-sulfinyl imines: A mild and highly efficient access to N-sulfonyl aldimines, ketimines and α-ketiminoesters. Tetrahedron Lett. 2020, 61, 152587. [Google Scholar] [CrossRef]
- Chidara, V.K.; Stadem, S.; Webster, D.C.; Du, G. Survey of several catalytic systems for the epoxidation of a biobased ester sucrose soyate. Catal. Commun. 2018, 111, 31–35. [Google Scholar] [CrossRef]
- Abou Shama, M.A.; Xu, Q. Optimal Design of Gas-Expanded Liquid Ethylene Oxide Production with Zero Carbon Dioxide Byproduct. Ind. Eng. Chem. Res. 2018, 57, 5351–5358. [Google Scholar] [CrossRef]
- Kühn, F.E.; Scherbaum, A.; Herrmann, W.A. Methyltrioxorhenium and its applications in olefin oxidation, metathesis and aldehyde olefination. J. Organomet. Chem. 2004, 689, 4149–4164. [Google Scholar] [CrossRef]
- Beattie, I.R.; Jones, P.J. Methyltrioxorhenium. An Air-stable compound containing a carbon-rhenium bond. Inorg. Chem. 1979, 18, 2318–2319. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Kuchler, J.G.; Felixberger, J.K.; Herdtweck, E.; Wagner, W. Methylrhenium Oxides: Synthesis from R2O7 and Catalytic Activity in Olefin Metathesis†. Angew. Chem. Int. Ed. Engl. 1988, 27, 394–396. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Kuehn, F.E.; Fischer, R.W.; Thiel, W.R.; Romao, C.C. Simple and efficient synthesis of methyltrioxorhenium(VII): A general method. Inorg. Chem. 1992, 31, 4431–4432. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Kratzer, R.M.; Fischer, R.W. Alkylrhenium Oxides from Perrhenates: A New, Economical Access to Organometallic Oxide Catalysts†. Angew. Chem. Inr. Ed. Engl. 1997, 36, 2652–2654. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Rost, A.M.; Mitterpleininger, J.K.; Szesni, N.; Sturm, S.; Fischer, R.W.; Kuhn, F.E. A cheap, efficient, and environmentally benign synthesis of the versatile catalyst methyltrioxorhenium (MTO). Angew. Chem.—Int. Ed. 2007, 46, 7301–7303. [Google Scholar] [CrossRef]
- Li, M.; Espenson, J.H. Kinetic study of epoxidations by urea-hydrogen peroxide catalyzed by methyltrioxorhenium(VII) on niobia. J. Mol. Catal. A Chem. 2004, 208, 123–128. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Fischer, R.W.; Marz, D.W. Methyltrioxorhenium as Catalyst for Olefin Oxidation. Angew. Chem. Int. Ed. Engl. 1991, 30, 1638–1641. [Google Scholar] [CrossRef]
- Rudolph, J.; Reddy, K.L.; Chiang, J.P.; Sharpless, B.K. Highly Efficient Epoxidation of Olefins Using Aqueous H2O2 and Catalytic Methyltrioxorhenium/Pyridine: Pyridine-Mediated Ligand Acceleration. J. Am. Chem. Soc. 1997, 119, 6189–6190. [Google Scholar] [CrossRef]
- Wang, W.D.; Espenson, J.H. Effects of Pyridine and Its Derivatives on the Equilibria and Kinetics Pertaining to Epoxidation Reactions Catalyzed by Methyltrioxorhenium. J. Am. Chem. Soc. 1998, 120, 11335–11341. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Qiu, C.; Zhao, J. Synthesis of di-nitrogen Schiff base complexes of methyltrioxorhenium(VII) and their application in epoxidation with aqueous hydrogen peroxide as oxidant. Appl. Organomet. Chem. 2010, 25, 54–60. [Google Scholar] [CrossRef]
- Aguado, J.; Escola, J.M.; Castro, M.C.; Paredes, B. Metathesis of 1-hexene over rhenium oxide supported on ordered mesoporous aluminas: Comparison with Re2O7/γ-Al2O3. Appl. Catal. A Gen. 2005, 284, 47–57. [Google Scholar] [CrossRef]
- Mol, J.C. Olefin metathesis over supported rhenium oxide catalysts. Catal. Today 1999, 51, 289–299. [Google Scholar] [CrossRef]
- Doledec, G.; Commereuc, D. Synthesis and properties of homogeneous models of the Re2O7/γ-Al2O3 metathesis catalyst. J. Mol. Catal. A Chem. 2000, 161, 125–140. [Google Scholar] [CrossRef]
- Vorakitkanvasin, S.; Ayudhya, S.K.N.; Suriye, K.; Praserthdam, P.; Panpranot, J. Enhanced metathesis activity of low loading Re2O7/Al2O3 catalysts for propylene production by using aluminum nitrate as Al2O3 precursor. Appl. Catal. A Gen. 2016, 517, 39–46. [Google Scholar] [CrossRef]
- Ivin, K.J.; Mol, J.C. Olefin Metathesis and Metathesis Polimerization; Academic Press: Cambridge, MA, USA, 1997; Available online: www.sciencedirect.com/science/book/9780123770455 (accessed on 30 June 2025).
- Finkel’shtein, E.S.; Bykov, V.I.; Portnykh, E.B. The olefin metathesis reaction—A versatile tool for fine organic synthesis. J. Mol. Catal. 1992, 76, 33–52. [Google Scholar] [CrossRef]
- Maksimov, Y.V.; Kushnerev, M.Y.; Dumesic, J.A.; Nechitailo, A.E.; Fridman, R.A.R.A. Structural investigation of Re2O7γ-Al2O3 catalysts for olefin disproportionation. J. Catal. 1976, 45, 114–117. [Google Scholar] [CrossRef]
- Edreva-Kardjieva, R.M.; Andreev, A.A. Formation and structure of the Re2O7γ-Al2O3 system under precatalysis conditions. J. Catal. 1985, 94, 97–107. [Google Scholar] [CrossRef]
- Ellison, A.; Bickerstaffe, A.; Diakun, G.; Worthington, P. Characterisation of rhenium oxide-alumina metathesis catalysts. J. Mol. Catal. 1986, 36, 67–77. [Google Scholar] [CrossRef]
- Xiaoding, X.; Boelhouwer, C.; Benecke, J.I.; Vonk, D.; Mol, J.C. Re2O7/Al2O3·B2O3 metathesis catalysts. J. Chem. Soc. Faraday Trans. 1 1986, 82, 1945–1953. [Google Scholar] [CrossRef]
- Xiaoding, X.; Mol, J.C. Re2O7/SiO2·Al2O3–SnR4 or –PbR4, a highly active catalyst for the metathesis of functionalized alkenes. J. Chem. Soc. Chem. Commun. 1985, 1, 631–633. [Google Scholar] [CrossRef]
- Marvey, B.B.; du Plessis, J.A.K.; Vosloo, H.C.M.; Mol, J.C. Metathesis of unsaturated fatty acid esters derived from South African sunflower oil in the presence of a 3 wt.% Re2O7/SiO2–Al2O3/SnBu4 catalyst. J. Mol. Catal. A Chem. 2003, 201, 297–308. [Google Scholar] [CrossRef]
- Andreini, A.; Xiaoding, X.; Mol, J.C. Activity of Re2O7/SiO2·Al2O3 catalysts for propene metathesis and the influence of alkyltin promotors. Appl. Catal. 1986, 27, 31–40. [Google Scholar] [CrossRef]
- Williams, K.P.J.; Harrison, K. Raman spectroscopic studies of the effects of tin promotion on a rhenium/alumina dismutation catalyst. J. Chem. Soc. Faraday Trans. 1990, 86, 1603–1606. [Google Scholar] [CrossRef]
- Spronk, R.; Mol, J.C. Regeneration of rhenium-based catalysts for the metathesis of propene. Appl. Catal. 1991, 76, 143–152. [Google Scholar] [CrossRef]
- Xiaoding, X.; Imhoff, P.; van den Aardweg, G.C.N.; Mol, J.C. Mixed-oxide catalysts for the metathesis of functionalized alkenes. J. Chem. Soc. Chem. Commun. 1985, 5, 273–275. [Google Scholar] [CrossRef]
- Xiaoding, X.; Boelhouwer, C.; Vonk, D.; Benecke, J.I.; Mol, J.C. A model for the generation of active sites of rhenium-containing metathesis catalysts. J. Mol. Catal. 1986, 36, 47–66. [Google Scholar] [CrossRef]
- Lwin, S.; Wachs, I.E. Olefin Metathesis by Supported Metal Oxide Catalysts. ACS Catal. 2014, 4, 2505–2520. [Google Scholar] [CrossRef]
- Mol, J.C.; Andreini, A. Activity and selectivity of rhenium-based catalysts for alkene metathesis. J. Mol. Catal. 1988, 46, 151–156. [Google Scholar] [CrossRef]
- Phongsawat, W.; Netiworaruksa, B.; Suriye, K.; Dokjampa, S.; Praserthdam, P.; Panpranot, J. Role of support nature (γ-Al2O3 and SiO2-Al2O3) on the performances of rhenium oxide catalysts in the metathesis of ethylene and 2-pentene. J. Nat. Gas Chem. 2012, 21, 158–164. [Google Scholar] [CrossRef]
- Phongsawat, W.; Netiworaruksa, B.; Suriye, K.; Praserthdam, P.; Panpranot, J. Influence of preparation method on the catalytic performances of Re2O7/SiO2-Al2O3 catalysts in the metathesis of ethylene and 2-pentene. J. Ind. Eng. Chem. 2014, 20, 145–152. [Google Scholar] [CrossRef]
- Phongsawat, W.; Netiworaruksa, B.; Suriye, K.; Praserthdam, P.; Panpranot, J. Effect of SiO2-Al2O3 Composition on the Catalytic Performance of the Re2O7/SiO2-Al2O3 Catalysts in the Metathesis of Ethylene and 2-Pentene for Propylene Production. Catal. Lett. 2012, 142, 1141–1149. [Google Scholar] [CrossRef]
- Rodella, C.B.; Cavalcante, J.A.M.; Buffon, R. Metathesis of methyl oleate over rhenium oxide-based catalysts supported on borated silica-alumina: Catalyst recycling. Appl. Catal. A Gen. 2004, 274, 213–217. [Google Scholar] [CrossRef]
- Mol, J.C. Metathesis of unsaturated fatty acid esters and fatty oils. J. Mol. Catal. A Cem. 1994, 90, 185–199. [Google Scholar] [CrossRef]
- Yelchuri, V.; Srikanth, K.; Prasad, R.B.N.; Karuna, M.S.L. Olefin metathesis of fatty acids and vegetable oils. J. Chem. Sci. 2019, 131, 131–139. [Google Scholar] [CrossRef]
- Sibeijn, M.; Spronk, R.; Mol, J.C. IR studies of Re2O7 metathesis catalysts supported on alumina and phosphated alumina. Catal. Lett. 1991, 8, 201–208. [Google Scholar] [CrossRef]
- Kawai, T.; Yamazaki, Y.; Tokumura, A. Metathesis of 1-Hexene over Re2O7-Al2O3 Catalysts in Liquid Phase. J. Jpn. Petrol. Inst. 1983, 26, 332–338. [Google Scholar] [CrossRef]
- Tarasov, A.L.; Shelimov, B.N.; Kazansky, V.B.; Mol, J.C. Olefin metathesis on supported rhenium catalysts activated by γ-irradiation. J. Mol. Catal. A Chem. 1997, 115, 219–228. [Google Scholar] [CrossRef]
- Amigues, P.; Chauvin, Y.; Commereuc, D.; Hong, C.T.; Lai, C.C.; Liu, Y.H. Methathesis of ethylene-butene mixtures to propylene with rhenium on alumina catalysts. J. Mol. Catal. 1991, 65, 39–50. [Google Scholar] [CrossRef]
- Kustov, L.M.; Furman, D.B.; Barkova, A.P. Metathesis of C5–C8 Terminal Olefins on Re2O7/Al2O3 Catalysts. Catal. Lett. 2016, 146, 1033–1039. [Google Scholar] [CrossRef]
- Sang, L.; Chen, S.-L.; Yuan, G.; Zheng, M.; You, J.; Chen, A.; Li, R.; Chen, L. Metathesis of 1-butene and 2-butene to propene over Re2O7 supported on macro-mesoporous γ-alumina prepared via a dual template method. J. Nat. Gas Chem. 2012, 21, 105–108. [Google Scholar] [CrossRef]
- Sang, L.; Chen, S.-L.; Yuan, G.; Zheng, Z.; Li, R.; Cen, A.; Zheng, M.; You, J. Preparation of mesoporous alumina with large pore size and their supported rhenium oxide catalysts in metathesis of 1-butene and 2-butene to propene. J. Nat. Gas Chem. 2012, 21, 352–359. [Google Scholar] [CrossRef]
- Verkuijlen, E.; Kapteijn, F.; Mol, J.C.; Boelhouwer, C. Heterogeneous metathesis of unsaturated fatty acid esters. J. Chem. Soc. Chem. Commun. 1977, 7, 198–199. [Google Scholar] [CrossRef]
- Boelhouwer, C.; Mol, J.C. Metathesis reactions of fatty acid esters. Prog. Lipid Res. 1985, 24, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.; Coverdale, A.K.; Dearing, P.F. The metathesis of unsaturated esters over Re2O7/Al2O3 catalysts. J. Mol. Catal. 1985, 28, 141–167. [Google Scholar] [CrossRef]
- Sibeijn, M.; Mol, J.C. Activity of supported Re2O7 catalysts for the metathesis of methyl oleate. Appl. Catal. 1990, 67, 279–295. [Google Scholar] [CrossRef]
- Mol, J.C. Metathesis of functionalizes acyclic olefins. J. Mol. Catal. 1991, 65, 145–162. [Google Scholar] [CrossRef]
- Kawai, T.; Uejima, S.; Suzuki, T.; Iyoda, T. Metathesis of halogen-containing olefin over Re2O7/Al2O3 catalyst promoted with alkylmetal as a cocatalyst. J. Mol. Catal. A Chem. 1998, 133, 51–59. [Google Scholar] [CrossRef]
- Chaumont, P.; John, C.S. Olefin disproportionation technology (FEAST)—A challenge for process development. J. Mol. Catal. 1988, 46, 317–328. [Google Scholar] [CrossRef]
- Lefebvre, F. Applications of the olefin metathesis reaction to industrial processes. In Ring Opening Metathesis Polymerisation and Related Chemistry. NATO Science Series: II Mathematics, Physics and Chemistry—Vol. 56; Khosravi, E., Szymańska-Buzar, T., Eds.; Springer: Dordrecht, The Netherlands; Polaica-Zdroj, Poland, 2000; pp. 247–261. [Google Scholar] [CrossRef]
- Dwyer, C.L. Chapter 6: Metathesis of Olefins. In Metal-Catalysis in Industrial Organic Processes. The Royal Society of Chemistry; Chiusoli, G.P., Maitlis, P.M., Eds.; RSC Publishing: Cambridge, UK, 2006; pp. 201–217. ISBN 978-1-84755-532-8. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A.; Krompiec, S.; Michalik, S.; Krompiec, M. Synthesis of perrhenic acid using ion exchange method. Hydrometallurgy 2007, 89, 289–296. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Malarz, J.; Ciszewski, M.; Kopyto, D.; Goc, K.; Grzybek, A.; Kowalik, P.; Orda, S.; Pianowska, K.; Turczyńska, A.; et al. Hydrometallurgical Technology for Producing Rhenium(VII) and Cobalt(II) from Waste. Crystals 2024, 14, 783. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Kopyto, D.; Malarz, J.; Ciszewski, M.; Goc, K. A New Method of Obtaining High Purity Nickel(II) Perrhenate from Waste. Crystals 2023, 13, 1465. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Malarz, J.; Kopyto, D.; Goc, K.; Grzybek, A.; Ciszewski, M.; Palmowski, A.; Benke, G.; Pianowska, K. Recovery of Zinc and Rhenium for the Production of Zinc Perrhenates. Crystals 2024, 14, 725. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Anyszkiewicz, K.; Chmielarz, A.; Machelska, G.; Witman, K. Sposób Wytwarzania Drobnokrystalicznego Renianu (VII) Srebra. Polish Patent PL 214591, 17 December 2012. [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Ciszewski, M.; Drzazga, M.; Malarz, J.; Machelska, G.; Witman, K.; Krompiec, S.; Szłapa-Kula, A.; Kula, S. Sposób Otrzymywania Bezwodnego Renianu(VII) Miedzi(II). Polish Patent PL 228989, 13 December 2017. [Google Scholar]
- Moulijn, J.A.; Mol, J.C. Structure and activity of rhenium-based metathesis catalysts. J. Mol. Catal. 1988, 46, 1–14. [Google Scholar] [CrossRef]
- Grubbs, R.H. Handbook of Metathesis: Catalyst Development; WILEY-VCH Verlag GmbH & Co. KgaA: Weinheim, The Netherlands, 2003; ISBN 9783527619481. [Google Scholar] [CrossRef]
- Grubbs, R.H.; Wenzel, A.G.; O’Leary, D.J.; Khosravi, E. Handbook of Metathesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, The Netherlands, 2015; ISBN 9783527674107. [Google Scholar] [CrossRef]






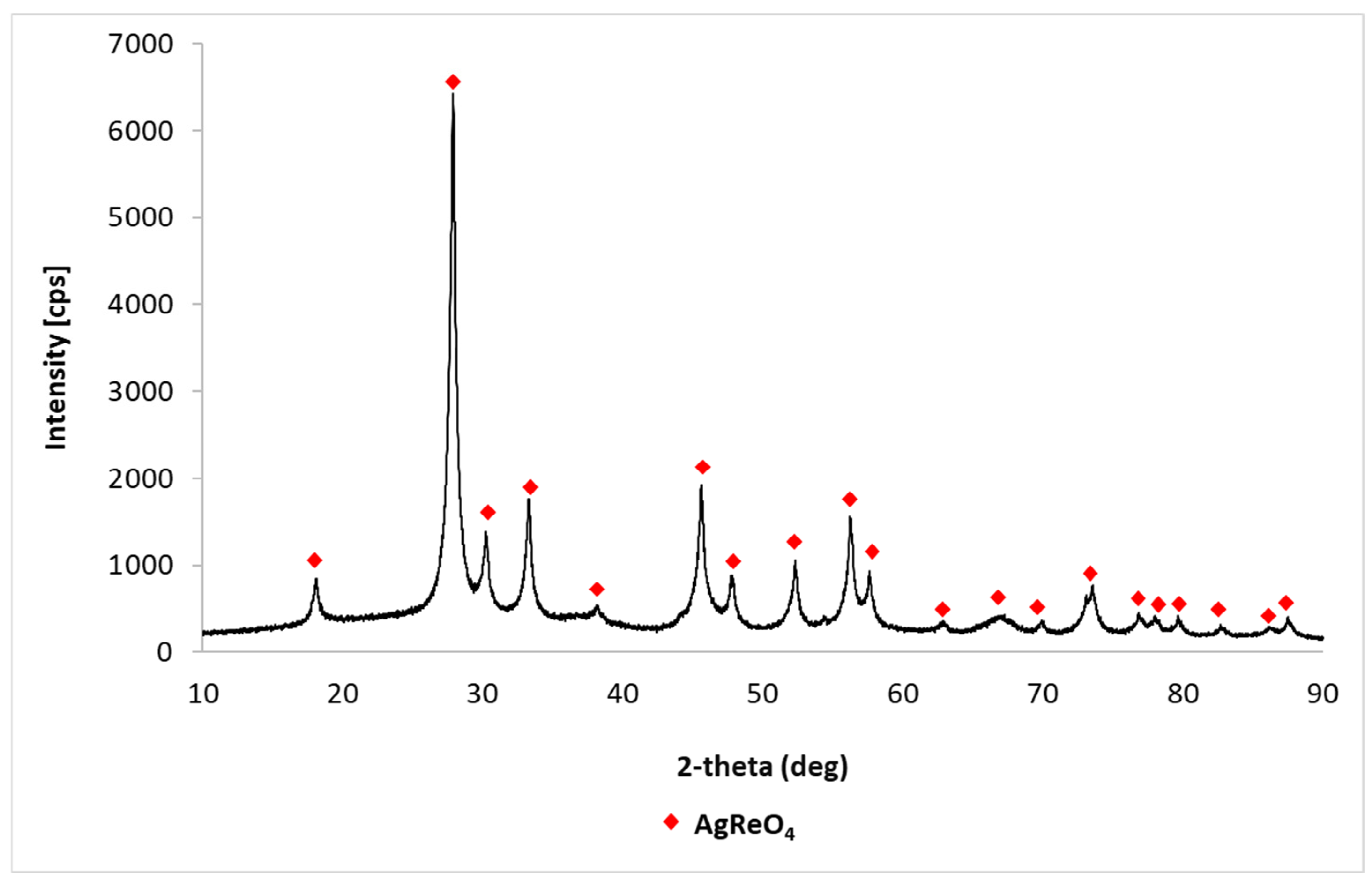
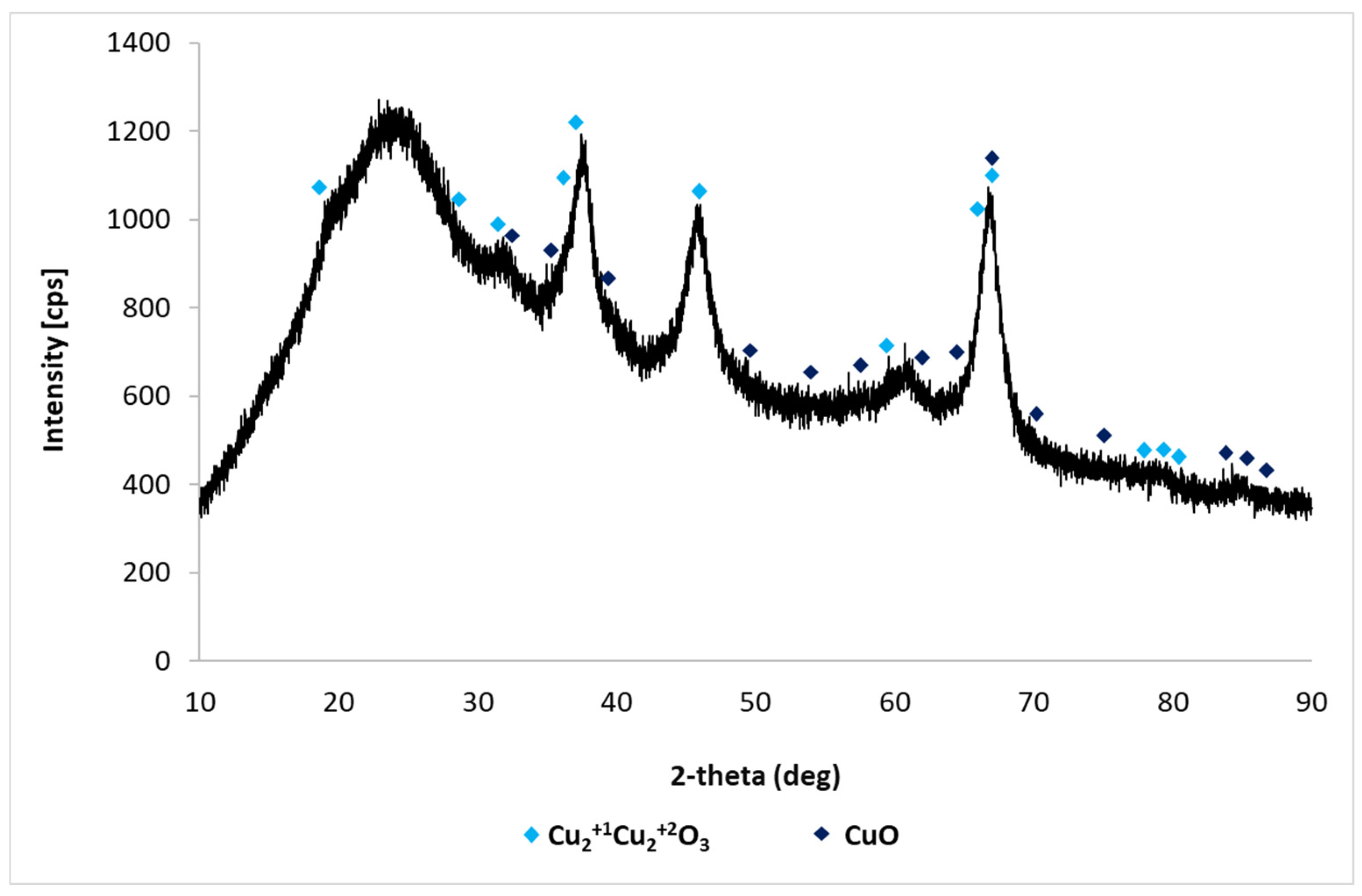





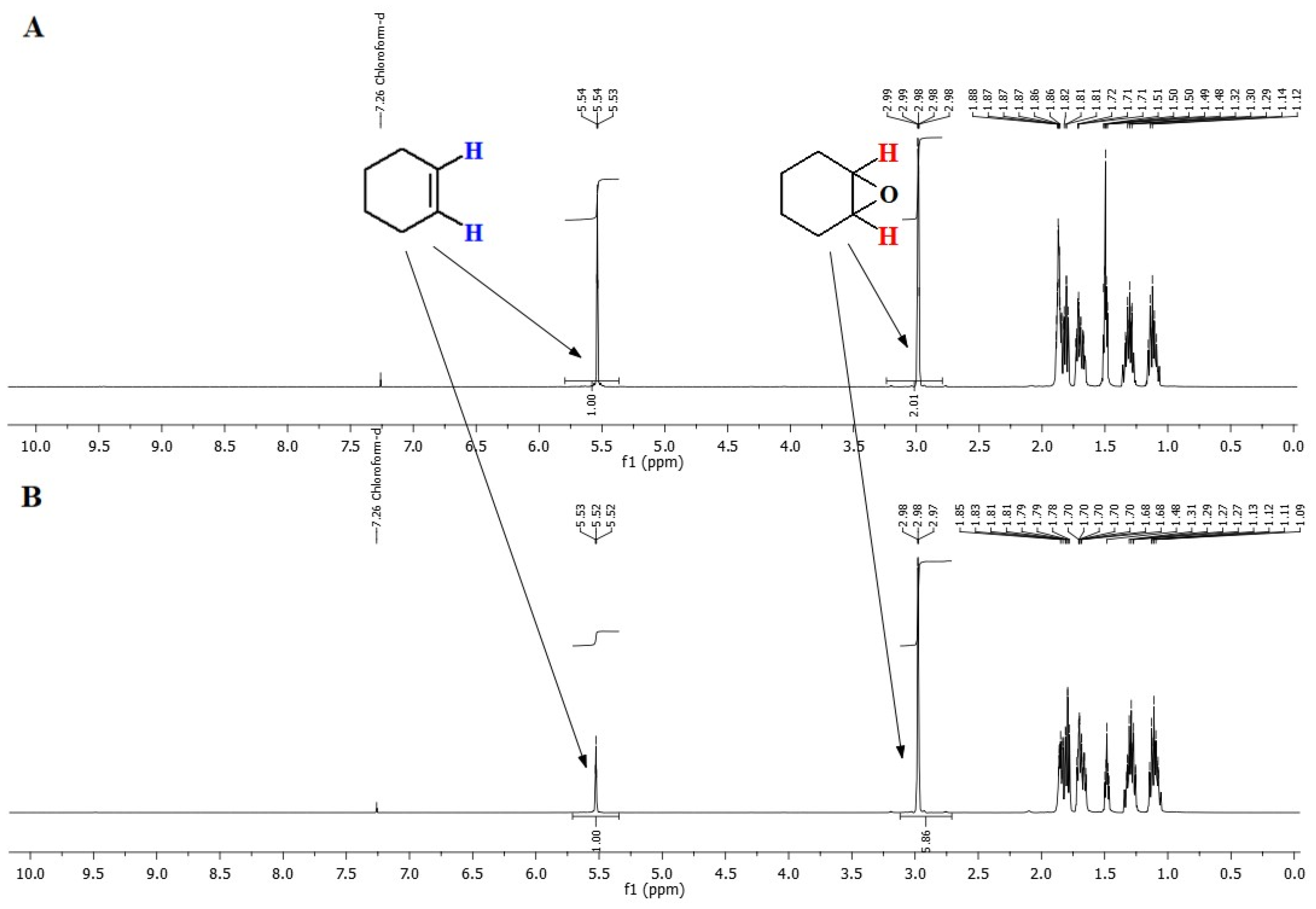







| Rhenium Compound | Composition [wt%], * [g/dm3] | |
|---|---|---|
| Re | M | |
| AgReO4 | 51.8 | 30.3 |
| Ni(ReO4)2 | 65.5 | 11.3 |
| Cu(ReO4)2 | 65.6 | 10.9 |
| Co(ReO4)2 | 66.8 | 10.4 |
| Zn(ReO4)2 | 64.0 | 12.5 |
| HReO4 | 295.02 * | - |
| MTO-M | Mass of M[ReO4] [g] | Content of Re in M[ReO4] [wt%] | Mass of MTO [g] | Yield [%] 1 |
|---|---|---|---|---|
| MTO-Ag | 1.00 | 51.8 | 0.62 | 89 |
| MTO-Ag 2 | 3.00 | 51.8 | 1.79 | 86 |
| MTO-Ni | 1.56 | 65.5 | 0.18 3 | 13 |
| MTO-Zn | 1.57 | 65.6 | 0.23 3 | 17 |
| MTO-Co | 1.56 | 66.8 | 0.21 3 | 15 |
| Catalysts | wt% Re 1 | wt% M 1 | Yield [%] 2 |
|---|---|---|---|
| Re2O7/Al2O3_4 3 | 20.9 | - | 70 |
| Re2O7/Al2O3 | 24.8 | - | 83 |
| Ag-Re2O7/Al2O3 | 27.7 | 14.8 | 92 |
| Cu-Re2O7/Al2O3 | 19.2 | 5.02 | 64 |
| Co-Re2O7/Al2O3 | 24.6 | 4.15 | 82 |
| Ni-Re2O7/Al2O3 | 24.1 | 4.11 | 80 |
| H-Re2O7/Al2O3 | 22.3 | - | 83 |
| Catalysts | wt% Re | Specific Surface Area [m2/g] | Volume Size [cm3/g] | Pore Size [Å] |
|---|---|---|---|---|
| Re2O7/Al2O3_4 1 | 20.9 | 80.3521 ± 1.7353 | 0.028979 | 7.148 |
| Re2O7/Al2O3 | 24.8 | 74.1301 ± 1.9455 | 0.026899 | 7.418 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malarz, J.; Goc, K.; Ciszewski, M.; Pianowska, K.; Wróbel, P.; Hawełek, Ł.; Kopyto, D.; Leszczyńska-Sejda, K. Research on the Production of Methyltrioxorhenium and Heterogenous Catalysts from Waste Materials. Crystals 2025, 15, 717. https://doi.org/10.3390/cryst15080717
Malarz J, Goc K, Ciszewski M, Pianowska K, Wróbel P, Hawełek Ł, Kopyto D, Leszczyńska-Sejda K. Research on the Production of Methyltrioxorhenium and Heterogenous Catalysts from Waste Materials. Crystals. 2025; 15(8):717. https://doi.org/10.3390/cryst15080717
Chicago/Turabian StyleMalarz, Joanna, Karolina Goc, Mateusz Ciszewski, Karolina Pianowska, Patrycja Wróbel, Łukasz Hawełek, Dorota Kopyto, and Katarzyna Leszczyńska-Sejda. 2025. "Research on the Production of Methyltrioxorhenium and Heterogenous Catalysts from Waste Materials" Crystals 15, no. 8: 717. https://doi.org/10.3390/cryst15080717
APA StyleMalarz, J., Goc, K., Ciszewski, M., Pianowska, K., Wróbel, P., Hawełek, Ł., Kopyto, D., & Leszczyńska-Sejda, K. (2025). Research on the Production of Methyltrioxorhenium and Heterogenous Catalysts from Waste Materials. Crystals, 15(8), 717. https://doi.org/10.3390/cryst15080717








