Abstract
Megacrystalline uraninite within Neoproterozoic migmatites in the Haita area of the Kangdian region of China provides a unique condition for the investigation of uraninite typomorphism under high-temperature conditions. The present study represents the first systematic characterization of the typomorphic signatures and genetic significance of megacrystalline uraninite via optical microscopy, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XRS), and electron probe microanalysis (EPMA). The results show that uranium mineralization occurs as euhedral megacrystalline uraninite (black grains ≤ 10 mm) hosted in quartz veins, exhibiting frequent rhombic dodecahedral and subordinate cubic–octahedral morphologies. The paragenetic assemblage is quartz–uraninite–titanite–apatite–molybdenite. The investigated uraninite is characterized by elevated unit-cell parameters and a reduced oxygen index, with complex chemical compositions enriched in ThO2 and Y2O3. These typomorphic characteristics indicate crystallization under high-temperature reducing conditions with gradual cooling. Post-crystallization tectonic fragmentation and uplift-facilitated oxidation occur, generating secondary uranium minerals with concentric color zonation (orange–red to yellow–green halos). Mineralization was jointly controlled by migmatization and late-stage tectonism, with the breakup of the Rodinia supercontinent serving as the key driver of fluid mobilization and ore deposition. The data materialized in the present study improve our knowledge about uranium mineralization during continental breakup events.
1. Introduction
As the primary industrial source of uranium, uraninite has been the focus of extensive research in recent decades, particularly regarding its (1) geochronology and tectonic significance [1,2,3,4,5]; (2) geochemical evolution, including compositional variability, alteration processes, and trace-element distributions [6,7,8,9,10,11]; and (3) geochronometry (e.g., thermal ionization mass spectrometry (TIMS), secondary ion mass spectrometry (SIMS), laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) U–Th–Pb dating) and U–Pb substitution mechanisms [12,13,14,15,16,17]. However, typomorphic analysis of natural uraninite remains challenging because of its typical submillimeter grain size (<1 mm), dispersed occurrence, and imperfect crystal development. Consequently, our current understanding of uraninite typomorphism relies heavily on synthetic analogs [18,19,20,21].
The recent discovery of megacrystalline uraninite in the Neoproterozoic migmatites of the Haita area of the Kangdian region (southwestern China) has provided a unique natural laboratory. These crystals exhibit characteristics that have rarely been documented anywhere in the world, including exceptional crystallinity and large grain sizes (black grains ≤ 10 mm) [22,23,24,25,26,27]. Therefore, the present study presents the first systematic investigation of uraninite typomorphism under natural high-temperature conditions. The typomorphic signatures of Haita megacrystalline uraninite are characterized through integrated microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and microprobe analysis to elucidate their implications for uranium mineralization during continental breakup events.
2. Geological Background
The Kangdian region in southwestern China (Figure 1a) represents a polydeformed Precambrian terrane with a complex structural architecture, diverse lithological assemblages, and world-class polymetallic mineralization [28,29,30]. As a critical segment of the Yangtze Craton, it exposes extensive Meso-Neoproterozoic (1.6–0.8 Ga) strata recording multi-stage orogenesis. Polyphase tectono-thermal events have generated composite structural domains hosting economically significant Cu–Fe–U metallogenic belts [30]. Recent exploration has revealed ultra-rich uranium occurrences dominated by megacrystalline uraninite along a 300 km NNW–SSE corridor stretching from Haita (Miyi County) to Shujie (Mouding County) via the Datian area of Panzhihua (Figure 1b). These uranium occurrences contain exceptionally coarse uraninite crystals (≤10 mm)—surpassing global average sizes by an order of magnitude—highlighting the favorable uranium mineralization conditions of the Kangdian region [22].
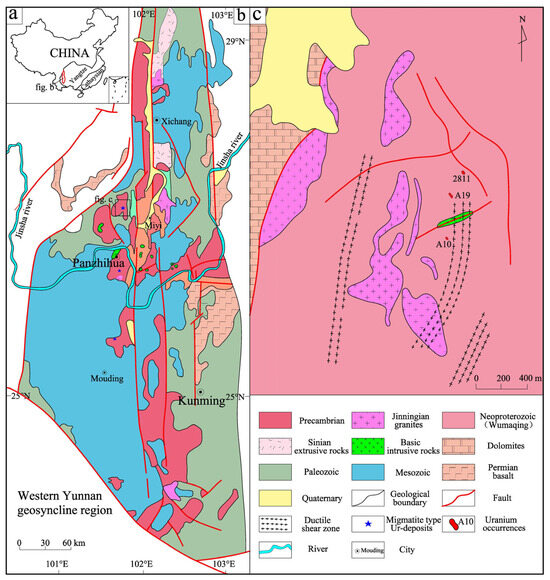
Figure 1.
(a) Position of the Kangdian region in China. (b) Brief geological map of the Kangdian region. (c) Geological map of the Haita area (modified after [26]).
The study area, located 8.7 km southwest of Miyi County (Figure 1c), experienced extensive metamorphism and migration. The lithology is dominated by medium- to high-grade metamorphic rocks of the Proterozoic Wumaqing strata, with widespread exposure of these metamorphic series, along with carbonate and clastic rocks of the Sinian–Cambrian Dengying Formation. Substantial multi-stage magmatism was widely distributed in the region, characterized by intermediate to felsic compositions from the Jinningian period as well as alkaline intrusives from the Hercynian–Indochina period. The area displays complex folds, ductile shear zones, brittle faults, and ductile faults, primarily controlled by north–south-trending deep faults (e.g., the Mopan Mountain and Anning River faults), with dominant north–south and northeast–southwest tectonic trends. Significant deformation and metamorphism have influenced the rock formations, although surface weathering obscures crystal-related tectonic traces. The Wumaqing Group metamorphic rocks and migmatites host three uranium occurrences (A10, A19, and 2811), with ore-hosting rocks classified according to their degree of migmatization as follows: (1) vein material, (2) migmatized metamorphic rock, (3) migmatite, and (4) vein body, where two-mica quartz schists serve as protoliths for the migmatitic vein material.
A uraninite-rich quartz vein (Haita 2811 Occurrence), which lies beneath the Quaternary cover, formed within dense fissure zones during the migmatization of extensively weathered two-mica quartz schists. The orientation of this vein, with a width of 35–50 cm, aligns with the schistosity of the surrounding rock. This vein contains more than 100 giant uraninite grains with well-faceted euhedral morphologies and large particle sizes; such uraninite-rich quartz veins are rare worldwide. Within the vein and adjacent fissure zones, the maximum γ-ray intensity reaches 6800 Sv, decreasing laterally (Figure 2).
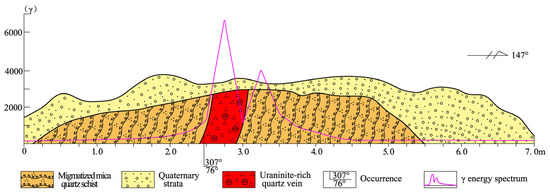
Figure 2.
Geological profile and γ-ray energy spectrum of the uraninite-rich quartz vein.
3. Samples and Analytical Methods
Five samples were collected from the uraninite-rich quartz vein for megacrystalline uraninite extraction. Thin sections, polished sections, and electron probe samples were prepared at the Geochemistry Department of Chengdu University of Technology.
Rock/mineral identification, reflectance measurements, and microhardness tests were conducted at the Comprehensive Rock and Mineral Identification Laboratory of Chengdu University of Technology using the following instruments: an Eclipse LV100 POL polarizing microscope (Nikon Corporation, Tokyo, Japan); an AxioScope A1 Pol reflective polarized light microscope (Carl Zeiss AG, Oberkochen, Germany); and an HV-1000NDT Micro Hardness tester (Truer Corporation, Shanghai, China).
XRD analysis was performed using a Bruker D8 Advance diffractometer (Bruker Corporation, Billerica, MA, USA) at Chengdu University of Technology. The instrument was operated with Cu Kα radiation (λ = 1.5406 Å) at 40 kV and 40 mA. Data were collected over a 2θ range of 5–80° with a step size of 0.02° and a scanning speed of 2°/min, using a LynxEye array detector (also by Bruker Corporation). The uraninite (UO2) sample was finely ground and homogeneously loaded into a glass sample holder, with continuous sample rotation during measurement to ensure data representativeness. Phase identification was conducted using MDI Jade 6.5 software with the ICDD-PDF2 database, achieving a measurement precision of ±0.01° 2θ as verified by the NIST SRM 640c silicon standard.
Electron Probe Microanalysis (EPMA) was conducted at the State Key Laboratory of Nuclear Resources and Environment at East China University of Technology using a JXA-8230 microanalyzer (JEOL Corporation, Tokyo, Japan). All measurements were performed under high vacuum conditions with an accelerating voltage of 15.0 kV, beam current of 20.0 nA, and probe diameter of 1–2 µm. For elemental analysis, peak counting times were set at 10 s for major elements (Al, Mg, Ca, Na, K, Si, Fe) with 5 s background measurements, while trace elements (P, Ti, U, Th, REEs, Y, Pb) employed 20 s peak counting and 10 s background measurements. Calibration standards included natural minerals (garnet for Si, rutile for Ti, dolomite for Ca, hematite for Fe, biotite for Al, galena for Pb, jadeite for Na, orthoclase for K, and monazite for La/Ce/P) and synthetic U-Th-Pb oxides. Analytical uncertainties were <1% for major elements and <10% for trace elements. Data were processed using the ZAF correction procedures following the Chinese National Standard GB/T 15617-2025. Elemental mapping was performed using a EPMA-1600 (Shimadzu Corporation, Kyoto, Japan)instrument at the Tianjin Center of China Geological Survey.
X-ray photoelectron spectroscopy analysis was performed by the Chengdu Scientific Compass Analysis and Testing Company using a Thermo Scientific K-Alpha+ spectrometer with the following parameters: chamber vacuum, ~5 × 10−9 mbar; monochromated Al Kα source (1486.6 eV); voltage, 15 kV; beam current, 15 mA; CAE analyzer mode; work function, 4.2; and beam diameter, 100 µm. Avantage software 6.9 was used to process the data and determine the uranium valence ratios.
4. Typomorphic Characteristics of Uraninite
4.1. Morphological Characteristics
The naturally formed uraninite exhibits well-faceted euhedral morphologies and large grain sizes (≤1 cm), occurring as discrete crystals within quartz veins (Figure 3a–d). Megacrystalline uraninite displays predominantly euhedral morphologies; rhombic dodecahedra and cubic–octahedral combinations dominate, with occasional pure cubic forms. Crystals are isometric and visibly discernible in the hand specimen (Figure 3a–d). The host quartz appeared smoky gray. Uraninite occurred as isometric granules distributed throughout the veins, with most grains measuring ~0.5 cm and the largest grains reaching ~1 cm. The uranium content amounts typically to 1–5 vol.% but occasionally exceeds 20 vol.% (the uranium content within the quartz vein). The limited sampling (to ~1 m depth) revealed an increasing abundance of uraninite grains with depth, suggesting higher uranium concentrations in deeper zones.
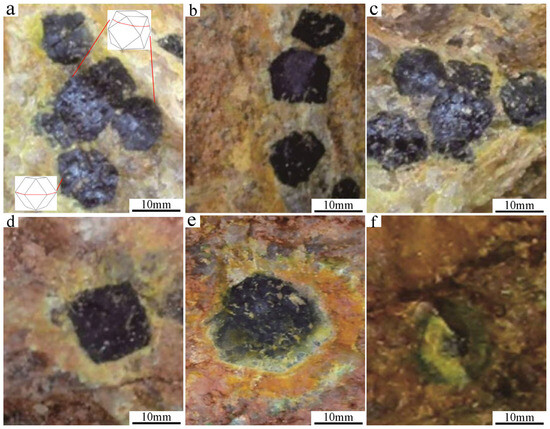
Figure 3.
Occurrence shapes of megacrystalline uraninite. (a–d) euhedral uraninite, and (e,f) secondary uranium minerals.
4.2. Physical Properties
The physical typomorphic properties of uraninite reflect its formation conditions through attributes such as color, hardness, and reflectance. These manifestations are fundamentally derived from the chemical composition and crystal structure. Field observations show black megacrystalline uraninite with a metallic luster, steel-gray streaks, and black striations. Strong oxidation results in secondary uranium minerals on the crystal surfaces (Figure 3e), including uranyl hydroxide, uranyl silicate, and uranyl phosphate. These minerals were distributed in concentric bands around the uraninite edges, displaying the characteristic color zonation of orange–red → orange → orange–yellow → yellow → yellow–green from the inner to the outer region, respectively. This sequence reflects progressive oxidation from uranyl hydroxide to uranyl silicates/phosphate, which is consistent with U4+ → U6+ transformation. Under intense oxidation, complete conversion to U6+ minerals preserved the original morphology (Figure 3f). The reflectance measurements (Table 1) yielded maximum, minimum, and average values of 14.572, 14.309, and 14.300, respectively. Microhardness tests indicated a relatively low hardness of ~330 HV, which was below the typical reference values.

Table 1.
Reflectance of megacrystalline uraninite samples.
4.3. Crystal Structure
Powder XRD identified the uranium mineral as uraninite (Figure 4). The diffraction intensity is strongest for {111}, followed by {220}, {200}, and {311}, with {222} showing the lowest intensity (Table 2). The unit-cell parameters for five single grains range from 5.457 to 5.462 Å.
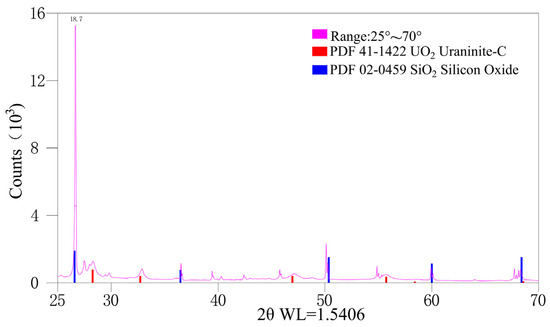
Figure 4.
X-Ray diffraction pattern of the investigated megacrystalline uraninite.

Table 2.
Unit-cell parameters and oxygen coefficients of megacrystalline uraninite samples.
In the crystallochemical formula (U4+, U6+, Th, rare earth elements (REEs), Pb)Ox, the typical oxygen index (x) is 2.17–2.92 [31,32,33]. According to the relationship between the unit-cell parameters and the oxidation degree (Figure 5), the oxygen index for the megacrystalline uraninite was 2.0600–2.0975.
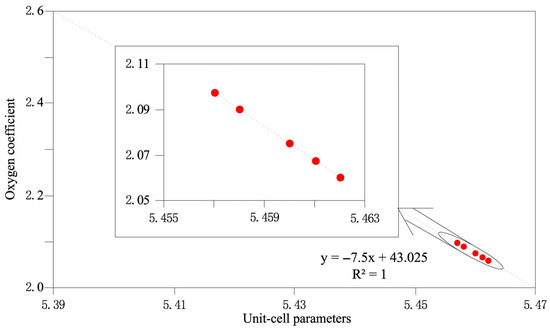
Figure 5.
Diagram of the relationship between the unit-cell parameter of the uraninite and its oxidation degree (modified after [31]).
4.4. Chemical Composition
The compositional ranges of UO2, PbO, ThO2, and Y2O3 in five megacrystalline uraninite particles were 67.63–83.67% (mean = 77.99%), 0.12–4.42% (mean = 2.64%), 2.50–17.93% (mean = 8.77%), and 0.03–1.72% (mean = 0.43%), respectively (Table S1). The contents of the other elements were below the detection limit of 0.01%. Correlation and linear fitting analyses (Figure 6) indicated negative correlations between UO2 and SiO2 (R2 =−0.53) and between UO2 and ThO2 (R2 =−0.87), but positive correlations for PbO (R2 =0.32) and Y2O3 (R2 =0.41). EPMA mapping (Figure 7) shows smaller fragmented particles but a homogeneous U–Th distribution.
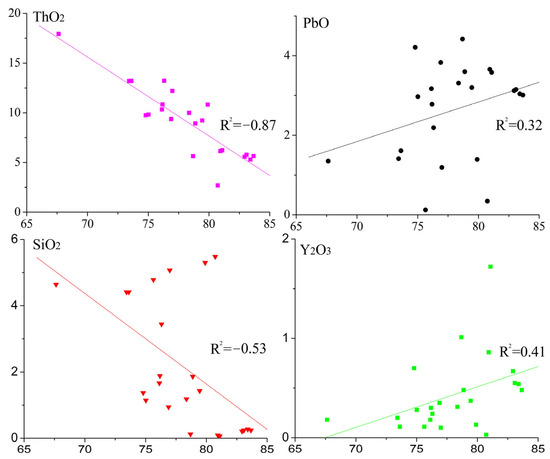
Figure 6.
Diagrams of the correlation coefficients and linear relationship between the content of UO2 and each of the shown chemical components.
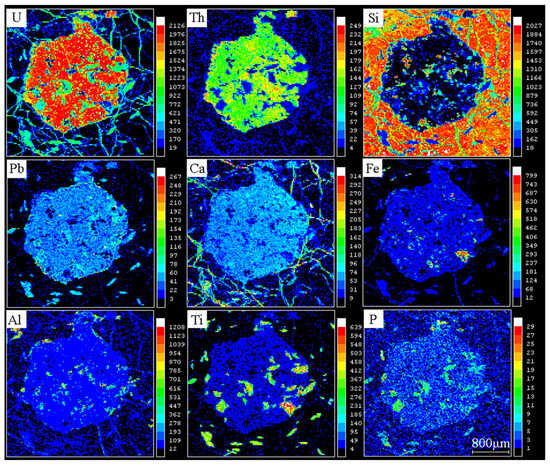
Figure 7.
Element distribution diagrams of each element in megacrystalline uraninite [34].
4.5. Mineral Assemblage
Integrated field, microscopic, and electron probe analyses revealed a simple mineral assemblage in the uraninite-rich quartz vein. Beyond uraninite and its oxidation-derived secondary minerals, the composition primarily comprised titanite and apatite, with minor amounts of molybdenite and xenotime (Figure 8). Titanite occurred as clustered euhedral stubby prisms (predominantly crystalline, rarely subhedral), with the largest crystals (reaching several millimeters) coexisting with the megacrystalline uraninite. Apatite displays distinctive equant hexagonal crystals (crystalline to subhedral), whereas acicular or elongated forms are absent. Low birefringence resulted in dark interference colors under cross-polarized light, with some grains exhibiting near extinction. Given the scarcity of molybdenite and xenotime, the paragenetic sequence was defined as quartz–uraninite–titanite–apatite–molybdenite [26].
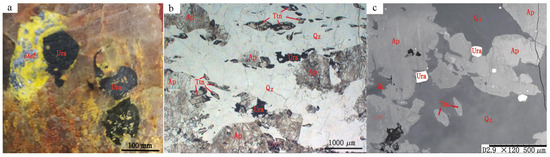
Figure 8.
Symbiotic mineral assemblages of uraninite: (a) Molybdenite with uraninite; (b,c) Euhedral uraninite with quartz and apatite [26]. Abbreviations: Ura, Uraninite; Mol, Molybdenite; Ap, Apatite; Ttn, Titanite; Qz, Quartz.
4.6. Valence State Characteristics
Table 3 presents the XPS-derived valence state characteristics of two representative uraninite samples (101 and 102). The data show consistent U4+ dominance (90.32–91.57 atomic%) with minor but measurable U6+ components (8.43–9.68 atomic%). The U4+ binding energy peaks at 380.42–380.46 eV with peak widths of 1.99–2.03 eV, while the U6+ peaks occur at 381.99–382.12 eV (widths 1.80–2.05 eV). This ~2 eV chemical shift between oxidation states agrees well with the established XPS standards for uranium oxides. The consistent ~10% U6+ content across samples suggests this minor oxidized component represents an intrinsic feature of the primary uraninite crystallization rather than a post-formational alteration.

Table 3.
Valence state characteristics of two representative uraninite samples.
As a simple uranium oxide, uraninite primarily occurs as tetravalent uranium (U4+) with minor U6+. Valence state variations and their proportions serve as critical proxies for oxygen fugacity (fO2) in ore-forming melts/fluids. Our data confirmed the exclusive presence of U4+ within the Haita 2811 occurrence.
5. Discussion
5.1. Geological Significance of Uraninite Typomorphic Characteristics
Crystal morphology and mineral grain size are valuable indicators for elucidating the genetic environment of uraninite [18]. For example, uraninite crystallized in pegmatites predominantly displays a combination of cubic and rhombic dodecahedral crystal forms; uraninite formed in hypothermal deposits primarily exhibits cubic and octahedral crystals, whereas uraninite formed in mesothermal deposits typically yields cubic crystals. The particle size distribution of minerals reflects the stability of the physicochemical conditions during their formation. The fundamental distinction between uraninite and pitchblende lies in the physicochemical gradient during mineralization, with the rate of temperature decline critically controlling mineral morphology. When the temperature decreases slowly, the crystallization rates decrease, favoring uraninite development, whereas rapid cooling promotes pitchblende formation [19]. The synthesis experiments producing octahedral uraninite (2.5 × 3.5 mm) demonstrate that optimal growth requires strongly reducing conditions, a temperature > 450 °C, pressure > 120 MPa, high acidity (pH < 4), and diluted solutions (U concentration < 23.8 mg/mL) [20]. The ratio of tetravalent (U4+)-to-hexavalent (U6+) uranium in uraninite (ideal formula UO2) carries significant geological implications, particularly as a robust indicator of redox conditions. A predominance of U4+ typically reflects reducing environments, such as those found in deep-seated granites or the primary zones of uranium deposits. This valence state is commonly associated with low oxygen fugacity and high-temperature magmatic or hydrothermal processes [35]. The valence analysis of uraninite from the Haita 2811 occurrence confirms the predominant presence of tetravalent uranium, indicating formation under reducing conditions. The well-developed crystal forms suggest stable physicochemical conditions during growth, which is consistent with crystallization from high-temperature, slightly acidic fluids under slow cooling, implying formation in a deep-seated, high-temperature reducing environment.
Uraninite typically appears black or brownish black. As the formation temperature and pressure decrease, the U4+/U6+ ratio progressively declines, causing gradual lightening of the mineral color. For example, uranyl minerals in supergene zones exhibit paler hues than those in redox transition zones. Uranyl hydroxide signals proximal to primary uranium mineralization spatially correlate with uraninite/pitchblende as products of in situ oxidation under weakly basic to alkaline conditions. These uranyl hydroxide minerals form only in high-grade ores with sharp concentration gradients, primarily within hydrothermal or pegmatitic uranium deposits [18,31,32,33]. The non-weathered surfaces of the Haita megacrystalline uraninite retained a uniform black coloration, indicating stable formation conditions. Color zonation (orange–red → orange → orange–yellow → yellow → yellow–green) in the peripheral uranyl hydroxides reflects post-crystallization uplift and oxidation in near-surface environments. Furthermore, the presence of uranyl hydroxide suggests an increase in oxidation intensity at shallower depths. Notably, the measured reflectance and hardness values for megacrystalline uraninite were lower than those of the standard references. Potential causes include the following: (1) analytical errors or sample surface imperfections, (2) reflectance reduction by secondary uranyl minerals from surface oxidation, and (3) late-stage tectonic fracturing, which is consistent with the EPMA mapping observations.
The typical unit-cell parameter range for major Chinese uranium minerals is 5.347–5.488 Å. Uraninite and pitchblende typically exhibit values of 5.423–5.488 Å (mean = 5.456 Å) and 5.346–5.477 Å (mean = 5.405 Å), respectively [31,32,33]. The factors influencing these parameters include temperature, chemical composition, and post-formational oxidation. Elevated temperatures promote the complete reduction of U6+ to U4+, establishing a positive correlation between unit-cell parameters and formation temperatures. These parameters also positively correlate with UO2 and impurity contents (Th, Pb, Y, and rare-earth elements (REEs)), although this relationship remains thermally controlled [18]. Post-crystallization oxidation (U4+ → U6+) induces lattice defects and unit-cell shrinkage. The high unit-cell parameters observed in this study (5.457–5.462 Å) confirm high-temperature uraninite formation. Th4+, Y3+, and REE3+ exhibit ionic radii similar to U4+ in hydrothermal fluids, facilitating substitution and lattice expansion, as verified by EPMA. The oxygen index reflects the formation conditions, longevity, and pre- and post-formation alterations; therefore, the values vary with the formation environment and temperature. For example, deep-seated pitchblende has lower oxygen index values than its low-temperature shallow equivalents; endogenic pitchblende shows low values, whereas exogenous varieties display high values; late-stage oxidation increases the oxygen index values; and synthetic U-oxides exhibit values of 2.00–3.00, whereas natural specimens exhibit values of 2.17–2.92 [31,32,33] (Figure 5). The oxygen index values obtained in the present study (2.0600–2.0975) indicate an endogenic origin without late oxidation (Table 2).
Uraninite is highly susceptible to fluid alteration [6,36,37]. REEs, Si, Ca, Fe, Al, and K serve as critical typomorphic indicators for physicochemical conditions [13,15,38,39,40]. Elevated Th contents are observed in metasomatized uraninite because Th and U ions coexist stably at high temperatures during deposit formation, when Th4+ ion activity is lower than that of U4+. Upon cooling, Th4+–U4+ decoupling occurs, enabling Th4+ incorporation into mineral structures. Thus, high-temperature uraninite shows Th enrichment. The positive correlations among U, Th, and Y, along with their elevated contents, provide further evidence for the relatively slow growth of uraninite crystals [40]. The U/Th ratio is a key geothermometer: >1000 indicates low crystallization temperatures; <1000 implies high temperatures; and <100 signifies magmatic conditions [10,41,42]. Yttrium behavior parallels that of thorium; its content tracks crystallization/recrystallization processes and correlates positively with the formation temperature [40,43]. U–Pb decay systems enable age determination [44], making U–Pb dating the primary method of isotopic chronology. Consequently, U and Pb contents should theoretically show negative correlations. However, owing to crystal–chemical incompatibility, Pb2+ tends to migrate from uraninite during fluid events, and is replaced by Ca2+, Si4+, and Fe2+/3+ [10,40,45], although these cations do not always participate equally in substitution.
Thus, megacrystalline uraninite shows elevated unit-cell parameters and a reduced oxygen index. According to EPMA data, the high unit-cell parameters can be attributed to the isomorphic incorporation of Th4+, Pb2+, and REEs. U/Th ratios <100 and significant negative U–Th correlations confirm high-temperature Th enrichment. The consistently high Y concentrations further support this interpretation. The observed positive U–Pb correlation may indicate an elevated initial common Pb content and an absence of post-formational fluid alteration. Although mapping suggests late tectonic effects, the EPMA results revealed homogeneous U–Th distributions, confirming the absence of fluid-induced recrystallization.
Titanite and apatite are diagnostic high-temperature coexisting minerals with uraninite that typically occur in pegmatites and high-temperature hydrothermal deposits but rarely in low-temperature hydrothermal systems. Thus, their direct crystallization from ore-forming fluids confirms high-temperature conditions. U–Mo associations typically develop in volcanic-related epithermal deposits such as pitchblende–molybdenite pairs. The rare uraninite–molybdenite assemblage observed in the present study suggests co-precipitation under slow cooling [19], where uraninite–molybdenite pairs form during gradual temperature decline, and the pitchblende–sulfide molybdenum associations indicate rapid quenching. This slow cooling environment is compatible with the other typomorphic characteristics of uraninite.
5.2. Metallogenic Background and Genesis of Uraninite
Previous studies have attributed uranium mineralization in the study area to post-migmatization hydrothermal fluids and superimposed hydrothermal events, emphasizing the key role of migmatization [46]. However, uraninite-hosting quartz veins occur within the tectonic fissures of migmatized schists, indicating dual control by migmatization and structural fracturing. The migmatite basement comprises paragneiss-derived mica quartz schists and gneisses, exhibiting U concentrations of 5.64–12.9 × 10−6 (avg. 8.89 × 10−6) and Th/U = 2.97 (U-rich protolith). The migmatization in Haita produced granitoids via anatexis with elevated U (12.9–30 × 10−6; avg. 21 × 10−6) concentrations, Th (avg. 59.13 × 10−6) concentrations, and Th/U = 3.2, indicating a regional preconcentration of uranium [47]. Regional migmatization occurred at 830–780 Ma, whereas uraninite–titanite–molybdenite crystallization occurred at ~780 Ma, marking the late stage of migmatization [48,49]. Refs. [27,50] linked Neoproterozoic uranium mineralization in the Kangdian region to the breakup of the Rodinia assembly. Thus, examining the mineralization of Haita within the Rodinia tectonic framework offers critical insights. Following the Rodinia assembly, orogenesis ceased, transitioning to extension via intracontinental subduction and supercontinent breakup, which manifests regionally as migmatization.
After collisional orogeny, the Kangdian region underwent intraplate extension. Crustal stretching generated intense faulting, which created structural traps and ore-hosting spaces. Intermediate–felsic magmas provided both thermal energy and fluids. Tectonism critically enhanced permeability and facilitated the infiltration of meteoric water. Heated meteoric solutions dissolved wall-rock sulfates, carbonates, and halogens, forming high-fO2 fluids that leached uranium [51]. These high-fO2 fluids oxidized U4+ to U6+, mobilizing uranium as UO2(CO3)22−, UO2(CO3)34−, [UO2F3]−, and [UO2F4]2− complexes. Fluid migration is governed by fO2 and tectonics, whereas pH controls U-complex speciation [51,52]. Fluid mixing between meteoric water and deep-sourced tectonic-thermal fluids likely occurred [53,54,55,56], with H-O isotopes confirming the meteoric contribution that maintained high fO2 conditions. A subsequent reduction of these oxidized fluids ultimately precipitated megacrystalline uraninite in optimal structural–geochemical settings.
6. Conclusions
The typomorphic characteristics and geological significance of megacrystalline uraninite from the Haita area of the Kangdian region can be summarized as follows:
- (1)
- Exceptional crystallinity and size. Naturally occurring uraninite exhibits well-developed euhedral morphologies with anomalously large grain sizes (≤10 mm).
- (2)
- High-temperature crystallization signatures. The typomorphic features reflect stable physicochemical conditions, indicating formation in a reduced environment (>450 °C) with gradual cooling.
- (3)
- Dual genetic controls. Mineralization is jointly governed by migmatization (uranium preconcentration) and tectonism (fluid pathways/ore deposition).
As an important typomorphic characterization of natural uraninite, this study advances our understanding of uranium mineralization during continental breakup events.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst15080718/s1. Table S1 Analyzes results of EMPA.
Author Contributions
Conceptualization, M.Y. and Z.X.; methodology, J.Y.; software, B.X.; validation, M.Y. and Z.X.; formal analysis, M.Y.; investigation, C.Z.; resources, Z.X.; data curation, M.Y.; writing—original draft preparation, M.Y.; writing—review and editing, M.Y.; visualization, J.Y.; supervision, Z.X.; project administration, Z.X.; funding acquisition, Z.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Opening Fund of the State Key Laboratory of Nuclear Resources and Environment (No. 2022NRE01), the Sichuan Science and Technology Program (No. 2025ZNSFSC0311, 2024NSFSC0801), the Doctoral Scientific Startup Foundation of Mianyang Normal University (No. QD2023A07), the Doctoral Scientific Startup Foundation of Southwest University of Science and Technology (No. 23ZX7148), and the Opening Fund of the Provincial Key Lab of Applied Nuclear Techniques in Geosciences (No. 202301).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We greatly appreciate the help of Jiamin Tian and Tao Li during daily discussion work.
Conflicts of Interest
Jian Yao was employed by the China National Nuclear Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Molnar, F.; O’Brien, H.; Stein, H.; Cook, N.D. Geochronology of hydrothermal processes leading to the formation of the Au–U mineralization at the Rompas prospect, Peräpohja belt, Northern Finland: Application of paired U–Pb dating of uraninite and Re–Os dating of molybdenite to the identification of multiple hydrothermal events in a metamorphic terrane. Minerals 2017, 7, 171. [Google Scholar]
- Wu, Y.; Qin, M.; Guo, D.; Fan, G.; Liu, Z.; Guo, G. The latest in-situ uraninite U-Pb age of the Guangshigou uranium deposit, Northern Qinling orogen, China: Constraint on the metallogenic mechanism. Acta Geol. Sin.-Engl. 2018, 92, 2445–2447. [Google Scholar]
- Cheng, L.; Zhang, C.; Song, H.; Cheng, Q. In-Situ LA-ICP-MS Uraninite U–Pb Dating and Genesis of the Datian Migmatite-Hosted Uranium Deposit, South China. Minerals 2021, 11, 1098. [Google Scholar] [CrossRef]
- Zhong, F.; Yan, J.; Xia, F.; Pan, J.; Liu, W.; Lai, J.; Zhao, Q. In-situ U-Pb isotope geochronology of uraninite for Changjiang granite-type uranium ore field in northern Guangdong, China: Implications for uranium mineralization. Acta Petrol. Sin. 2019, 35, 2727–2744, (In Chinese with English Abstract). [Google Scholar]
- Xu, K.; Li, G.; Zhang, H.; Dong, W.; Liu, X.; Zhang, X.; Wang, R. Uranium and U-bearing minerals in the Husab uranium deposit in Namibia: Occurrence, composition, age, and implications for uranium mineralization process. Ore Geol. Rev. 2025, 182, 106642. [Google Scholar] [CrossRef]
- Macmillan, E.; Ciobanu, C.L.; Ehrig, K.; Cook, N.J.; Pring, A. Chemical zoning and lattice distortion in uraninite from Olympic Dam, South Australia. Am. Mineral. 2016, 101, 2351–2354. [Google Scholar] [CrossRef]
- Spano, T.L.; Simonetti, A.; Balboni, E.; Dorais, C.; Burns, P.C. Trace element and U isotope analysis of uraninite and ore concentrate: Applications for nuclear forensic investigations. Appl. Geochem. 2017, 84, 277–285. [Google Scholar] [CrossRef]
- Yuan, F.; Jiang, S.Y.; Liu, J.; Zhang, S.; Xiao, Z.; Liu, G.; Hu, X. Geochronology and Geochemistry of Uraninite and Coffinite: Insights into Ore-Forming Process in the Pegmatite-Hosted Uraniferous Province, North Qinling, Central China. Minerals 2019, 9, 552. [Google Scholar] [CrossRef]
- Ozha, M.K.; Pal, D.C.; Mishra, B.; Desapati, T.; Shaji, T.S. Geochemistry and chemical dating of uraninite in the Samarkiya area, central Rajasthan, northwestern India—Implication for geochemical and temporal evolution of uranium mineralization. Ore Geol. Rev. 2017, 88, 23–42. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Wang, F.; White, N.C.; Zhou, T. Release of uranium from uraninite in granites through alteration: Implications for the source of granite-related uranium ores. Econ. Geol. 2021, 116, 1115–1139. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Zhou, T.; Zhu, J.; Cao, C. Metallogenesis of uranium deposits in China: Perspectives from uraninite chemistry. Ore Geol. Rev. 2024, 173, 106251. [Google Scholar] [CrossRef]
- Bowles, J.F. Age dating of individual grains of uraninite in rocks from electron microprobe analyses. Chem. Geol. 1990, 83, 47–53. [Google Scholar] [CrossRef]
- Alexandre, P.; Kyser, T.K. Effects of cationic substitutions and alteration in uraninite, and implications for the dating of uranium deposits. Can. Mineral. 2005, 43, 1005–1017. [Google Scholar] [CrossRef]
- Schmitt, A.K. Uranium series accessory crystal dating of magmatic processes. Annu. Rev. Earth Planet. Sci. 2011, 39, 321–349. [Google Scholar] [CrossRef]
- Pal, D.C.; Rhede, D. Geochemistry and chemical dating of uraninite in the Jaduguda Uranium Deposit, Singhbhum Shear Zone, India—Implications for uranium mineralization and geochemical evolution of uraninite. Econ. Geol. 2013, 108, 1499–1515. [Google Scholar] [CrossRef]
- Waitzinger, M.; Finger, F. In-situ U–Th–Pb geochronometry with submicron-scale resolution: Low-voltage electron-beam dating of complexly zoned polygenetic uraninite microcrystals. Geol. Carpath. 2018, 69, 558–572. [Google Scholar] [CrossRef]
- Corcoran, L.; Simonetti, A. Geochronology of uraninite revisited. Minerals 2020, 10, 205. [Google Scholar] [CrossRef]
- Song, H.; Xiao, T.; Chi, G.; Wang, Z.; Xu, Z.; Hou, M. CAUM: A software for calculating and assessing chemical ages of uranium minerals. Geosci. Front. 2025, 16, 102031. [Google Scholar] [CrossRef]
- Min, M.; Zhang, F. Genetic Uranium Mineralogy, 1st ed.; Atomic Energy Press: Beijing, China, 1992; pp. 1–354. (In Chinese) [Google Scholar]
- Yao, L.Y.; Zhang, B.J. Experimental Geochemistry of the Xiangshan Hydrothermal Uranium Deposit; Atomic Energy Press: Beijing, China, 2014; pp. 1–54. (In Chinese) [Google Scholar]
- Shen, C.Q.; Zhao, F.M. The experimental study of synthetic octagonal uraninite. Uranium Geol. 2014, 30, 252–256, (In Chinese with English Abstract). [Google Scholar]
- DeVetter, B.M.; Myers, T.L.; Cannon, B.D.; Scharko, N.K.; Kelly-Gorham, M.R.K.; Corbey, J.F.; Johnson, T.J. Optical and chemical characterization of uranium dioxide (UO2) and uraninite mineral: Calculation of the fundamental optical constants. J. Phys. Chem. A 2018, 122, 7062–7070. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Li, J.; Xu, Z.; Yao, J. The discovery of coarse-grained uraninite in Kangdian Axis and its geological significance. Geol. Bull. China 2015, 34, 2219–2226, (In Chinese with English Abstract). [Google Scholar]
- Xu, Z.; Yin, M.; Chen, Y.; Xiang, L.; Song, H.; Zhang, C.; Yao, J.; Guo, H. Genesis of megacrystalline uraninite: A case study of the Haita area of the western margin of the Yangtze block, China. Minerals 2021, 11, 1173. [Google Scholar] [CrossRef]
- Yin, M.; Xu, Z.; Song, H.; Zhang, C.; Zhang, S.; Tian, J.; Guo, H. Constraints of the geochemical characteristics of apatite on uranium mineralization in a uraninite-rich quartz vein in the Haita area of the Kangdian region, China. Geochem. J. 2021, 55, 301–312. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Y.; Yao, J.; Ye, J. Study on characteristics of gaint grain uraninite in Haita area of Miyi county, Sichuan. World Nucl. Geosci. 2017, 34, 187–193, (In Chinese with English Abstract). [Google Scholar]
- Yin, M.; Zhang, S.; Xu, Z.; Song, H.; Chen, Y.; Yao, J.; Wang, F. Neoproterozoic uranium mineralization in the Kangdian region of the eastern Tibetan Plateau, China. Geosyst. Geoenviron. 2024, 3, 100135. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, J.; Zhang, X.; Zheng, H.; Yang, X.; Zhou, G.; Tao, D. Teconics and Rifting in Kangdian Region; Chongqing Publishing House: Chongqing, China, 1987; pp. 13–15. (In Chinese) [Google Scholar]
- Zhou, M.F.; Zhao, X.F.; Chen, W.T.; Li, X.C.; Wang, W.; Yan, D.P.; Qiu, H.N. Proterozoic Fe–Cu metallogeny and supercontinental cycles of the southwestern Yangtze Block, southern China and northern Vietnam. Earth-Sci. Rev. 2014, 139, 59–82. [Google Scholar] [CrossRef]
- Song, H.; Chi, G.; Zhang, C.; Xu, D.; Xu, Z.; Fan, G.; Zhang, G. Uranium enrichment in the Lala Cu-Fe deposit, Kangdian region, China: A new case of uranium mineralization associated with an IOCG system. Ore Geol. Rev. 2020, 121, 103463. [Google Scholar] [CrossRef]
- Wang, D.Y.; Fu, Y.Q. Uranium Mineralogy, 1st ed.; Atomic Energy Press: Beijing, China, 1986; pp. 1–442. (In Chinese) [Google Scholar]
- Finch, R.; Murakami, T. Systematics and paragenesis of uranium minerals. In Reviews in Mineralogy; Mineralogical Society of America: Washington, DC, USA, 1999; Volume 38, pp. 91–179. [Google Scholar]
- Xu, G.Q.; Wang, A.Z.; Gu, Y.F.; Zhang, J.Y.; Zhang, S.M.; Huang, Y.Z. Some mineralogical characteristics of uraninte and pitchbleede in China. Acta Mineral. Sin. 1982, 3, 193–200, (In Chinese with English Abstract). [Google Scholar]
- Xu, Z.; Yin, M.; Song, H. Characteristics and Genesis of Megacrystalline Uraninite on the Kangdian Region, Southwestern China; Science Press: Beijing, China, 2024; p. 67. (In Chinese) [Google Scholar]
- Li, Z.; Huang, Z.; Li, X.; Fan, C. The discovery of natural native uranium and its significance. Acta Geol. Sin.-Engl. 2015, 89, 1561–1567. [Google Scholar]
- Clauer, N.; Mercadier, J.; Patrier, P.; Laverret, E.; Bruneton, P. Relating unconformity-type uranium mineralization of the Alligator Rivers Uranium Field (Northern Territory, Australia) to the regional Proterozoic tectono-thermal activity: An illite K–Ar dating approach. Precambrian Res. 2015, 269, 107–121. [Google Scholar] [CrossRef]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Pring, A. Uraninite from the Olympic Dam IOCG-U-Ag deposit: Linking textural and compositional variation to temporal evolution. Am. Mineral. 2016, 101, 1295–1320. [Google Scholar] [CrossRef]
- Ram, R.; Charalambous, F.A.; McMaster, S.; Pownceby, M.I.; Tardio, J.; Bhargava, S.K. Chemical and micro-structural characterisation studies on natural uraninite and associated gangue minerals. Miner. Eng. 2013, 45, 159–169. [Google Scholar] [CrossRef]
- Ram, R.; Charalambous, F.A.; McMaster, S.; Pownceby, M.I.; Tardio, J.; Bhargava, S.K. An investigation on the dissolution of natural uraninite ores. Miner. Eng. 2013, 50, 83–92. [Google Scholar] [CrossRef]
- Alexandre, P.; Kyser, K.; Layton-Matthews, D.; Joy, B.; Uvarova, Y. Chemical compositions of natural uraninite. Can. Mineral. 2015, 53, 595–622. [Google Scholar] [CrossRef]
- Cuney, M. Evolution of uranium fractionation processes through time: Driving the secular variation of uranium deposit types. Econ. Geol. 2010, 105, 553–569. [Google Scholar] [CrossRef]
- Frimmel, H.E.; Schedel, S.; Brätz, H. Uraninite chemistry as forensic tool for provenance analysis. Appl. Geochem. 2014, 48, 104–121. [Google Scholar] [CrossRef]
- Eglinger, A.; André-Mayer, A.S.; Vanderhaeghe, O.; Mercadier, J.; Cuney, M.; Decrée, S.; Milesi, J.P. Geochemical signatures of uranium oxides in the Lufilian belt: From unconformity-related to syn-metamorphic uranium deposits during the Pan-African orogenic cycle. Ore Geol. Rev. 2013, 54, 197–213. [Google Scholar] [CrossRef]
- Tu, J.R.; Xiao, Z.B.; Zhou, H.Y.; An, S.Q.; Li, G.Z.; Cui, Y.R.; Li, H.M. U–Pb dating of single-grain uraninite by isotope dilution thermal ionization mass spectrometry. Ore Geol. Rev. 2019, 109, 407–412. [Google Scholar] [CrossRef]
- Janeczek, J.; Ewing, R.C. Structural formula of uraninite. J. Nucl. Mater. 1992, 190, 128–132. [Google Scholar] [CrossRef]
- Liu, K.P. Mineralization Physical and Chemical Conditions of Uranium Deposit in Haita Area, Miyi County, Sichuan Province. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2017. (In Chinese with English Abstract). [Google Scholar]
- Chang, D. Geological and Geochemical Characteristics of Migmatite Type Uranium Deposit in the Haita Area, Miyi Town, Sichuan Province. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2016. (In Chinese with English Abstract). [Google Scholar]
- Xiang, L.; Guo, J.; Yin, M.; Song, H.; Xu, Z.; Duan, Z.; Wang, R. Polygenetic titanites constraining the genesis of Neoproterozoic leucocratic-dyke-hosted U mineralization at the western margin of the Yangtze Block. Lithos 2023, 438, 107008. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Ouyang, X.; Yao, J.; Sun, K.; Yin, M. Chronology Characteristics and Significance of Datian Uranium Deposit in Panzhihua. Uranium Geol. 2017, 33, 280–287, (In Chinese with English Abstract). [Google Scholar]
- Ni, S.; Zhang, C.; Xu, Z.; Chen, Y.; Li, J.C.; Sun, Z.X. Major Geological Events and Uranium Mineralization in Southwest China; Science Press: Beijing, China, 2014; pp. 1–50. (In Chinese) [Google Scholar]
- Wang, K.; Deng, J.; Hao, X. The geochemical behavior of uranium and mineralization: South China uranium province as an example. Acta Petrol. Sin. 2020, 36, 35–43. [Google Scholar]
- Kyser, K. Uranium ore deposits. Treatise Geochem. 2014, 13, 489–513. [Google Scholar]
- Hu, R.Z.; Bi, X.W.; Zhou, M.F.; Peng, J.T.; Su, W.C.; Liu, S.; Qi, H.W. Uranium metallogenesis in South China and its relationship to crustal extension during the Cretaceous to Tertiary. Econ. Geol. 2008, 103, 583–598. [Google Scholar] [CrossRef]
- Hu, R.Z.; Bi, X.W.; Su, W.C.; Peng, J.T.; Li, C.Y. The relationship between uranium metallogenesis and crustal extension during the cretaceous-tertiary in south China. Geosci. Front. 2004, 11, 153–160, (In Chinese with English Abstract). [Google Scholar]
- Yan, B.; Yan, H.; Wei, W.F.; Cao, Y.; Li, J. Helium—Argon isotopic characters and geological significance of the shazhou uranium deposit, Xiangshan Orefield, Jiangxi Province. Geol. Rev. 2014, 60, 624–634, (In Chinese with English Abstract). [Google Scholar]
- Brugger, J.; Liu, W.; Etschmann, B.; Mei, Y.; Sherman, D.M.; Testemale, D. A review of the coordination chemistry of hydrothermal systems, or do coordination changes make ore deposits? Chem. Geol. 2016, 447, 219–253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).