Abstract
Titanium alloys are of significant value in aerospace, biomedical, and marine engineering applications due to their excellent specific strength and favorable biocompatibility. As a crucial interstitial solute, oxygen significantly influences the mechanical properties of titanium alloys. However, excessive oxygen content can lead to severe embrittlement and a significant reduction in ductility. This paper systematically reviews the mechanisms of microstructural evolution induced by oxygen in conventionally manufactured titanium alloys and their impact on mechanical properties, highlighting that conventional processes require complex post-treatments (PT) to achieve a balance between strength and plasticity. This assessment further explores the regulatory mechanisms of oxygen on the microstructure and mechanical properties of laser additive manufactured (LAM) titanium alloys, elucidating the fundamental phenomena regarding the oxygen–microstructure–property relationship. Finally, based on the current research progress, this paper provides an outlook on the future development directions and key research priorities in this field. This review offers valuable insights into the role of oxygen in titanium alloys and the development of high-performance titanium alloys.
1. Introduction
Titanium alloys are widely applied in aerospace, biomedical, and marine industries due to their exceptional specific strength, good corrosion resistance, and outstanding fatigue performance [1,2,3]. The precise regulation of their mechanical properties through compositional design remains a challenge in advanced materials research. Among various elements, oxygen garnered significant scientific interest owing to its unique dual role in modifying the mechanical behavior of materials by occupying octahedral interstitial sites and inducing an asymmetric lattice distortion. The resulting three-dimensional elastic stress field interacts with the dislocation strain fields, impeding dislocation glide, and thereby achieving solid solution strengthening. With increasing oxygen content, this strengthening mechanism transitions toward detrimental effects: a high oxygen content suppresses the activation of multiple slip systems and restricts the dislocation cross-slip capability, hindering the formation and propagation of shear bands. This leads to a significant reduction in the capacity of the material for coordinated plastic deformation. The failure of this coordination mechanism initiates strain localization at the microscale level and is macroscopically manifested as the progressive deterioration of ductility. When the oxygen content exceeds a critical threshold, the stress concentration effects dominate the deformation process, ultimately inducing a ductile-to-brittle fracture behavior.
Research demonstrated that oxygen atoms occupy octahedral interstitial sites near the core of screw dislocations [4]. The lattice distortion at the dislocation core causes a substantial reduction in the interstitial volume, thereby generating strong short-range repulsive interactions. This intense interaction forces screw dislocation motion to occur through two distinct mechanisms: ① The interstitial shuffling mechanism, through which oxygen atoms must overcome an activation energy barrier to migrate from octahedral interstitial sites on the slip plane to new basal plane positions (Figure 1a). ② The local cross-slip induced by oxygen interstitials, through which segments shift to adjacent prismatic planes, forming immobile edge dislocation segments (Figure 1b). However, the strengthening effect is accompanied by a deterioration in plasticity. The strong pinning effect of oxygen inhibits dislocation glide (Figure 1c–e), thereby reducing the ductility of the material. In summary, as the oxygen content increased from 0.1 wt.% to 0.3 wt.%, and oxygen atoms acting as interstitial solutes significantly enhance their pinning effect on dislocations at the microscale (Figure 1c,e), thereby restricting dislocation slip. The elevated oxygen concentration simultaneously suppresses shear band formation during deformation, leading to progressive deterioration of ductility. From a macroscopic perspective, this microstructural evolution fundamentally alters the deformation mechanism, and the material loses its ability to effectively coordinate plastic deformation through the conventional dislocation motion and shear band formation. This mechanistic transition results in high stress concentration during deformation. With a further increase in oxygen content, the stress concentration effect becomes critically intensified, triggering a sequential degradation process: initial ductility loss (quantified by reduced elongation) followed by complete embrittlement (evidenced by catastrophic brittle fracture).
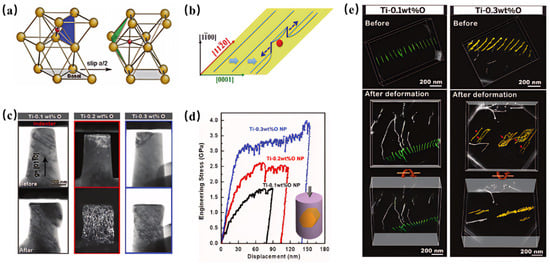
Figure 1.
(a) Sliding path of oxygen atoms, (b) schematic of local dislocation cross-slip when <a> screw dislocation encounters oxygen interstitials, (c) corresponding transmission electron microscopy (TEM) images of the pillars before and after compression, and (d) engineering displacement curves of pillar compression tests at different oxygen concentrations. A schematic of the sample and crystallographic orientation is shown bottom right, where the loading direction is along []; (e) tomograms showing the 3D evolution of screw dislocation arrays in Ti-0.1 wt.% O and Ti-0.3 wt.% O [4].
Based on the competing mechanisms of interstitial solid solution strengthening versus embrittlement by oxygen, titanium alloy engineering components experience progressive oxygen enrichment during long-term service, thereby inducing service-induced degradation of titanium alloy parts across multiple application domains. In the biomedical field, titanium alloys are commonly used for joint implantation. If an implant undergoes prolonged oxidation due to certain biological electrochemical factors, its relatively low-oxidation resistance leads to the formation of micro-cracks. This causes the fragments to detach, potentially triggering inflammation or implant loosening. In the aerospace sector, titanium alloys are frequently employed in engine blades in the aerospace sector. High oxygen content can cause a drastic reduction in the fatigue life of alloy components, loss of plasticity, and even oxygen embrittlement. In marine engineering, titanium alloys are used in submersible pressure hulls and seawater piping systems. These applications can lead to in-service chemical alteration of the oxygen content in the alloys, leading to fatigue failure and embrittlement.
Previous studies demonstrated that titanium alloys prepared through various processing routes exhibit a consistent decline in elongation with increasing oxygen content. For instance, the critical oxygen threshold was approximately 0.46 wt.% for pure Ti, ~0.33 wt.% for powder metallurgy-fabricated Ti-6Al-4V, and ~0.5 wt.% for β-type titanium alloys due to their superior oxygen tolerance compared to other titanium alloys [5]. Beyond these thresholds, the room-temperature elongation of titanium and its alloys undergoes a drastic deterioration. This phenomenon primarily originates from embrittlement of the hexagonal close-packed (HCP) α phase when the oxygen content exceeds 0.4 wt.%. In commercial titanium alloys, the maximum permissible oxygen content is typically restricted to 0.2 wt.% to ensure satisfactory ductility. Consequently, substantial research efforts focused on enhancing the mechanical properties by incorporating deoxidizers to reduce the oxygen content in solid solutions [6,7,8,9,10,11,12,13]. For example, Pan et al. [6] achieved strength–ductility synergy (yield stress, YS = 508 ± 15 MPa; ultimate tensile stress, UTS = 621 ± 25 MPa; and elongation, El = 29.3 ± 2.6%) through the addition of 0.4 wt.% CaC2 to raw powders, effectively reducing oxygen content in solid solutions from 0.40 to 0.19 wt.%. This reduction mechanism involves the thermal decomposition of CaC2 at elevated temperatures, which generates active calcium (Ca). The reactive Ca combines with intergranular oxygen in titanium powder to form stable CaO compounds, thereby immobilizing and removing oxygen atoms dissolved in the titanium lattice and significantly lowering the dissolved oxygen concentration. However, while calcium-based deoxidizers (e.g., CaC2) effectively stabilize oxygen during powder metallurgy processing, their deoxidation products, CaO and potential Ca-Ti intermetallic compounds (such as CaTi and CaTi2), formed due to local calcium excess or titanium reactions, persist in the titanium matrix as brittle residual phases. These rigid second-phase particles tend to serve as stress concentration sites and microcrack initiation points under mechanical loading during subsequent processing or service, thus compromising the fatigue resistance of titanium alloys. To balance oxygen reduction and fatigue performance, the following measures are recommended: precise control of deoxidizer dosage in PT. In addition, the effective application of the CaC2 deoxidizer and the abovementioned optimization strategies (especially the precise control of temperature, atmosphere, and implementation of PT) increased the process complexity and cost.
Emerging strategies exploit the dual role of oxygen through advanced manufacturing and PT. Techniques such as high-pressure torsion (HPT) and hot isostatic pressing (HIP) redistribute oxygen atoms and modify dislocation patterns to mitigate embrittlement. Such “active regulation” approaches replace traditional deoxidation, enabling concurrent strength–ductility enhancement through controlled oxygen-mediated strengthening. Novel alloy design strategies based on solute element synergy have been developed. Chong et al. [14] systematically investigated the multiscale regulation mechanism of deformation behavior by co-alloying titanium with 6 wt.% Al and 0.3 wt.% O. Their work revealed that the synergistic interplay between Al-induced short-range ordering (SRO) domains and the interstitial pinning effect of oxygen effectively suppresses the planar slip mode dominated by oxygen-mediated “mechanical shuffling” in conventional titanium alloys. The synergistic effect of interstitial oxygen and the SRO of Al hinders dislocation movement and promotes dislocation entanglement. Consequently, the activation energy for cross-slip decreases, resulting in an increase in dislocations on multiple slip surfaces. The three-dimensional network formed by cross-slip activation offsets the strain concentration (Figure 2a,b). Consequently, the alloy exhibited enhanced strain-hardening capability (Figure 2c) and delayed strain localization, establishing a new pathway for oxygen-utilizing titanium-alloy design.
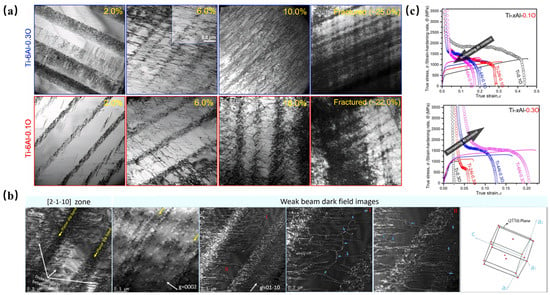
Figure 2.
(a) Typical dislocation morphologies of Ti-6Al-0.1O and Ti-6Al-0.3O alloys after tensile deformation at cryogenic temperatures under selected strains and eventual fracture. (b) Detailed analysis of dislocation morphologies in Ti-6Al-0.1O alloy tensile deformed to 6.0% strain under cryogenic conditions. (c) True stress–strain curves and strain-hardening rate curves of Ti-xAl-0.1O and Ti-xAl-0.3O alloy systems in liquid nitrogen [14].
Recent studies revealed that oxygen not only alters dislocation motion patterns through lattice distortion, but also significantly influences the phase transformation kinetics and non-equilibrium microstructure evolution in titanium alloys. Compared to conventional manufacturing, additive manufacturing significantly promotes a supersaturated interstitial solid solution of oxygen within the titanium alloy lattice owing to its inherent rapid solidification, cyclic thermal history, and nonequilibrium phase transformations. This dissolved oxygen occupies octahedral interstitial sites in α-Ti, inducing an asymmetric lattice distortion. Through strong pinning effects, it impedes dislocation motion while substantially reducing stacking fault energy to diminish the activation energy for cross-slip. Consequently, this promotes coordinated activation of multiple slip systems, leading to the formation of three-dimensional dislocation networks. At the phase transformation kinetics level, oxygen acts as a potent β-phase stabilizer, reducing the driving force for the β→α phase transformation and suppressing diffusional phase transformation processes. This results in non-equilibrium microstructural characteristics, such as the significant retention of the metastable β-phase co-evolving with shear-type α’ martensite.
LAM, an emerging processing technology, overcomes the thermodynamic equilibrium constraints of conventional methods through its characteristic rapid solidification, effectively increasing oxygen solubility while suppressing oxygen segregation at grain boundaries and crystalline defects. Importantly, high-concentration oxygen solid solutions stabilize metastable face-centered cubic (FCC) phases that are inaccessible via traditional processing routes, simultaneously promoting the formation of high-density dislocation configurations and nanoscale precipitates to achieve synergistic strengthening and ductility enhancement. When integrated with an in situ alloying technique, this approach eliminates the requirement for complex PT typically needed in conventional manufacturing, thereby enabling the production of high-strength, high-ductility oxygen-containing titanium alloys. This breakthrough provides an efficient and cost-effective strategy for developing advanced, high-oxygen titanium materials.
This review comprehensively summarizes the multiscale mechanisms of interstitial oxygen on the microstructure and mechanical properties of titanium alloys, systematically analyzing existing research achievements using two principal frameworks: PT-manufactured titanium alloy systems and LAM-fabricated titanium alloy systems. By comparatively examining the similarities and differences in the functional mechanisms of oxygen across various processing techniques, this study elucidates the structure–process–property correlations, offering theoretical guidance and technological references for overcoming the strength–ductility trade-off dilemma in titanium alloys.
2. The Influence of Interstitial Oxygen on Conventionally Manufactured and PT Titanium Alloys
Oxygen, a critical interstitial solute, governs microstructural evolution and mechanical behavior in titanium alloys. While enhancing the α-phase strength via octahedral site occupation [15], oxygen concurrently suppresses plasticity by impeding dislocation dynamics. The exceptional solubility of C in α-Ti (~14.5 wt.%) versus the β phase (~2 wt.% at 1273 K) drives microstructural heterogeneities, including twin inhibition and lattice distortion. Oxygen segregation generates localized stress fields that restrict dislocation mobility, transforming the deformation mechanisms from cross-slip in oxygen-lean matrices to planar slip dominance in oxygen-enriched systems [16]. This transition, coupled with suppressed twinning, synergistically reduces ductility and strain hardening capacity [17].
In recent years, a paradigm shift occurred in research strategies addressing the oxygen embrittlement of titanium alloys, transitioning from the conventional “passive deoxidation” approach to diversified “active regulation” methodologies. This transformation fundamentally redefines the mechanistic understanding of interstitial oxygen, which is no longer regarded as a detrimental element requiring elimination, but rather, is strategically manipulated through precision process control, innovative alloy design, and advanced manufacturing technologies. By synergistically integrating the solid solution strengthening effects of oxygen with microstructural optimization, researchers aim to suppress strain localization while achieving concurrent strength–ductility enhancement.
This section classifies PT titanium alloys into three primary categories based on their metastable phase constituents: α-type, α+β-type, and β-type alloys, and systematically analyzes the impact of oxygen content on their mechanical properties.
2.1. Influence of Interstitial Oxygen on α-Type Titanium Alloys
α-type titanium alloys are systematically categorized into fully α-phase alloys (exemplified by commercially pure titanium) and near-α variants based on their phase constitution characteristics. This alloy design philosophy incorporates trace additions of α-stabilizing elements (e.g., Al and Sn) while strictly limiting beta-stabilizing constituents (Mo and V), thereby establishing α-phase-dominated microstructures at ambient temperatures. Such compositional optimization confers exceptional elevated-temperature stability and balanced mechanical performance, particularly manifesting as superior creep resistance and endurance strength. From an engineering perspective, these alloys have become the material of choice for high-temperature load-bearing components in aerospace systems (e.g., compressor blades and combustion chamber assemblies in aero-engines) owing to their distinctive solid solution strengthening effects, exceptional corrosion resistance, and favorable manufacturing compatibility. Furthermore, their extensive utilization in extreme service environments, including chemical processing reactors, deep-sea structural components, and orthopedic implants, underscores their remarkable adaptability across multidisciplinary engineering applications. The following Table 1 summarizes the mechanical properties of the α titanium alloy after PT with oxygen:

Table 1.
Summary of the mechanical properties of α-titanium alloy after PT.
Chong et al. [15] found that the addition of oxygen reduced the plasticity, whereas the addition of Al enhanced the strength and plasticity of the material. Atomic-scale analyses confirmed that Al substitution induces Al-O SRO, altering the metastable mechanical shuffling mechanism of oxygen. Enhanced covalent Al-O bonding alters the oxygen rearrangement energy barriers by 35–50%, suppressing localized deformation while enabling cross-slip-dominated plasticity. In Ti-6Al-0.3O, dislocation delocalization and inhibited twinning preserve oxygen strengthening (Δσ ≈ 220 MPa) while improving ductility by 40–60%. Mechanistically, Al reduces oxygen solubility via substitution-induced lattice strain (>0.8 eV/atom) and forms stable Al-O nanoclusters (1.2–1.8 nm) that impede crack nucleation. This strategy decouples oxygen embrittlement from strengthening, thereby advancing the design of damage-tolerant titanium alloys.
Kang et al. [23] systematically investigated the thermomechanical processing of Ti-(0.082–0.268 wt.%) O alloy ingots through hot rolling in both α-phase and β-phase regions, followed by annealing and cold rolling procedures within the α-phase domain, concluding with solution treatment at 873 K for 1 h and subsequent water quenching. Crucially, their findings reveal that elevated oxygen content induced a transition in deformation mechanisms, with <a+c> dislocations progressively dominating mechanical twinning as the primary plastic accommodation mode. While the Hall–Petch effect typically predicts diminished work-hardening rates accompanying twin boundary reduction, researchers demonstrated that oxygen-mediated lattice distortion generates asymmetric strain fields. These fields effectively confined dislocations to specific crystallographic planes, thereby simultaneously enhancing the work-hardening rate (dσ/dε:0.28→0.40 GPa) and ductility. This typical strengthening–toughening synergy was attributed to the dual role of oxygen in solid solution strengthening through localized lattice distortion and dislocation dynamics modification [24].
Xiang et al. [25] performed the homogenization of Ti-4Zr-xO (wt.%) alloys (x = 0, 0.15, 0.20, 0.25, and 0.30) in a vacuum tube furnace at 1000 °C for 5 h. When the oxygen content was less than 0.25%, the α phase exhibited a basket-weave microstructure. TEM characterization of the Ti-4Zr-0.25O alloy revealed the presence of dislocation pileups along the grain boundaries and the composition of nanoscale twins. Localized stress concentrations arising from dislocation blockage at the grain boundaries were found to trigger deformation twinning. The resultant nanotwins not only provide supplementary slip pathways for dislocation motion, but also facilitate crystallographic reorientation, thereby promoting the activation of the cross-slip systems. This coordinated mechanism satisfies the Von Mises criterion by enabling the simultaneous operation of five or more independent slip systems, thereby improving the strain accommodation capacity and enhancing homogeneous plastic deformation through twin-induced dislocation reconfiguration.
Chong et al. [26] demonstrated that Ti-0.3O achieves significant grain refinement relative to pure titanium under equivalent thermomechanical treatments, which is attributed to oxygen-induced boundary pinning effects (Figure 3), a finding corroborated by Cai et al. [27]. This refinement mechanism primarily originates from the dynamic dragging effect induced by interstitial oxygen atoms through their pinning and interaction with grain boundary migration, thereby effectively restraining grain coarsening. Notably, the ultrafine-grained microstructure facilitated homogeneous oxygen distribution within the intragranular regions while promoting dispersed dislocation arrangements, which completely eliminated the planar slip patterns typically observed in conventional titanium alloys. TEM observations revealed the predominant activation of high-density <c + a> pyramidal dislocations in the Ti-0.3O alloy, a phenomenon attributed to the intensified lattice distortion and elevated stacking fault energy caused by oxygen interstitials. This dislocation-mediated plasticity mechanism not only suppresses deformation twinning through oxygen-induced crystalline symmetry modification, but also delays microcrack nucleation and void propagation via synergistic motion across multiple slip systems, ultimately enhancing the plastic deformability of the alloy. As the grain size of Ti-0.3O decreased, its El increased from 1.5% to 14.0% (from 68 μm to 2 μm), while its UTS increased from 1.0 GPa to 1.25 GPa.
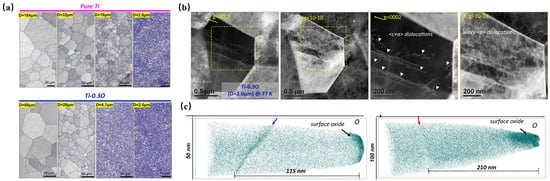
Figure 3.
(a) Grain boundary maps of pure Ti and Ti-0.3O, where red and blue indicate low-angle grain boundaries (LAGBs, 2° < θ ≤ 15°) and high-angle grain boundaries (HAGBs, θ > 15°); (b) dislocation distribution map of UFG Ti-0.3 O observed by TEM; and (c) oxygen atom distribution maps of CG and UFG Ti-0.3O obtained by APT [26].
Amann et al. [18] demonstrated that in Ti-xO alloys, increasing oxygen content induces progressive grain refinement while simultaneously modulating deformation mechanisms. TEM characterization revealed a dual-phase strengthening architecture comprising ordered nanoprecipitates and intensified cross-slip activity, which substantially enhanced the work-hardening capacity. The improved mechanical performance was attributed to the synergistic interplay between oxygen-mediated solid solution strengthening (via asymmetric lattice distortion) and dynamic dislocation storage enabled by these microstructural features. Specifically, the Ti-0.6O alloy exhibited an optimal strength–ductility balance of 800 MPa UTS with 29% El. Notably, the extrapolation of the alloy system to Ti-4.5Zr-xO compositions revealed enhanced oxygen tolerance, where the Ti-4.5Zr-0.8O variant achieved exceptional mechanical properties (UTS: 1075 MPa, El: 28%) owing to the catalytic effect of zirconium on oxygen redistribution and the stabilization of dislocation multiplication mechanisms.
Chong et al. [28] systematically elucidated the oxygen-mediated deformation transition in Ti-O alloys through a comparative investigation of pure Ti, Ti-0.1O, and Ti-0.3O (wt.%) systems. Their work established a critical interdependence between the oxygen concentration, thermal/mechanical loading parameters, and slip mode evolution, as quantified in Figure 4a. The study revealed that pure titanium necessitates extreme cryogenic temperatures (>77 K) coupled with high strain rates (>103 s−1) to activate planar slip, whereas oxygen alloying progressively lowers the activation threshold through a novel interstitial shuffling mechanism (ISM). This atomic-scale process involves the dynamic rearrangement of oxygen interstitials from octahedral to tetrahedral lattice sites during a <a>-type dislocation glide along the prismatic planes (Figure 4b). Crucially, the ISM induces slip plane softening through two competitive pathways: ① reduced lattice friction via oxygen redistribution along dislocation cores and ② thermal recovery via reverse site occupation driven by stochastic atomic fluctuations. The kinetics of these counteracting processes, governed by Arrhenius-type temperature dependence and oxygen diffusivity, fundamentally dictate the cross-slip to planar slip transition. Furthermore, oxygen significantly inhibits conventional deformation twins (such as <011> twins), but the synergistic effect of high oxygen content (0.88 at.%) and low temperature can induce abnormal twins. The interaction between such twins and grain boundaries is prone to cause microcracks (Figure 4c–e), resulting in low-temperature brittle fracture. Based on the ISM model, it is proposed to improve the oxygen tolerance of titanium alloys by regulating lattice parameters or introducing displacement solutes to enhance the stability of hexahedral oxygen.
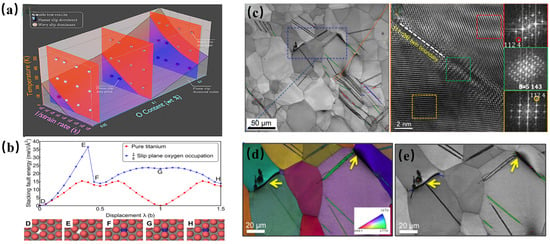
Figure 4.
(a) A three-dimensional (3D) diagram demonstrating the combined analysis of the temperature, strain rate, and oxygen content dependences of the dislocation morphology; (b) modified GSF energy on the prismatic plane calculated with density functional theory (DFT) and the dynamic migration path of oxygen atoms during the sliding process; and (c–e) the nucleation, propagation of abnormal twins, and their association mechanism with brittle fracture in titanium alloys with high oxygen content (0.88 at %) (Ti-0.3O) during tensile fracture at a low temperature (~100 K) [28].
2.2. The Influence of Interstitial Oxygen on β-Type Titanium Alloys
Among titanium and its alloys, β-titanium alloys have been extensively studied for biomedical applications owing to their excellent biocompatibility, corrosion resistance, machinability, and fatigue resistance [29,30,31]. When alloying, it is important to avoid using potentially harmful elements (such as Al, V, and Cu) [32], and the added elements are generally those that are completely biocompatible (such as Ta, Zr, and Nb). β-titanium alloys consist of a body-centered cubic (BCC) phase microstructure, which makes them suitable for cold processing, and they typically exhibit a lower Young’s modulus than other titanium alloys. This helps reduce the stress shielding effect and enhances the compatibility between implants and human bones. Specifically, the Young’s modulus of biomedical β-titanium alloys typically ranges from 50 to 110 GPa, with advanced compositions achieving values as low as 50–60 GPa after optimized processing. In contrast, conventional Ti-6Al-4V (an α+β alloy) exhibits an elastic modulus of ~110 GPa, which is substantially higher than that of human bone (10–35 GPa) [33,34,35,36]. The reduced elastic modulus of β-titanium alloys mitigates the risk of stress shielding effects, which typically arise when stiffer materials are used to replace natural hard tissue. This closer mechanical match between β-titanium alloys and biological tissues enhances biocompatibility during hard tissue replacement, preserving bone integrity and physiological function.
Increasing the content of interstitial elements (O and N) is beneficial because it significantly improves the tensile strength without substantially increasing the Young’s modulus [37]. However, an increase in the oxygen content of the alloy may lead to a reduction in ductility [6,38,39]. After complex PT, the oxygen resistance of β-titanium alloy has been significantly improved. The following Table 2 summarizes the mechanical properties of β titanium alloy after PT with oxygen:

Table 2.
Summary of mechanical Properties of β-titanium alloy after PT.
Bartáková et al. [40] revealed oxygen’s unique decoupling effect on strength and modulus in Ti-Nb β-phase titanium alloys. Incorporating 0.4 wt.% oxygen enhances strength through three mechanisms: ① octahedral lattice distortion by interstitial oxygen, which generates 30% higher strain fields than C/N, ② β-phase stabilization, which elevates ω-phase nucleation energy by 18% via oxygen adsorption, suppressing brittle phase formation, and ③ increased critical shear stress (450 MPa) for {332} <113> twinning, which shifts deformation to dislocation slip with sustained hardening. This multiscale control enables Ti-35Nb-2Zr-0.4O alloy to achieve 1.2 GPa tensile strength while maintaining a β-phase modulus below 100 GPa, demonstrating a viable pathway for biomedical alloy optimization.
Chong et al. [41] systematically investigated the thermomechanical processing of Ti-12Mo and Ti-12Mo-0.3O alloys through a multi-step protocol involving hot forging (920℃), homogenization, quenching, and high-pressure torsion, achieving a refined grain size of 4.5 μm in the oxygen-containing alloy. The Ti-12Mo-0.3O ternary alloy exhibited improved mechanical characteristics (YS = 826 MPa, UTS = 1064 MPa, and El = 24%), effectively addressing the strength–ductility trade-off common in metallic biomaterials. This notable combination of strength and ductility makes it a promising candidate for applications requiring a balanced load-bearing capacity and deformation tolerance. Microstructural analysis highlighted the critical role of oxygen in suppressing strain-induced α’’ martensitic phase formation and restricting {332} <113> deformation twin thickening. Mechanistically, interstitial oxygen stabilizes the β-phase matrix via solid solution strengthening, reducing the thermodynamic driving force for displacive martensitic transformation, while simultaneously impeding twin boundary migration through solute drag effects. This dual inhibition preserves the strain-hardening capacity by maintaining controlled dislocation and defect densities, enabling concurrent improvements in strength and ductility. These findings underscore the dual functionality of oxygen as a microstructural stabilizer and deformation behavior modulator in titanium alloys.
Kozlík et al. [45] fabricated Ti-Nb-Cr alloys via electric FAST, with selected samples subjected to solution treatment at 1000 °C for 2 h in a vacuum tube furnace (10−3 Pa) followed by water quenching. Elevated oxygen content significantly increased Young’s modulus owing to lattice distortion strengthening from interstitial oxygen atoms, which enhanced electron cloud density and interatomic bonding forces, thereby inhibiting dislocation motion. Notably, a higher Nb content in the solution-treated and quenched alloys yielded a unique mechanical profile: the Young’s modulus decreased to 64 GPa, whereas the microhardness reached 337 HV. This stems from Nb’s dual role in stabilizing the β-Ti phase during solution treatment, suppressing brittle ω-phase formation, and retaining a supersaturated solid solution structure via non-equilibrium quenching. The inherently low modulus of the β phase synergized with Nb-induced solid solution strengthening through lattice distortion and short-range ordering, achieving simultaneous modulus reduction and hardness enhancement.
Wang et al. [47] systematically investigated oxygen-modified Ti-5Al-4Mo-4V-4Cr-3Zr (wt.%) alloys through β-phase solution treatment (1000 °C) and subsequent isothermal oxidation in an oxygen-argon atmosphere. The optimal performance was observed in the 4-hour oxidized sample (OC4h-β-Ti), which exhibited a surface oxygen concentration of 3.4 wt.% with exceptional hardness (1125 HV) and compressive strength (1.7 GPa). This superior performance originates from a dual mechanism: ① Oxygen diffusion creates a gradient microstructure featuring an oxygen-enriched surface layer and oxygen-gradient transition zones, where interstitial oxygen atoms induce lattice distortion and solution strengthening through electron density redistribution. ② The oxygen concentration gradient establishes coordinated strain hardening [49,50,51], enabling sequential plastic deformation from the high-oxygen (hardened) surface to the low-oxygen (ductile) core, effectively delaying crack initiation and propagation.
Gao et al. [48] investigated oxygen-modulated deformation mechanisms in Ti-32Nb-xO (x = 0.2–0.7 wt.%) alloys fabricated via arc melting. Oxygen doping induced β-phase stabilization by suppressing ω-phase formation while modifying deformation pathway activation thresholds, enabling tailored TRIP/TWIP effect regulation. The 0.3O alloy exhibited sequential stress-induced α″ martensite nucleation (<5% strain) followed by α″-variant reorientation and multi-stage twinning, achieving exceptional strain partitioning. In contrast, 0.5O alloy prioritized {332} <113> twinning initiation due to elevated β stability, with subsequent multi-level twinning and strain-induced α″ martensite (SIM) synergistically enhancing strain hardening. Critical oxygen content (0.3 wt.%) optimized β-phase metastability, balancing stress partitioning between deformation modes: martensitic transformation accommodated early plasticity while twinning sustained hardening, culminating in 887 MPa ultimate strength with 53% ductility, realizing a synergistic improvement in mechanical properties.
2.3. The Influence of Interstitial Oxygen on α+β Dual-Phase Titanium Alloys
The α+β dual-phase titanium alloy system is generally synthesized through strategic incorporation of β-stabilizers (e.g., Cr, Mo, V, and Nb) into the α-Ti matrix, which facilitates the formation of a dual-phase architecture comprising HCP-phase and BCC-phase domains. Due to their highly controllable phase ratio and morphology, α+β titanium alloys offer superior mechanical properties compared to α titanium and β titanium alloys, as well as enhanced ability to reduce crack propagation rates. Therefore, α+β titanium alloys occupy the largest proportion in the practical production and application of titanium alloys [52]. The following Table 3 summarizes the mechanical properties of duplex titanium alloys after PT due to interstitial oxygen:

Table 3.
Summary of mechanical properties of α+β-titanium alloy after PT.
Gao et al. [54] examined the impact of oxygen in powder metallurgy-processed Ti-3Al-5Mo-4.5V and found that oxygen, as interstitial atoms, preferentially occupies octahedral interstitial sites within the α-phase lattice. This occupation induces lattice expansion along the c-axis direction, increasing the c/a ratio to 1.593 Å (Figure 5a–c), and significantly enhances the hardness of the α phase from 4.03 GPa to 5.05 GPa, thereby improving the overall strength of the alloy by approximately 150 MPa. However, elevated oxygen content exacerbates the hardness disparity between the α-phases and β-phases, weakening their coordinated deformation capability (Figure 5d). This leads to dislocation pileups and stress concentration at phase interfaces, initiating rapid crack propagation. When the oxygen content is reduced to 1500 ppm, the activation of abundant dislocations within both phases and the formation of subgrain boundaries promote intragranular stress relief. Concurrently, the interfacial resistance to dislocation migration decreases, substantially enhancing the plastic compatibility of the equiaxed microstructure.
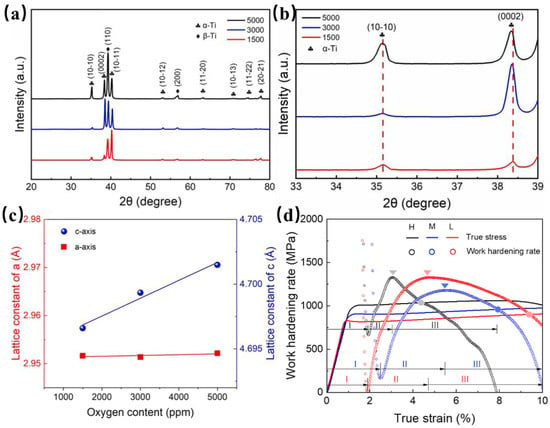
Figure 5.
(a,b) X-ray diffractometer (XRD) and the corresponding magnified pattern; (c) lattice parameter evolution (c/a ratio) with increasing oxygen content; and (d) work-hardening behavior under varying oxygen levels [54].
Fu et al. [56] performed five remelting processes on Ti-8.56Nb-2.08Fe-0.66O ingots and heat-treated them at 1000 °C for 2 h. The ingots underwent rolling at 750 °C (4 mm) and 650 °C (1.5 mm), followed by 725 °C/30 min annealing and water quenching to achieve full recrystallization. Under tensile loading, the yield strength reached 1386 MPa, with a relatively high ductility of 10.8 ± 0.6%. The calculated solid solution-strengthening contributions in the α and β phases reached 590 MPa and 732 MPa, respectively, demonstrating interstitial oxygen-dominated strengthening. This solute-induced enhancement suppressed β→α″ martensitic transformation via stress field redistribution while activating <c + a> dislocations through interstitial rearrangement. Concurrently, equiaxed fine-grained structures combined with intense basal α-texture synergistically facilitated <c + a> dislocation coordination, thereby improving ductility [58], ultimately resulting in excellent mechanical properties.
The attainment of balanced mechanical characteristics in conventionally cast α+β titanium alloys fundamentally relies on precisely controlled PT coupled with multistage post-solidification heat treatment protocols. This metallurgical strategy effectively addresses the inherent microstructural deficiencies of as-cast alloys, which typically exhibit coarse primary β grains (300–800 μm diameter) and thick α colony structures (50–150 μm thickness) containing lamellar α+β phases with interlamellar spacing exceeding 2 μm. Sequential hot working operations (F, R) were conducted in the α+β phase field (typically 750–950 °C for Ti-6Al-4V), achieving refined equiaxed α grains (5–15 μm) embedded in transformed β matrices with submicron-scale β-phase distributions. Ultimately, the synergistic effect of strength and plasticity is achieved.
In summary, interstitial oxygen has different mechanisms of action on the three types of titanium alloys to affect their mechanical properties. In α-type alloys (e.g., commercially pure titanium and Ti-Al-Zr systems), oxygen atoms occupying octahedral sites in the α phase generate potent solid solution strengthening (e.g., Ti-0.3O achieves UTS of 1.25 GPa), but severely suppress plastic deformation mechanisms such as twinning and planar slip dominance. This leads to ductility plummeting below 2% at high oxygen contents (>0.8 wt.%). Incorporating Al to form Al-O short-range ordering (SRO) clusters can synergistically enhance strength–plasticity balance (e.g., Ti-6Al-0.3O gains ~220 MPa strength with 40–60% ductility improvement).
In contrast, β-type alloys (e.g., biomedical Ti-Nb and Ti-Mo systems) demonstrate unique “strength–modulus decoupling” behavior under oxygen addition. Oxygen significantly increases strength (e.g., Ti-35Nb-0.4O exhibits YS of 1170 MPa) while maintaining low Young’s modulus (<100 GPa), effectively mitigating stress shielding effects in implants. This stems from oxygen’s capacity to suppress ω-phase embrittlement and stress-induced martensitic transformation, promoting dislocation slip-dominated plasticity (e.g., Ti-12Mo-0.3O retains 24% El). Oxygen gradient design further enables “α+β oxygen-dissolved zone + β oxygen-dissolved zone + β non-oxygen zone” architectures (surface hardness: 1125 HV, modulus ≈ 60 GPa).
Regarding α+β dual-phase alloys (e.g., Ti-Al-Mo-V systems), oxygen’s preferential strengthening of α-phase (25% hardness increase) exacerbates α/β-phase hardness mismatch. This induces interfacial stress concentration and premature cracking (ductility decreases 50% at >0.3 wt.% O). Optimizing oxygen distribution via powder metallurgy or thermomechanical processing maintains high ductility (El >20%) at low oxygen levels (≤0.15 wt.%), where oxygen contributes 590 MPa (α-phase) and 732 MPa (β-phase) solid solution strengthening (e.g., Ti-8Nb-2Fe-0.66O). While all three alloy types rely on plastic deformation (PT) techniques for microstructural optimization to enhance oxygen tolerance, their underlying mechanisms diverge substantially due to phase constitution differences.
3. The Effect of Interstitial Oxygen on LAM of Titanium Alloys
3.1. LAM Technology
LAM is an advanced fabrication technique that constructs multilayered metallic components through incremental material deposition, guided by computer-aided design [59,60]. As industries prioritize agile production, this technology transitioned from experimental research to industrial implementation. Its core mechanism employs intense laser irradiation for the localized melting of feedstock (powder), bypassing the geometric constraints inherent in subtractive methods [61]. By integrating digital modeling with direct energy processing, LAM eliminates reliance on conventional fixtures, cutting tools, and multi-step machining, thereby enabling rapid prototyping of intricate geometries with enhanced resource efficiency [62,63].
Within the LAM ecosystem, two dominant approaches to titanium alloy processing emerged: powder bed fusion (PBF) and directed energy deposition (DED). Their distinction lies in feedstock delivery dynamics—PBF selectively bonds pre-laid powder layers, while DED synchronizes coaxial powder/wire feeding with laser melting for in situ deposition [64].
The favorable thermophysical properties of titanium alloys, such as low thermal conductivity and minimal thermal expansion, significantly suppress the solidification cracking susceptibility during LAM. These materials further demonstrate enhanced energy utilization efficiency due to their high laser absorption rates. Such synergistic advantages propelled LAM-processed titanium alloys into a research hotspot over the last decade, with scholarly investigations and industrial adoption advancing in parallel [65]. This technology supports practical applications in aerospace propulsion systems, automotive lightweighting, precision industrial tooling, and biomedical implants [66]. Among the prevalent LAM variants, two principal methodologies dominate titanium processing.
Laser powder bed fusion (L-PBF), an advanced additive manufacturing technology, utilizes a focused laser beam (wavelength: 1064–1080 nm) to selectively fuse metallic powder layers, enabling the layer-by-layer fabrication of intricate three-dimensional components with dimensional accuracy ≤ 50 μm (Figure 6a). The technique gained prominence in titanium alloy processing due to its unique capacity to engineer multiscale heterostructures, characterized by α’/β phase mixtures with nanoscale lath spacing (50–200 nm) that simultaneously enhance UTS (1100–1350 MPa) and El (8–12%) [67,68]. Such microstructural control facilitates the production of geometrically complex parts, including lattice-structured biomedical implants (porosity: 60–80%), aerofoil-shaped turbine blades (wall thickness: 200–500 μm), and conformal cooling systems for thermal management applications. The forming quality of samples produced by L-PBF is influenced by numerous factors [65,69], including processing parameters such as laser power (P), scan speed (v), scan spacing (h), layer thickness (t), and powder quality [70]. High-quality powders should have good sphericity and a uniform particle size distribution to ensure uniform melting under the influence of the laser, which results in ideal microstructure and mechanical properties [71,72]. Previous studies have shown that the forming quality of samples produced using L-PBF is typically related to the volumetric energy density, which can be calculated using the following formula [73,74]:
Zhao et al. [75] found that for the preparation of Ti-6Al-4V by L-PBF, the volumetric energy density is effective in optimizing the porosity, but has certain limitations in controlling the microstructure. The same EV can have different processing windows, and finding an optimized processing window is a critical step in achieving the final required performance of the material and component [76].
Laser-directed energy deposition (L-DED): The core principle of L-DED involves the use of a high-energy laser beam or other forms of heat sources to directly act on the surface of a substrate, creating a local molten zone known as a micro-melt pool. During processing, the material (metal powder and/or wire) is precisely fed into the molten zone, rapidly melted, and then fused with the surrounding material, gradually building up the desired complex three-dimensional structures or components layer by layer. Its schematic diagram is shown in Figure 6b. High-energy lasers are used as the heat source, L-DED technology not only achieves faster deposition rates, but also maintains higher precision and controllability during processing, especially suitable for manufacturing large or medium-sized components that often have rough surface features. Similar to L-PBF, one of the major advantages of L-DED is its flexibility, as key process parameters such as laser power, scanning speed, and powder feed rate can be customized to a large extent, allowing for the control of the material deposition process. Compared with L-PBF, L-DED has unique advantages in the following aspects: ① higher deposition rates; ② manufacturing of large-scale components; ③ repair of high-value parts; ④ high flexibility in producing graded multi-materials; and ⑤ greater integration flexibility with other technologies, such as machining and rolling [71,77,78,79]. Thus, the L-DED technology is well suited for applications with high manufacturing demands, such as complex component manufacturing in the aerospace industry, rapid prototyping in the automotive industry, and complex geometric shape processing in mold manufacturing.

Figure 6.
(a) L-PBF schematic diagram; (b) L-DED schematic diagram [80,81,82].
In LAM, the characteristic directional thermal gradient combined with accelerated cooling rates suppresses nucleation ahead of the solidification front, promoting preferential epitaxial growth of columnar grains aligned with the build direction (BD). This phenomenon results in pronounced crystallographic texture and mechanical anisotropy. The current strategies for microstructural refinement during LAM processing primarily focus on two approaches that do not require PT. The first methodology involves parameter optimization to achieve reduced thermal gradients and elevated solidification rates, which enhances undercooling to modify the microstructural characteristics. However, this approach demonstrates limited efficacy in altering the fundamental epitaxial growth mechanism responsible for columnar grain formation.
An alternative strategy incorporates exogenous elements or particulate additives to augment heterogeneous nucleation sites. This method is particularly effective in LAM because of the abbreviated thermal cycles of the process, which enable metastable phases to persist through solidification and serve as active nucleation substrates [83].
Notably, oxygen management emerged as a critical consideration in LAM processes, as it is prone to induce the segregation of β-stabilizing elements (such as Fe, Cr, and Mo) in titanium alloys, thereby damaging their mechanical properties. The segregation of alloying elements can be regulated and controlled by adding rare earth oxides, optimizing the process window, post-treatment, etc. Oxygen not only affects the kinetics and thermal history of the molten pool, but also regulates phase transformation in the subsequent treatment process, ultimately determining the properties of the alloy [84]. While environmental oxygen contamination remains an inherent challenge comparable to conventional manufacturing, stringent deoxygenation protocols substantially increase the production costs. This economic constraint has driven investigations into controlled oxygen gradient engineering coupled with parametric adjustments to develop titanium alloys with optimized oxygen content for strength–ductility synergy. The following Table 4 summarizes the mechanical properties of the titanium alloys fabricated by LAM.

Table 4.
Mechanical properties of the titanium alloys fabricated using LAM.
3.2. The Effect of Interstitial Oxygen on L-PBF Prepared Titanium Alloys
Ding et al. [86] fabricated customized high-oxygen titanium powder (0.65 wt.% O) via L-PBF technology, revealing the multiscale regulatory mechanisms of oxygen in titanium alloys during non-equilibrium solidification. The study demonstrates that a surface oxygen-enriched layer (~30 nm) synergizes with rapid solidification conditions (104–106 K/s) to induce localized thermal stress fields, triggering HCP→FCC solid-state phase transformation through coordinated slip of Shockley partial dislocations. This process generated nanoscale lamellar FCC phases (width: 79 ± 39 nm, length: 1044 ± 346 nm) uniformly distributed within the HCP matrix. Thermodynamic calculations based on molecular dynamics simulations indicate that oxygen exhibits 5.0 eV-lower formation energy at octahedral interstitial sites in the FCC phase than in the HCP phase. This unique coherent heterostructure achieves strength–ductility synergy through multilevel deformation mechanisms: ① continuous dislocation transmission across phase boundaries effectively mitigates stress concentration; ② dislocation pileups and subgrain boundary formation enhance work-hardening capacity; and ③ nanotwins act as secondary deformation carriers to coordinate macroscopic plasticity. These coupled effects yield exceptional mechanical properties with a UTS of 1119.3 ± 29.2 MPa and El of 23.3 ± 1.9%. The experimental results show that when the oxygen concentration exceeds 0.75 wt%, lattice distortion energy barriers develop in FCC phases, leading to interface decoherence and stacking fault networks that cause localized stress concentration and catastrophic ductility loss (<5%). Subsequent thermodynamic analysis elucidates oxygen diffusion pathways governing microstructural evolution: After 700 °C annealing, restricted oxygen diffusion preserves FCC phase fraction, where retained coherent interfaces maintain strength (963.0 ± 20.0 MPa) while enhancing ductility to 24.7 ± 1.4%. Conversely, 1000 °C treatment promotes oxygen homogenization, triggering FCC→HCP reverse transformation through reduced oxygen chemical potential in the FCC phases, ultimately forming a mechanically degraded single HCP structure.
Shota et al. [94] systematically explored the oxygen-strengthening mechanisms in L-PBF-processed Ti-O alloys through compositional gradient design. Their experimental matrix revealed a critical oxygen threshold of 0.15 wt.% and 0.67 wt.%, where UTS escalated from 962 MPa to 1025 MPa, while maintaining substantial ductility (15.1%), with merely 4.7% ductility reduction. Advanced characterization combining in situ tensile testing with scanning electron microscope–electron backscatter diffraction (SEM-EBSD) crystallographic analysis demonstrated that oxygen-induced lattice distortion promotes atypical deformation pathways. Specifically, the oxygen-stabilized α′-Ti phase exhibited spontaneous {100} ⟨110⟩ deformation twinning during plastic strain, a phenomenon rarely observed in conventional titanium alloys. This twinning mechanism facilitates coordinated grain rotation and strain accommodation, effectively circumventing the conventional dislocation-mediated failure modes. Oxygen-enhanced solid solution strengthening synergizes with this deformation twin activation, achieving unprecedented strength–ductility combinations without requiring PT thermomechanical treatments. This discovery highlights the dual functionality of oxygen as both a strengthening agent and a deformation-mode modulator in additive-manufactured titanium systems.
Akira et al. [95] revealed that Zr-O co-alloying in L-PBF-processed Ti alloys induces a multiscale synergistic strengthening mechanism, achieving an exceptional combination of high strength and ductility. The experimental results demonstrate that increasing the Zr and O contents from Ti-0.15O to Ti-0.74Zr-0.41O significantly enhanced the YS (363→826 MPa, +127%) and UTS (419→900 MPa, +115%), while simultaneously improving El by 8.8% (18.6%→27.4%). Further compositional optimization of Ti-1.48Zr-0.66O resulted in ultrahigh strength (YS: 1014 MPa, UTS: 1073 MPa) with retained ductility (El: 17%). This performance breakthrough originates from three interdependent mechanisms: oxygen atoms act as interstitial strengtheners through lattice distortion, whereas zirconium induces substitutional solid solution strengthening and modifies the phase transformation kinetics during rapid solidification. Their synergistic interaction promotes the formation of refined α’ martensite with an enhanced dislocation storage capacity, coupled with L-PBF-induced hierarchical microstructures containing nanoscale dislocation cells and oxygen-stabilized stacking faults. These multi-scale features collectively enhance the strain-hardening efficiency while maintaining deformation compatibility, thereby overcoming the conventional strength–ductility trade-off in metallic materials.
Wang et al. [96] successfully introduced an oxygen-rich FCC phase (designated as the C phase) into a Ti-6Al-4V alloy via L-PBF additive manufacturing, with a lattice parameter of 0.406 nm (Figure 7d). Oxygen stabilization of this phase was achieved through the preferential occupancy of octahedral interstices in the FCC structure (Figure 7c), significantly lowering the system energy. EBSD phase maps revealed progressive accumulation of the C phase from the top (0 vol. %) to the bottom layers (6.6 vol.%). APT and electron energy loss spectroscopy (EELS) analyses (Figure 7a,b) confirmed an oxygen concentration of 30–33 at.% in the C-phase, substantially higher than that in the surrounding α′ phase (2.8 at.%), with oxygen distribution modifying the chemical bonding environment to stabilize the FCC structure. The oxygen-enriched C phase exhibited a crystallographic orientation relationship with the α′ phase: [0001]α′//C and α′//C (Figure 7e). The enhancement in the mechanical properties was attributed to dislocation interactions and deformation twinning (Figure 7f). Micropillar compression tests demonstrated that dual-phase (α’+C) samples achieved a yield strength of 1.9 GPa, a 58% increase over single-phase α′ samples (1.2 GPa), while maintaining comparable plasticity. Tensile testing revealed improved uniform elongation (5.5%) and fracture strain (10%) in the dual-phase specimens compared with the α′-dominated samples. DFT calculations indicated that the ordered oxygen distribution in the FCC phase suppressed unfavorable first nearest-neighbor configurations, thereby optimizing the strengthening effects. In conclusion, oxygen not only refines the microstructure through solid solution strengthening and phase transformation control, but also enables synergistic strengthening–ductility enhancement via FCC/HCP phase interactions.
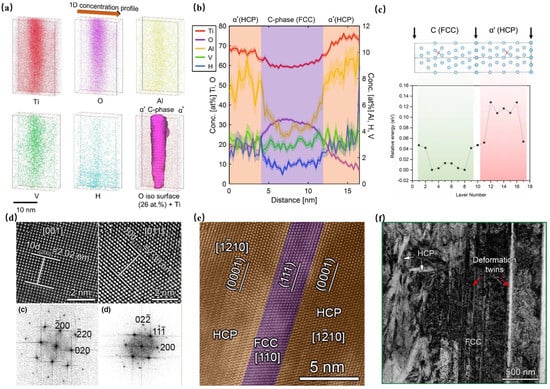
Figure 7.
(a) APT tomography and oxygen enrichment (26 at.%) in the C phase; (b) elemental concentration profiles with 95% confidence interval analysis; (c) DFT modeling of FCC/HCP interface and oxygen energy mapping; (d) C-phase crystallography via high-angle annular dark-field/fast Fourier transform (HAADF/FFT) imaging; (e) <>α′/<> zone axis; and (f) dislocation and deformation twin evolution in the C phase [96].
3.3. The Effect of Interstitial Oxygen on L-DED Prepared Titanium Alloys
Song et al. [92] used L-DED technology and added TiO2 to construct four alloys with varying oxygen contents (0.15 wt.%, 0.35 wt.%, 0.50 wt.%, and 0.70 wt.%) and 3 wt.% Fe. Representative alloys Ti-0.34O-3.25Fe and Ti-0.50O-3.17Fe exhibit UTS of 1157 ± 3 MPa and 1194 ± 8 MPa, respectively, with fracture strains (ε) maintaining 9.0 ± 0.8% and 9.0 ± 0.5%, demonstrating significant superiority over conventional cast alloys. The L-DED process, leveraging rapid solidification kinetics and dynamic thermal cycling modulation (Figure 8a,b), promotes the formation of refined equiaxed β grains with internal α-β lamellar structures. Oxygen atoms preferentially segregate at α-phase interfaces, creating a nanoscale chemically heterogeneous distribution characterized by high-oxygen interfacial regions (strengthening zones) and low-oxygen cores (ductile zones). Notably, β-phase regions remain oxygen-depleted (<0.03 at%) but exhibit iron enrichment. APT and scanning transmission electron microscopy (STEM) analyses reveal two critical mechanisms: ① α-phase strengthening: oxygen segregation at HCP interstitial sites within the α phase (Figure 8c,d) induces localized charge density gradients (Figure 8e) and generates Cottrell atmosphere pinning effects (Figure 8f), effectively hindering dislocation motion. ② β-phase strengthening: The heterogeneous distribution of Fe in β-phase (Figure 8g) induces lattice distortions, creating localized strain fields that reinforce the matrix. Thermodynamic partitioning during L-DED processing strongly stabilizes the α phase while suppressing β-phase volume fraction. This process-engineered nanoscale oxygen/iron partitioning strategy establishes a dual-phase strengthening paradigm, overcoming the traditional strength–ductility trade-off in titanium alloys.
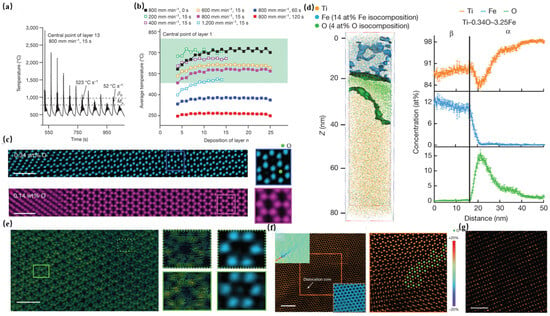
Figure 8.
Microstructural and chemical evolution in L-DED processed Ti-O-Fe alloys. (a) Simulated temperature profile at layer 13 midpoint in a 25-layer coupon; (b) simulated processing window (green zone); and (c) IDPC-STEM of α/β interfaces showing O enrichment in Ti-0.34O-3.25Fe (vs. Ti-0.14O-3.23Fe). Scale bars: 1 nm; (d) APT analysis of O segregation at α-phase edges near α/β interfaces; (e) tensor DPC-STEM mapping of electric field gradients (green = weak, yellow = strong; [000])α). Scale bar: 1 nm; (f) HAADF-STEM of O interstitials (green) pinning dislocation (insets: strain analysis and O array). Scale bar: 2 nm; (g) Fe heterogeneity in the β phase (HAADF-STEM, [110]β zone). Bright contrast: Fe-rich regions. Scale bar: 1 nm [92].
Yang et al. [93] exposed Ti-6Al-4V powder to oxygen concentrations ranging from 9 ppm to 9500 ppm, yielding in oxygen equivalent (OEQ) concentrations spanning 0.12–0.72 wt.% (calculated as shown in Formula (2) [2]). The powder was then processed using the L-DED technology. With rising OEQ (Figure 9b), the α lath width increased from ~0.5 μm to ~1.1 μm, and the microstructure transitioned from an initial partially martensitic structure to a fully lamellar α+β configuration (Figure 9d). This phenomenon stems from the oxygen-induced elevation of the β-transus temperature and martensite start temperature (Ms), which shortens the temporal window for the β→α solid-state phase transformation. Consequently, phase transformation occurred at higher temperatures with prolonged durations, accelerating α lath coarsening and martensite decomposition [97]. Furthermore, the thermal cycling effects of the L-DED process (Figure 9a) and its broad processing window optimized the phase transformation kinetics. At OEQ = 0.36 wt.%, the alloy achieved an exceptional combination of UTS (1160 MPa) and El (11%). Even at OEQ = 0.48 wt.%, the UTS remained as high as 1234 MPa (Figure 9c) with 6.5% elongation, demonstrating a synergistic balance between high strength and retained ductility.

Figure 9.
(a) Longitudinal section showing columnar grain structure formed by L-DED; (b) correlation between α lath thickness and oxygen exposure; (c) model of YS/UTS versus oxygen equivalent; and (d) lamellar α+β microstructure [93].
4. Conclusions and Outlook
As a critical interstitial element in titanium alloys, oxygen exerts a dual influence on their mechanical properties through interstitial solid solution strengthening and microstructural modulation. Systematic studies reveal that oxygen concentrations within the optimized range of 0.2–0.7 wt.% enable significant strength enhancement (via localized lattice distortion and dislocation pinning) while maintaining acceptable ductility. However, this delicate balance is intricately linked to manufacturing methodologies owing to the inherent diffusivity and segregation tendencies of oxygen. Conventional manufacturing processes face inherent limitations in controlling oxygen distribution, where thermal gradient-induced segregation necessitates complex PT strategies, such as multi-stage annealing or strategic solute additions (e.g., Al and V) to compensate for embrittlement risks. In contrast, LAM leverages its unique thermodynamics, characterized by ultrahigh cooling rates (~106 K/s) and micrometer-scale melt pool dimensions, to alter oxygen-mediated microstructural evolution. The rapid solidification kinetics in AM effectively suppress β-phase grain coarsening while confining oxygen diffusion to sub-micrometer domains, thereby achieving in situ microstructure refinement and oxygen gradient engineering without PT.
The strengthening effect of interstitial oxygen on titanium alloys is primarily achieved through solid solution strengthening. As the oxygen content increases, oxygen embrittlement may occur because of the hindrance of screw dislocations by interstitial oxygen, causing the cross-slip to transition into planar slip with increasing oxygen content. This severely degrades the ductility of the material, resulting in a sharp decline in ductility.
Conventionally fabricated titanium alloys require multi-stage deformation-based processing (e.g., hot/cold rolling and high-pressure torsion) combined with PT to optimize O-induced solid solution strengthening while maintaining ductility.
Compared with traditional fabrication methods, additive manufacturing offers greater flexibility, lower material waste, shorter development cycles, faster production times, well-controlled oxygen doping content, and significantly reduced manufacturing costs. Due to its unique high cooling rates, additive manufacturing can produce ultrafine grains and accommodate more oxygen, providing an advanced technique for low-cost, strong, and tough oxygen-containing titanium alloys with synergistic properties.
Future research should focus on establishing multiscale mechanistic frameworks to unravel the synergistic interplay between oxygen and impurity elements (interstitial N/C and substitutional species) across atomic-to-mesoscopic regimes, leveraging advanced atomic-scale characterization (APT and HR-TEM) integrated with CALPHAD-based thermodynamic modeling. Concurrently, harnessing machine learning-driven optimization of additive manufacturing parameters is critical for exploiting the unique thermal cycling dynamics of LAM processes, enabling programmable oxygen gradient architectures and precise metastable phase control through intelligent regulation of melt pool thermodynamics and solidification kinetics.
Author Contributions
Conceptualization, Y.R.; methodology, J.X.; writing—original draft preparation, J.X.; writing—review and editing, Y.R., Y.W., Y.L. and J.Z.; supervision, S.L.; project administration, Y.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Postdoctoral Fellowship Program (Grade C) of China Postdoctoral Science Foundation”, “China Postdoctoral Science Foundation” and “Natural Science Basic Research Plan in Shaanxi Province of China” OF FUNDER, grant number “GZC20241335”, “2024MD753962” and “2025JC-YBQN-580”.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Zhao, S.T.; Zhang, R.P.; Yu, Q.; Ell, J.; Ritchie, R.O.; Minor, A.M. Cryoforged nanotwinned titanium with ultrahigh strength and ductility. Science 2021, 373, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wu, H.; Agbedor, S.-O.; Lu, Y.; Zhang, Y.; Fang, Q.; Li, J.; Tian, Y.; Baker, I. Microstructure, mechanical and tribological properties of a Ti-5Cu alloy and a B4C/Ti-5Cu in situ composite fabricated by laser powder bed fusion. Mater. Charact. 2022, 192, 112217. [Google Scholar] [CrossRef]
- Yu, Q.; Qi, L.; Tsuru, T.; Traylor, R.; Rugg, D.; Morris, J.W.; Asta, M.; Chrzan, D.C.; Minor, A.M. Origin of dramatic oxygen solute strengthening effect in titanium. Science 2015, 347, 635–639. [Google Scholar] [CrossRef]
- Yan, M.; Xu, W.; Dargusch, M.S.; Tang, H.P.; Brandt, M.; Qian, M. Review of effect of oxygen on room temperature ductility of titanium and titanium alloys. Powder Metall. 2014, 57, 251–257. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, Y.; Zhou, Q.; Qu, X.; Cao, P.; Lu, X. Achieving synergy of strength and ductility in powder metallurgy commercially pure titanium by a unique oxygen scavenger. Acta Mater. 2024, 263, 119485. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Sun, J.; Liu, Y.; Zhang, C.; Li, R.; Kuang, F.; Wu, X.; Lu, X. Enhanced strength and ductility in a powder metallurgy Ti material by the oxygen scavenger of CaB6. J. Mater. Sci. Technol. 2023, 137, 132–142. [Google Scholar] [CrossRef]
- Yang, Y.F.; Li, S.F.; Qian, M.; Zhu, Q.S.; Hu, C.Q.; Shi, Y. Enabling the development of ductile powder metallurgy titanium alloys by a unique scavenger of oxygen and chlorine. J. Alloys Compd. 2018, 764, 467–475. [Google Scholar] [CrossRef]
- Okabe, T.H.; Zheng, C.; Taninouchi, Y.-k. Thermodynamic Considerations of Direct Oxygen Removal from Titanium by Utilizing the Deoxidation Capability of Rare Earth Metals. Metall. Mater. Trans. B 2018, 49, 1056–1066. [Google Scholar] [CrossRef]
- Yang, Y.F.; Luo, S.D.; Qian, M. The effect of lanthanum boride on the sintering, sintered microstructure and mechanical properties of titanium and titanium alloys. Mater. Sci. Eng. A 2014, 618, 447–455. [Google Scholar] [CrossRef]
- Liu, Y.; Yong, L.; Bin, W.; Jingwen, Q.; Bin, L.; Tang, H. Microstructures Evolution and Mechanical Properties of a Powder Metallurgical Titanium Alloy with Yttrium Addition. Mater. Manuf. Process. 2010, 25, 735–739. [Google Scholar] [CrossRef]
- Yang, Y.F.; Luo, S.D.; Schaffer, G.B.; Qian, M. Impurity scavenging, microstructural refinement and mechanical properties of powder metallurgy titanium and titanium alloys by a small addition of cerium silicide. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2013, 573, 166–174. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, F.; Guo, Z.; Wang, H.; Lu, B. Oxygen scavenging, grain refinement and mechanical properties improvement in powder metallurgy titanium and titanium alloys with CaB6. Powder Technol. 2018, 340, 362–369. [Google Scholar] [CrossRef]
- Chong, Y.; Zhang, R.; Hooshmand, M.S.; Zhao, S.; Chrzan, D.C.; Asta, M.; Morris, J.W.; Minor, A.M. Elimination of oxygen sensitivity in α-titanium by substitutional alloying with Al. Nat. Commun. 2021, 12, 6158. [Google Scholar] [CrossRef]
- Ghazisaeidi, M.; Trinkle, D.R. Interaction of oxygen interstitials with lattice faults in Ti. Acta Mater. 2014, 76, 82–86. [Google Scholar] [CrossRef]
- Feng, X.; Liang, Y.L.; Sun, H.; Wang, S. Effect of Dislocation Slip Mechanism under the Control of Oxygen Concentration in Alpha-Case on Strength and Ductility of TC4 Alloy. Metals 2021, 11, 1057. [Google Scholar] [CrossRef]
- Ueki, S.; Mine, Y.; Chiu, Y.-L.; Bowen, P.; Takashima, K. Effects of crystallographic orientation and lamellar configuration on fatigue crack propagation in single-colony structures of Ti–6Al–4V alloy: Alternating shear crack growth vs. damage accumulation crack propagation. Mater. Sci. Eng. A 2024, 890, 145885. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, F.; Chen, C.; Shao, Y.; Lu, B.; Lu, T.; Sui, Y.; Guo, Z. Mechanical property enhancement of high-plasticity powder metallurgy titanium with a high oxygen concentration. J. Alloys Compd. 2021, 885, 161006. [Google Scholar] [CrossRef]
- Sun, B.; Li, S.; Imai, H.; Mimoto, T.; Umeda, J.; Kondoh, K. Fabrication of high-strength Ti materials by in-process solid solution strengthening of oxygen via P/M methods. Mater. Sci. Eng. A 2013, 563, 95–100. [Google Scholar] [CrossRef]
- Sinha, P.K. Influence of an oxygen-enriched layer on the tensile properties of an alpha titanium alloy. Mater. Today Commun. 2024, 38, 107698. [Google Scholar] [CrossRef]
- Zhang, H.R.; Niu, H.Z.; Zhang, Y.H.; Zang, M.C.; Zhang, D.L. Achieving a high strength and tensile ductility synergy of a high-oxygen powder metallurgy near alpha titanium alloy by importing βt domains into lamellar structures. J. Alloys Compd. 2022, 894, 162517. [Google Scholar] [CrossRef]
- Kang, D.S.; Lee, K.J.; Kwon, E.P.; Tsuchiyama, T.; Takaki, S. Variation of work hardening rate by oxygen contents in pure titanium alloy. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2015, 632, 120–126. [Google Scholar] [CrossRef]
- Wei, Q.Q.; Wang, L.Q.; Fu, Y.F.; Qin, J.N.; Lu, W.J.; Zhang, D. Influence of oxygen content on microstructure and mechanical properties of Ti-Nb-Ta-Zr alloy. Mater. Des. 2011, 32, 2934–2939. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, T.; Miao, R.j.; Fei, Y. Influence of oxygen on microstructures and tensile properties of hot-rolled Ti-4Zr-xO alloys. Mater. Charact. 2021, 171, 110681. [Google Scholar] [CrossRef]
- Chong, Y.; Gholizadeh, R.; Tsuru, T.; Zhang, R.; Inoue, K.; Gao, W.; Godfrey, A.; Mitsuhara, M.; Morris, J.W.; Minor, A.M.; et al. Grain refinement in titanium prevents low temperature oxygen embrittlement. Nat. Commun. 2023, 14, 404. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Cheng, X.; Chen, J.Y.; Xiang, T.; Xie, G.Q. Optimized mechanical properties of titanium-oxygen alloys by powder metallurgy. J. Mater. Res. Technol.-JMRT 2022, 21, 4151–4163. [Google Scholar] [CrossRef]
- Amann, F.; Poulain, R.; Delannoy, S.; Couzinié, J.P.; Clouet, E.; Guillot, I.; Prima, F. An improved combination of tensile strength and ductility in titanium alloys via oxygen ordering. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2023, 867, 144720. [Google Scholar] [CrossRef]
- Chong, Y.; Poschmann, M.; Zhang, R.P.; Zhao, S.T.; Hooshmand, M.S.; Rothchild, E.; Olmsted, D.L.; Morris, J.W.; Chrzan, D.C.; Asta, M.; et al. Mechanistic basis of oxygen sensitivity in titanium. Sci. Adv. 2020, 6, eabc4060. [Google Scholar] [CrossRef]
- Sarraf, M.; Ghomi, E.R.; Alipour, S.; Ramakrishna, S.; Sukiman, N.L. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-Des. Manuf. 2022, 5, 371–395. [Google Scholar] [CrossRef]
- Özcan, M.; Hämmerle, C. Titanium as a Reconstruction and Implant Material in Dentistry: Advantages and Pitfalls. Materials 2012, 5, 1528–1545. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, C.; Tan, C.; Wan, M.; Zhao, Y.; Ye, J.; Zeng, W. Influence of microstructure on cyclic deformation response and micromechanics of Ti–55531 alloy. Mater. Sci. Eng. A 2021, 803, 140505. [Google Scholar] [CrossRef]
- Haghighi, S.E.; Lu, H.B.; Jian, G.Y.; Cao, G.H.; Habibi, D.; Zhang, L.C. Effect of α” martensite on the microstructure and mechanical properties of beta-type Ti-Fe-Ta alloys. Mater. Des. 2015, 76, 47–54. [Google Scholar] [CrossRef]
- Zhang, L.C.; Klemm, D.; Eckert, J.; Hao, Y.L.; Sercombe, T.B. Manufacture by selective laser melting and mechanical behavior of a biomedical Ti-24Nb-4Zr-8Sn alloy. Scr. Mater. 2011, 65, 21–24. [Google Scholar] [CrossRef]
- Kanayama, M.; Cunningham, B.W.; Haggerty, C.J.; Abumi, K.; Kaneda, K.; McAfee, P.C. In vitro biomechanical investigation of the stability and stress-shielding effect of lumbar interbody fusion devices. J. Neurosurg. 2000, 93, 259–265. [Google Scholar] [CrossRef]
- Engh, C.A.; Bobyn, J.D.; Glassman, A.H. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J. Bone Jt. Surg. Br. Vol. 1987, 69, 45–55. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Li, X.; Zhang, X.; Gong, X.; Zhu, Y.; Ren, Z.; Zhang, B.; Cheng, J. Biocompatibility and osseointegration properties of a novel high strength and low modulus β- Ti10Mo6Zr4Sn3Nb alloy. Front. Bioeng. Biotechnol. 2023, 11, 1127929. [Google Scholar] [CrossRef]
- Geng, F.; Niinomi, M.; Nakai, M. Observation of yielding and strain hardening in a titanium alloy having high oxygen content. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2011, 528, 5435–5445. [Google Scholar] [CrossRef]
- Kang, L.M.; Yang, C. A Review on High-Strength Titanium Alloys: Microstructure, Strengthening, and Properties. Adv. Eng. Mater. 2019, 21, 1801359. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546–550. [Google Scholar] [CrossRef]
- Bartáková, S.; Málek, J.; Prachár, P. The Effect of Oxygen Addition on Microstructure and Mechanical Properties of Various Beta-Titanium Alloys. JOM 2020, 72, 1656–1663. [Google Scholar] [CrossRef]
- Chong, Y.; Gholizadeh, R.; Guo, B.; Tsuru, T.; Zhao, G.; Yoshida, S.; Mitsuhara, M.; Godfrey, A.; Tsuji, N. Oxygen interstitials make metastable β titanium alloys strong and ductile. Acta Mater. 2023, 257, 119165. [Google Scholar] [CrossRef]
- Han, C.-B.; Lee, D.-G. Effect of Oxygen on Static Recrystallization Behaviors of Biomedical Ti-Nb-Zr Alloys. Metals 2024, 14, 333. [Google Scholar] [CrossRef]
- Qazi, J.I.; Rack, H.J.; Marquardt, B. High-strength metastable beta-titanium alloys for biomedical applications. JOM 2004, 56, 49–51. [Google Scholar] [CrossRef]
- Stráský, J.; Harcuba, P.; Václavová, K.; Horváth, K.; Landa, M.; Srba, O.; Janeček, M. Increasing strength of a biomedical Ti-Nb-Ta-Zr alloy by alloying with Fe, Si and O. J. Mech. Behav. Biomed. Mater. 2017, 71, 329–336. [Google Scholar] [CrossRef]
- Kozlík, J.; Preisler, D.; Stráský, J.; Košutová, T.; Corrêa, C.A.; Veselý, J.; Bodnárová, L.; Lukáč, F.; Chráska, T.; Janeček, M. Manufacturing of biomedical Ti alloys with controlled oxygen content by blended elemental powder metallurgy. J. Alloys Compd. 2022, 905, 164259. [Google Scholar] [CrossRef]
- Yokota, K.; Bahador, A.; Shitara, K.; Umeda, J.; Kondoh, K. Mechanisms of tensile strengthening and oxygen solid solution in single β-phase Ti-35 at.%Ta+O alloys. Mater. Sci. Eng. A 2021, 802, 140677. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zhang, Y.-S.; Han, W.-Z. Design of high strength and wear-resistance β-Ti alloy via oxygen-charging. Acta Mater. 2022, 227, 117686. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, W.L.; Kent, D.; Wang, J.S.; Jiang, W.T.; Meng, F.Q.; Peng, Z.L.; Fu, Y.; Ma, C.L. Manipulating TWIP/TRIP via oxygen-doping to synergistically enhance strength and ductility of metastable beta titanium alloys. J. Mater. Sci. Technol. 2025, 215, 58–70. [Google Scholar] [CrossRef]
- Yang, P.J.; Li, Q.J.; Tsuru, T.; Ogata, S.; Zhang, J.W.; Sheng, H.W.; Shan, Z.W.; Sha, G.; Han, W.Z.; Li, J.; et al. Mechanism of hardening and damage initiation in oxygen embrittlement of body-centred-cubic niobium. Acta Mater. 2019, 168, 331–342. [Google Scholar] [CrossRef]
- Yang, P.J.; Li, Q.J.; Han, W.Z.; Li, J.; Ma, E. Designing solid solution hardening to retain uniform ductility while quadrupling yield strength. Acta Mater. 2019, 179, 107–118. [Google Scholar] [CrossRef]
- Zhang, J.; Han, W.Z. Oxygen solutes induced anomalous hardening, toughening and embrittlement in body-centered cubic vanadium. Acta Mater. 2020, 196, 122–132. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.B.; Liu, J.R.; Wang, L.; Yang, G.; Gong, S.L.; Wang, Q.J.; Yang, R. Effect of α texture on the tensile deformation behavior of Ti-6Al-4V alloy produced via electron beam rapid manufacturing. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2019, 742, 508–516. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Li, S.; Zhang, J.; Li, J.; Liu, G.; Sun, J. Oxygen-dislocation interaction-mediated nanotwinned nanomartensites in ultra-strong and ductile titanium alloys. Mater. Today 2024, 75, 85–96. [Google Scholar] [CrossRef]
- Gao, S.; Pan, K.; Chen, D.; Wang, B.; Wu, S.; Luo, X.; Sun, M.; Zhao, C.; Li, N. Mechanism of oxygen content on impact toughness of α + β powder metallurgy titanium alloy. J. Mater. Res. Technol. 2024, 33, 318–334. [Google Scholar] [CrossRef]
- Naydenkin, E.V.; Mishin, I.P.; Ratochka, I.V.; Lykova, O.N.; Zabudchenko, O.V. The effect of alpha-case formation on plastic deformation and fracture of near β titanium alloy. Mater. Sci. Eng. A 2020, 769, 138495. [Google Scholar] [CrossRef]
- Fu, Y.; Xiao, W.; Zhao, S.; Ren, L.; Wang, J.; Rong, J.; Li, J.; Zhao, X.; Ma, C. Substantially strengthening a dual-phase titanium alloy by moderate oxygen doping. Scr. Mater. 2023, 226, 115236. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.Y.; Hou, J.P.; Zhang, D.D.; Yue, Y.H.; Liu, G.; Sun, J. Making a low-cost duplex titanium alloy ultra-strong and ductile via interstitial solutes. Acta Mater. 2022, 241, 118411. [Google Scholar] [CrossRef]
- Zhang, H.R.; Niu, H.Z.; Liu, S.; Zang, M.C.; Zhang, D.L. Significantly enhanced tensile ductility and its origin of a <0001> micro-textured extrusion bar of a powder metallurgy near alpha titanium alloy. Scr. Mater. 2022, 213, 114633. [Google Scholar] [CrossRef]
- Gong, G.H.; Ye, J.J.; Chi, Y.M.; Zhao, Z.H.; Wang, Z.F.; Xia, G.; Du, X.Y.; Tian, H.F.; Yu, H.J.; Chen, C.Z. Research status of laser additive manufacturing for metal: A review. J. Mater. Res. Technol.-JMRT 2021, 15, 855–884. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhao, D.D.; Wang, P.; Yan, M.; Yang, C.; Chen, Z.W.; Lu, J.; Lu, Z.P. Additive manufacturing of metals: Microstructure evolution and multistage control. J. Mater. Sci. Technol. 2022, 100, 224–236. [Google Scholar] [CrossRef]
- Su, E.P.; Justin, D.E.; Pratt, C.R.; Sarin, V.K.; Nguyen, V.S.; Oh, S.; Jin, S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100B, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal Fabrication by Additive Manufacturing Using Laser and Electron Beam Melting Technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Bremer, S.J.L.; Luckabauer, M.; Römer, G.-w.R.B.E. Laser intensity profile as a means to steer microstructure of deposited tracks in Directed Energy Deposition. Mater. Des. 2023, 227, 111725. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, Z.; Seet, H.L.; Liu, T.; Liao, W.; Ramamurty, U.; Ling Nai, S.M. Recent progress on the additive manufacturing of aluminum alloys and aluminum matrix composites: Microstructure, properties, and applications. Int. J. Mach. Tools Manuf. 2023, 190, 104047. [Google Scholar] [CrossRef]
- Kranz, J.; Herzog, D.; Emmelmann, C. Design guidelines for laser additive manufacturing of lightweight structures in TiAl6V4. J. Laser Appl. 2015, 27, S14001. [Google Scholar] [CrossRef]
- Tan, C.; Weng, F.; Sui, S.; Chew, Y.; Bi, G. Progress and perspectives in laser additive manufacturing of key aeroengine materials. Int. J. Mach. Tools Manuf. 2021, 170, 103804. [Google Scholar] [CrossRef]
- Ren, Y.; Han, B.; Wu, H.; Wang, J.; Liu, B.; Wei, B.; Jiao, Z.; Baker, I. Copper segregation-mediated formation of nanotwins and 9R phase in titanium alloys produced by laser powder bed fusion. Scr. Mater. 2023, 224, 115115. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Z.; Wang, Q.; Liu, J.; Zhang, L.; Song, M.; Liu, S.; Guo, S.; Jiao, Z.; Baker, I.; et al. Effect of Nb content on microstructural evolution, mechanical and tribological properties of in situ alloyed copper-modified titanium produced using laser powder bed fusion. J. Mater. Sci. Technol. 2025, 219, 257–270. [Google Scholar] [CrossRef]
- Alrbaey, K.; Wimpenny, D.; Tosi, R.; Manning, W.; Moroz, A. On Optimization of Surface Roughness of Selective Laser Melted Stainless Steel Parts: A Statistical Study. J. Mater. Eng. Perform. 2014, 23, 2139–2148. [Google Scholar] [CrossRef]
- Attar, H.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Manufacture by selective laser melting and mechanical behavior of commercially pure titanium. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2014, 593, 170–177. [Google Scholar] [CrossRef]
- Gradl, P.; Tinker, D.C.; Park, A.; Mireles, O.R.; Garcia, M.; Wilkerson, R.; McKinney, C. Robust Metal Additive Manufacturing Process Selection and Development for Aerospace Components. J. Mater. Eng. Perform. 2022, 31, 6013–6044. [Google Scholar] [CrossRef]
- Ladani, L.; Sadeghilaridjani, M. Review of Powder Bed Fusion Additive Manufacturing for Metals. Metals 2021, 11, 1391. [Google Scholar] [CrossRef]
- Spierings, A.B.; Herres, N.; Levy, G. Influence of the particle size distribution on surface quality and mechanical properties in AM steel parts. Rapid Prototyp. J. 2011, 17, 195–202. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.T.; Liao, W.H.; MacDonald, E.; Wei, H.L.; Chen, X.Y.; Jiang, L.Y. The influence of process parameters on vertical surface roughness of the AlSi10Mg parts fabricated by selective laser melting. J. Mater. Process. Technol. 2019, 266, 26–36. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, C.; Wang, W.; Cao, T.; Shuai, S.; Xu, S.; Hu, T.; Liao, H.; Wang, J.; Ren, Z. On the role of volumetric energy density in the microstructure and mechanical properties of laser powder bed fusion Ti-6Al-4V alloy. Addit. Manuf. 2022, 51, 102605. [Google Scholar] [CrossRef]
- Gordon, J.V.; Narra, S.P.; Cunningham, R.W.; Liu, H.; Chen, H.; Suter, R.M.; Beuth, J.L.; Rollett, A.D. Defect structure process maps for laser powder bed fusion additive manufacturing. Addit. Manuf. 2020, 36, 101552. [Google Scholar] [CrossRef]
- Svetlizky, D.; Zheng, B.; Vyatskikh, A.; Das, M.; Bose, S.; Bandyopadhyay, A.; Schoenung, J.M.; Lavernia, E.J.; Eliaz, N. Laser-based directed energy deposition (DED-LB) of advanced materials. Mater. Sci. Eng. A 2022, 840, 142967. [Google Scholar] [CrossRef]
- Fu, R.; Liang, Y.; Han, Q.; Guo, Y.; Lei, H.; Liu, C. Strengthening and fracturing mechanisms of laser-directed energy deposited Al-7075 alloy. Mater. Sci. Eng. A 2023, 881, 145433. [Google Scholar] [CrossRef]
- Tan, C.; Chew, Y.; Weng, F.; Sui, S.; Ng, F.L.; Liu, T.; Bi, G. Laser aided additive manufacturing of spatially heterostructured steels. Int. J. Mach. Tools Manuf. 2022, 172, 103817. [Google Scholar] [CrossRef]
- Yue, W.; Zhang, Y.; Zheng, Z.; Lai, Y. Hybrid Laser Additive Manufacturing of Metals: A Review. Coatings 2024, 14, 315. [Google Scholar] [CrossRef]
- Spears, T.G.; Gold, S.A. In-process sensing in selective laser melting (SLM) additive manufacturing. Integr. Mater. Manuf. Innov. 2016, 5, 16–40. [Google Scholar] [CrossRef]
- Razavykia, A.; Brusa, E.; Delprete, C.; Yavari, R. An Overview of Additive Manufacturing Technologies—A Review to Technical Synthesis in Numerical Study of Selective Laser Melting. Materials 2020, 13, 3895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, K.; Qiu, D.; Niu, W. Additive manufacturing of high-strength commercially pure titanium through lanthanum oxide addition. Mater. Charact. 2021, 176, 111074. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Cao, X.; Wanjara, P.; Medraj, M. Oxide films in laser additive manufactured Inconel 718. Acta Mater. 2013, 61, 6562–6576. [Google Scholar] [CrossRef]
- Kondoh, K.; Ichikawa, E.; Issariyapat, A.; Shitara, K.; Umeda, J.; Chen, B.; Li, S. Tensile property enhancement by oxygen solutes in selectively laser melted titanium materials fabricated from pre-mixed pure Ti and TiO2 powder. Mater. Sci. Eng. A 2020, 795, 139983. [Google Scholar] [CrossRef]
- Ding, W.; Tao, Q.; Liu, C.; Chen, G.; Yoo, S.; Cai, W.; Cao, P.; Jia, B.; Wu, H.; Zhang, D.; et al. Lean design of a strong and ductile dual-phase titanium–oxygen alloy. Nat. Mater. 2025, 24, 506–512. [Google Scholar] [CrossRef]
- Quintana, O.A.; Tong, W. Effects of Oxygen Content on Tensile and Fatigue Performance of Ti-6Al-4 V Manufactured by Selective Laser Melting. JOM 2017, 69, 2693–2697. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, J.; Bi, X.; Chen, J.; Chen, W.; Ren, Y.; Niu, Y.; Li, C.; Qiu, W.; Yuan, T. Effect of scanning strategies on the microstructure and mechanical properties of Ti–15Mo alloy fabricated by selective laser melting. Vacuum 2022, 205, 111454. [Google Scholar] [CrossRef]
- Prandi, G.V.; Rodrigues, J.F.Q.; Valentim, M.; Sangali, M.; Starck, L.F.; Soyama, J.; Caram, R. HYbrid Titanium Alloys produced by laser powder bed Fusion using Ti-5553 and Ti–42Nb powder recycling. J. Mater. Res. Technol. 2023, 27, 8281–8291. [Google Scholar] [CrossRef]
- Dong, Y.P.; Wang, D.W.; Li, Q.Z.; Luo, X.P.; Zhang, J.; Prashanth, K.G.; Wang, P.; Eckert, J.; Mädler, L.; Okulov, I.V.; et al. Strong and ductile titanium via additive manufacturing under a reactive atmosphere. Mater. Today Adv. 2023, 17, 100347. [Google Scholar] [CrossRef]
- Zhang, X.; Ran, X.; Wang, Z.; Xu, W.; Zhu, X.; Du, Z.; Zhang, J.; Li, R.; Li, Y.; Lu, X. Designing laser powder bed fusion low-alloyed titanium with superior strength-ductility trade-off via machine learning. J. Mater. Sci. Technol. 2025, 237, 323–330. [Google Scholar] [CrossRef]
- Song, T.; Chen, Z.; Cui, X.; Lu, S.; Chen, H.; Wang, H.; Dong, T.; Qin, B.; Chan, K.C.; Brandt, M.; et al. Strong and ductile titanium–oxygen–iron alloys by additive manufacturing. Nature 2023, 618, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.V.; Looze, G.R.d.; Nguyen, V.; Wilson, R.S. Directed-energy deposition of Ti-6Al-4V alloy using fresh and recycled feedstock powders under reactive atmosphere. Addit. Manuf. 2022, 58, 103043. [Google Scholar] [CrossRef]
- Kariya, S.; Issariyapat, A.; Bahador, A.; Umeda, J.; Shen, J.; Yamanaka, K.; Chiba, A.; Kondoh, K. Novel tensile deformation mode in laser powder bed fusion prepared Ti–O alloy. Mater. Sci. Eng. A 2024, 892, 146057. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, D.; Gibson, M.A.; Zheng, Y.; Fraser, H.L.; StJohn, D.H.; Easton, M.A. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature 2019, 576, 91–95. [Google Scholar] [CrossRef]
- Wang, H.; Chao, Q.; Cui, X.Y.; Chen, Z.B.; Breen, A.J.; Cabral, M.; Haghdadi, N.; Huang, Q.W.; Niu, R.; Chen, H.S.; et al. Introducing C phase in additively manufactured Ti-6Al-4V: A new oxygen-stabilized face-centred cubic solid solution with improved mechanical properties. Mater. Today 2022, 61, 11–21. [Google Scholar] [CrossRef]
- Cao, S.; Chu, R.K.; Zhou, X.G.; Yang, K.; Jia, Q.B.; Lim, C.V.S.; Huang, A.J.; Wu, X.H. Role of martensite decomposition in tensile properties of selective laser melted Ti-6Al-4V. J. Alloys Compd. 2018, 744, 357–363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).