Crystal Structure of 4′-Phenyl-1′,4′-Dihydro-2,2′:6′,2″-Terpyridine: An Intermediate from the Synthesis of Phenylterpyridine
Abstract
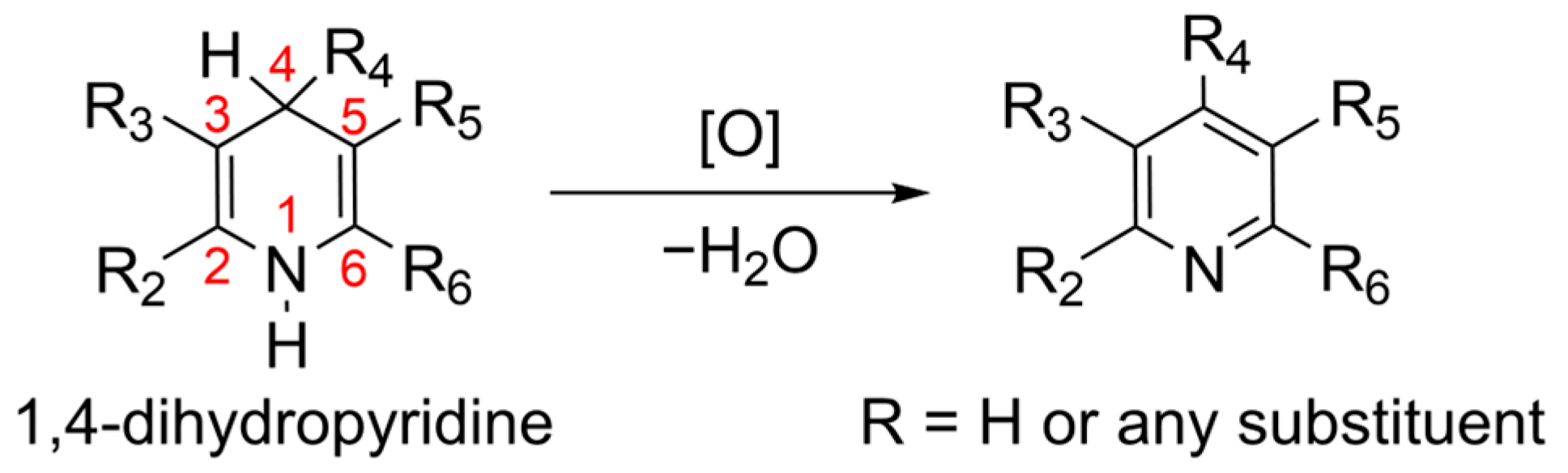
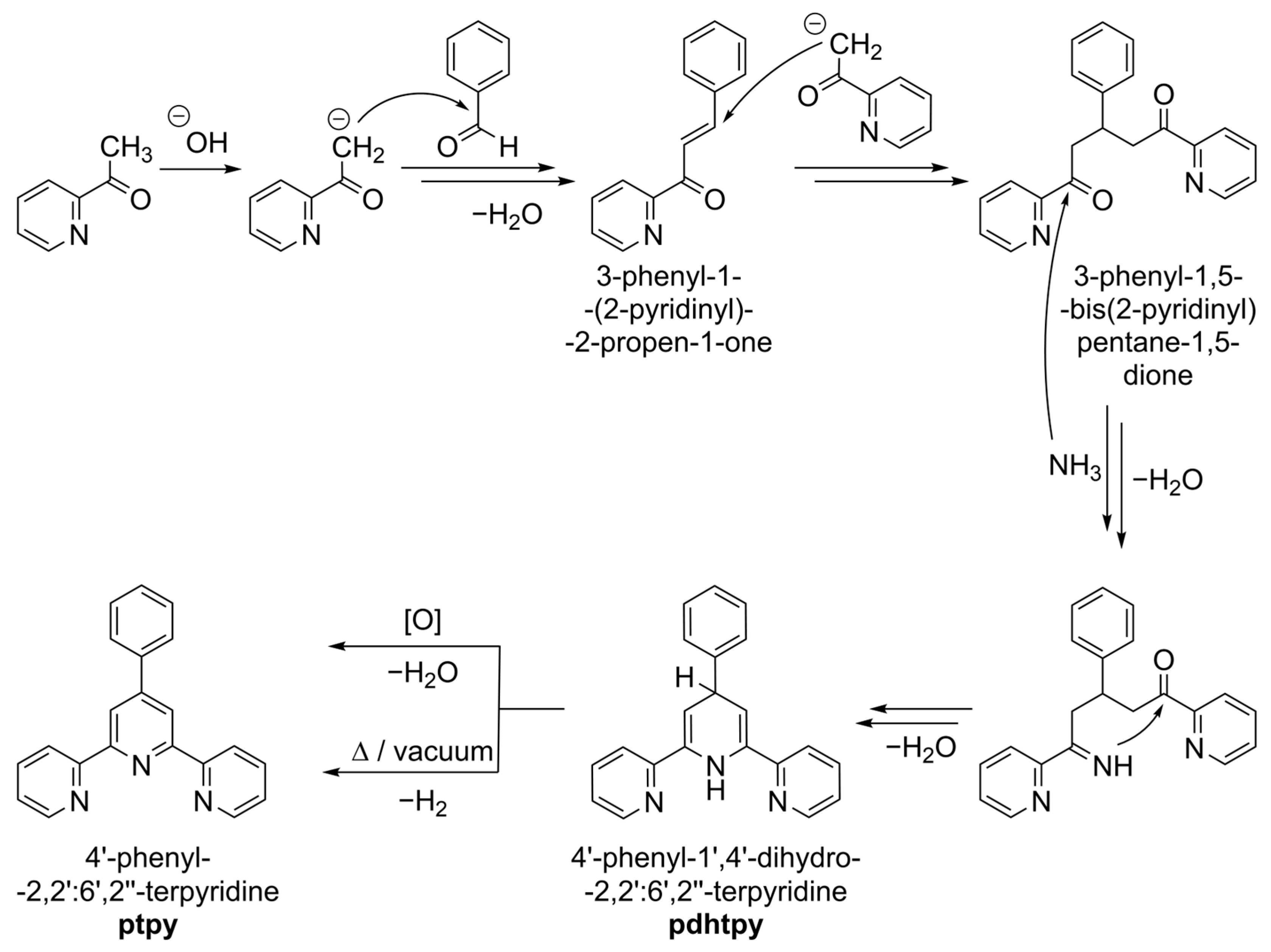
1. Introduction
2. Materials and Methods
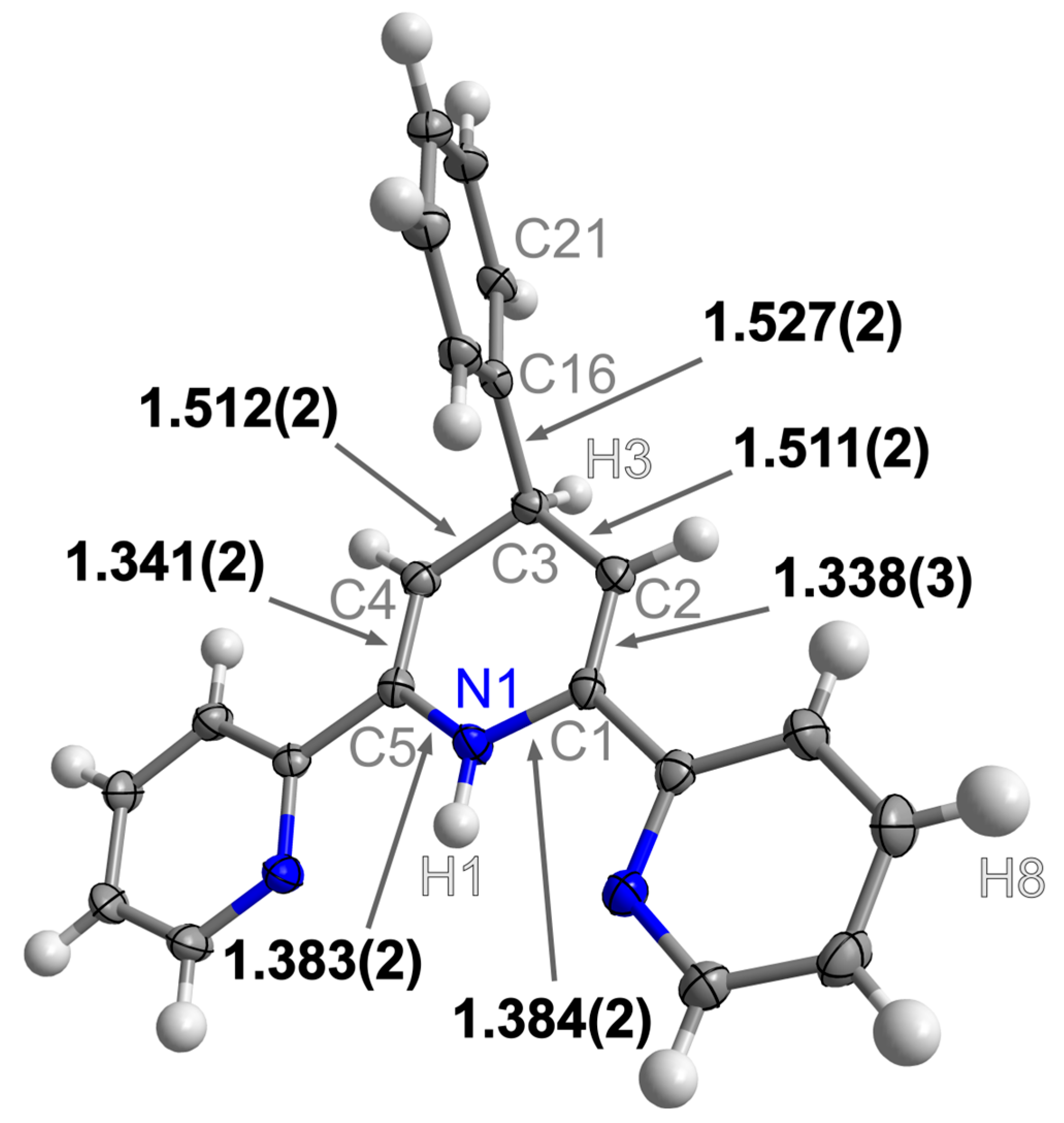
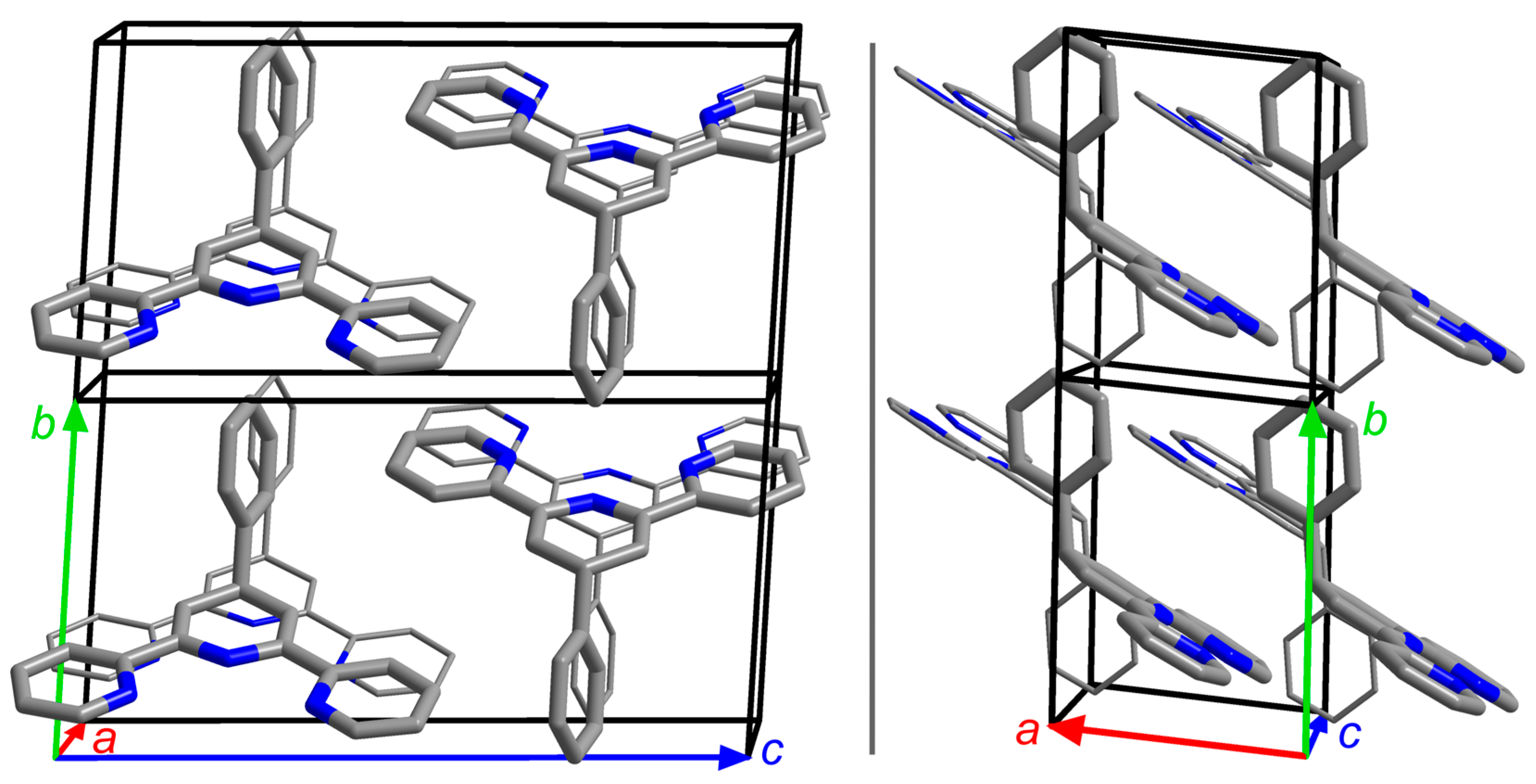
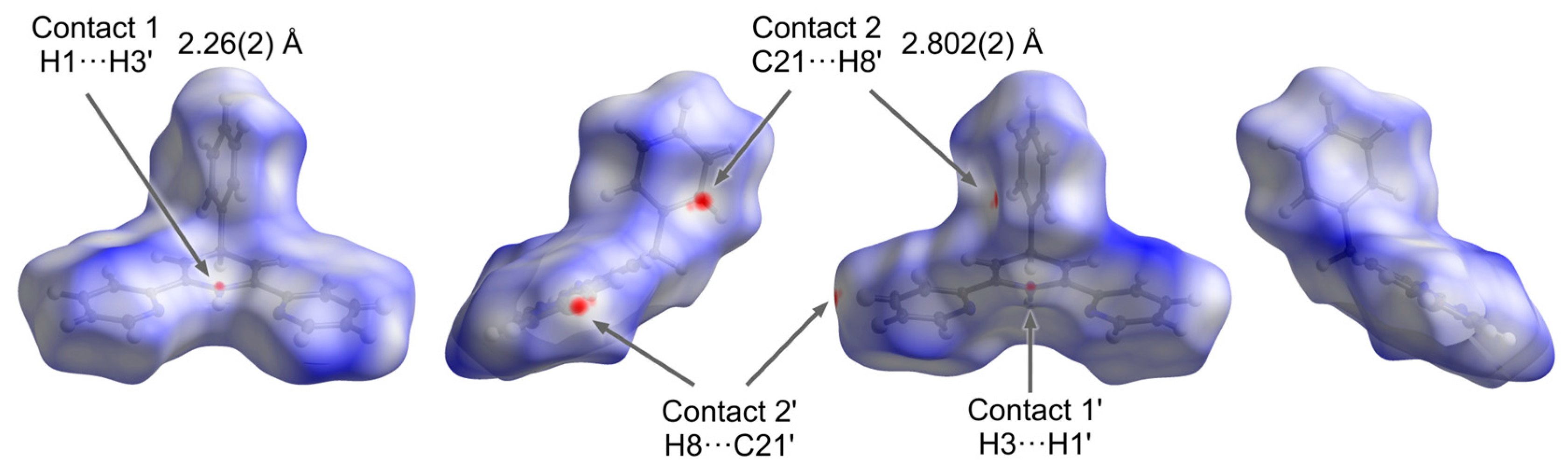
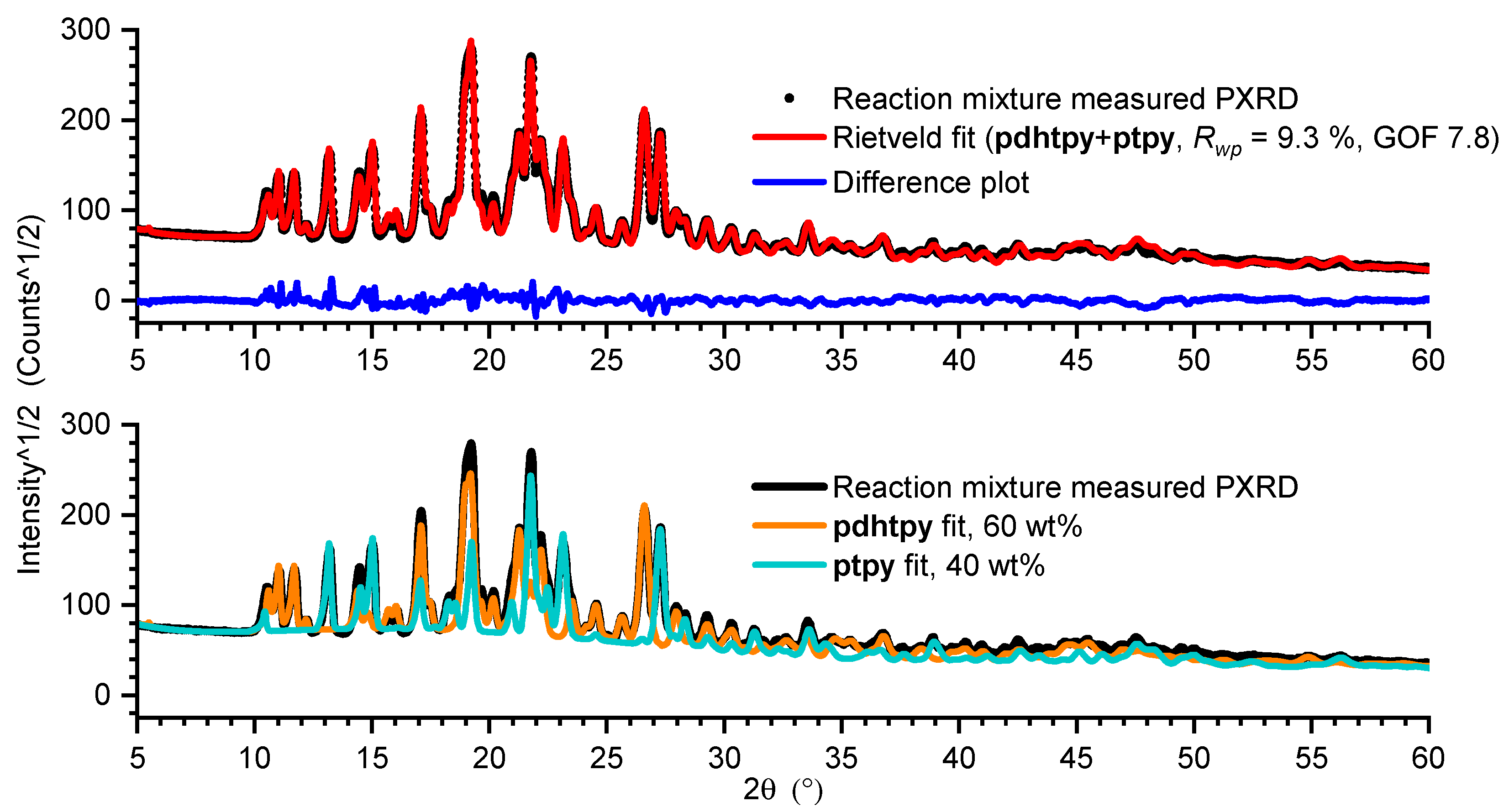
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hantzsch, A. Condensationsprodukte aus Aldehydammoniak und ketonartigen Verbindungen. Berichte Dtsch. Chem. Gesellschaft 1881, 14, 1637–1638. [Google Scholar] [CrossRef]
- Li, J.J. Name Reactions; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Weiss, M. Acetic Acid—Ammonium Acetate Reactions. An Improved Chichibabin Pyridine Synthesis 1. J. Am. Chem. Soc. 1952, 74, 200–202. [Google Scholar] [CrossRef]
- Kröhnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1976, 1–24. [Google Scholar] [CrossRef]
- Morigi, R.; Locatelli, A.; Rambaldi, M.; Consorti, A.; Leoni, A. Diethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate and ethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank 2012, 2012, M787. [Google Scholar] [CrossRef]
- Rucins, M.; Pajuste, K.; Plotniece, A.; Pikun, N.; Rodik, R.; Vyshnevskiy, S.; Sobolev, A. Synthesis and Characterisation of 1,1′-{[3,5-Bis(dodecyloxy-carbonyl)-4-(thiophen-3-yl)-1,4-dihydropyridine-2,6-diyl]bis-(methylene)}bis(pyridin-1-ium) Dibromide. Molbank 2021, 2022, M1311. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G. A Facile Route to Sterically Hindered and Non-Hindered 4′-Aryl-2,2′:6′,2′′-Terpyridines. Synlett 2005, 2005, 1251–1254. [Google Scholar] [CrossRef]
- Tu, S.; Jia, R.; Jiang, B.; Zhang, J.; Zhang, Y.; Yao, C.; Ji, S. Kröhnke reaction in aqueous media: One-pot clean synthesis of 4′-aryl-2,2′:6′,2″-terpyridines. Tetrahedron 2007, 63, 381–388. [Google Scholar] [CrossRef]
- Winter, A.; Van Den Berg, A.M.J.; Hoogenboom, R.; Kickelbick, G.; Schubert, U.S. A green and straightforward synthesis of 4’-substituted terpyridines. Synthesis 2006, 2873–2878. [Google Scholar] [CrossRef]
- Rocco, D.; Housecroft, C.E.; Constable, E.C. Synthesis of terpyridines: Simple reactions-what could possibly go wrong? Molecules 2019, 24, 1799. [Google Scholar] [CrossRef]
- Zhu, S.L.; Ji, S.J.; Su, X.M.; Sun, C.; Liu, Y. Facile and efficient synthesis of a new class of bis(3′-indolyl)pyridine derivatives via one-pot multicomponent reactions. Tetrahedron Lett. 2008, 49, 1777–1781. [Google Scholar] [CrossRef]
- Purushothaman, M.; Thanigaimani, K.; Arshad, S.; Silambarasan, S.; Razak, I.A.; Ali, K.M.S. 2,6-Diamino-4-(4-chlorophenyl)-1-methyl-1,4-dihydropyridine-3, 5-dicarbonitrile. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, o812–o813. [Google Scholar] [CrossRef]
- Sun, C.W.; Chen, Y.X.; Liu, T.Y. 2-Amino-6-{[(6-chloropyridin-3-yl)methyl](ethyl)amino}-1-methyl-5-nitro-4-phenyl-1,4-dihydro-pyridine-3-carbonitrile ethanol monosolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o1146. [Google Scholar] [CrossRef] [PubMed]
- Bolte, M. Dimorphism of ethyl 1,4-dihydro-2,4,6-triphenylpyridine-3-carboxylate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2001, 57, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Subbiahpandi, A.; Thirumurugan, P.; Perumal, P.T.; Ponnuswamy, M.N. 4-(2,4-Dichlorophenyl)-2-(1H-indol-3-yl)-6-(2-pyridyl)-1, 4-dihydropyridine-4-carbonitrile. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, 2697–2704. [Google Scholar] [CrossRef]
- Gong, Y.; Ward, J.S.; Rissanen, K.; Mulks, F.F. Tributyl(1-((dimethylamino)(dimethyliminio)methyl)-1,4-dihydropyridin-4-yl)phosphonium Ditrifluoromethanesulfonate. Molbank 2023, 2023, M1710. [Google Scholar] [CrossRef]
- Zhu, Z.B.; Gao, S.; Ng, S.W. Hydronium 4-oxo-1,4-dihydro-pyridine-3-sulfonate dihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o2687. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Z.Y.; Huo, L.H.; Zhao, J.G.; Zain, S.M.; Ng, S.W. (4-Oxo-1,4-dihydropyridin-1-yl)acetic acid. Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60, 1006–1008. [Google Scholar] [CrossRef]
- Rucins, M.; Plotniece, A.; Bernotiene, E.; Tsai, W.-B.; Sobolev, A. Recent Approaches to Chiral 1,4-Dihydropyridines and their Fused Analogues. Catalysts 2020, 10, 1019. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Ragno, G. 1,4-Dihydropyridine Antihypertensive Drugs: Recent Advances in Photostabilization Strategies. Pharmaceutics 2019, 11, 85. [Google Scholar] [CrossRef]
- Parthiban, A.; Makam, P. 1,4-Dihydropyridine: Synthetic advances, medicinal and insecticidal properties. RSC Adv. 2022, 12, 29253–29290. [Google Scholar] [CrossRef]
- Sedykh, A.E.; Kurth, D.G.; Müller-Buschbaum, K. Two Series of Lanthanide Coordination Polymers and Complexes with 4′-Phenylterpyridine and their Luminescence Properties. Eur. J. Inorg. Chem. 2019, 2019, 4564–4571. [Google Scholar] [CrossRef]
- Sedykh, A.E.; Maxeiner, M.; Seuffert, M.T.; Heuler, D.; Kurth, D.G.; Müller-Buschbaum, K. Enhancing the Analysis of Eu3+ Photoluminescence in Coordination Compounds in the Solid State by Determining their Refractive Index. Eur. J. Inorg. Chem. 2024, 27, e202400078. [Google Scholar] [CrossRef]
- Sedykh, A.E.; Pflug, J.J.; Schäfer, T.C.; Bissert, R.; Kurth, D.G.; Müller-Buschbaum, K. Rapid Spectrometer-Free Luminescence-Based Detection of Tb 3+ and Eu 3+ in Aqueous Solution for Recovery and Urban Mining. ACS Sustain. Chem. Eng. 2022, 10, 5101–5109. [Google Scholar] [CrossRef]
- Sedykh, A.E.; Becker, M.; Seuffert, M.T.; Heuler, D.; Maxeiner, M.; Kurth, D.G.; Housecroft, C.E.; Constable, E.C.; Müller-Buschbaum, K. Air-Stable Solid-State Photoluminescence Standards for Quantitative Measurements Based on 4′-Phenyl-2,2′:6′,2′′-Terpyridine Complexes with Trivalent Lanthanides. ChemPhotoChem 2023, 7, e202200244. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimisation program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Constable, E.C.; Lewis, J.; Liptrot, M.C.; Raithby, P.R. The coordination chemistry of 4’-phenyl-2,2’:6’,2”-terpyridine; the synthesis, crystal and molecular structures of 4’-phenyl-2,2’:6’,2”-terpyridine and bis(4’-phenyl-2,2’:6’,2”-terpyridine)nickel(II) chloride decahydrate. Inorganica Chim. Acta 1990, 178, 47–54. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Reddy, P.P.; Vijayakumar, V.; Suresh, J.; Narasimhamurthy, T.; Lakshman, P.L.N. Diethyl 4-(2,4-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o363. [Google Scholar] [CrossRef]
- Metcalf, S.K.; Holt, E.M. Three methoxy-substituted diethyl 4-phenyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate compounds. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2000, 56, 1228–1231. [Google Scholar] [CrossRef]
- Rathore, R.S.; Reddy, B.P.; Vijayakumar, V.; Ragavan, R.V.; Narasimhamurthy, T. Hantzsch 1,4-dihydropyridine esters and analogs: Candidates for generating reproducible one-dimensional packing motifs. Acta Crystallogr. Sect. B Struct. Sci. 2009, 65, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Caignan, G.A.; Holt, E.M. New 1,4-dihydropyridine derivatives with C4 heterocyclic substituents. Z. Fur. Krist. 2000, 215, 122–126. [Google Scholar] [CrossRef]
- Vrabel, V.; Oktavec, D.; Marchalín, Š. 2,3-Dimethyl 5-isopropyl 1-ethoxymethyl-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-2,3,5-tricarboxylate. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, o306–o307. [Google Scholar] [CrossRef]
- Constable, E.C.; Phillips, D.; Raithby, P.R. Nickel(II) chloride adducts of 4′-phenyl-2,2′:6′,2″-terpyridine. Inorg. Chem. Commun. 2002, 5, 519–521. [Google Scholar] [CrossRef]
- Fu, W.-W.; Ye, S.-Q.; Liu, Y.; Chen, M.-S.; Kuang, D.-Z. Syntheses, crystal structures and luminescence properties of two copper(II) complexes with terpyridine and dicarboxylate ligands. Transit. Met. Chem. 2015, 40, 227–233. [Google Scholar] [CrossRef]
- Frei, A.; Rubbiani, R.; Tubafard, S.; Blacque, O.; Anstaett, P.; Felgenträger, A.; Maisch, T.; Spiccia, L.; Gasser, G. Synthesis, characterisation, and biological evaluation of new Ru(II) polypyridyl photosensitisers for photodynamic therapy Supporting Information. J. Med. Chem. 2014, 57, 7280–7292. [Google Scholar] [CrossRef]
- Cai, L.L.; Hu, Y.T.; Li, Y.; Wang, K.; Zhang, X.Q.; Muller, G.; Li, X.M.; Wang, G.X. Solid-state luminescence properties, Hirshfeld surface analysis and DFT calculations of mononuclear lanthanide complexes (Ln = EuIII, GdIII, TbIII, DyIII) containing 4′-phenyl-2,2′:6′,2″-terpyridine. Inorganica Chim. Acta 2019, 489, 85–92. [Google Scholar] [CrossRef]
- Hussain, A.; Somyajit, K.; Banik, B.; Banerjee, S.; Nagaraju, G.; Chakravarty, A.R. Enhancing the photocytotoxic potential of curcumin on terpyridyl lanthanide(III) complex formation. Dalt. Trans. 2013, 42, 182–195. [Google Scholar] [CrossRef]
- Caignan, G.A.; Holt, E.M. New 1,4-dihydropyridine derivatives with hetero and saturated B rings. J. Chem. Crystallogr. 2002, 32, 315–323. [Google Scholar] [CrossRef]
- Shashi, R.; Prasad, N.L.; Begum, N.S. One-Pot Synthesis of 1,4-Dihydropyridine Derivatives and Their X-Ray Crystal Structures: Role of Fluorine in Weak Interactions. J. Struct. Chem. 2020, 61, 938–947. [Google Scholar] [CrossRef]
- Yıldırım, S.Ö.; Akkurt, M.; Pehlivanlar, E.; Çetin, G.; Şimşek, R.; Butcher, R.J.; Bhattarai, A. Syntheses, characterizations, crystal structures and Hirshfeld surface analyses of methyl 4-[4-(difluoro-methoxy)phenyl]-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, isopropyl 4-[4-(difluoromethoxy)phenyl]-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate and tert-butyl 4-[4-(difluoromethoxy)phenyl]-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2024, 80, 281–288. [Google Scholar] [CrossRef]
- Metherall, J.P.; Corner, P.A.; McCabe, J.F.; Hall, M.J.; Probert, M.R. High-throughput nanoscale crystallisation of dihydropyridine active pharmaceutical ingredients. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2024, 80 Pt 1, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Sedykh, A.E.; Kurth, D.G.; Müller-Buschbaum, K. Phosphorescence Afterglow and Thermal Properties of [ScCl3(ptpy)] (ptpy: 4’-phenyl-2,2’,6’,2’’-terpyridine). Z. Für Anorg. Und Allg. Chem. 2021, 647, 359–364. [Google Scholar] [CrossRef]
- Turowska-Tyrk, I.; Grześniak, K.; Trzop, E.; Zych, T. Monitoring structural transformations in crystals. Part 4. Monitoring structural changes in crystals of pyridine analogs of chalcone during [2+2]-photodimerisation and possibilities of the reaction in hydroxy derivatives. J. Solid State Chem. 2003, 174, 459–465. [Google Scholar] [CrossRef]
- James, L.; Maguire, G.E.M.; Martincigh, B.S.; McKee, V.; Ndlovu, N. 3-Phenyl-1,5-di-2-pyridylpentane-1,5-dione. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, o153–o155. [Google Scholar] [CrossRef]
- Yadav, J.S.; Subba Reddy, B.V.; Sabitha, G.; Kiran Kumar Reddy, G.S. Aromatization of Hantzsch 1,4-dihydropyridines with I2-MeOH. Synthesis 2000, 1532–1534. [Google Scholar] [CrossRef]
- Shalev, O.; Shtein, M. Effect of crystal density on sublimation properties of molecular organic semiconductors. Org. Electron. 2013, 14, 94–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedykh, A.; Zhernakov, M.; Becker, M.; Kurth, D.G.; Müller-Buschbaum, K. Crystal Structure of 4′-Phenyl-1′,4′-Dihydro-2,2′:6′,2″-Terpyridine: An Intermediate from the Synthesis of Phenylterpyridine. Crystals 2025, 15, 619. https://doi.org/10.3390/cryst15070619
Sedykh A, Zhernakov M, Becker M, Kurth DG, Müller-Buschbaum K. Crystal Structure of 4′-Phenyl-1′,4′-Dihydro-2,2′:6′,2″-Terpyridine: An Intermediate from the Synthesis of Phenylterpyridine. Crystals. 2025; 15(7):619. https://doi.org/10.3390/cryst15070619
Chicago/Turabian StyleSedykh, Alexander, Maksim Zhernakov, Mariia Becker, Dirk G. Kurth, and Klaus Müller-Buschbaum. 2025. "Crystal Structure of 4′-Phenyl-1′,4′-Dihydro-2,2′:6′,2″-Terpyridine: An Intermediate from the Synthesis of Phenylterpyridine" Crystals 15, no. 7: 619. https://doi.org/10.3390/cryst15070619
APA StyleSedykh, A., Zhernakov, M., Becker, M., Kurth, D. G., & Müller-Buschbaum, K. (2025). Crystal Structure of 4′-Phenyl-1′,4′-Dihydro-2,2′:6′,2″-Terpyridine: An Intermediate from the Synthesis of Phenylterpyridine. Crystals, 15(7), 619. https://doi.org/10.3390/cryst15070619







