Abstract
Lead–bismuth eutectic alloy (LBE, Pb44.5Bi55.5) has emerged as a promising candidate for use in advanced nuclear and solar energy systems due to its favorable thermophysical characteristics and radiation shielding capabilities. The aim of this research is to assess the applicability of the induction melting technique to synthesize LBE. This paper presents a comprehensive evaluation of the structural, thermophysical, and radiation shielding properties of the obtained LBE sample. Various techniques were employed to investigate the solid-to-liquid eutectic transformation, phase composition, morphology, and homogeneity of the obtained material. Experimental and theoretical determinations on density, void, molar volume, thermal conductivity, heat capacity, thermal diffusivity, and electrical conductivity were performed. Radiation shielding performance over photon energies ranging from 0.015 to 15 MeV was simulated using the Phy-X/PSD program. The results revealed the eutectic structure comprising Pb7Bi3 and Bi phases with near-ideal stoichiometry and a melting point of 127.6 °C. The alloy demonstrated a small void that corresponds to a high degree of sample compaction, high specific heat capacity, moderate thermal conductivity, low thermal diffusivity, and effective radiation shielding. These findings confirm that LBE obtained by the induction melting technique possesses the necessary structural stability and functional properties for integration into nuclear reactor and solar thermal technologies.
1. Introduction
Liquid-metal coolants have been investigated for several decades as potential heat transfer fluids (HTFs) in both nuclear applications, such as nuclear power plants and submarine propulsion, and renewable energy systems such as concentrated solar power, (CSP) owing to their favorable thermophysical properties [1]: high volumetric heat capacity, excellent thermal conductivity, suitable thermal expansion coefficient, and low chemical reactivity with other coolants [2,3,4,5].
With the increasing global demand for sustainable energy, interest in advanced nuclear technologies, such as Generation IV Fast Reactors, which employ non-water coolants, has made liquid-metal coolants a focal point of international research. Lead–bismuth eutectic (LBE) serves as a cooling agent in fast reactors (LFRs) and is a promising candidate for accelerator-driven subcritical (ADS) reactors [6,7]. Additionally, research on liquid-metal coolants as high-temperature HTFs in concentrated solar power (CSP) and thermal energy storage systems has grown significantly in recent years [8,9,10].
Concentrated solar power systems offer a viable solution to meet global electricity demands while reducing greenhouse gas emissions. In these systems, concentrated solar radiation is transferred through a heat exchanger to an HTF, which drives a thermodynamic cycle. CSP systems can achieve high temperatures, enabling more efficient thermodynamic cycles and reducing power generation costs [11]. Additionally, HTFs operating above 900 °C can facilitate direct hydrogen synthesis from water. By integrating CSP with thermal or hydrogen storage, continuous energy production is feasible, ensuring a stable electricity supply during nighttime [12]. However, the performance of CSP systems relies on developing HTFs capable of operating at high temperatures.
Conventional fluids, such as water or commercial molten salts [13,14,15,16], cannot withstand these elevated temperatures. Consequently, liquid metals, including sodium (Na), lead (Pb), and lead–bismuth eutectic (LBE), are considered promising HTFs for CSP systems. These liquid metals exhibit desirable properties: (i) low melting points and high boiling points, enabling a wide operating temperature range; (ii) high thermal conductivity and heat capacity, ensuring efficient heat transfer; and (iii) low viscosity and thermal expansion, reducing pumping power requirements [17,18]. Also, several studies have been reported on the synthesis and thermophysical properties of different alloys as liquid-metal coolants [19,20].
Previous reports have theoretically studied some of the thermophysical and shielding properties of LBE [21,22,23]; however, few have addressed the experimental synthesis, structural characterization, and simultaneous evaluation of the thermophysical and radiation shielding performance of LBE. Notably, most studies lack a detailed description of the sample preparation process, especially in the solid state, which significantly hampers the reproducibility and interpretation of experimental results [24].
The novelty of this study is the synthesis of LBE via induction melting, a technique that provides advantages such as uniform heating and reduced contamination compared to conventional melting methods. This method is particularly effective for obtaining LBE, which is highly sensitive to alloy stoichiometry and requires precise composition control to ensure the formation of the compound.
This study focuses on lead–bismuth eutectic (LBE), an alloy with a composition of Pb44.5Bi55.5, as indicated by its phase diagram in Figure 1 [25].
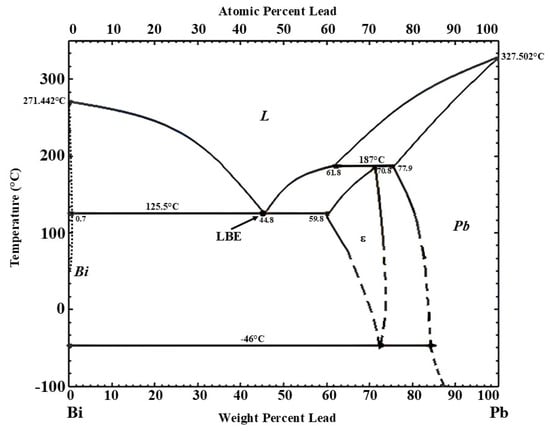
Figure 1.
Lead–bismuth phase diagram [25].
The primary advantage of LBE as an HTF lies in its significant temperature range, with a low melting point of 125 °C and a high boiling point of 1670 °C, making it suitable for diverse applications. This work details the synthesis of LBE via induction melting, alongside a comprehensive analysis of its structural and thermophysical properties.
Furthermore, among different alloy systems, lead–bismuth alloy in solid state has attained significant interest due to its interesting electronic, superconducting [26], and radiation shielding properties [27]. In nuclear reactors operated at high temperatures, radiation shielding is a crucial property. Both lead and LBE are effective gamma-ray shielding materials and can adsorb and suppress fission products, especially some volatile fission products and actinides [28,29].
A comprehensive evaluation of the structural and thermophysical properties of Pb-Bi alloy provides promising avenues for its application prospects.
2. Materials and Methods
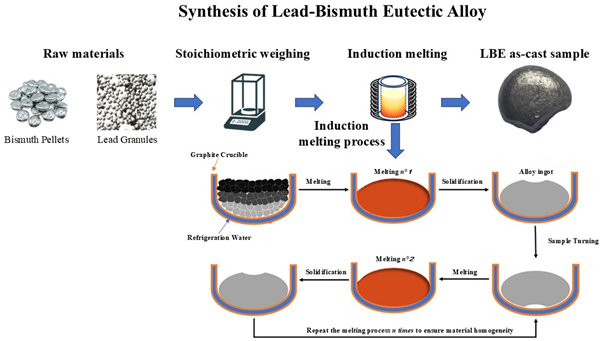
The Pb-Bi alloy in eutectic composition Pb44.5Bi55.5 was prepared by induction melting to reduce the defects including void and cracking, essential parameters for the thermophysical properties of materials. Elemental constituents Pb and Bi with high purity (>99.9%) were used as starting materials. The materials were weighed in the specified weight ratio (44.5:55.5) and melted in a graphite crucible under vacuum conditions (10−4 mbar). To ensure homogeneity, the sample was turned and re-melted 6 times. The as-cast sample was obtained by cooling the crucible using a water-cooling system, with water temperature at approximately 5 °C, until the sample reached room temperature. This process resulted in a mass loss of less than 1%. The experimental conditions for the induction melting process are summarized in Table 1. A schematic representation of the alloy synthesis process is shown in the below figure, illustrating the sequence of weighing, melting, mixing, and cooling steps.

Table 1.
The experimental conditions for the induction melting process.
The Brüker D8 ADVANCE diffractometer, with CuKα radiation and wavelength of 1.54 Å, was used to analyze the crystal structure of the alloy. The XRD measurements were performed at a source voltage of 40 kV and a current of 40 mA. The sample was scanned over a 2θ range of 20–90° with a step size of 0.03° and a scanning time of 2 s per step. This scanning time was chosen to optimize the signal-to-noise ratio, ensuring sufficient data quality for the accurate determination of peak positions and intensities while maintaining reasonable measurement duration.
The POWDER CELL program was used for indexing the diffraction patterns [30]. To compare the results obtained from XRD measurements, the stoichiometry of the sample was investigated using X-ray analysis by energy dispersion (EDX). At the same time, the homogeneity of the sample was estimated from Scanning Electron Microscopy (SEM) measurements.
Differential thermal analysis (DTA) was carried out under Ar atmosphere to study structural transformations and to determine the melting point of the investigated sample. The DTA measurement was performed with a TA Instruments Q600 in the temperature range 100–250 °C and the heating rate was fixed at 10 °C/min.
The Hot Disk TPS 2500S R&D instrument was used to measure the thermal transport properties of the investigation sample. The instrument employs the transient plane source (TPS) technique for thermal characterization. It measures thermal conductivity, thermal diffusivity, and specific heat capacity with reproducibility better than 2% for thermal conductivity and 5% for thermal diffusivity and specific heat capacity.
Triplicate measurements were performed at room temperature. The electrical conductivity (σ) was deduced from thermal conductivity value according to the Wiedemann–Franz law:
where k, L, and T are thermal conductivity, Lorenz number (2.443 × 10−8 W·Ω·K−2), and absolute temperature (K) [16].
k/σ = L·T; σ = k/(L∙T)
Photon Shielding and Dosimetry online software https://phy-x.net/PSD (accessed on 30 April 2025) was used for theoretical determination of various shielding parameters: mass and linear attenuation coefficients, attenuation coefficient (MAC, LAC), half value layer (HVL), tenth value layer (TVL), and mean free path (MFP). The effectiveness of Pb-Bi alloy for attenuating radiation was tested in the energy range 0.015–15 MeV.
3. Results and Discussion
3.1. Structure and Stoichiometry of LBE
The X-ray diffraction pattern for eutectic Pb-Bi alloy obtained by induction melting is shown in Figure 2.
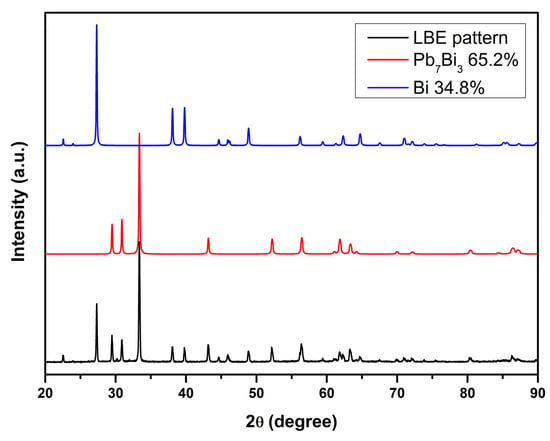
Figure 2.
XRD pattern for Pb-Bi alloy.
By indexing the characteristic reflections, the sample crystallizes in a mixture of two structural phases, corresponding to the ε-Pb7Bi3 and Bi structures in accordance with the phase diagram of the binary Pb-Bi. The ε-Pb7Bi3 (hexagonal crystal structure) and Bi (trigonal crystal structure) phases, identified by XRD, confirm the formation of the eutectic Pb-Bi alloy.
The lattice parameters of both structural phases, as well as the concentration values obtained from XRD pattern refinement, are presented in Table 2. The literature values of the lattice parameters are provided for comparison. Using the concentration values of the Pb7Bi3 and Bi phases, the stoichiometry of the sample was estimated. The stoichiometry determined from XRD diffractogram refinements was Pb45.64Bi54.36. A slight deviation from the ideal stoichiometry (Pb44.5Bi55.5) is attributed to measurement errors and possible evaporation of elements during the melting process.

Table 2.
The concentration values, lattice parameters, and stoichiometry obtained from XRD measurement. For comparison, the lattice parameters from the literature are given.
The sample was investigated by EDX spectroscopy in order to compare the stoichiometry from the XRD results. The EDX measurements of the Pb-Bi alloy are presented in Figure 3 and the microscopic surface morphology is shown in Figure 4a. The quantity for each element of the EDX measurement is reported in atomic percentages. As can be seen, the stoichiometry indicated by EDX measurement and that derived by XRD measurement are comparable. The slight difference can be attributed to experimental errors between the two methodologies. The variation from perfect stoichiometry for both situations is less than 4%, which is well within the allowable measurement errors. We examined the distribution of each component element of the studied Pb-Bi sample by using the SEM method. Figure 4b shows that the distribution of each element is uniform. This validates the homogeneity of the material obtained by the induction melting method.
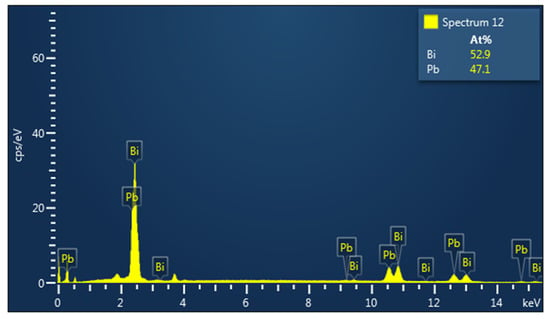
Figure 3.
EDX spectra of Pb-Bi alloy.
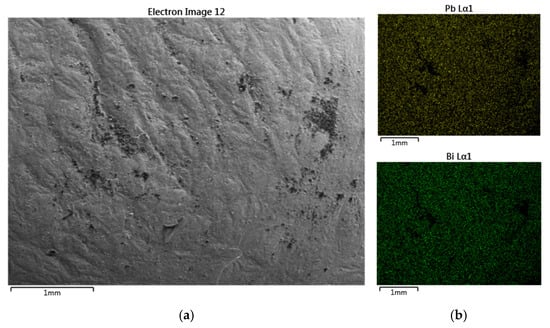
Figure 4.
(a) Microscope surface morphology of Pb-Bi alloy; (b) SEM images and mapping of Pb and Bi.
Figure 5 presents the DTA plot of the Pb-Bi alloy. Within the measured temperature range, a single endothermic transition is observed on the DTA curve.
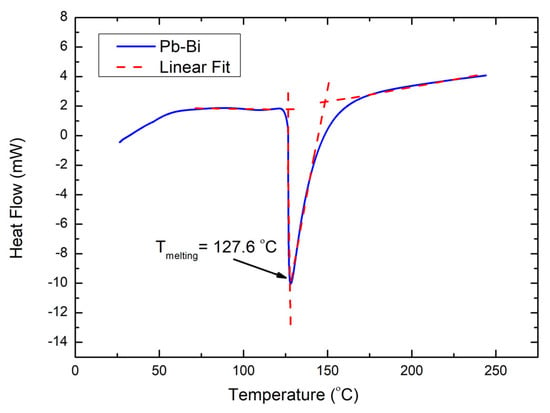
Figure 5.
DTA curve for Pb-Bi sample.
The temperature of this transition, consistent with the phase diagram, indicates a solid-to-liquid eutectic transformation of the Pb-Bi alloy. As a result, the melting point calculated using the tangent approach [33] is 127.6 °C. This value is close to the eutectic temperature of 125.5 °C reported in the phase diagram for the Pb-Bi binary system [25]. The experimentally obtained melting point confirms that the stoichiometry of the studied sample is close to the ideal value of the Pb-Bi eutectic.
3.2. Thermophysical Properties of LBE
The experimental density (ρ) of the Pb-Bi alloy was evaluated using Archimedes’ Principle using Equation (2). The experiments were performed at room temperature in distilled water on five distinct samples.
where ρi, Wa, and Wl represent density of the immersion fluid and weight of the sample in air and in immersion fluid, respectively.
The theoretical density is calculated by the following equation:
where wti% is the weight percentage, and ρi is the densities of the constituents, respectively.
The percentage of voids in the Pb-Bi alloy was determined by the following relation:
The molar volume (Vm) was estimated according to the following formula:
where ρ is the density and M represents the molecular weight of the sample.
The experimental density and Vm determined for the Pb-Bi sample with the values 10.45 g/cm3 and 19.86 cm3/ mole, respectively, are found to be similar to previously reported results for LBE in the solid state obtained by the conventional melting technique [2,34]. At the same time, the small voids value, 0.19%, shows a small deviation from the theoretical density value, 10.47 g/cm3, indicating a high degree of LBE compaction obtained by the induction melting method.
Thermal conductivity, thermal diffusivity, specific heat capacity, and electrical conductivity for the Pb-Bi alloy recorded under ambient temperature are plotted in Figure 6.
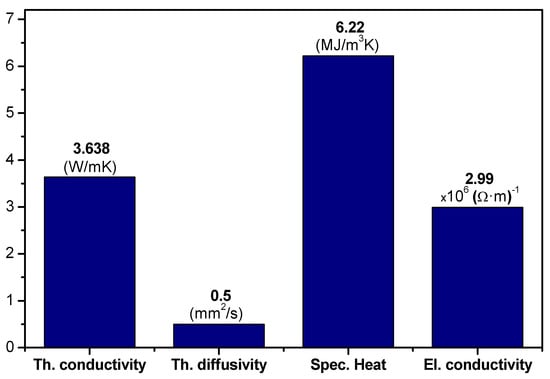
Figure 6.
Thermal parameters and electrical conductivity of Pb-Bi alloy.
The thermal conductivity of the Pb-Bi alloy was found to be 3.638 W/(mK) at room temperature. The heat capacity of the Pb-Bi alloy sample is significantly high, resulting in reduced thermal diffusivity. Low thermal diffusivity impedes temperature changes and delays heat propagation [35]. The heat transfer properties of LBE make it a promising HTF for concentrated solar power (CSP) systems, where efficient heat transfer at temperatures exceeding 900 °C is critical for optimizing thermodynamic cycles. The estimated value of electrical conductivity of Pb-Bi alloys was found to be 2.99 × 106 (Ωm)−1, significantly less than that of pure Pb, which is 4.55 × 106 (Ωm)−1.
3.3. Radiation Shielding Properties of LBE
The effectiveness of the Pb-Bi alloy in attenuating radiation was tested in the energy range 0.015–15 MeV using Phy-X/PSD software [36].
The mass attenuation coefficient (MAC) (cm2/g) is a critical parameter for evaluating the radiation shielding properties of a material. The theoretical MAC values for the Pb-Bi alloy are plotted in logarithmic-scale as a function of energy in Figure 7a.
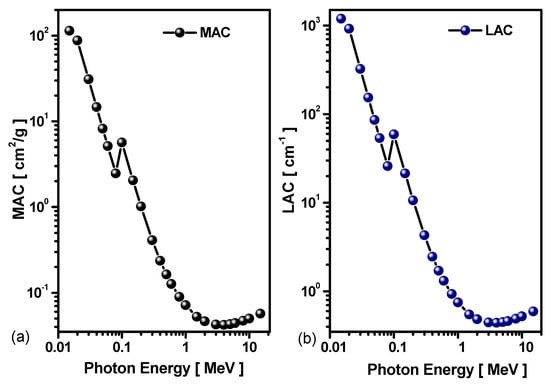
Figure 7.
(a) MAC and (b) LAC of Pb-Bi alloy.
The results indicate that the MAC values decreased exponentially with increasing photon energy from 114.01cm2/g at 0.015 MeV to 2.476 cm2/g at 0.08 MeV and attained a minimum value of 0.0422 at 4 MeV due to photon interaction mechanisms with matter. At low energies, E < 0.1 MeV, where the photoelectric effect predominates [37], MAC demonstrates enhanced shielding capacity. The MAC increases abruptly at energies slightly above the binding energies for K- and L-shell electrons of the constituent atoms (summarized in Table 3), around 0.1 MeV. In the 0.3–4 MeV energy range, MAC values decrease rapidly with increasing energy, as the Compton scattering effect becomes the main interaction process.

Table 3.
K- and L-shell absorption edge for Bi and Pb elements.
At high energies, E ≥ 4 MeV, electron/position pair production process becomes significant, and the MAC is low and almost constant. A higher MAC at a given energy demonstrated more effective attenuating photons at that energy. The LBE sample is therefore an effective shielding material for low energy radiation applications.
Another critical parameter for studying the gamma-ray interaction with materials is the linear attenuation coefficient, LAC (μ). Figure 8b) shows the variation in LAC (μ) with photon energy for the Pb-Bi alloy, within the 0.015–15 MeV energy range. The LAC values decrease nearly linearly from 1191.38 to 0.422 cm−1 with increasing photon energy from 0.015 to 4 MeV, then slowly rise from 0.429 to 0.569 cm−1 at higher energy. Additionally, at photon energy around 0.08 MeV, a sudden jump appears. As can be seen, the LAC exhibits changes across different energy regions, similar to the behavior of the MAC, which can be correlated through the same photon–matter interaction processes.
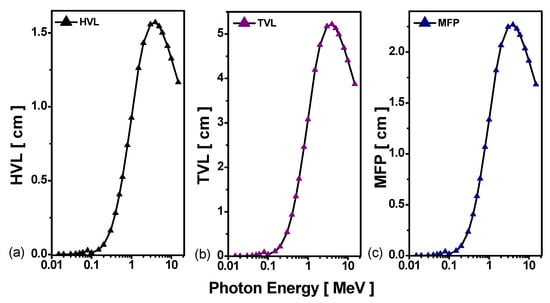
Figure 8.
The (a) HVL, (b) TVL, and (c) MFP of the Pb-Bi alloy.
Half value layer (HVL), tenth value layer (TVL), and mean free path (MFP) are essential parameters in the evaluation of the gamma-ray shielding properties of materials [38]. These parameters allow for determining the thickness of shielding material needed to reduce the initial radiation intensity to certain levels: 50%, 90%, and 64.2%, respectively. The low HVL, TVL, and MFP values reveal the superior radiation attenuation properties of the material [39].
The simulated HVL, TVL, and MFP parameters are displayed in Figure 8.
It is found that as the photon energy increases, the HVL, TVL, and MFP values increase, the probability of interactions decreases, and therefore the required material thickness rises. The maxima of HVL, TVL, and MFP thickness are found at 4 MeV, with values of 1.571 cm for HVL, 5.218 cm for TVL, and 2.266 cm for MFP. Again, these results show that LBE is the most effective for shielding at low energies, i.e., E < 0.1 MeV.
4. Conclusions
This study synthesized a homogeneous Pb-Bi eutectic alloy (Pb44.5Bi55.5) using induction melting. The crystal structure, confirmed as Pb7Bi3 and Bi phases, and stoichiometry were validated. The melting point (~125.5 °C) aligned with reported values, and thermophysical properties (thermal conductivity, diffusivity, specific heat) were measured, supporting the alloy’s suitability for high-temperature applications like lead-cooled fast reactors (LFRs) and concentrated solar power (CSP) systems due to its wide temperature range.
Electrical conductivity suggests potential in electronics. The alloy’s density and simulated radiation shielding properties indicate effectiveness for nuclear components and protection systems.
These findings confirm the reliability of the induction melting method for producing high-quality Pb-Bi eutectic alloys with desirable structural, thermophysical, and radiation shielding properties. The results are intended to provide reference data for future design and implementation of LBE in both nuclear and solar thermal systems.
Author Contributions
Conceptualization, R.C.G. and V.R.; methodology, R.C.G., M.Z. and V.R.; validation, R.C.G. and V.R.; investigation, R.C.G., E.S. and R.H.; data curation, R.C.G., M.Z. and V.R.; writing—original draft preparation, R.C.G. and M.Z.; writing—review and editing, R.C.G., M.Z. and V.R.; visualization, R.C.G., E.S., R.H., M.Z. and V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education and Scientific Research through the Nucleu Programme within the National Plan for Research Development and Innovation Plan 2022–2027, Project No. 27N/3 January 2023, component project code PN 23 24 02 01.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the technical support provided by Teodora Maria RADU (I.N.C.D.T.I.M. Cluj-Napoca) in the thermal conductivity, thermal diffusivity, and specific heat capacity measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, W.; Yang, C.; You, Y.; Yin, H. A Review of Corrosion Behavior of Structural Steel in Liquid Lead–Bismuth Eutectic. Crystals 2023, 13, 968. [Google Scholar] [CrossRef]
- Amer, T.Z.; Saleh, S.E.; El Shazly, R.M.; Gomma, N.S.; Bahgat, A.A. Study of the physical and nuclear properties of liquid PbBiCd alloy coolant in nuclear fast reactor. J. Nucl. Mater. 2019, 522, 226–235. [Google Scholar] [CrossRef]
- Gong, X.; Short, M.P.; Auger, T.; Charalampopoulou, E.; Lambrinou, K. Environmental degradation of structural materials in liquid lead and lead-bismuth eutectic-cooled reactors. Prog. Mater. Sci. 2022, 126, 100920. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Q.; Tan, J.; Wu, X.; Ma, H.; Zhang, Z.; Ren, Q.; Han, E.-H.; Wang, X. Corrosion behavior of T91 steel in liquid lead-bismuth eutectic at 550 °C: Effects of exposure time and dissolved oxygen concentration. Corros. Sci. 2022, 204, 110405. [Google Scholar] [CrossRef]
- Qiu, J.; Han, J.; Schoell, R.; Popovic, M.; Ghanbari, E.; Kaoumi, D.; Scully, J.R.; Macdonald, D.D.; Hosemann, P. Electrical properties of thermal oxide scales on pure iron in liquid lead–bismuth eutectic. Corros. Sci. 2020, 176, 109052. [Google Scholar] [CrossRef]
- Xue, H.; Xu, P.; Yu, G.; Guo, Y.; Liao, Z.; Chen, Z. Determination of 21 Po generation from lead–bismuth eutectic irradiated with neutrons. J. Radioanal. Nucl. Chem. 2023, 332, 1345–1351. [Google Scholar] [CrossRef]
- Montanari, R.; Varone, A.; Gregoratti, L.; Kaciulis, S.; Mezzi, A. Lead-Bismuth Eutectic: Atomic and Micro-Scale Melt Evolution. Materials 2019, 12, 3158. [Google Scholar] [CrossRef]
- Wang, G.; Pang, S.; Jiang, T. A brief review of liquid heat transfer materials used in concentrated solar power systems and thermal energy storage devices of concentrated solar power systems. Eng. Rep. 2023, 5, e12576. [Google Scholar] [CrossRef]
- Wang, G.; Wang, T. Effect Evaluation of Filling Medium Parameters on Operating and Mechanical Performances of Liquid Heavy Metal Heat Storage Tank. Sustainability 2022, 14, 14551. [Google Scholar] [CrossRef]
- Popović, M.P.; Olmsted, D.L.; Bolind, A.M.; Asta, M.; Sohn, S.; Schroers, J.; Shao, R.; Hosemann, P. A study of the effects of minor additives to Pb-Bi eutectic: Designing novel Pb-Bi-X liquid alloys for heat transfer applications. Mater. Des. 2018, 159, 240–251. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, S.; An, B.; Dai, X. Performance and parameter sensitivity comparison of CSP power cycles under wide solar energy temperature ranges and multiple working conditions. Energy Convers. Manag. 2020, 218, 112996. [Google Scholar] [CrossRef]
- Lorenzin, N.; Abánades, A. A review on the application of liquid metals as heat transfer fluid in Concentrated Solar Power technologies. Int. J. Hydrogen Energy 2016, 41, 6990–6995. [Google Scholar] [CrossRef]
- de Figueiredo Luiz, D.; Boon, J.; Rodriguez, G.O.; van Sint Annaland, M. Review of the molten salt technology and assessment of its potential to achieve an energy efficient heat management in a decarbonized chemical industry. Chem. Eng. J. 2024, 498, 155819. [Google Scholar] [CrossRef]
- Caraballo, A.; Galán-Casado, S.; Caballero, Á.; Serena, S. Molten salts for sensible thermal energy storage: A review and an energy performance analysis. Energies 2021, 14, 1197. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xinhai, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat transfer fluids for concentrating solar power systems—A review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Pacio, J.; Wetzel, T. Assessment of liquid metal technology status and research paths for their use as efficient heat transfer fluids in solar central receiver systems. Sol. Energy 2013, 93, 11–22. [Google Scholar] [CrossRef]
- Flesch, J.; Niedermeier, K.; Fritsch, A.; Musaeva, D.; Marocco, L.; Uhlig, R.; Baake, E.; Buck, R.; Wetzel, T. Liquid metals for solar power systems. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 228, p. 012012. [Google Scholar]
- Gancarz, T. The thermophysical properties of Bi-Ga alloys. J. Mol. Liq. 2022, 363, 119912. [Google Scholar] [CrossRef]
- Novakovic, R.; Giuranno, D.; Lee, J.; Mohr, M.; Delsante, S.; Borzone, G.; Miani, F.; Fecht, H.-J. Thermophysical Properties of Fe-Si and Cu-Pb Melts and Their Effects on Solidification Related Processes. Metals 2022, 12, 336. [Google Scholar] [CrossRef]
- Saad, M.; ALMohiy, H.; Alqahtani, M.S.; Alshihri, A.A.; Shalaby, R.M. Study of structural, physical, characteristics and radiation shielding parameters of Bi50-Pb40-Sn10 and Bi40-Pb40-Sn10-Cd10 alloys used for radiation therapy. Radiat. Eff. Defects Solids 2022, 177, 545–555. [Google Scholar] [CrossRef]
- Herreros, A.F. Overview and History of Lead and Lead-Bismuth Fast Reactors (LFRs); Stanford University: Stanford, CA, USA, 2024. [Google Scholar]
- Grauer, M.; Benndorf, C.; Rohr, V.; Paulmann, C.; Oeckler, O. Investigations of Some Disordered Quaternary Compounds in the Systems Ag/Pb/Sb/Se and Ag/Pb/Sb/Te. Crystals 2024, 14, 789. [Google Scholar] [CrossRef]
- Agazhanov, A.S.; Stankus, S.V.; Savchenko, I.V.; Samoshkin, D.A. Thermal conductivity of lead and bismuth-lead eutectic melts up to 1300 K. Nucl. Eng. Des. 2024, 423, 113166. [Google Scholar] [CrossRef]
- Concetta, F.; Sobolev, V.P.; Aerts, A.; Gavrilov, S.; Lambrinou, K.; Schuurmans, P.; Gessi, A.; Agostini, P.; Ciampichetti, A.; Martinelli, L.; et al. Handbook on Lead-Bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermal-Hydraulics and Technologies; OECD/NEA: Paris, France, 2015. [Google Scholar]
- Wei, Y.; La, P.; Zheng, Y.; Zhan, F.; Yu, H.; Yang, P.; Zhu, M.; Bai, Z.; Gao, Y. Review of Molten Salt Corrosion in Stainless Steels and Superalloys. Crystals 2025, 15, 237. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Siddiqui, S.; Sreedhar, I.; Parameshwaran, R. Molten salts: Potential candidates for thermal energy. Int. J. Energy Res. 2022, 46, 17755–17785. [Google Scholar] [CrossRef]
- Jo, S.; Kim, S.; Cho, J. A Fast Variance Reduction Technique for Efficient Radiation Shielding Calculations in Nuclear Reactors. Energies 2024, 17, 5695. [Google Scholar] [CrossRef]
- Xing, M.; Fan, J.; Shen, F.; Lu, D.; Li, L.; Yu, H.; Fan, J. Comparative Analysis on the Characteristics of Liquid Lead and Lead–Bismuth Eutectic as Coolants for Fast Reactors. Energies 2025, 18, 596. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A programfor the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Rasmussen, S.E.; Lundtoft, B. Crystal data for Pb7Bi3, a superconducting ε-phase in the Pb-Bi system. Powder Diffr. 1987, 2, 28. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystal Structures; Interscience Publishers: New York, NY, USA, 1963; Volume 1. [Google Scholar]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Morita, K.; Maschek, W.; Flad, M.; Yamano, H.; Tobita, Y. Thermophysical Properties of Lead-Bismuth Eutectic Alloyin Reactor Safety Analyses. J. Nucl. Sci. Technol. 2006, 43, 526–536. [Google Scholar] [CrossRef]
- Xiong, F.; Zhou, J.; Jin, Y.; Zhang, Z.; Qin, M.; Han, H.; Shen, Z.; Han, S.; Geng, X.; Jia, K.; et al. Thermal shock protection with scalable heat-absorbing aerogels. Nat. Commun. 2024, 15, 7125. [Google Scholar] [CrossRef] [PubMed]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar] [CrossRef]
- Zagrai, M.; Suciu, R.-C.; Gavrea, R.C.; Rednic, V. Evolution of the Radiation Shielding, Optical, and Luminescence Properties of PbO2-SiO2 Glass Systems and the Influence of Rare Earth Elements (Eu, Ce, Yb). Appl. Sci. 2025, 15, 2076–3417. [Google Scholar] [CrossRef]
- Yin, S.; Wang, H.; Wang, S.; Zhang, J.; Zhu, Y. Effect of B2O3 on the Radiation Shielding Performance of Telluride Lead Glass System. Crystals 2022, 12, 178. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S.; Saleh, E.E.; Alresheedi, F. Synthesis of Novel Li2O-CuO-Bi2O3-B2O3 Glasses for Radiation Protection: An Experimental and Theoretical Study. Inorganics 2023, 11, 27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).