1. Introduction
Zinc-ion batteries (ZIBs) offer a promising alternative to lithium-ion systems, thanks to their safety, low cost, and use of aqueous electrolytes [
1,
2]. Using zinc metal anodes enables high-capacity and stable cycling, but developing effective cathode materials remains challenging. Vanadium-based oxides stand out for their multi-electron redox behavior, structural tunability, and efficient Zn
2+ intercalation. Among various candidates, vanadium-based oxides and vanadates stand out for their high redox activity involving multiple oxidation states (V
3+/V
4+/V
5+), enabling high Zn
2+ storage capacities through both intercalation and pseudocapacitive processes [
3,
4,
5]. When synthesized from metal–organic frameworks (MOFs), these compounds often exhibit nanoscale or porous morphologies that promote rapid ion diffusion and efficient charge transfer [
6,
7,
8]. During thermal treatment, the MOF’s organic ligands can convert into nitrogen-doped carbon, enhancing the electrical conductivity and supporting high-rate performance. Additionally, MOF-derived vanadates offer improved structural stability, helping to suppress vanadium dissolution and mechanical degradation during repeated cycling. The resulting carbon-integrated architecture not only stabilizes the active material but also contributes to long-term cycling durability. For example, recent studies have demonstrated the effectiveness of V
2O
5 nanoplates, VO
2 composites, and ZnV
2O
4 spinels in achieving capacities above 300 mAh/g under moderate cycling conditions. These systems, however, often suffer from vanadium dissolution, phase instability, or sluggish ion diffusion when used in thick electrode formats [
6].
To overcome these challenges, MOF-derived vanadium oxides have emerged as a powerful strategy, combining structural tunability, porosity, and in situ carbon formation. MOF-derived materials such as V
2O
5@C, VO
x@NC, and ZnV
2O
4/C composites have demonstrated enhanced rate capabilities and cycling durability, as the carbon matrix provides electronic conductivity and buffers volume changes [
7,
8,
9]. For instance, Ding et al. synthesized V-MOF-derived V
2O
5 nanoplates with superior capacity retention, while Wu et al. developed MOF-driven vanadium–carbon hybrids with tailored interfaces to improve the diffusion kinetics [
5,
6].
Zeolitic imidazolate framework 8 (ZIF-8), a zinc-based MOF, is particularly attractive due to its high thermal stability, uniform porosity, and ability to form nitrogen-doped carbon upon annealing [
9]. Its decomposition not only provides a conductive matrix but also allows the spatial confinement of active phases, leading to enhanced particle dispersion and a preserved nanoscale morphology [
10,
11]. This approach yields materials with controlled morphologies, improved conductivity, and stable frameworks—key features for efficient Zn
2+ storage and long-term cycling stability. Although many studies have focused on V
2O
5 or VO
2 derived from MOFs, investigations into MOF-derived V
2O
3 are still limited. V
2O
3 offers unique advantages, such as a lower working voltage and stronger intercalation pseudocapacitance, making it a compelling candidate for high-rate Zn-ion storage [
12,
13].
Here, we fabricated the MOF-derived (V2O3/carbon) V2O3/C composite cathode. ZIF-8 serves multiple valuable roles in the synthesis of V2O3-based composites, even when the formation of Zn-containing phases like ZnV2O4 is absent. During annealing, the organic linkers in ZIF-8 (2-methylimidazole) undergo carbonization, producing a porous nitrogen-doped carbon matrix. This carbon structure confines the growth of V2O3 particles, prevents agglomeration, and enhances the overall electrical conductivity, which is particularly beneficial for applications in batteries and catalysis. The high surface area and uniform distribution of metal ions in ZIF-8 provide a templating effect, enabling the formation of uniformly dispersed V2O3 nanocrystals with a controlled morphology and reduced particle size. These nanoscale features contribute to improved reactivity and enhanced electrochemical performance.
In energy storage systems such as Zn-ion, Li-ion, or Na-ion batteries, V2O3/carbon composites derived from ZIF-8 often demonstrate superior cycling stability, attributed to the carbon matrix buffering the volume changes during charge/discharge. The conductive carbon network also facilitates faster ion and electron transport, leading to better rate capabilities. Moreover, the robust carbon framework derived from ZIF-8 improves the structural stability by mitigating the degradation and agglomeration of V2O3 during repeated cycling or catalytic reactions. ZIF-8 effectively functions as a sacrificial template, a carbon source, and a nanoscale reactor, making it a highly advantageous precursor for the synthesis of V2O3 with an improved microstructure, conductivity, and long-term performance.
In this study, we synthesized a V2O3/carbon composite by mixing ZIF-8 with NH4VO3 and annealing the mixture at 800 °C. Although ZnV2O4 was not formed, the decomposition of ZIF-8 produced a porous, nitrogen-doped carbon matrix that confined V2O3 particles and enhanced the conductivity. The resulting composite was fabricated into a cathode using AC-EPD and tested in an aqueous zinc-ion battery. Electrochemical analysis, including scan rate-dependent CV, revealed favorable kinetics with mixed capacitive and diffusion-controlled charge storage behavior.
3. Results and Discussion
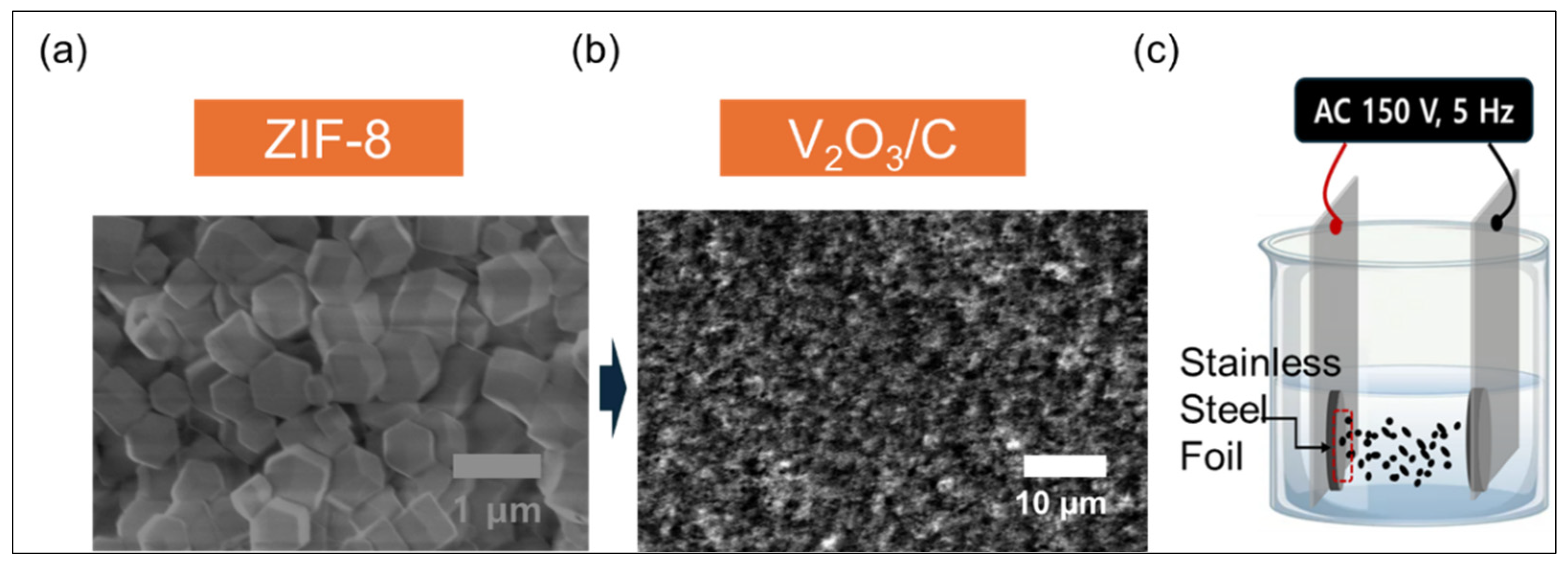
Figure 1 illustrates the synthesis process of a V
2O
3/carbon (V
2O
3/C) composite using ZIF-8 and NH
4VO
3 as precursors, accompanied by scanning electron microscopy (SEM) images that capture the morphological evolution during the transformation.
Figure 1a shows the as-synthesized ZIF-8, appearing as a white crystalline powder. The corresponding SEM image reveals well-defined polyhedral particles with smooth surfaces and sharp edges, characteristic of the highly crystalline and uniform morphology typical of ZIF-8. This ordered structure serves as an excellent sacrificial template for the production of nanostructured composites. The schematic shows that ZIF-8 was mixed with NH
4VO
3 and subsequently annealed at 800 °C under an argon atmosphere. During this thermal treatment, ZIF-8 decomposed, yielding a nitrogen-doped porous carbon matrix, while NH
4VO
3 thermally decomposed to generate vanadium oxides. The solid-state reaction between the components led to the formation of a V
2O
3 phase embedded within a conductive carbon framework. Due to high-temperature processing, volatile Zn species likely evaporated, resulting in the absence of ZnV
2O
4 in the final product.
Figure 1b shows the annealed product as a fine black powder, indicating successful carbonization and vanadium oxide formation. The accompanying SEM image reveals a drastic change in morphology compared to the original ZIF-8. The polyhedral shape disappears, giving way to a rough, fine-grained surface composed of nanoscale V
2O
3 particles uniformly dispersed in the carbon matrix.
Figure 1c illustrates the fabrication of a thin, uniform cathode layer using an AC-EPD method [
14,
15,
16]. A stainless steel (SS) substrate was coated with a V
2O
3/C composite film by applying an AC field of 150 V at 5 Hz, which promoted homogeneous deposition and minimized particle aggregation. Following deposition, the film was annealed at 200 °C for one hour under an argon atmosphere to strengthen particle adhesion and enhance the film’s structural integrity. The use of an alternating current during AC-EPD plays a crucial role in producing a smooth, crack-free film with a consistent thickness across the substrate surface. Such controlled deposition enables excellent interface contact between the active material and electrolyte, which is critical for efficient ion transport and charge transfer. The resulting ~300 nm film offers high surface accessibility and a stable electrode architecture, contributing to an improved capacity, superior rate capabilities, and a prolonged cycling life in zinc-ion battery systems.
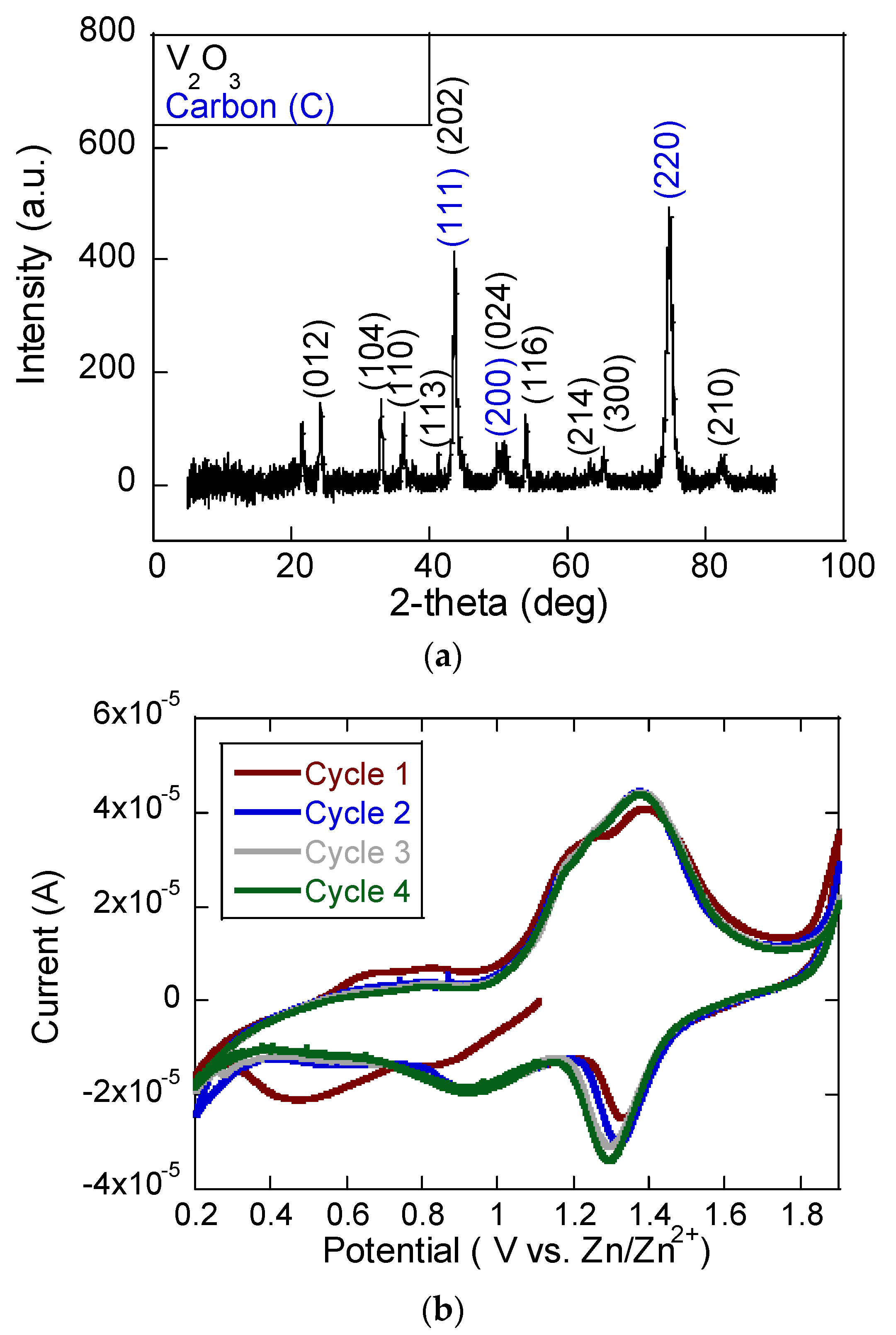
The XRD pattern in
Figure 2a presented for the V
2O
3/C composite confirms the successful formation of crystalline V
2O
3 alongside the presence of carbon. The sharp and well-defined diffraction peaks correspond to the rhombohedral phase of V
2O
3, with characteristic reflections indexed to planes such as (012), (104), (110), (113), (202), (024), (116), (214), (300), and (210). These peak positions and intensities align well with the standard JCPDS data (no. 34-0187) for V
2O
3, indicating the good crystallinity of the vanadium oxide phase after annealing. In addition to the V
2O
3 peaks, several peaks are assigned to the (111), (200), and (220) planes of graphitic carbon. This carbon likely originates from the thermal decomposition of the organic ligands in ZIF-8, which contributes to the conductive matrix in the composite. The coexistence of these two sets of reflections confirms the formation of a V
2O
3/C hybrid structure, which is beneficial for electrochemical applications due to the synergistic combination of active V
2O
3 and conductive carbon.
Figure 2b presents the CV profile of the V
2O
3/C cathode, revealing well-defined redox peaks that confirm the reversible electrochemical process involving Zn
2+ ions. During the anodic scan, distinct oxidation peaks appeared in the range of approximately 1.2 to 1.5 V, corresponding to the release of Zn
2+ ions from the cathode structure [
12,
17]. This process is associated with the oxidation of vanadium species, likely transitioning from V
3+ to higher oxidation states such as V
4+ or V
5+. In the cathodic sweep, reduction peaks at 0.9 and 1.3 V indicate the re-insertion of Zn
2+ ions into the host material, accompanied by the reduction of vanadium back to lower valence states. The consistency of the peak positions and intensities over successive cycles (from the first to the fourth) reflects the electrochemical stability and good reversibility of the Zn
2+ intercalation/deintercalation mechanism within the V
2O
3/C matrix.
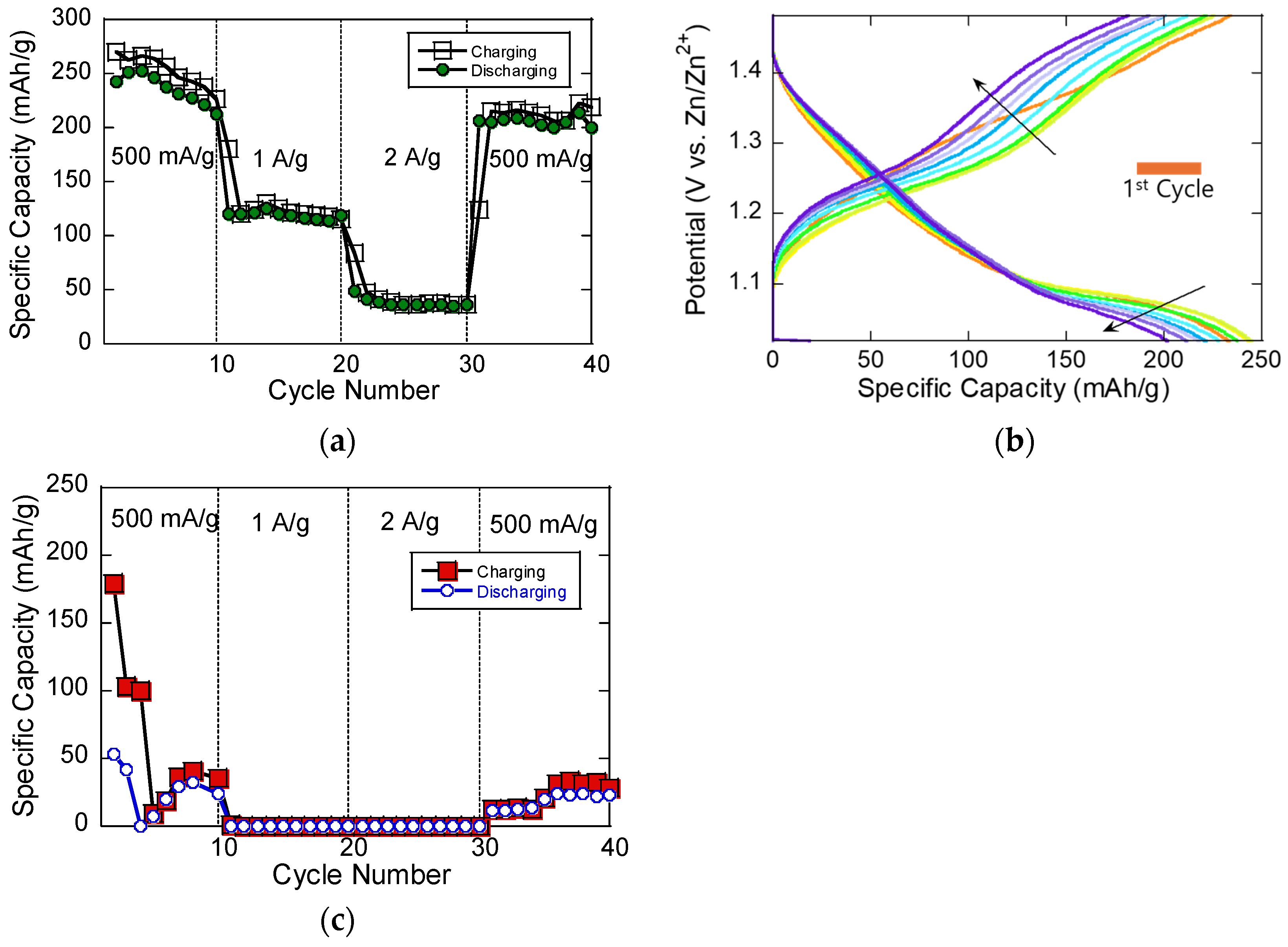
The electrochemical evaluation of the V
2O
3/C composite cathode in a ZIB is shown in
Figure 3, highlighting its cycling performance (
Figure 3a) and corresponding charge–discharge voltage profiles (
Figure 3b). In the initial cycle, the composite delivers a relatively high specific capacity of approximately 270 mAh/g, which can be attributed to surface-dominated capacitive contributions and the electrochemical activation of the V
2O
3/C cathode structure. This elevated capacity in the first cycle may also result from side reactions such as electrolyte decomposition or Zn
2+ insertion into structural defects. Upon increasing the current density to 1 and 2 A/g, the capacity decreases significantly, stabilizing between 50 and 120 mAh/g, suggesting that, at higher rates, Zn
2+ ion diffusion is hindered, limiting the material’s intercalation capabilities. However, when the current density is returned to 500 mA/g, the capacity partially recovers to 210 mAh/g, demonstrating that the material retains its structural stability and electrochemical reversibility even after high-rate cycling.
The charge–discharge profiles in
Figure 3b exhibit sloped voltage curves rather than distinct plateaus, indicative of a mixed charge storage mechanism involving both ion diffusion and pseudocapacitive processes. The subtle plateaus observed may correspond to the stepwise redox transitions of vanadium (e.g., V
5+/V
4+ and V
4+/V
3+), in agreement with the redox peaks previously identified in the CV curves shown in
Figure 2. The larger voltage gap seen in the first cycle likely arises from initial material activation and irreversible side reactions. In subsequent cycles, the reduced polarization suggests improved kinetics and the stabilization of the electrode–electrolyte interface over time.
We compared the rate performance of the MOF-derived V
2O
3/C cathode fabricated via AC-EPD with that of a conventionally prepared electrode using slurry casting, which incorporated the same synthesized V
2O
3/C composite mixed with carbon black and a PVDF binder, as shown in
Figure 3c. The results reveal that the slurry-casted V
2O
3 electrode delivers significantly a lower specific capacity across all current densities. In contrast, the binder-free MOF-derived V
2O
3/C cathode demonstrates a much higher capacity, highlighting its superior charge storage behavior. The significantly lower specific capacity observed in the slurry-casted V
2O
3 cathode, as shown in
Figure 3c, compared to the MOF-derived V
2O
3/C cathode in
Figure 3a, highlights the clear advantage of the MOF-based design and fabrication strategy. This performance difference can be attributed to several key factors. First, the MOF-derived V
2O
3/C composite benefits from an in situ-formed nitrogen-doped carbon matrix, which originates from the decomposition of the organic ligands in ZIF-8 during annealing. This conductive carbon framework not only enhances electron transport but also promotes a highly porous structure, offering abundant active sites and shortened Zn
2+ ion diffusion pathways. In contrast, the conventionally prepared slurry cathode—containing V
2O
3, super carbon, and a PVDF binder—suffers from poor connectivity between the active material and conductive additives, and the electrochemically inactive binder further dilutes the energy-storing phase, reducing the overall capacity. Second, the MOF-derived cathode was fabricated using AC-EPD, which produced a uniform, binder-free, ultrathin film (~300 nm) directly on the current collector. This method ensures close contact between the active material and current collector, minimizing resistance and improving the reaction kinetics. In contrast, the slurry-casted electrode tends to have thicker and less uniform films, where increased tortuosity hinders ion transport, especially at high rates. Lastly, the nanoscale dispersion of V
2O
3 in the carbon matrix achieved through MOF templating provides superior structural integrity during cycling, which maintains capacity retention and improves the reversibility. The slurry-based V
2O
3 electrode, on the other hand, may experience particle agglomeration and poor mechanical cohesion, leading to performance degradation.
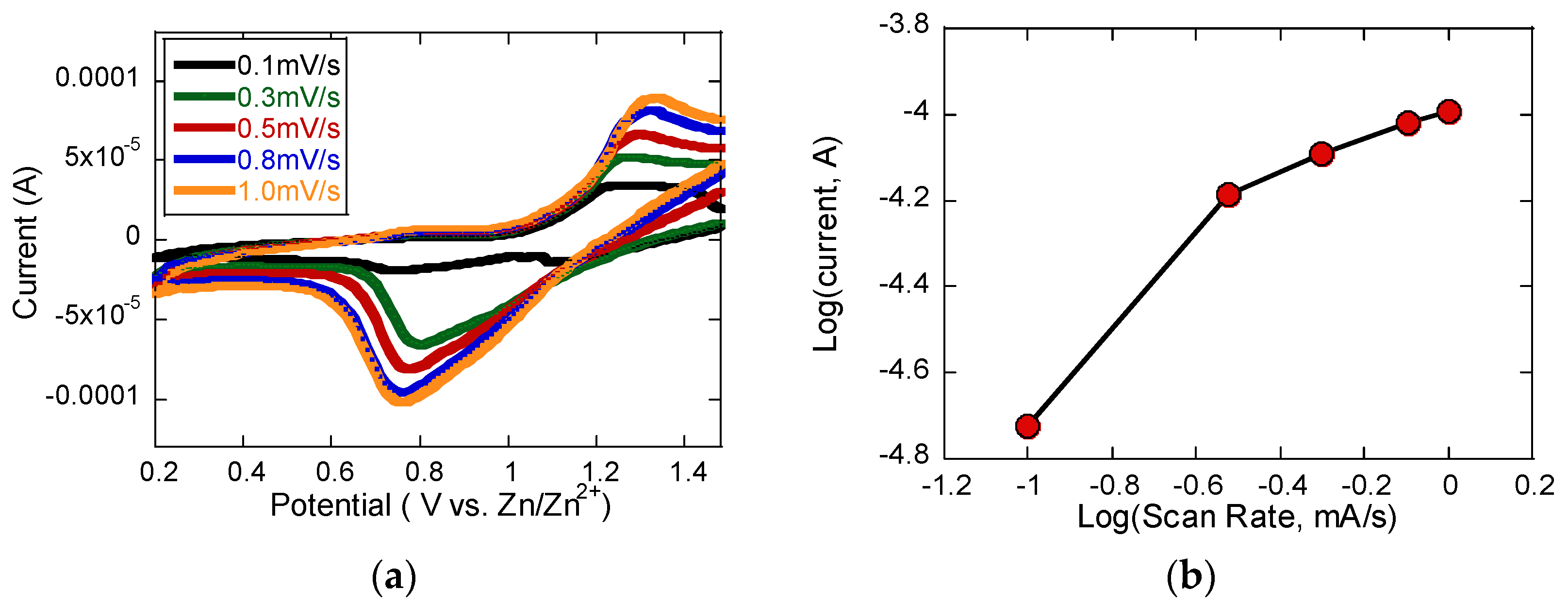
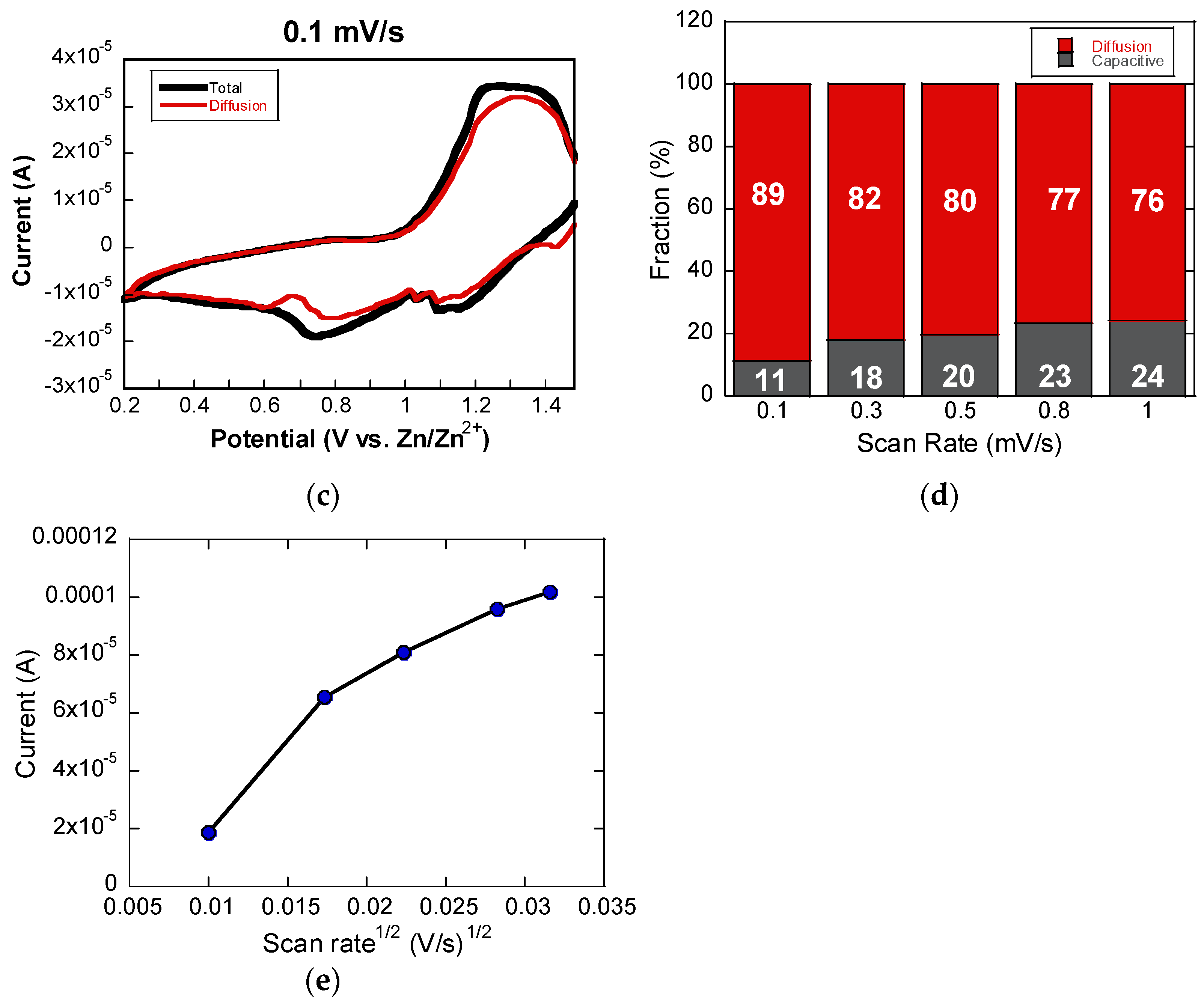
Figure 4 presents the charge storage analysis of the V
2O
3/C composite cathode based on CV measurements conducted at varying scan rates. As shown in
Figure 4a, the CV curves exhibit a clear redox peak approximately between 0.7 and 0.9 V, and the relationship between the peak current and scan rate was used to determine the
b-value, as plotted in
Figure 4b. By applying the power-law relationship
ip = avb., where
ip is the peak current and
v is the scan rate, the calculated
b-value was found to be 0.72 [
18,
19]. This value was obtained from the slope of the
log(
i) versus
log(
v) plot, which is commonly used to assess the dominant charge storage mechanism. A
b-value of 0.5 suggests diffusion-limited ion intercalation, while a value of 1.0 indicates surface-controlled pseudocapacitive behavior. The intermediate value of 0.72 observed in this study indicates a mixed mechanism, where both Zn
2+ intercalation into the bulk and pseudocapacitive surface reactions contribute to the overall charge storage process. The dominance of the diffusion-limited process, given that the
b-value is closer to 0.5, suggests that ion transport into the electrode structure remains a significant contributor, but the presence of surface redox activity enhances the rate performance and power handling.
To further distinguish the nature of charge storage, the current response was analyzed using the relationship
i(
V)
= k1ν + k2ν1⁄2, where
k1ν accounts for the capacitive component and
k2ν1⁄2 represents the diffusion-limited contribution. This equation was linearized to separate the two components by plotting
i(
V)
⁄ν1⁄2 against
ν2⁄1, allowing the extraction of
k1 and
k2 values from the slope and intercept. At a low scan rate of 0.1 mV/s, the diffusion-controlled process dominates the charge storage behavior, as indicated by the larger shaded area, as shown in
Figure 4c. However, as the scan rate increases from 0.1 to 1.0 mV/s, the capacitive contribution becomes more pronounced (
Figure 4d). This shift reflects the enhanced role of surface redox reactions at higher scan rates, which supports faster charge/discharge responses. Overall, the scan rate-dependent CV analysis confirms that the V
2O
3/C composite operates through a hybrid mechanism, combining the high energy density of diffusion-controlled intercalation with the high-rate capabilities of surface-driven pseudocapacitive processes.
The Zn
2+ ion diffusion coefficient within the ZIB was determined using CV data collected at varying scan rates, applying the Randles–Ševčík equation [
20]:
In this expression,
Ip represents the peak current,
ν is the scan rate, and
DZn denotes the Zn
2+ diffusion coefficient. The other parameters are standard electrochemical constants:
n is the number of electrons involved in the redox reaction,
F is Faraday’s constant,
A is the electrode’s surface area,
CZn is the Zn
2+ concentration in the electrolyte,
R is the universal gas constant, and
T is the temperature in Kelvin. By plotting the peak current against the square root of the scan rate (
ν1/2), as illustrated in
Figure 4e, a linear relationship was observed, from which the Zn
2+ diffusion coefficient was extracted. For the reduction process, the diffusion coefficient was calculated to be approximately 6.2 × 10
−11 cm
2/s. This value reflects the favorable ion transport characteristics of the ultrathin V
2O
3/C composite cathode, indicating its ability to support rapid Zn
2+ diffusion and efficient electrochemical performance in ZIB applications.
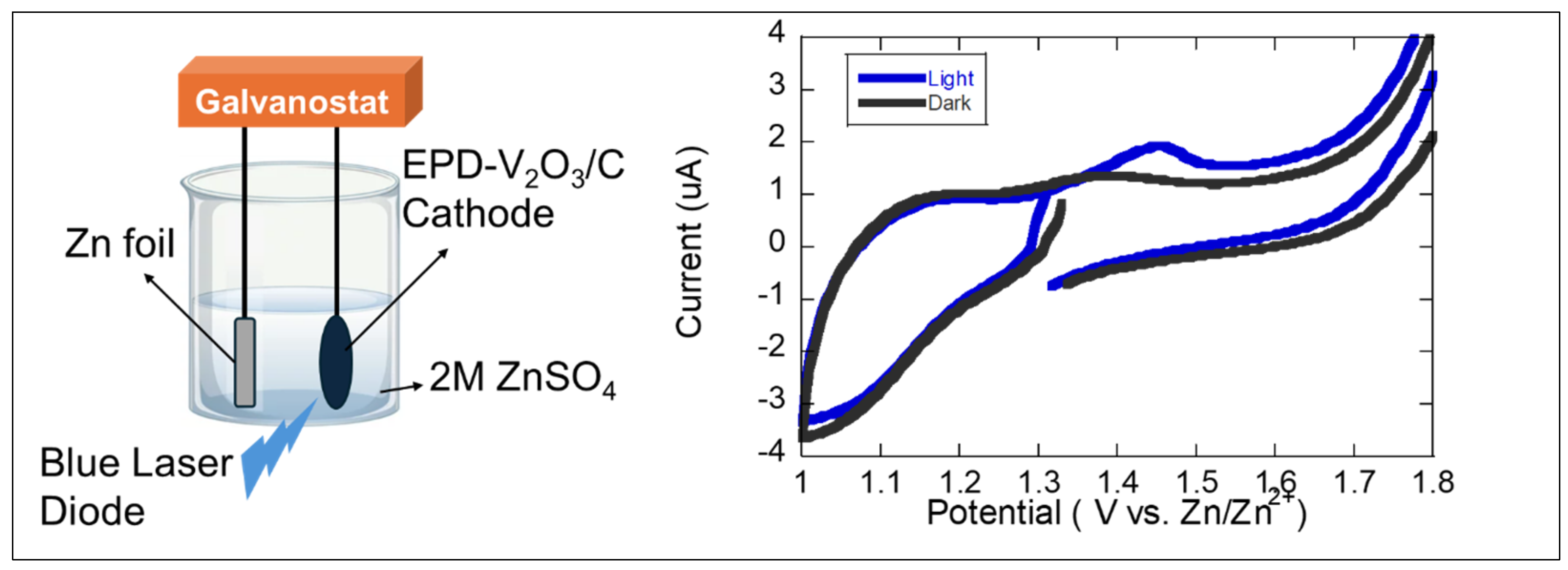
Figure 5 presents the photoelectrochemical characterization of the MOF-derived V
2O
3/C composite cathode. During the CV measurements, the cathode—placed in a vial-type electrochemical cell—was exposed to blue laser illumination (20 mW/cm
2) at a wavelength of 450 nm, as illustrated in
Figure 5. The appearance of an enhanced or newly formed peak in the CV curve under illumination—absent or weaker in the dark—suggests that light exposure is actively influencing the redox behavior of the MOF-derived V
2O
3/C cathode. This peak enhancement under illumination is most likely associated with the photo-induced acceleration of the redox reaction involving vanadium ions. Specifically, in the V
2O
3/C composite, vanadium undergoes redox transitions such as V
3+ ↔ V
4+ or V
4+ ↔ V
5+ during Zn
2+ intercalation and deintercalation. Under illumination, photons absorbed by the conductive carbon matrix or V
2O
3 itself may generate electron–hole pairs. These additional carriers reduce the overpotential required for redox transitions, leading to an increased current response and more pronounced redox peaks. The presence of photoexcited carriers can enhance charge transfer at the electrode–electrolyte interface, making the redox reaction more efficient. This manifests as sharper or higher peaks in the CV curve under light. In
Figure 5, the light-induced peak is located around the region where vanadium redox activity is typically observed (~1.3–1.5 V vs. Zn/Zn
2+). The fact that this peak is enhanced or more distinct under illumination confirms that the redox process benefits from photoactivation. This is further supported by the absence of such behavior in the slurry-casted V
2O
3 electrode, which lacks the conductive and photoactive architecture of the MOF-derived composite.
This enhancement in the electrochemical response under light exposure suggests that the MOF-derived structure possesses photoactive characteristics, likely due to the close integration of V2O3 with the conductive, nitrogen-doped carbon formed during MOF decomposition. The conductive carbon matrix may facilitate light-induced charge carrier generation and separation, leading to enhanced redox kinetics and current responses. In contrast, no noticeable difference was observed for the conventionally slurry-casted V2O3 cathode under light and dark conditions, indicating the absence of photoresponsiveness. This stark contrast implies that the conventional electrode structure lacks the electronic connectivity, nanoscale interface engineering, and possibly light-absorbing properties that are inherently built into the MOF-derived composite.