Pd-Gated N-Polar GaN/AlGaN High-Electron-Mobility Transistor for High-Sensitivity Hydrogen Gas Detection
Abstract
1. Introduction
2. Experiments
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ishibe, T.; Kozuki, S.; Komatsubara, Y.; Uematsu, Y.; Yoshizaki, T.; Yamashita, Y.; Naruse, N.; Mera, Y.; Kobayashi, E.; Nakamura, Y. Selective Anion Manipulation for Controlling the Thermoelectric Properties of Epitaxial SnO2 Films On R-Al2O3. Acs Appl. Energ. Mater. 2025, 8, 4411–4417. [Google Scholar] [CrossRef]
- Mahapatra, A.; Ajimsha, R.S.; Deepak, D.; Misra, P. Tuning Zno-Based Piezoelectric Nanogenerator Efficiency through N-Zno/P-Nio Bulk Interfacing. Sci. Rep. 2024, 14, 11871. [Google Scholar] [CrossRef]
- Meng, M.; Qin, N.; Sun, L.; Chen, Y.; Xu, K.; Zhang, Y.; Liu, M.; Du, S.; Liu, K.; Feng, Y. Lightweight 3D-TiO2 Nanotube Arrays On Ti Mesh for Promoted Photoelectrochemical Water Splitting. J. Nanoelectron. Optoelectron. 2021, 16, 1342–1347. [Google Scholar] [CrossRef]
- Meng, M.; Qin, W.; Li, C.; Xu, K.; Xu, L.; Li, J.; Ma, L.; Liu, K.; Li, J.; Qin, N. Synergistic Effect of Photonic Crystals and Oxygen Vacancies On Photoelectrochemical Water Splitting of TiO2 Nanotube. J. Nanoelectron. Optoelectron. 2020, 15, 226–230. [Google Scholar] [CrossRef]
- Menon, S.K.; Kumar, A.; Mondal, S. Advancements in Hydrogen Gas Leakage Detection Sensor Technologies and Safety Measures. Clean Energy 2025, 9, 263–277. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Luo, C.; Wang, X.; Yang, M.; Xiong, Z.; Gu, J.; Gong, Z.; Wei, Z.; Qian, F. SnO2-Based Resistive Hydrogen Gas Sensor: A Comprehensive Review From Performance to Function Optimization. Mater. Sci. Semicond. Process. 2025, 188, 109209. [Google Scholar] [CrossRef]
- Koo, W.; Cho, H.; Kim, D.; Kim, Y.H.; Shin, H.; Penner, R.M.; Kim, I. Chemiresistive Hydrogen Sensors: Fundamentals, Recent Advances, and Challenges. ACS Nano 2020, 14, 14284–14322. [Google Scholar] [CrossRef]
- Wright, J.S.; Lim, W.; Gila, B.P.; Pearton, S.J.; Johnson, J.L.; Ural, A.; Ren, F. Hydrogen Sensing with Pt-Functionalized GaN Nanowires. Sens. Actuator B-Chem. 2009, 140, 196–199. [Google Scholar] [CrossRef]
- Chiu, S.; Huang, H.; Huang, T.; Liang, K.; Liu, K.; Tsai, J.; Lour, W. Comprehensive Investigation On Planar Type of Pd–GaN Hydrogen Sensors. Int. J. Hydrog. Energy 2009, 34, 5604–5615. [Google Scholar] [CrossRef]
- Anderson, T.; Ren, F.; Pearton, S.; Kang, B.S.; Wang, H.; Chang, C.; Lin, J. Advances in Hydrogen, Carbon Dioxide, and Hydrocarbon Gas Sensor Technology Using GaN and ZnO-Based Devices. Sensors 2009, 9, 4669–4694. [Google Scholar] [CrossRef]
- Guo, H.; Jia, X.L.; Dong, Y.; Ye, J.D.; Chen, D.J.; Zhang, R.; Zheng, Y.D. Applications of AlGaN/GaN high electron mobility transistor-based sensors in water quality monitoring. Semicond. Sci. Technol. 2020, 35, 123001. [Google Scholar] [CrossRef]
- Jung, S.; Baik, K.H.; Ren, F.; Pearton, S.J.; Jang, S. Pt-AlGaN/GaN Hydrogen Sensor with Water-Blocking PMMA Layer. IEEE Electron. Device Lett. 2017, 38, 657–660. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, L.; Hao, Z.; Luo, Y. Modeling and Experimental Study On Sensing Response of an AlGaN/GaN HEMT-Based Hydrogen Sensor. Sens. Actuator B-Chem. 2013, 176, 241–247. [Google Scholar] [CrossRef]
- Guo, H.; Gong, H.H.; Shao, P.F.; Yu, X.X.; Wang, J.; Wang, R.; Yu, L.; Ye, J.D.; Chen, D.J.; Lu, H.; et al. Over 1200 V Normally-OFF p-NiO Gated AlGaN/GaN HEMTs on Si With a Small Threshold Voltage Shift. IEEE Electron. Device Lett. 2022, 43, 268–271. [Google Scholar] [CrossRef]
- Li, W.; Sokolovskij, R.; Jiang, Y.; Wen, K.; Hu, Q.; Deng, C.; Wang, Q.; Yu, H. Hydrogen Detection Performance of a Pt-AlGaN/GaN HEMT Sensor at High Temperatures in Air Ambient. J. Electrochem. Soc. 2024, 171, 127513. [Google Scholar] [CrossRef]
- Taher, M.I.B.; Kumar, M.; Halfaya, Y.; Lazerges, M.; Sama, N.Y.; Bouzid, K.; Moudakir, T.; Ngo, T.H.; Bouhnane, H.; Othmani, S. High Electron Mobility Transistor (HEMT) Based Hydrogen Sensor for Deep-Surface Applications: Effect of Air and N2 Atmosphere. Int. J. Hydrog. Energy 2024, 55, 1514–1522. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Chen, Y.; Cai, Z.; He, Z.; Lai, P. Effect of Hydrogen On Electrical Characteristics of AlGaN/GaN HEMTs After HTO Stress. IEEE Trans. Electron. Devices 2024, 71, 5895–5900. [Google Scholar] [CrossRef]
- Sokolovskij, R.; Zhang, J.; Zheng, H.; Li, W.; Jiang, Y.; Yang, G.; Yu, H.; Sarro, P.M.; Zhang, G.Q. Recessed Gate Pt-Algan/Gan Hemt H2 Sensor. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Chahdi, H.O.; Helli, O.; Bourzgui, N.; Breuil, L.; Danovitch, D.; Voss, P.L.; Sundaram, S.; Aubry, V.; Halfaya, Y.; Ougazzaden, A. Sensors Based On Algan/Gan Hemt for Fast H2 and O2 Detection and Measurement at High Temperature. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Wang, Y.; Ren, F.; Zhang, U.; Sun, Q.; Yerino, C.D.; Ko, T.S.; Cho, Y.S.; Lee, I.H.; Han, J.; Pearton, S.J. Improved Hydrogen Detection Sensitivity in N-Polar GaN Schottky Diodes. Appl. Phys. Lett. 2009, 94, 212108. [Google Scholar] [CrossRef]
- Hyeon Baik, K.; Kim, H.; Lee, S.; Lim, E.; Pearton, S.J.; Ren, F.; Jang, S. Hydrogen Sensing Characteristics of Semipolar (112¯ 2) Gan Schottky Diodes. Appl. Phys. Lett. 2014, 104, 072103. [Google Scholar] [CrossRef]
- Cho, S.; Ahn, H.; Park, K.; Choi, J.; Kang, H.; Jung, H. Ultrasmall Grained Pd Nanopattern H2 Sensor. Acs Sens. 2018, 3, 1876–1883. [Google Scholar] [CrossRef]
- Northrup, J.E.; Neugebauer, J. Strong Affinity of Hydrogen for the GaN(000-1) Surface: Implications for Molecular Beam Epitaxy and Metalorganic Chemical Vapor Deposition. Appl. Phys. Lett. 2004, 85, 3429–3431. [Google Scholar] [CrossRef]
- Chung, G.H.; Vuong, T.A.; Kim, H. Demonstration of Hydrogen Sensing Operation of AlGaN/GaN HEMT Gas Sensors in Extreme Environment. Results Phys. 2019, 12, 83–84. [Google Scholar] [CrossRef]
- Watanabe, A.; Nakamura, S.; Okumura, T. Selective Hydrogen Detection of Pd/AlGaN/GaN HEMT-Type Sensors by Temperature Sweep Operation. In Proceedings of the SENSORS, 2012 IEEE, Taipei, Taiwan, 28–31 October 2012; pp. 1–4. [Google Scholar]
- Sokolovskij, R.; Iervolino, E.; Zhao, C.; Wang, F.; Yu, H.; Santagata, F.; Sarro, P.M.; Zhang, G.Q. Pt-AlGaN/GaN HEMT-Sensor Layout Optimization for Enhancement of Hydrogen Detection. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Sokolovskij, R.; Zhang, J.; Zheng, H.; Li, W.; Jiang, Y.; Yang, G.; Yu, H.; Sarro, P.M.; Zhang, G. The Impact of Gate Recess On the H₂ Detection Properties of Pt-AlGaN/GaN HEMT Sensors. IEEE Sens. J. 2020, 20, 8947–8955. [Google Scholar] [CrossRef]
- Huang, C.; Chen, H.; Liu, I.; Chen, C.; Chou, P.; Liou, J.; Liu, W. Comprehensive Study of Hydrogen Sensing Phenomena of an Electroless Plating (Ep)-Based Pd/Algan/Gan Heterostructure Field-Effect Transistor (Hfet). Sens. Actuator B-Chem. 2014, 190, 913–921. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Liu, I.; Chou, P.; Liou, J.; Huang, C.; Liu, W. Hydrogen Sensing Characteristics of a Pt/Algan/Gan Heterostructure Field-Effect Transistor (Hfet) Prepared by Sensitization, Activation, and Electroless Plating (Ep) Approaches. Sens. Actuator B-Chem. 2015, 212, 127–136. [Google Scholar] [CrossRef]

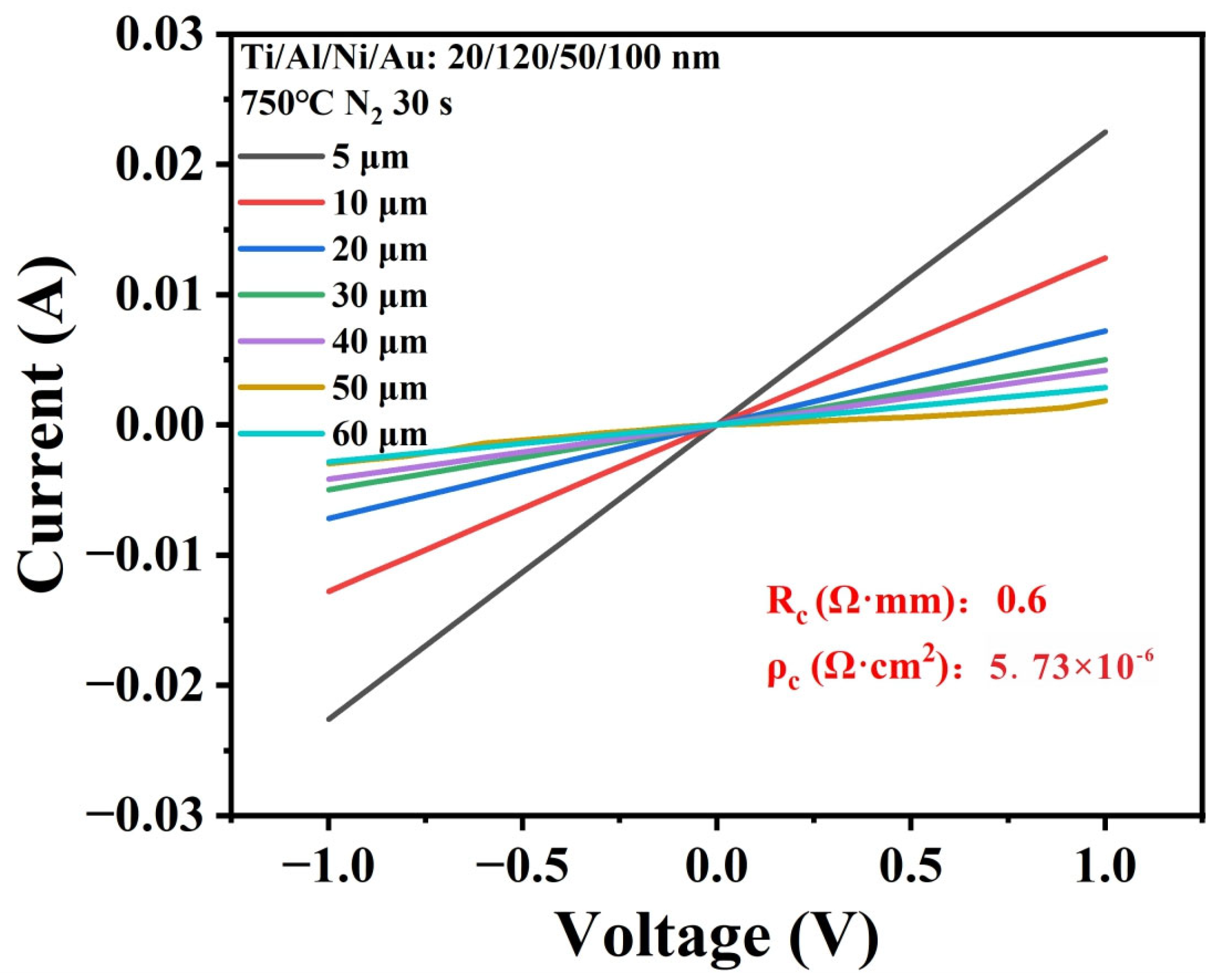
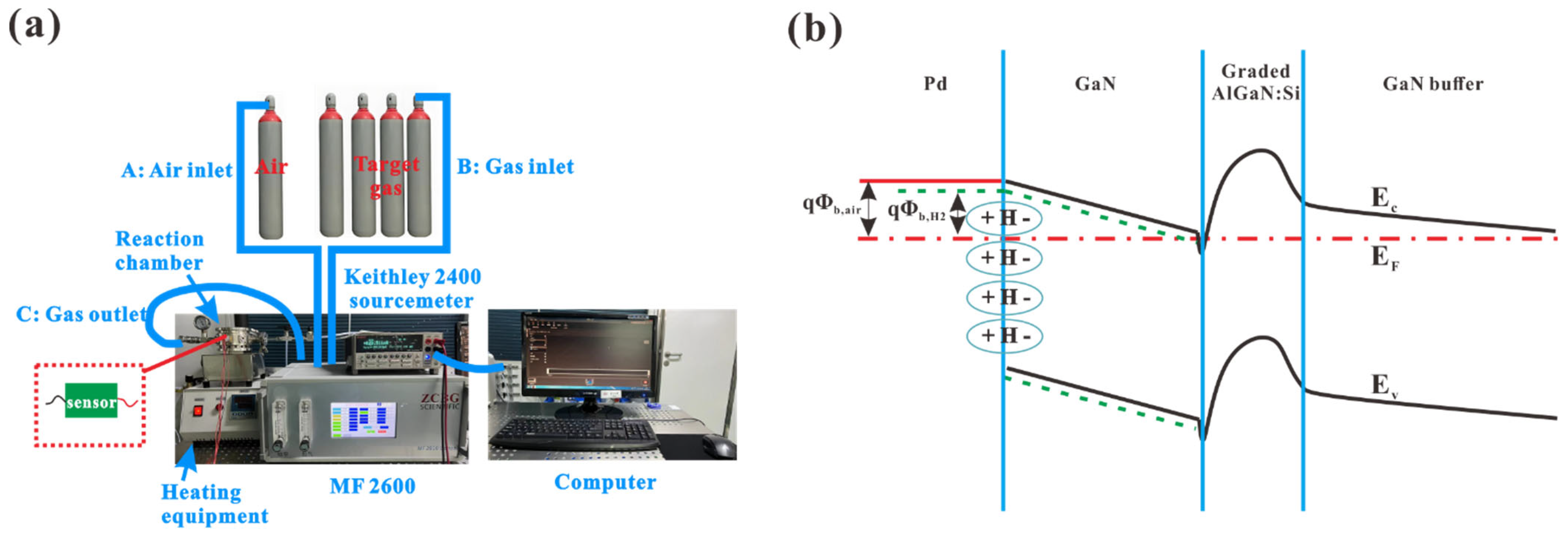
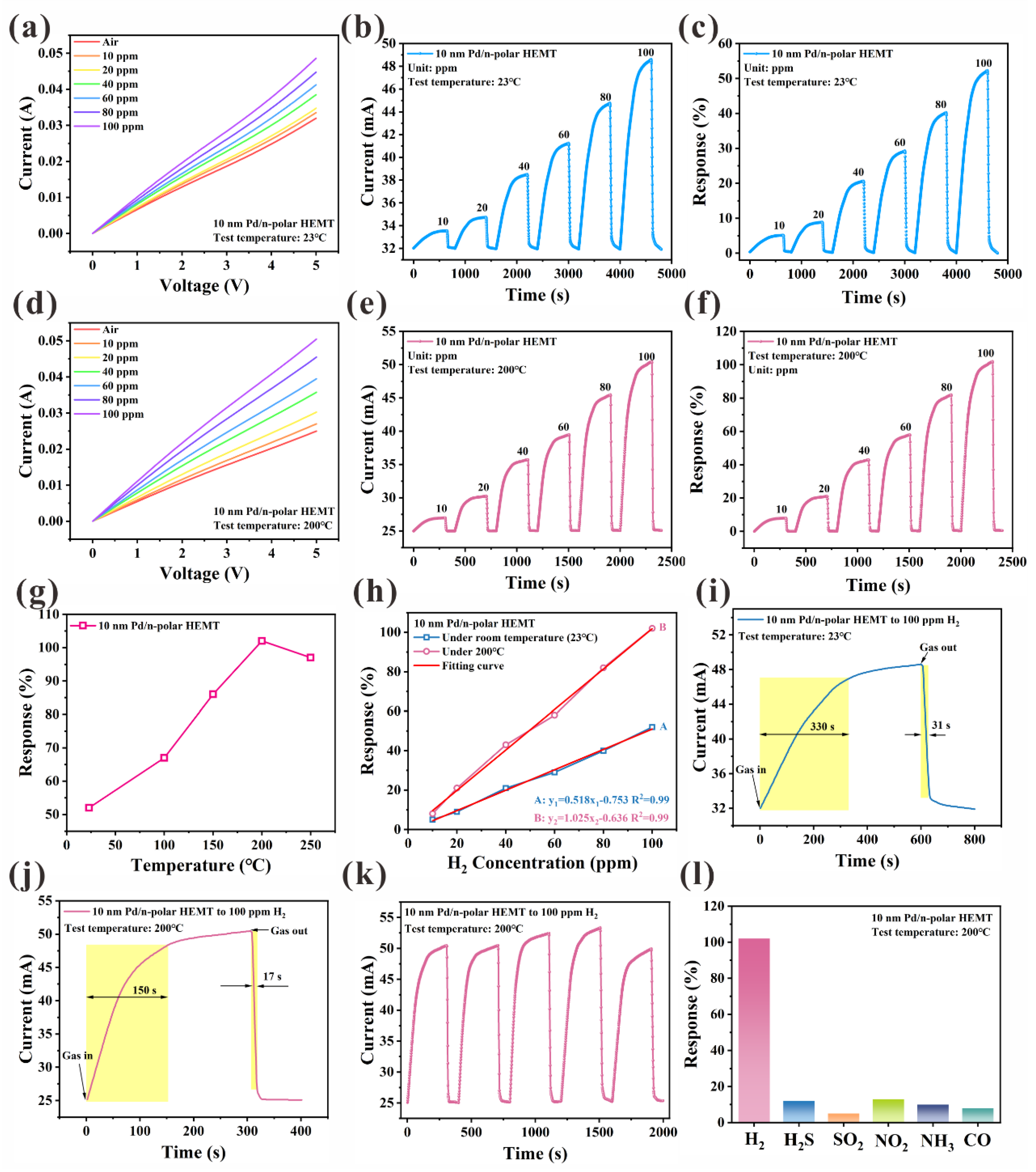
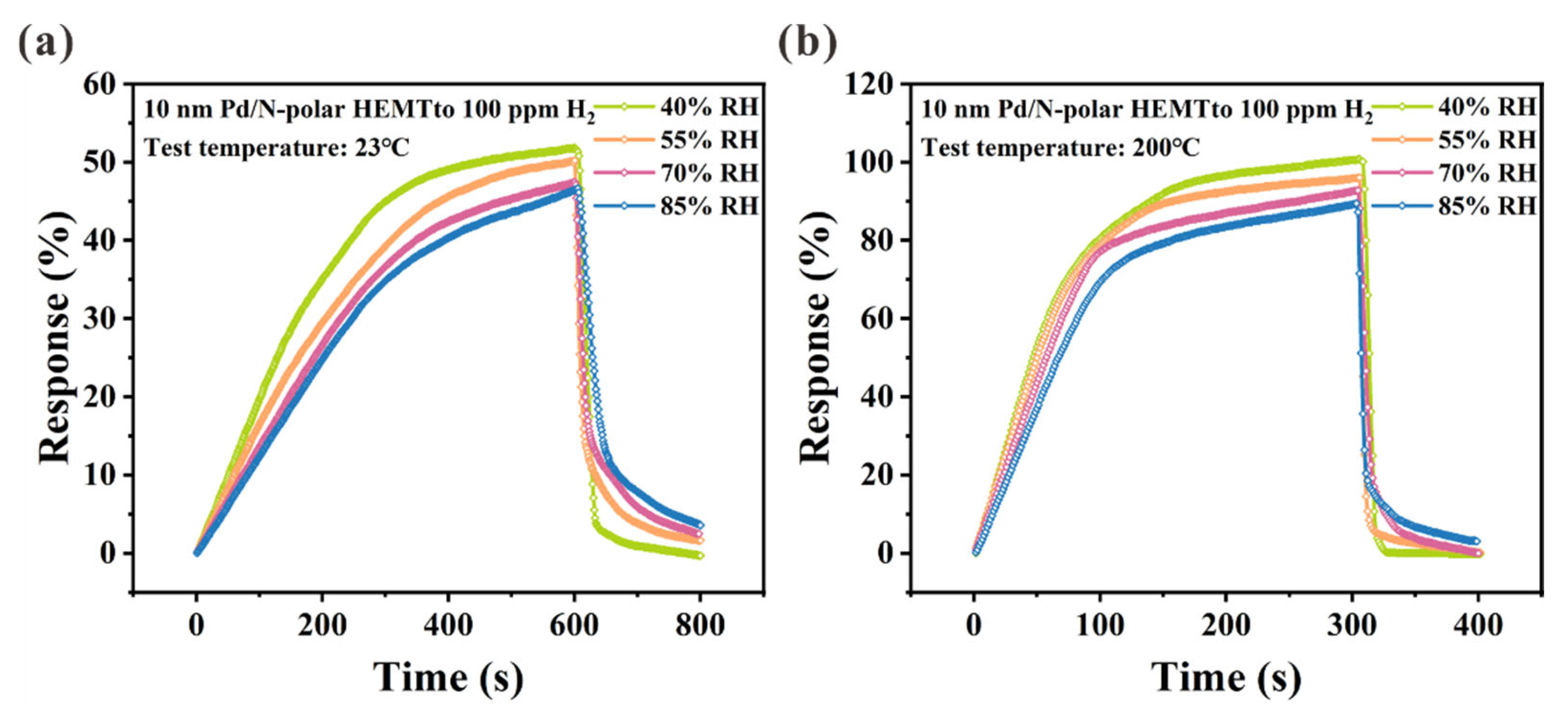
| Hydrogen Gas Sensor | H2 Conc. | Temp. (°C) | S | Tres/Trec | Ref. |
|---|---|---|---|---|---|
| Pt-AlGaN/GaN | 250 ppm | 240 | 42.2% | 4.3 min/13.6 min | [18] |
| Pt/AlGaN/GaN | 4% | 350 | 33% | - | [24] |
| Pd/AlGaN/GaN | 1000 | 230 | 78% | - | [25] |
| Pt/AlGaN/GaN | 500 | 200 | 7.6% | 342 s/1539 s | [26] |
| Pt-AlGaN/GaN | 300 | 240 | 145.8% | 2.5 min/8.85 min | [27] |
| Pt/AlGaN/GaN | 10,000 ppm | 126 | 13.7% | 28 s/36 s | [28] |
| Pd/AlGaN/GaN | 10,000 ppm | 100 | 26.3% | 53 s/76 s | [29] |
| Pd-gated/N-polar GaN/AlGaN | 100 ppm | 200 | 52% | 330 s/31 s | This work |
| 100 ppm | 102% | 150 s/17 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, L.; Bai, H.; Teng, Y.; Yang, X. Pd-Gated N-Polar GaN/AlGaN High-Electron-Mobility Transistor for High-Sensitivity Hydrogen Gas Detection. Crystals 2025, 15, 578. https://doi.org/10.3390/cryst15060578
Ge L, Bai H, Teng Y, Yang X. Pd-Gated N-Polar GaN/AlGaN High-Electron-Mobility Transistor for High-Sensitivity Hydrogen Gas Detection. Crystals. 2025; 15(6):578. https://doi.org/10.3390/cryst15060578
Chicago/Turabian StyleGe, Long, Haineng Bai, Yidi Teng, and Xifeng Yang. 2025. "Pd-Gated N-Polar GaN/AlGaN High-Electron-Mobility Transistor for High-Sensitivity Hydrogen Gas Detection" Crystals 15, no. 6: 578. https://doi.org/10.3390/cryst15060578
APA StyleGe, L., Bai, H., Teng, Y., & Yang, X. (2025). Pd-Gated N-Polar GaN/AlGaN High-Electron-Mobility Transistor for High-Sensitivity Hydrogen Gas Detection. Crystals, 15(6), 578. https://doi.org/10.3390/cryst15060578





