Influence of Reflow Cycles of the Pb–Free/Pb Hybrid Assembly Process on the IMCs Growth Interface of Micro-Solder Joints
Abstract
1. Introduction
2. Experimental Materials and Methods
3. Result
3.1. Microstructural Changes
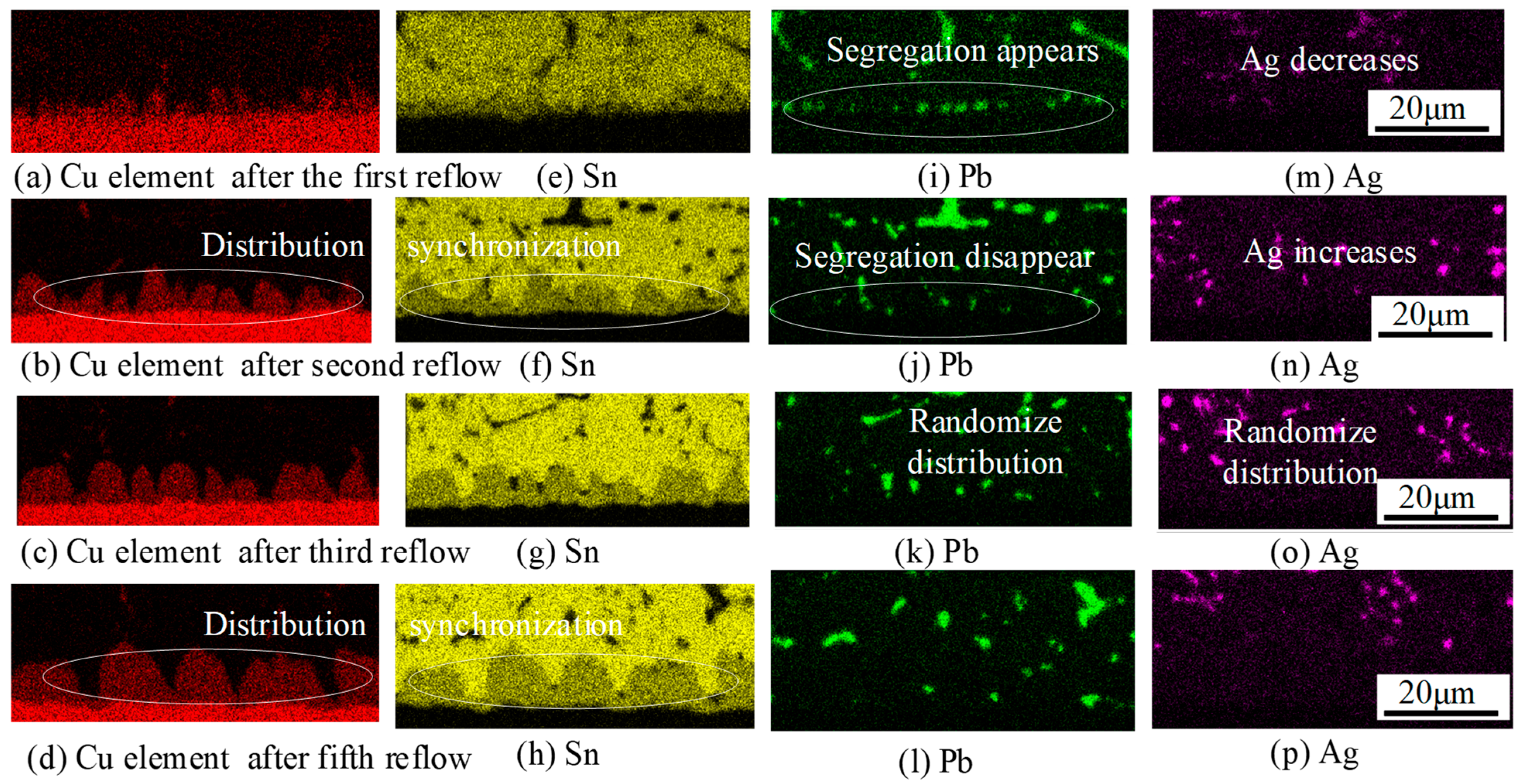
3.2. Changes in Elemental Distribution
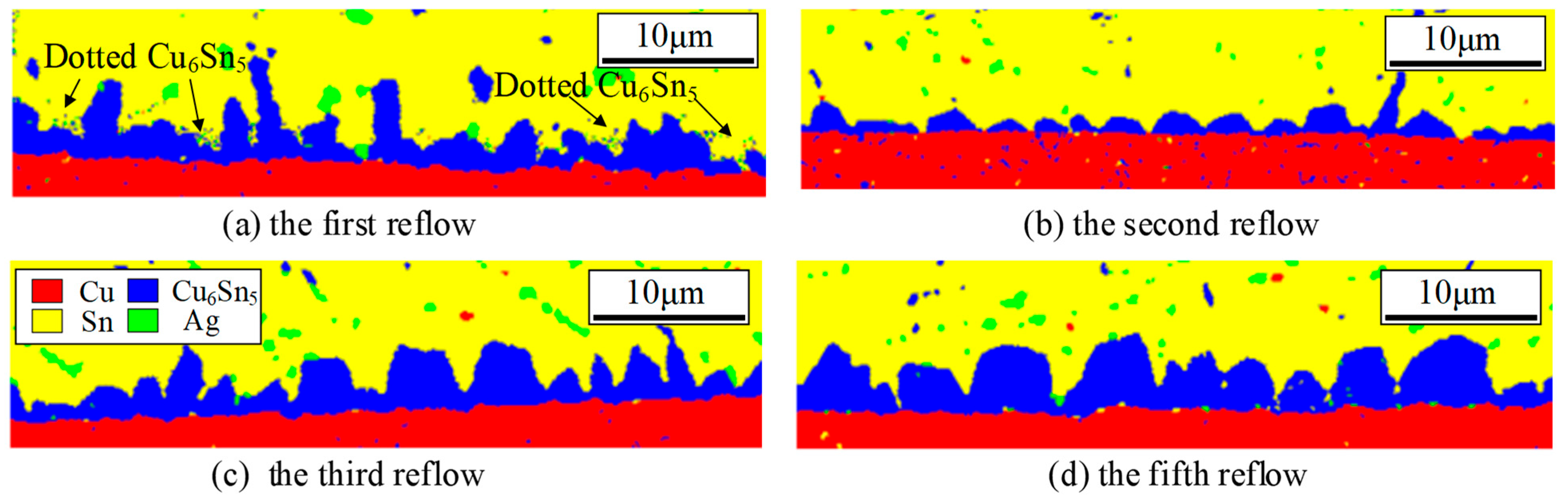
3.3. Changes in Phase Distribution
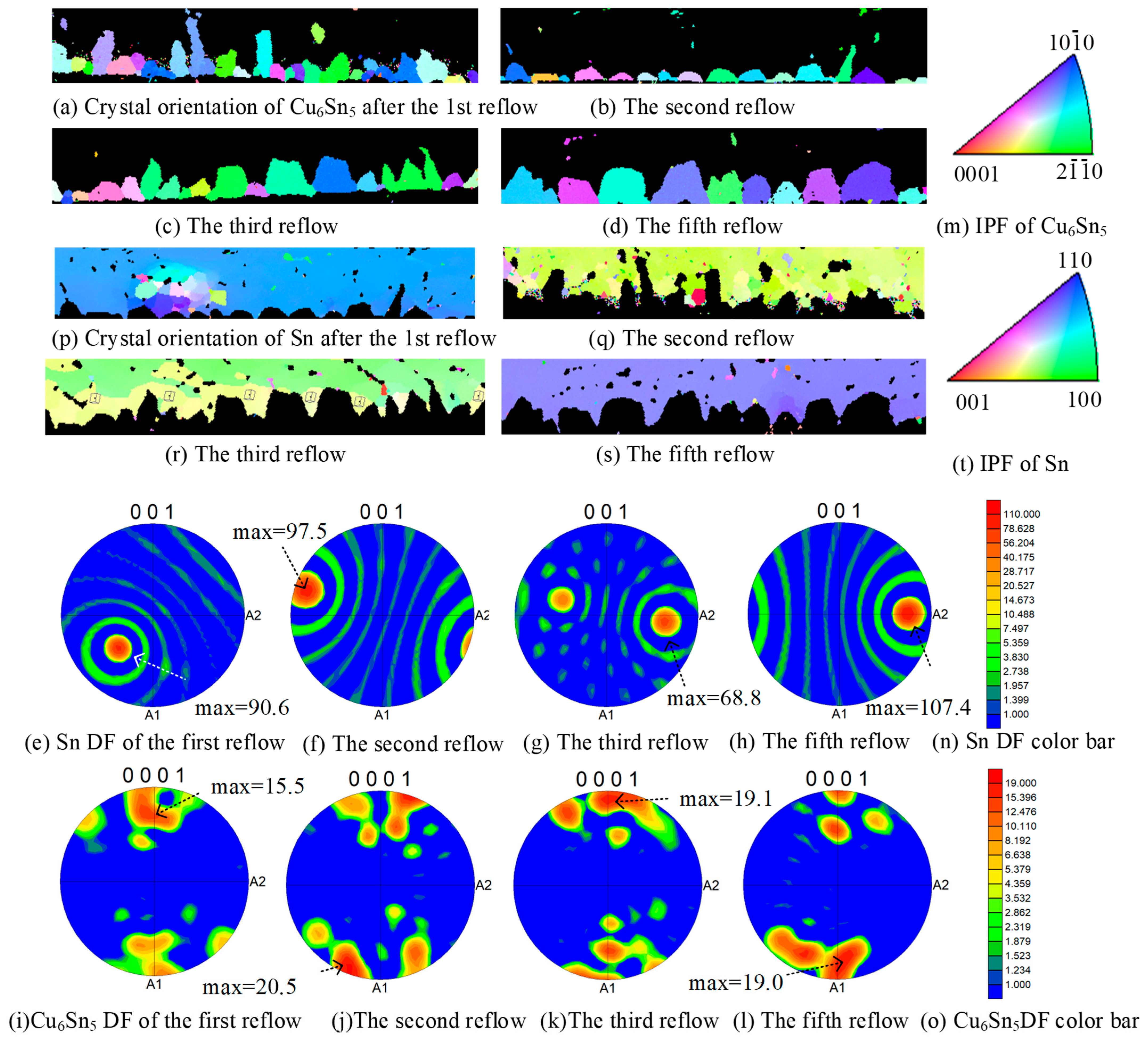
3.4. Change in Crystal Orientation
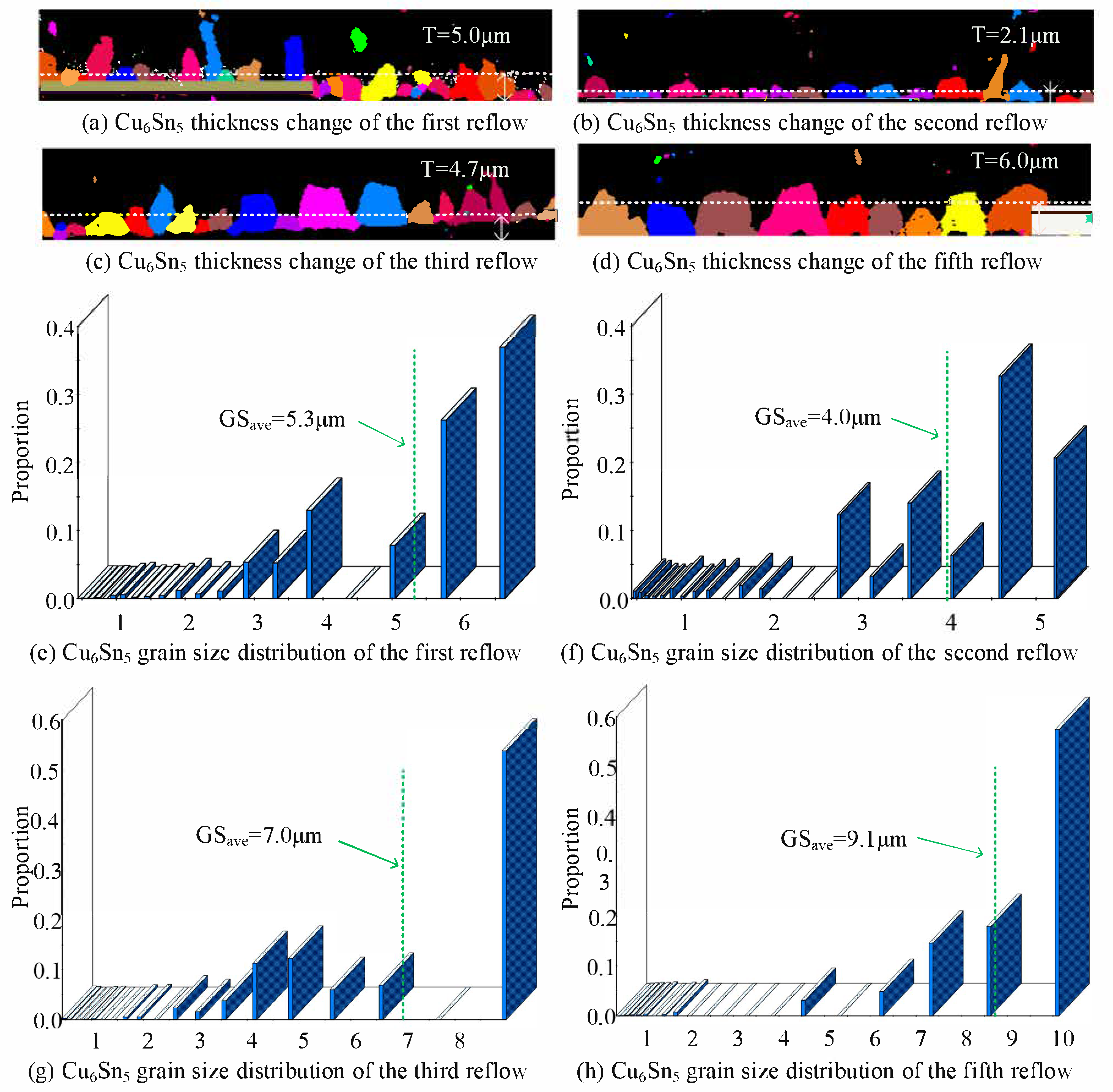
3.5. Size and Thickness Variations
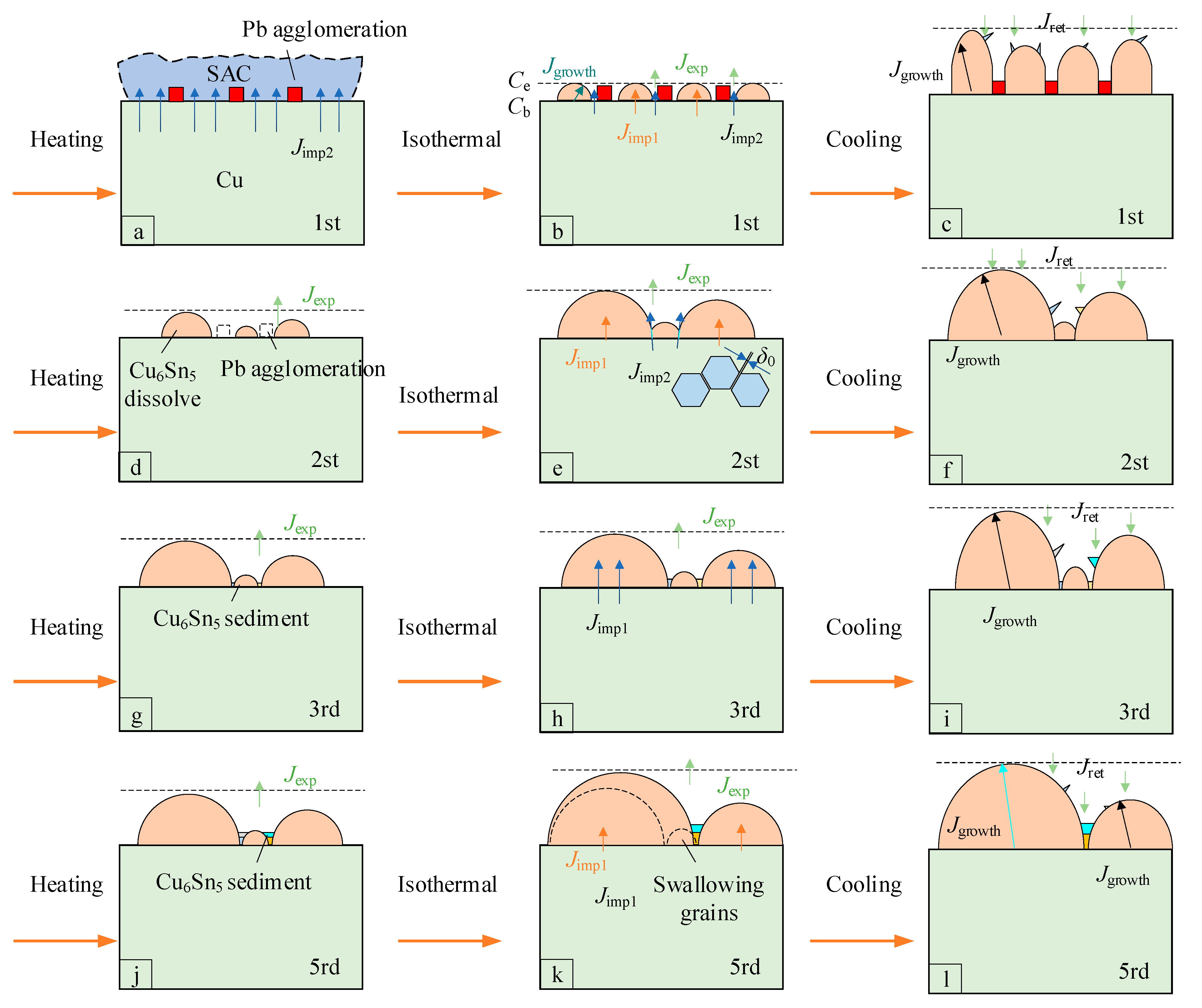
4. Discussion
5. Conclusions
- (1)
- The interfacial IMCs in hybrid solder joints predominantly consist of Cu6Sn5 grains. With increasing reflow cycles, Pb distribution transitions from clustered to random dispersion patterns, accompanied by the progressive transformation of prismatic Cu6Sn5 into scallop-shaped and semicircular configurations.
- (2)
- Cu6Sn5 grains exhibit pronounced preferential orientation along the horizontal crystallographic direction across all reflow cycles, with this orientation influencing Sn crystal alignment.
- (3)
- Quantitative EBSD analysis reveals microstructural evolution. The first reflow elongates Cu6Sn5 grains, exhibiting high aspect ratios, with observable fine crystalline protrusions indicating active growth, and the second reflow reduces both dimensions significantly. Scallop-type Cu6Sn5 of the third reflow demonstrates uniform growth with a stable grain population, and coarsening dominates after the fifth reflow, accompanied by pronounced grain coalescence.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le, F.; Lee, S.W.; Zhang, Q. 3D chip stacking with through-silicon-vias (TSVs) for vertical interconnect and underfill dispensing. J. Micromech. Microeng. 2017, 27, 045012. [Google Scholar] [CrossRef]
- Ma, H.R.; Kunwar, A.; Shang, S.Y.; Jiang, C.R.; Wang, Y.P.; Ma, H.T.; Zhao, N. Evolution behavior and growth kinetics of intermetallic compounds at Sn/Cu interface during multiple reflows. Intermetallics 2018, 96, 1–12. [Google Scholar] [CrossRef]
- Qu, L.; Zhao, N.; Zhao, H.; Huang, M.; Ma, H. In situ study of the real-time growth behavior of Cu6Sn5 at the Sn/Cu interface during the soldering reaction. Scr. Mater. 2014, 72, 43–46. [Google Scholar] [CrossRef]
- Park, M.S.; Arróyave, R. Early stages of intermetallic compound formation and growth during lead-free soldering. Acta Mater. 2010, 58, 4900–4910. [Google Scholar] [CrossRef]
- Chen, H.; Tsai, Y.-L.; Chang, Y.-T.; Wu, A.T. Effect of massive spalling on mechanical strength of solder joints in Pb-free solder reflowed on Co-based surface finishes. J. Alloys Compd. 2016, 671, 100–108. [Google Scholar] [CrossRef]
- Haseeb, A.S.; Arafat, M.M.; Johan, M.R. Stability of molybdenum nanoparticles in Sn-3.8Ag-0.7Cu solder during multiple reflow and their influence on interfacial intermetallic compounds. Mater. Charact. 2012, 64, 27–35. [Google Scholar] [CrossRef]
- Ha, S.S.; Jang, J.K. Effect of multiple reflows on interfacial reaction and shear strength ofSn-Ag electroplated solder bumps for fip chip package. Microelectron. Eng. 2010, 87, 517–521. [Google Scholar] [CrossRef]
- Noh, B.I.; Koo, J.M.; Kim, J.W.; Kim, D.G.; Nam, J.D.; Joo, J.; Jung, S.B. Effects of number of reflows on the mechanical andelectrical properties of BGA package. Intermetallics 2006, 14, 1375–1378. [Google Scholar] [CrossRef]
- Yao, P.; Liu, P.; Liu, J. Effects of multiple reflows on intermetallic morphology and shear strength of Sn Ag Cu-xNi composite solder joints on electrolytic Ni/Au metalized substrate. J. Alloys Compd. 2008, 462, 73–79. [Google Scholar] [CrossRef]
- Huebner, H.; Penka, S.; Barchmann, B.; Eigner, M.; Gruber, W.; Nobis, M.; Janka, S.; Kristen, G.; Schneegans, M. Microcontacts with sub-30um pitch for 3D chip-on-chip integration. Microelectron. Eng. 2006, 83, 2155–2162. [Google Scholar] [CrossRef]
- Darbandi, P.; Bieler, T.R.; Pourboghrat, F.; Lee, T.-K. The effect of cooling rate on grainorientation and misorientation microstructure of SAC105 solder joints before and after impact droptests. J. Electron. Mater. 2014, 43, 2521–2529. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Zhao, N.; Wu, C. Effects of cooling rate and joint size on Sn grain features inCu/Sn-3.5Ag/Cu solder joints. Materialia 2020, 14, 100929. [Google Scholar] [CrossRef]
- Ma, H.; Kunwar, A.; Huang, R.; Chen, J.; Wang, Y.; Zhao, N.; Ma, H. Size effect on lMC growth induced by Cu concentration gradient and pinning of Ag3Sn particles during multiple reflows. Intermetallics 2017, 90, 90–96. [Google Scholar] [CrossRef]
- Huang, M.; Yang, F.; Zhao, N.; Zhang, Z. In situ study on dissolution and growth mechanism of interfacial Cu6Sn5 in wetting reaction. Mater. Lett. 2015, 139, 42–45. [Google Scholar] [CrossRef]
- Ma, H.; Ma, H.; Kunwar, A.; Shang, S.; Wang, Y.; Chen, J.; Huang, M.; Zhao, N. Effect of initial Cu concentration on the lMC size and grain aspect ratio in Sn-xCu solders during multiple reflows. J. Mater. Sci. Mater. Electron. 2018, 29, 602–613. [Google Scholar] [CrossRef]
- Li, J.; Mannan, S.; Clode, M.; Whalley, D.; Hutt, D. Interfacial reactions between molten Sn-Bi-X soldersand Cu substrates for liquid solder interconnects. Acta Vaterialia 2006, 54, 2907–2922. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Zhang, Q.; Cui, Q.; Bai, Y.; Fu, L.; Zhao, Z.; Zou, C.; Wang, Y. Influence of Reflow Cycles of the Pb–Free/Pb Hybrid Assembly Process on the IMCs Growth Interface of Micro-Solder Joints. Crystals 2025, 15, 516. https://doi.org/10.3390/cryst15060516
He X, Zhang Q, Cui Q, Bai Y, Fu L, Zhao Z, Zou C, Wang Y. Influence of Reflow Cycles of the Pb–Free/Pb Hybrid Assembly Process on the IMCs Growth Interface of Micro-Solder Joints. Crystals. 2025; 15(6):516. https://doi.org/10.3390/cryst15060516
Chicago/Turabian StyleHe, Xinyuan, Qi Zhang, Qiming Cui, Yifan Bai, Lincheng Fu, Zicong Zhao, Chuanhang Zou, and Yong Wang. 2025. "Influence of Reflow Cycles of the Pb–Free/Pb Hybrid Assembly Process on the IMCs Growth Interface of Micro-Solder Joints" Crystals 15, no. 6: 516. https://doi.org/10.3390/cryst15060516
APA StyleHe, X., Zhang, Q., Cui, Q., Bai, Y., Fu, L., Zhao, Z., Zou, C., & Wang, Y. (2025). Influence of Reflow Cycles of the Pb–Free/Pb Hybrid Assembly Process on the IMCs Growth Interface of Micro-Solder Joints. Crystals, 15(6), 516. https://doi.org/10.3390/cryst15060516






