Bismuth(III) Sulfide Films by Chemical Bath Deposition Method Using L-Cysteine as a Novel Sulfur Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formation of the Bi2S3 Films
2.3. Characterization Methods of the Bi2S3 Films
3. Results and Discussion
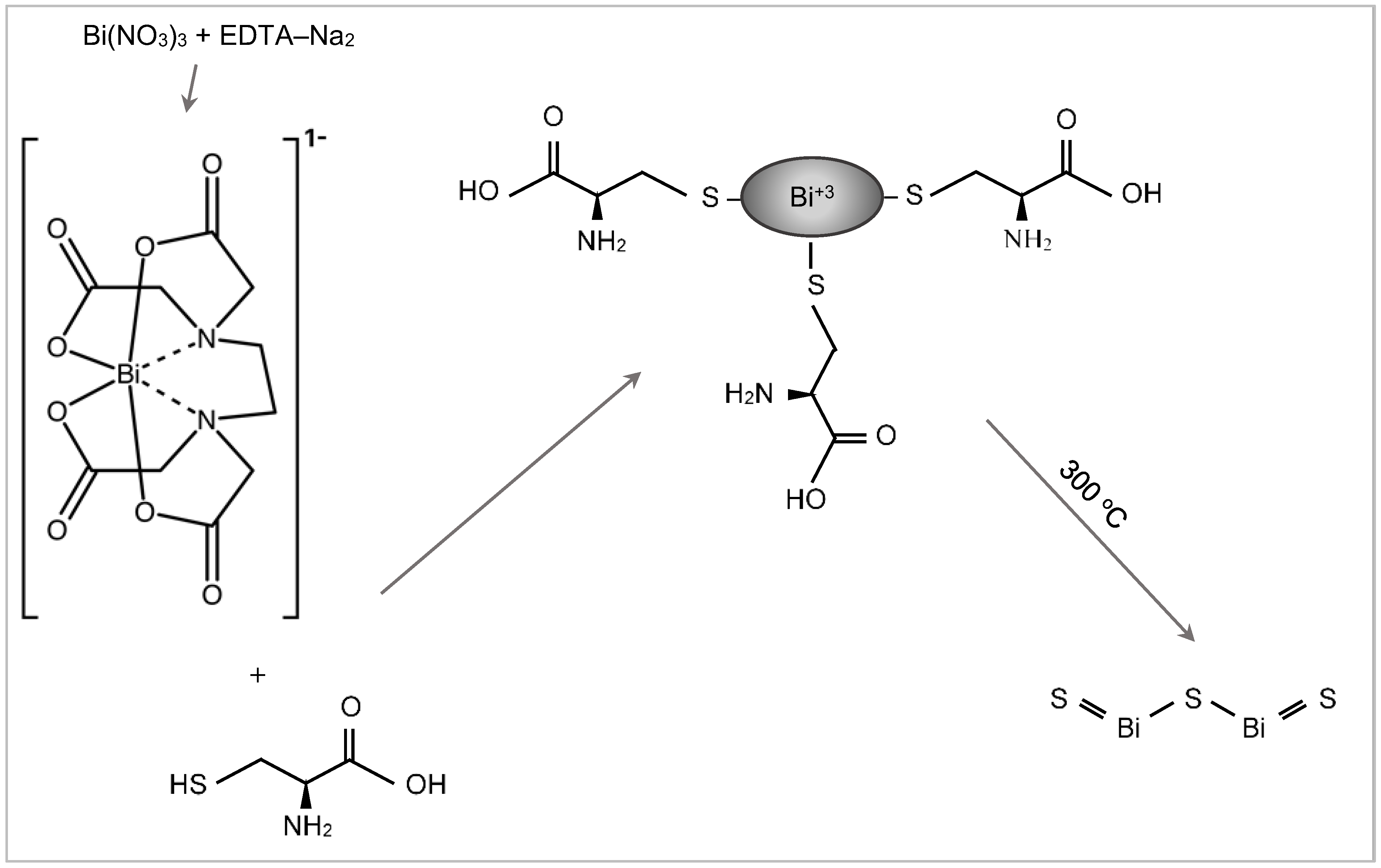
3.1. Bi2S3 Film Formation
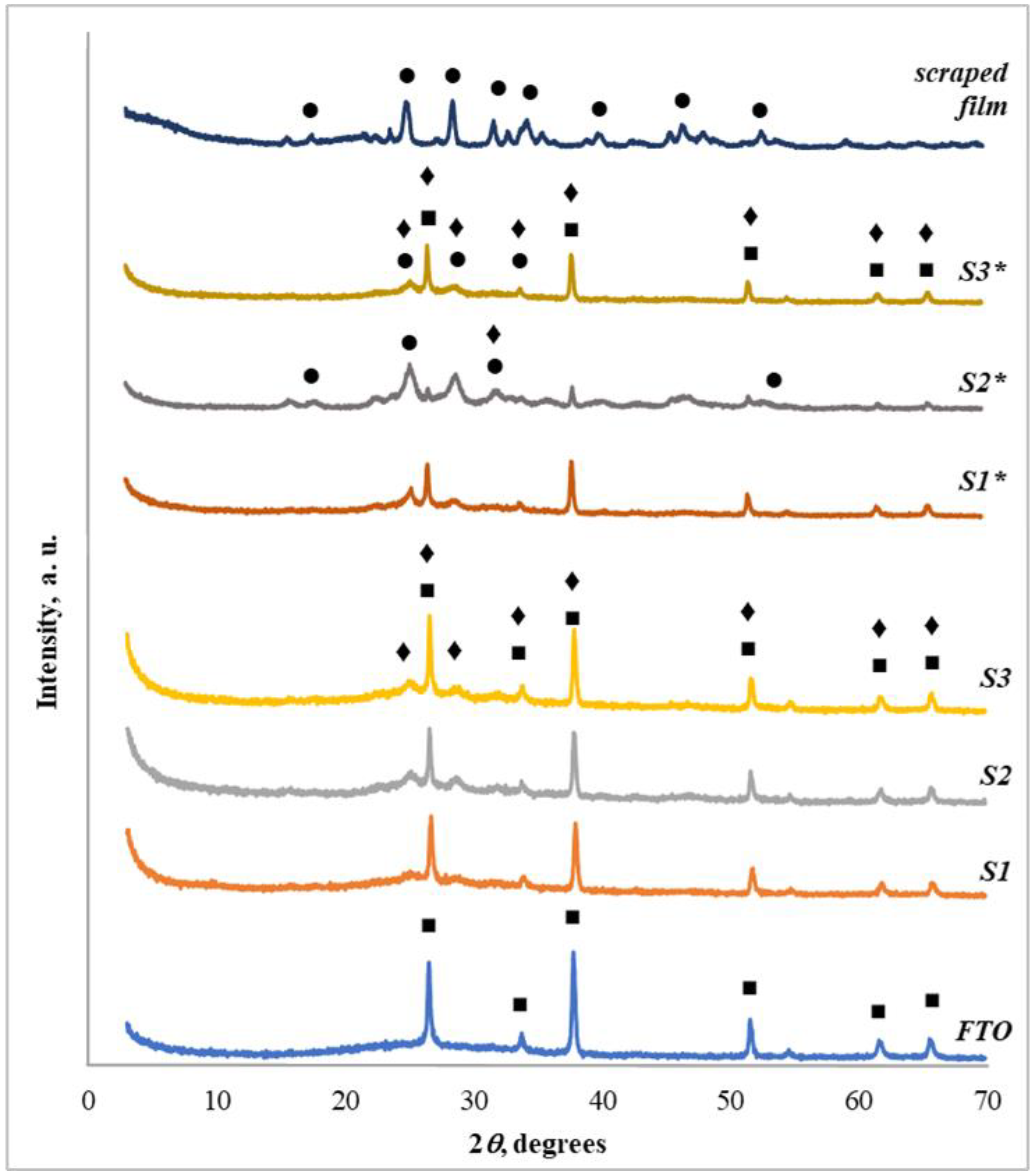
3.2. XRD Characterization
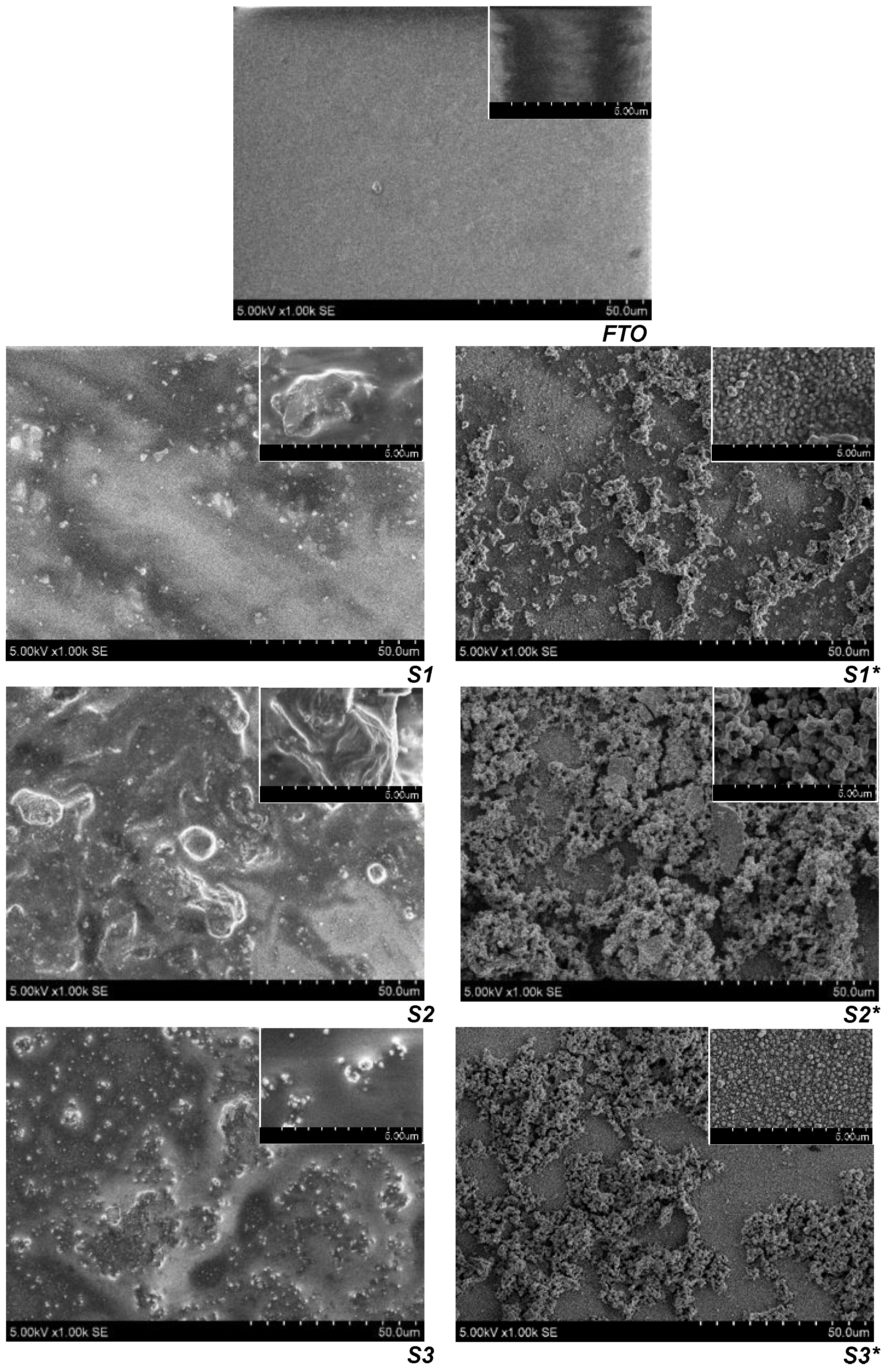
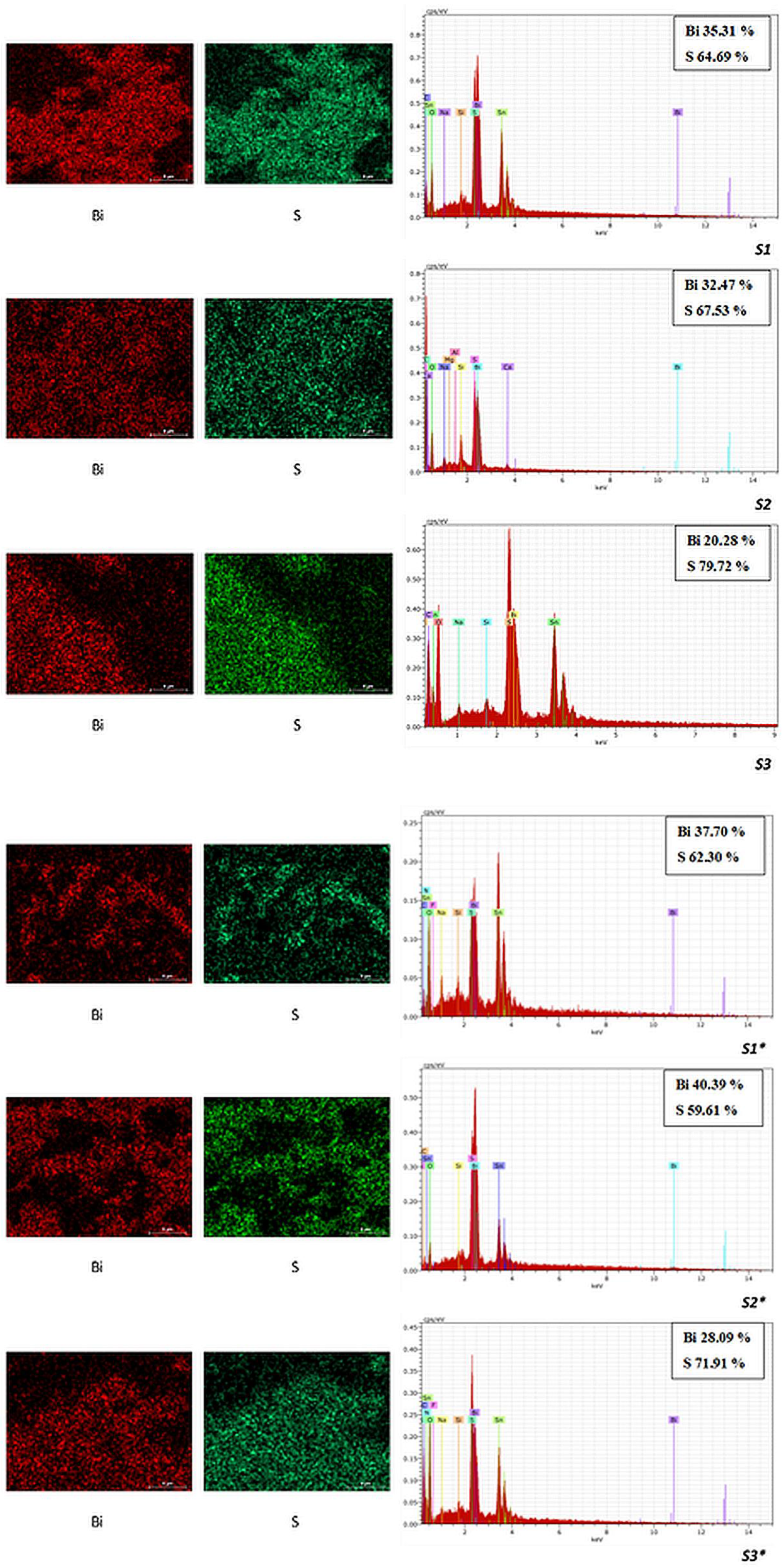
3.3. SEM/EDX Characterization
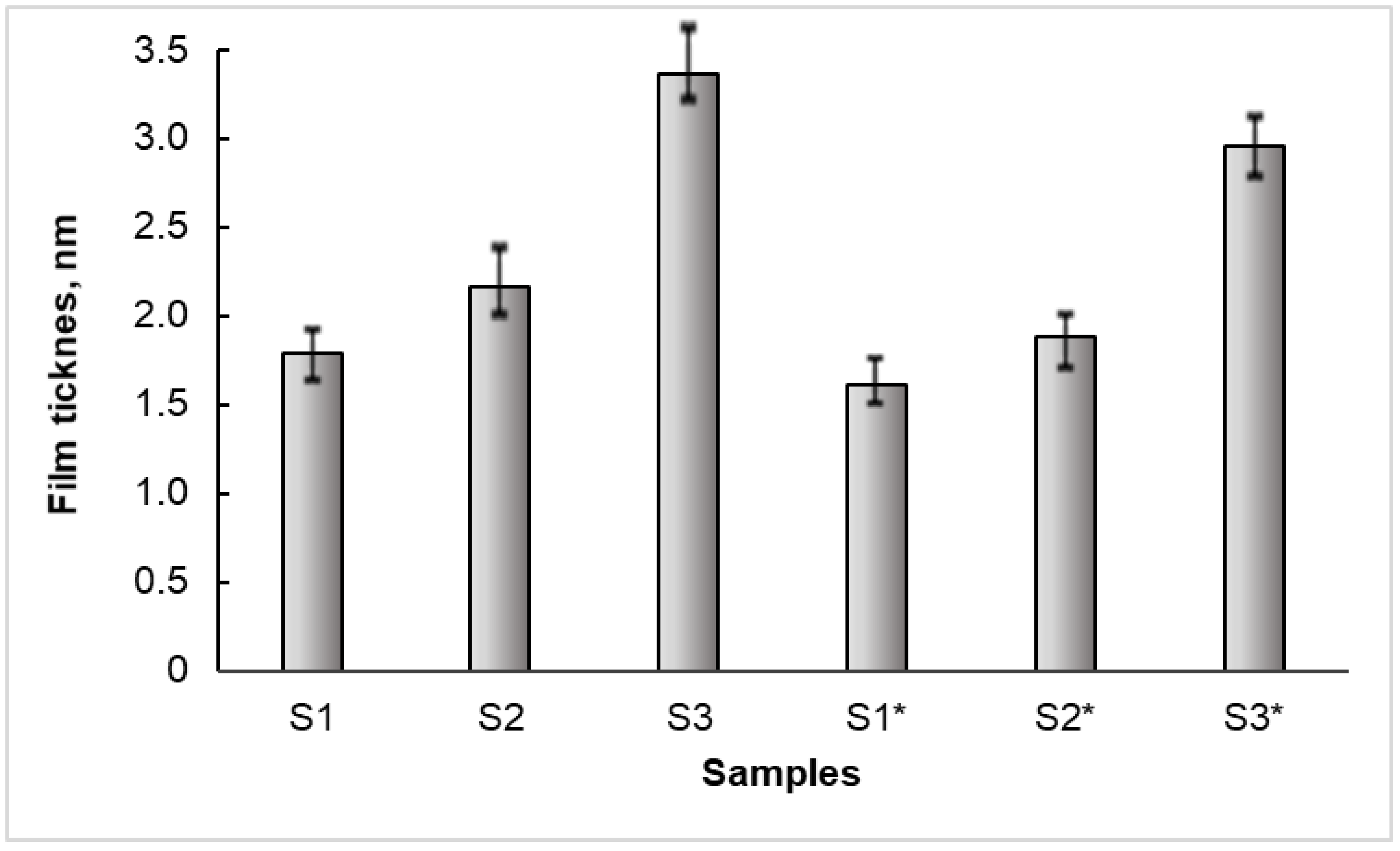
3.4. The Thickness of the Bi2S3 Films
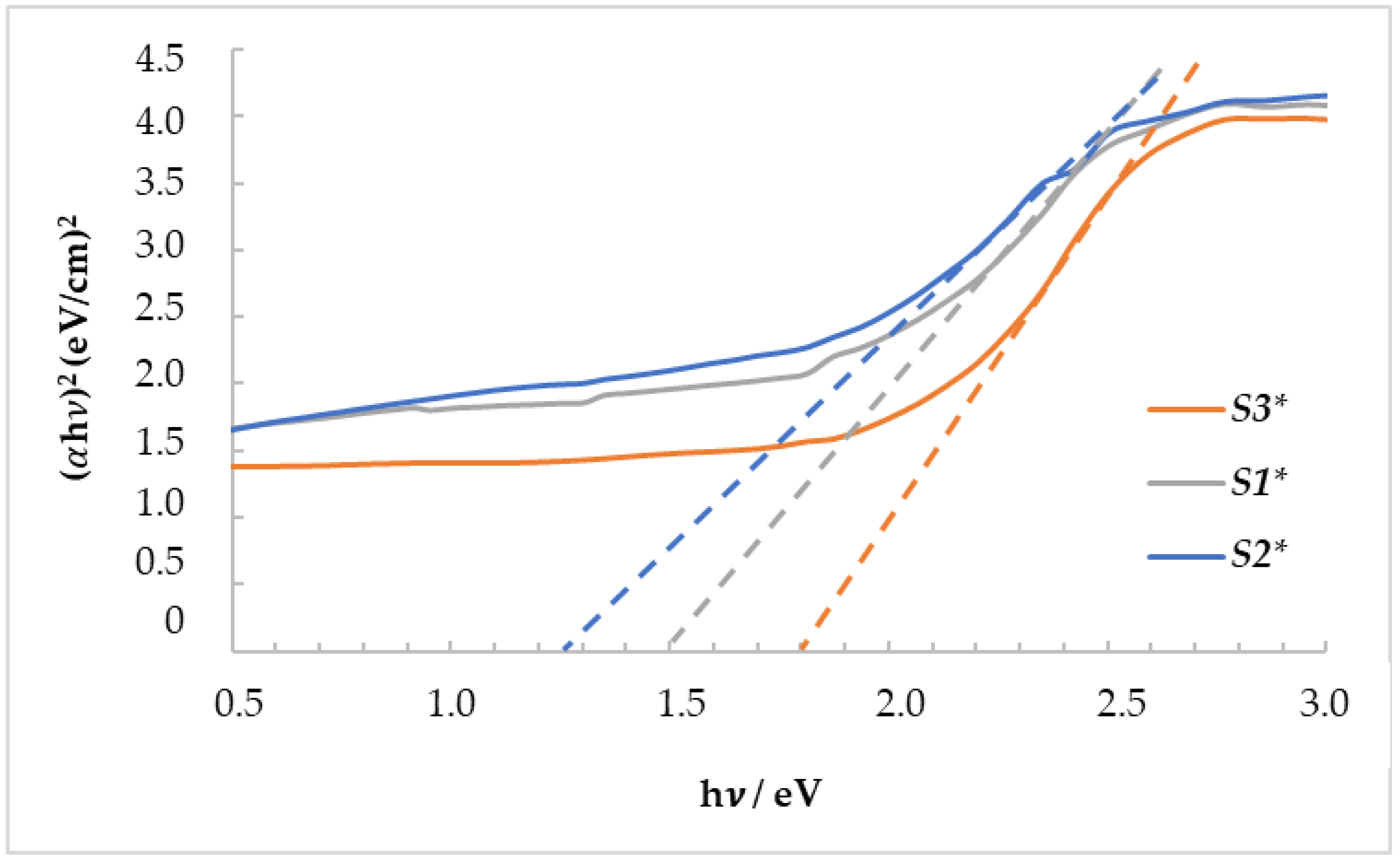
3.5. UV–Vis Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Supekar, A.T.; Bhujbal, P.K.; Salunke, S.A.; Rathod, S.M.; Patole, S.P.; Pathan, H.M. Bismuth Sulfide and Antimony Sulfide-Based Solar Cells: A Review. ES Energy Environ. 2023, 19, 848. [Google Scholar] [CrossRef]
- Fazal, T.; Ismail, B.; Shah, M.; Iqbal, S.; Elkaeed, E.B.; Awwad, N.S.; Ibrahium, H.A. Simplified Route for Deposition of Binary and Ternary Bismuth Sulphide Thin Films for Solar Cell Applications. Sustainability 2022, 14, 4603. [Google Scholar] [CrossRef]
- Renuka Devee, D.; Sivanesan, T.; Muthukrishnan, R.M.; Pourkodee, D.; Mohammed Yusuf Ansari, P.; Abdul Kader, S.M.; Ranjani, R. A novel photocatalytic activity of Bi2S3 nanoparticles for pharmaceutical and organic pollution removal in water remediation. Chem. Phys. Impact 2024, 8, 100605. [Google Scholar] [CrossRef]
- Razavi, F.S.; Mahdi, M.A.; Ghanbari, D.; Dawi, E.A.; Abed, M.J.; Ganduh, S.H.; Jasim, L.S.; Salavati-Niasari, M. Fabrication and design of four-component Bi2S3/CuFe2O4/CuO/Cu2O nanocomposite as new active materials for high performance electrochemical hydrogen storage application. J. Energy Storage 2024, 94, 112493. [Google Scholar] [CrossRef]
- Rong, P.; Gao, S.; Ren, S.; Lu, H.; Yan, J.; Li, L.; Zhang, M.; Han, Y.; Jiao, S.; Wang, J. Large-Area Freestanding Bi2S3 Nanofibrous Membranes for Fast Photoresponse Flexible IR Imaging Photodetector. Adv. Funct. Mater. 2023, 33, 2300159. [Google Scholar] [CrossRef]
- Singh, A.; Chauhan, P.; Verma, A.; Yadav, B.C. Interfacial engineering enables polyaniline-decorated bismuth sulfide nanorods towards ultrafast metal–semiconductor-metal UV-Vis broad spectra photodetector. Adv. Compos. Hybrid Mater. 2024, 7, 88. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Tang, Y.; Lu, Z.; Hu, J.; Wang, Y.; Cao, Y. Oxygen Self-Doping Bi2S3@C Spheric Successfully Enhanced Long-Term Performance in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 52423–52431. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, Z.; Lien, S.-Y.; Yu, Y.; Gao, P. Self-Powered Thermoelectric Hydrogen Sensors Based on Low-Cost Bismuth Sulfide Thin Films: Quick Response at Room Temperature. ACS Appl. Mater. Interfaces 2022, 14, 47696–47705. [Google Scholar] [CrossRef]
- Kan, H.; Yang, W.; Guo, Z.; Li, M. Highly sensitive room-temperture NO2 gas sensor based on Bi2S3 nanorods. J. Mater. Sci. Mater. Electron. 2024, 35, 331. [Google Scholar] [CrossRef]
- Terdalkar, P.; Kumbhar, D.D.; Pawar, S.D.; Nirmal, K.A.; Kim, T.G.; Mukherjee, S.; Khot, K.V.; Dongale, T.D. Revealing switching statistics and artificial synaptic properties of Bi2S3 memristor. Solid State Electron. 2025, 225, 109076. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, Y.; Huang, Q.; Huang, J.; Kuang, J.; Gu, R.; Zeng, P.; Li, H.-Y.; Liang, H.; Liu, H. Electrochemical Biosensor Employing Bi2S3 Nanocrystals-Modified Electrode for Bladder Cancer Biomarker Detection. Chemosensors 2022, 10, 48. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Zhang, J.; Liu, J.; Yu, X. Application of bismuth sulfide based nanomaterials in cancer diagnosis and treatment. Nano Today 2023, 49, 101799. [Google Scholar] [CrossRef]
- Bouachri, M.; Oubakalla, M.; El Farri, H.; Díaz-Guerra, C.; Mhalla, J.; Zimou, J.; El-Habib, A.; Beraich, M.; Nouneh, K.; Fahoume, M.; et al. Substrate temperature effects on the structural, morphological and optical properties of Bi2S3 thin films deposited by spray pyrolysis: An experimental and first-principles study. Opt. Mater. 2023, 135, 113215. [Google Scholar] [CrossRef]
- Rodríguez-Rosales, K.; Cruz-Gómez, J.; Cruz, J.S.; Guillén-Cervantes, A.; de Moure-Flores, F.; Villagrán-Muniz, M. Plasma emission spectroscopy for studying Bi2S3 produced by pulsed laser deposition and effects of substrate temperature on structural, morphological, and optical properties of thin films. Mater. Sci. Eng. B 2025, 312, 117867. [Google Scholar] [CrossRef]
- Atamtürk, U.; Jung, E.; Fischer, T.; Mathur, S. Tale of Two Bismuth Alkylthiolate Precursors’ Bifurcating Paths in Chemical Vapor Deposition. Chem. Mater. 2022, 34, 7344–7356. [Google Scholar] [CrossRef]
- Ran, Y.; Song, Y.; Jia, X.; Gu, P.; Cheng, Z.; Zhu, Y.; Wang, Q.; Pan, Y.; Li, Y.; Gao, Y.; et al. Large-Scale Vertically Interconnected Complementary Field-Effect Transistors Based on Thermal Evaporation. Small 2024, 20, 2309953. [Google Scholar] [CrossRef]
- Su, Y.-W.; Paul, B.K.; Chang, C.-H. Investigation of CdS nanoparticles formation and deposition by the continuous flow microreactor. Appl. Surf. Sci. 2019, 472, 158–164. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Onwudiwe, D.C. Bismuth sulfide based compounds: Properties, synthesis and applications. Results Chem. 2021, 3, 100151. [Google Scholar] [CrossRef]
- Mukurala, N.; Mishra, R.K.; Jin, S.H.; Kushwaha, A.K. Sulphur precursor dependent crystallinity and optical properties of solution grown Cu2FeSnS4 particles. Mater. Res. Express 2019, 6, 085099. [Google Scholar] [CrossRef]
- Behera, C.; Samal, R.; Panda, A.K.; Rout, C.S.; Samal, S.L. Synthesis of flower and biconcave shape CuS: Enhancement of super-capacitance properties via Ni–CuS nanocomposite formation. Solid. State Sci. 2021, 117, 106631. [Google Scholar] [CrossRef]
- Fazal, T.; Iqbal, S.; Shah, M.; Ismail, B.; Shaheen, N.; Alharthi, A.I.; Awwad, N.S.; Ibrahium, H.A. Correlation between structural, morphological and optical properties of Bi2S3 thin films deposited by various aqueous and non-aqueous chemical bath deposition methods. Results Phys. 2022, 40, 105817. [Google Scholar] [CrossRef]
- Koswattage, K.R.; Liyanage, C.J.; Maduwantha, G.D.K.V. Ultraviolet photoelectron spectroscopic study on the interface electronic structure of the L-cysteine on Pd surface. Surf. Interface Anal. 2022, 54, 561–566. [Google Scholar] [CrossRef]
- Toorbaf, M.; Moradi, L.; Dehghani, A. Preparation of GO/Cys-Cu(II) as a novel, effective and recoverable catalyst for the multi component synthesis of spirooxindoles under mild conditions. J. Mol. Struct. 2023, 1294, 136335. [Google Scholar] [CrossRef]
- Muralikrishna, S.; Sureshkumar, K.; Varley, T.S.; Nagaraju, D.H.; Ramakrishnappa, T. Ramakrishnappa, In situ reduction and functionalization of graphene oxide with L-cysteine for simultaneous electrochemical determination of cadmium(II), lead(II), copper(II), and mercury(II) ions. Anal. Methods 2014, 6, 8698–8705. [Google Scholar] [CrossRef]
- Melikyan, Y.; Gharagulyan, H.; Vasil’Ev, A.; Hayrapetyan, V.; Zhezhu, M.; Simonyan, A.; Ghazaryan, D.; Torosyan, M.; Kharatyan, A.; Michalicka, J.; et al. E-beam induced micropattern generation and amorphization of L-cysteine-functionalized graphene oxide nano-composites. Colloid Interface Sci. Commun. 2024, 58, 100766. [Google Scholar] [CrossRef]
- Javidparvar, A.A.; Naderi, R.; Ramezanzadeh, B. L-cysteine reduced/functionalized graphene oxide application as a smart/control release nanocarrier of sustainable cerium ions for epoxy coating anti-corrosion properties improvement. J. Hazard. Mater. 2020, 389, 122135. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-J.; Liao, X.-J.; Wang, H.; Ren, S.-W.; Cao, J.-T.; Liu, Y.-M. In Situ Formation of Bi2MoO6-Bi2S3 Heterostructure: A Proof-Of-Concept Study for Photoelectrochemical Bioassay of l-Cysteine. Front. Chem. 2022, 10, 845617. [Google Scholar] [CrossRef]
- Yan, Y.; Chang, K.; Ni, T.; Li, K. l-cysteine assisted synthesis of Bi2S3 hollow sphere with enhanced near-infrared light harvesting for photothermal conversion and drug delivery. Mater. Lett. 2019, 245, 158–161. [Google Scholar] [CrossRef]
- Tao, X.; Hu, X.; Wen, Z.; Ming, Y.; Li, J.; Liu, Y.; Chen, R. Highly efficient Cr(VI) removal from industrial electroplating wastewater over Bi2S3 nanostructures prepared by dual sulfur-precursors: Insights on the promotion effect of sulfate ions. J. Hazard. Mater. 2022, 424, 127423. [Google Scholar] [CrossRef]
- Guo, R.; Zhu, G.; Gao, Y.; Li, B.; Gou, J.; Cheng, X. Synthesis of 3D Bi2S3/TiO2 NTAs photocatalytic system and its high visible light driven photocatalytic performance for organic compound degradation. Sep. Purif. Technol. 2019, 226, 315–322. [Google Scholar] [CrossRef]
- Karsandık, Ö.; Özdal, T.; Kavak, H. Influence of thickness and annealing temperature on properties of solution processed bismuth sulfide thin films. J. Mater. Sci. Mater. Electron. 2022, 33, 18014–18027. [Google Scholar] [CrossRef]
- Hussain, A.; Begum, A.; Rahman, A. Effects of annealing on nanocrystalline Bi2S3 thin films prepared by chemical bath deposition. Mater. Sci. Semicond. Process 2014, 21, 74–81. [Google Scholar] [CrossRef]
- Ahire, R.; Deshpande, N.; Gudage, Y.; Sagade, A.; Chavhan, S.; Phase, D.; Sharma, R. A comparative study of the physical properties of CdS, Bi2S3 and composite CdS–Bi2S3 thin films for photosensor application. Sens. Actuators A Phys. 2007, 140, 207–214. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, L.; Li, J.; Yu, H. Adsorption of heavy metals by l-cysteine intercalated layered double hydroxide: Kinetic, isothermal and mechanistic studies. J. Colloid Interface Sci. 2020, 562, 149–158. [Google Scholar] [CrossRef]
- Khadka, D.B.; Kato, S.; Soga, T. Impact of annealing temperature on structural, optical and photovoltaic properties of bismuth oxysulfate thin films. Opt. Mater. 2024, 154, 115736. [Google Scholar] [CrossRef]
- El Adraa, K.; Georgelin, T.; Lambert, J.-F.; Jaber, F.; Tielens, F.; Jaber, M. Cysteine-montmorillonite composites for heavy metal cation complexation: A combined experimental and theoretical study. Chem. Eng. J. 2017, 314, 406–417. [Google Scholar] [CrossRef]
- Fazal, T.; Iqbal, S.; Shah, M.; Mahmood, Q.; Ismail, B.; Alzhrani, R.M.; Awwad, N.S.; Ibrahium, H.A.; Alam, S.; Yasir, M.; et al. Optoelectronic Analysis of Bismuth Sulfide and Copper-Doped Bismuth Sulfide Thin Films. JOM 2022, 74, 2809–2816. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Behfard, Z.; Maddahfar, M. Controllable synthesis of Bi2S3 via a simple hydrothermal approach starting from an inorganic precursor. J. Ind. Eng. Chem. 2014, 20, 4066–4075. [Google Scholar] [CrossRef]
- Coppens, P.; Yang, Y.W.; Blessing, R.H.; Copper, W.F.; Larsen, F.K. The Experimental Charge Distribution in Sulfur Containing Molecules. Analysis of Cyclic Octasulfur at 300 and 100 K. J. Am. Chem. Soc. 1977, 99, 760–766. [Google Scholar] [CrossRef]
- Pekoite, CuPbBi 11 S 18, a New Member of the Bismuthinite-Aikinite Mineral Series; Its Crystal Structure and Relationship with Naturally- and Synthetically-Formed Members | The Canadian Mineralogist | GeoScienceWorld, (n.d.). Available online: https://pubs.geoscienceworld.org/mac/canmin/article-abstract/14/3/322/11114/Pekoite-CuPbBi-11-S-18-a-new-member-of-the (accessed on 11 March 2025).
- Vishwakarma, S.R.; Upadhyay, J.P.; Prasad, H.C. Physical properties of arsenic-doped tin oxide thin films. Thin Solid Film 1989, 176, 99–110. [Google Scholar] [CrossRef]
- Ivanauskas, R.; Samardokas, L.; Mikolajunas, M.; Virzonis, D.; Baltrusaitis, J. Polyamide–thallium selenide composite materials via temperature and pH controlled adsorption–diffusion method. Appl. Surf. Sci. 2014, 317, 818–827. [Google Scholar] [CrossRef]
- Ivanauskas, A.; Ivanauskas, R.; Ancutiene, I. Effect of In-Incorporation and Annealing on CuxSe Thin Films. Materials 2021, 14, 3810. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, P.S.; Patil, L.A. Effect of nonstoichiometry on structural and optical properties of nanostructured Bi2S3 thin films prepared chemically at room temperature. Mater. Chem. Phys. 2007, 105, 157–161. [Google Scholar] [CrossRef]
- Riahi, M.; Martínez-Tomás, C.; Agouram, S.; Boukhachem, A.; Maghraoui-Meherzi, H. The effects of thermal treatment on structural, morphological and optical properties of electrochemically deposited Bi2S3 thin films. Thin Solid Film 2017, 626, 9–16. [Google Scholar] [CrossRef]
- Dachraoui, O.; Merino, J.M.; Mami, A.; León, M.; Caballero, R.; Maghraoui-Meherzi, H. Annealing study and thermal investigation on bismuth sulfide thin films prepared by chemical bath deposition in basic medium. Appl. Phys. A Mater. Sci. Process 2018, 124, 166. [Google Scholar] [CrossRef]
- Chanthong, T.; Intaratat, W.; Wichean, T.N. Effect of Thickness on Electrical and Optical Properties of ZnO:Al Films. Trends Sci. 2023, 20, 6372. [Google Scholar] [CrossRef]
- Moreno-García, H.; Messina, S.; Calixto-Rodriguez, M.; Martínez, H. Physical properties of chemically deposited Bi2S3 thin films using two post-deposition treatments. Appl. Surf. Sci. 2014, 311, 729–733. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Krishna, V.G.; Reddy, G.P.; Revathi, N.; Reddy, K.T.R. Comprehensive physical and chemical properties of sulfurized Bi2S3 films prepared by CBD process. Next Mater. 2025, 8, 100566. [Google Scholar] [CrossRef]
- Rincón, M.E.; Campos, J.; Suárez, R. A comparison of the various thermal treatments of chemically deposited bismuth sulfide thin films and the effect on the structural and electrical properties. J. Phys. Chem. Solids 1999, 60, 385–392. [Google Scholar] [CrossRef]
- Linhart, W.M.; Zelewski, S.J.; Scharoch, P.; Dybała, F.; Kudrawiec, R. Nesting-like band gap in bismuth sulfide Bi2S3. J. Mater. Chem. C Mater. 2021, 9, 13733–13738. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikas, A.; Ivanauskas, R.; Zalenkiene, S.; Mikolajūnas, M. Bismuth(III) Sulfide Films by Chemical Bath Deposition Method Using L-Cysteine as a Novel Sulfur Source. Crystals 2025, 15, 515. https://doi.org/10.3390/cryst15060515
Melnikas A, Ivanauskas R, Zalenkiene S, Mikolajūnas M. Bismuth(III) Sulfide Films by Chemical Bath Deposition Method Using L-Cysteine as a Novel Sulfur Source. Crystals. 2025; 15(6):515. https://doi.org/10.3390/cryst15060515
Chicago/Turabian StyleMelnikas, Aistis, Remigijus Ivanauskas, Skirma Zalenkiene, and Marius Mikolajūnas. 2025. "Bismuth(III) Sulfide Films by Chemical Bath Deposition Method Using L-Cysteine as a Novel Sulfur Source" Crystals 15, no. 6: 515. https://doi.org/10.3390/cryst15060515
APA StyleMelnikas, A., Ivanauskas, R., Zalenkiene, S., & Mikolajūnas, M. (2025). Bismuth(III) Sulfide Films by Chemical Bath Deposition Method Using L-Cysteine as a Novel Sulfur Source. Crystals, 15(6), 515. https://doi.org/10.3390/cryst15060515






