Abstract
In this study, the phase transformation mechanism during the decomposition of undercooled austenite and its effect on the deformation behavior of a high-strength medium Mn steel were studied. The results indicate that the austenite formation during heating (α → γ) is a relatively fast reaction. However, the transformation of undercooled prior austenite above the martensite start (Ms) temperature (γ → α) is difficult due to its high thermal stability. Only martensite transformation occurred during the final air-cooling stage following a 120-h isothermal treatment at 360 °C (slightly above Ms). The growth of martensite laths was limited by the boundaries of prior austenite grains and martensite packets. High-strength tensile properties were achieved, with a yield strength of 955 MPa, ultimate tensile strength of 1228 MPa, and total elongation of 11.6%. These properties result from the synergistic hardening effects of grain refinement, high-density lattice distortion, and an increased boundary length per unit area. The composition design with medium Mn content increased the processing window for high-strength martensite transformation, providing a theoretical basis for an energy-saving approach that depends on the decomposition transformation of undercooled austenite.
1. Introduction
With the growing demand for environmentally friendly and energy-efficient materials, advanced high-strength steels (AHSS) with short preparation processes show great potential for practical applications [1,2]. As a promisong candidate of the third generation of AHSS, medium Mn steels—with a wide range of mechanical properties—have great application potential in engineering machinery, automotive, and other sectors [3,4]. These steels usually produce an ultrafine microstructure consisting of a ferrite matrix (i.e., heavily tempered martensite) and C- and Mn-enriched metastable retained austenite (RA). Their excellent mechanical properties arise from the transformation-induced plasticity (TRIP) effect and/or the twinning-induced plasticity (TWIP) effect [5,6,7].
The austenite reversion treatment (ART) and/or quenching–partitioning (Q&P) process are recognized as the dominant heat treatment processes in medium Mn steels. In the ART process, the matrix (composed of nearly fully martensite or a mixture of martensite and pre-existing austenite) is annealed within the intercritical α + γ phase region. This treatment produces an ultrafine microstructure composed of ferrite and austenite, during which the thermal stability of the intercritical austenite is enhanced by the partitioning of C and Mn from the α phase into the γ phase [8,9,10]. The TRIP and/or TWIP effect is activated during deformation, resulting in localized work hardening and enabling a greater balance between strength and ductility. Kumat et al. [11] carried out intercritical annealing on 7.5 Mn steel at 760 °C for 1 h and obtained excellent tensile properties, with an ultimate tensile strength (UTS) of 1100 MPa and a total elongation (TEL) of 34.8%. However, the ferrite matrix obtained through intercritical isothermal treatment is softer than a martensite or tempered martensite matrix due to recovery and recrystallization, which leads to a reduction in yield strength [12,13,14]. In the Q&P process, a fully or partially austenitized state is first obtained in the steel, followed by quenching to a temperature (QT) to obtain supersaturated martensite and untransformed austenite. The material is then held at a specified partitioning temperature (PT; often ≥QT), during which C partitions from the martensite into the austenite [15,16,17]. Recently, Li et al. [18] achieved a UTS of 1650 MPa and a TEL of 11.06% in a 7.0 Mn steel after a partitioning treatment at 300 °C. However, these excellent mechanical properties depend on precise temperature control during the first quenching process, which limits processability in industrial production. The key to RA in ART and Q&P processes is effective partitioning of austenite-stabilizing elements.
Based on research into the partitioning behavior of austenite stabilizers, Somani et al. carried out an energy-saving strategy called direct quenching and partitioning (DQ&P) in 0.2C steels [19,20]. The prior austenite was directly quenched to PT (PT = QT), followed by slow furnace cooling over 30 h. Dynamic carbon partitioning from the preferentially transformed martensite to the nearby untransformed austenite occurred utilizing the residual heat from the hot-rolled steel coil [21]. The short preparation process provides a pathway for the production of medium Mn steels. In fact, Mn is also a potent hardenability-promoting element in steel, i.e., high-strength martensite can be obtained during a relatively slow cooling process. Research on the undercooled austenite transformation mechanism in medium Mn steel is expected to address the challenge of controlling the PT/QT in the industrial implementation of the DQ&P process. Thus, it is essential to study the evolution of the microstructure and its effect on the deformation behavior of medium Mn steels.
In this study, the undercooled austenite transformation mechanism during continuous cooling and low-temperature zone isothermal heat treatment was explored. The austenite thermal stability, characteristics of the resulting transformation products, and the deformation behavior were systematically discussed. The difference between austenite reversion transformation and undercooled austenite transformation was also studied. This work provides theoretical support for process design strategies that depend on the decomposition transformation of undercooled austenite.
2. Experimental Procedures and Methods
A 150 kg ingot of the experimental steel was cast in a vacuum induction furnace and then hot forged to a thickness of 60 m. The chemical composition of 5.5 Mn steel is listed in Table 1.

Table 1.
Chemical composition of the experimental steel (mass fraction/%).
The phase transformation temperatures were measured using a Formastor-FII dilatometer (Fuji Denki Kogyo Co., Ltd., Osaka, Japan). For the continuous cooling transformation (CCT) process, the samples were reheated to 1200 °C at a linear rate of 10 °C/s, held for 180 s to achieve full austenitization, and finally cooled to 50 °C rates ranging from 0.5 to 30 °C/s. A schematic of the process is shown in Figure 1a. For the slow cooling rate process, the sample was first cooled to 900 °C (a fully austenitization state) at a rate of 5 °C/s after homogenization treatment. t was then cooled slowly to 450 °C at a rate of 0.05 °C/s to measure the Ar1 temperature (the austenite-to-ferrite transformation finish temperature during cooling). Finally, the sample was cooled to 50 °C at a rate of 30 °C/s. The process is shown in Figure 1b (Process 1). For the isothermal heat treatment simulation process, the samples were cooled to 550, 450, and 400 °C at a rate of 5 °C/s after homogenization treatment. The samples were then maintained in low-temperature zone for 2 h to study the phase transformation behavior. The samples were finally cooled to 50 °C at a rate of 30 °C/s. This process is shown in Figure 1b (Process 2). The corresponding samples are referred to as I550, I450, and I400, respectively.
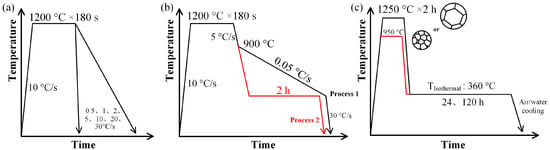
Figure 1.
Schematic of the phase transformation temperature determination and heating processes. (a) Continuous cooling transformation processes; (b) slow cooling and isothermal heat treatment simulation processes; (c) isothermal heat treatment processes.
Figure 1c shows the isothermal heat treatment processes that were produced to investigate the structure–property relationships of the experimental steels. The steels were reheated to the austenitization temperature and held for 2 h to homogenize. Then, they were air cooled to 360 °C and isothermally held for 24 h or 120 h. Finally, the samples were cooled to room temperature. The parameters of the treatment processes are provided in Table 2. Austenitization temperatures of 950 °C and 1250 °C were used to obtain different types of undercooled prior austenite. The isothermal temperature of 360 °C (slightly higher than the martensite start temperature (Ms) and soaking times of 24 h and 120 h were used to study the thermal stability of prior austenite. Air cooling and water quenching were selected as the final cooling methods to study the characteristics of microstructure evolution.

Table 2.
Parameters of the isothermal treatment process in Figure 1c.
The equilibrium phase transformation temperatures were calculated using Thermo-Calc software 2023. The microstructure morphology and tensile fracture surfaces were observed using a Zeiss Ultra 55 scanning electron microscope (SEM, Carl Zeiss, Oberkochen, Germany). The crystallographic information was examined using an electron backscattered diffractometer (EBSD, Gemini 300, Carl Zeiss, Oberkochen, Germany) and AztecCrystal processing software v2.1.259. For the SEM micrograph observation, the specimens were mechanically ground and polished, and then etched with a 4 vol% nital solution for ~15 s. The EBSD specimens were mechanically ground and then electro-polished with an electrolyte of 92 vol% CH3CH2OH and 8 vol% HClO4 at an electric current of 0.5 A for ~20 s.
The SANS UTM5105 (SUNS Technology Stock Co., Ltd., Shenzhen, China) standard testing machine was used for tensile tests at a crosshead speed of 3 mm/min at room temperature. Standard tensile samples were prepared with a gage length of 25 mm and thickness off 2 mm. Vickers hardness tests were conducted using a MHV 1000Z (Truer, Shanghai, China) microhardness tester with a 500 gf load and load time of 15 s. Tensile properties and hardness values for each condition were obtained from three and five tests, respectively.
3. Results and Discussion
3.1. Phase Transformation Characteristic of Undercooled Prior Austenite
Figure 2 shows the phase transformation temperature and SEM micrographs of the samples subjected to the continuous cooling processes in Figure 1a. The phase transformation temperatures of undercooled prior austenite were determined from temperature–dilation curves using the tangent method. The rise and fall of the upper curve in Figure 2a,b show the austenite transformations during the heating stage. The range of Ms temperature for these samples was 308–323 °C. The results indicate that martensite transformation as the only phase change observed within the cooling rate range of 0.5–30 °C/s. During the cooling process, the medium Mn content expanded the austenite region. The high-temperature phase transformation before martensite transformation was inhibited due to the high thermal stability of the undercooled prior austenite. The black dotted lines in Figure 2d represent the prior austenite grain boundaries (PAGBs). The martensite packets with different orientations were observed in a prior austenite grain. The martensite solid solution matrix was obtained in the 30 °C/s sample. The element diffusion was inhibited due to the quick cooling rate and insufficient diffusion time, resulting in the formation of supersaturated martensite. For the 0.5 °C/s sample, the short rod-like cementite was observed in the lath-like martensite, as observed in [9,22]. During the slow cooling process, the supersaturated carbon atoms in the preferentially transformed martensite and the alloy atoms were precipitated as cementite in chemical form. The solid solubility provided the driving force for C diffusion.
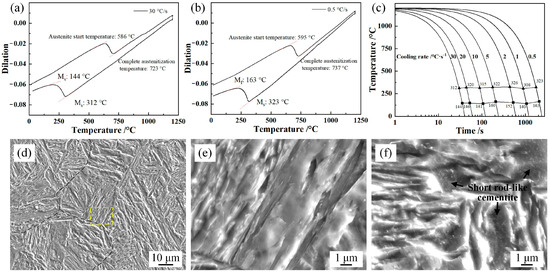
Figure 2.
Temperature–dilation curves, CCT curves, and SEM micrographs of the samples subjected to the processes in Figure 1a: (a) 30 °C/s sample; (b) 0.5 °C/s sample; (c) CCT curves; (d) SEM micrograph of 30 °C/s sample; (e) SEM micrograph of the dashed area in (d); (f) SEM micrograph of the 0.5 °C/s sample.
Figure 3 shows the temperature–dilation curves of the samples subjected to the heat treatment processes in Figure 1b. During the heating stage of Process 1, austenite formation (α → γ) occurred over ~14 s, from the start temperature (591 °C) to complete austenitization (733 °C), as shown in Figure 3a. The equilibrium transformation temperature of complete austenitizing was 720 °C (Figure 3b), which was lower than 733 °C because of the superheat degree of phase transformation in the heating stage. During the slow cooling stage at a rate of 0.05 °C/s, no phase transformation (γ → α) was observed within the temperature range of 720 °C to 450 °C and over a duration of 5400 s. In addition, there was also no phase transformation during the isothermal treatment at 550, 450, or 400 °C, as shown in Figure 3d–f. Only martensite transformation occurred in these samples during the final cooling stage. Thus, austenite formation (α → γ) during austenite reversion treatment is significantly faster than the undercooled austenite transformation (γ → α) during the cooling process [7,23,24,25].
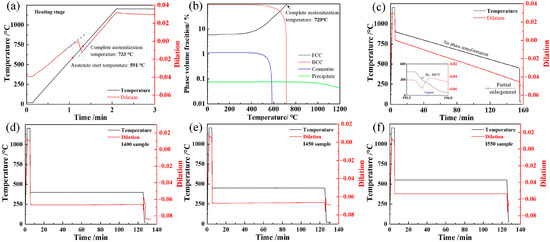
Figure 3.
Temperature–dilation curves of the samples subjected to the processes in Figure 1b and the equilibrium phase diagram. (a) SC450 sample during the heating stage; (b) equilibrium phase diagram; (c) SC450 sample; (d) I400 sample; (e) I450 sample; (f) I550 sample.
3.2. Microstructure Evolution During Undercooled Prior Austenite Transformation
Isothermal treatment processes were conducted to investigate the effect of prior austenite stability on phase transformation behavior and its effect on mechanical properties. The SEM micrographs of variants A, B, and C are shown in Figure 4. In variant A, the lath martensite matrix with short rod-like cementite was observed, as shown in Figure 4a,d. In contrast, the lath martensite solid solution matrix without cementite was obtained in variant B, as shown in Figure 4b,e. The results indicate that no cementite formed during the air-cooling stage after austenitization and the subsequent isothermal treatment at 360 °C for 24 h. Cementite formed from lath martensite as a result of variant C supersaturation during the transformation from a face-centered cubic (FCC) to body-centered cubic (BCC) structure. That is, no isothermal phase transformation occurred in variants A and B. To reduce the thermal stability of undercooled prior austenite, a higher austenitization temperature of 1250 °C and longer isothermal time of 120 h were adopted for variant C. However, the lath martensite solid solution matrix in Figure 4f proves that undercooled prior austenite transformation (above Ms temperature) is difficult. Thus, the medium Mn composition design broadens the processing window for the formation of high-strength martensite. The grain boundaries (GBs) and martensite packet boundaries (PBs) are marked with black dotted lines and red dotted lines, respectively. The growth of martensite lath was limited by GBs and PBs [26]. Thus, refined martensite was obtained in variants A and B, which were subjected to relatively lower austenitization temperatures.
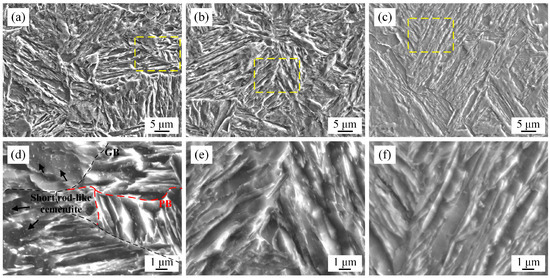
Figure 4.
SEM micrographs of experimental steels subjected to the heat treatment processes in Figure 1c. (a,d) Variant A; (b,e) variant B; (c,f) variant C; (d–f) the corresponding partial enlargement (yellow square) micrograph.
The EBSD characterization results are shown in Figure 5. The austenite phase is shown in red. Because of the high hardenability of the experimental 5.5 Mn steel, an almost fully martensite structure was obtained, with martensite contents of 99.6 vol.% (variant B) and 99.8 vol.% (variant C). High-angle boundaries (HAGs) with misorientation angles of 15° and above are represented by black lines and the low-angle boundaries (LAGs) with misorientation angles between 2 and 15° are represented by yellow lines. The boundary lengths per unit area of variant B and variant C are 4.1 and 3.34 μm−1, respectively. Based on the band contrast, boundary distribution, and inverse pole figure maps, the HAGs were mainly distributed at the GBs, PBs, and martensite block boundaries (BBs). The boundaries between the martensite laths in a block exhibited LAGs [27]. Thus, a relatively higher proportion of HAGs was observed in variant B, attributed to its refined prior austenite grain structure. During deformation, microcrack propagation could be deflected or terminated by HAGs. In addition, the high density of boundaries in variant B enhanced the work-hardening effect through intensified dislocation interactions [28,29].
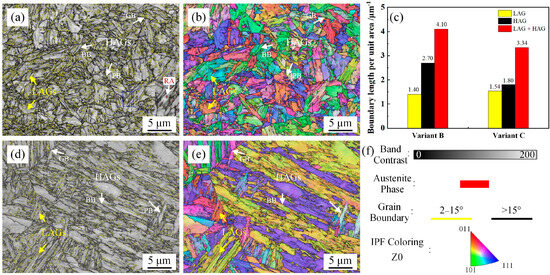
Figure 5.
EBSD results of variants B and C. (a,d) Combined map of the band contrast image and boundary distribution map; (b,e) Combined map of the inverse pole image and boundary distribution map; (c) boundary length per unit area; (f) the corresponding legends; (a,b) Variant B; (d,e) Variant C.
3.3. The Relationship Between the Microstructure and Mechanical Properties
The tensile properties and mean hardness are shown in Table 3, and the corresponding tensile curves are shown in Figure 6. The strength of Rp0.2 is determined as the yield strength (YS). High-strength tensile properties were obtained, with a combination of YS, UTS, and TEL exceeding 830 MPa, 1130 MPa, and 11.6%, respectively. Due to the high thermal stability of prior austenite, phase transformation during the air-cooling stage following austenitization and isothermal heat treatment was completely inhibited. As a result, undercooled austenite transformation occurred only during the final cooling process. Therefore, the lath martensite matrix was formed. In variant B, a refined martensite matrix was observed due to the lower austenitization temperature of 950 °C and the use of water quenching in the final cooling stage. The supersaturated C atoms in martensite exist in the form of the interstitial solid solution, resulting in a significant lattice distortion. The dislocation interaction was more severe at the initial plastic deformation stage, resulting in a higher work-hardening rate, as shown in Figure 6b. In variant A, the strength and Vickers hardness decreased due to the recovery of preferentially transformed martensite during the air-cooling process. For variant C, the coarse microstructure and relatively lower boundary length per unit area were obtained, leading to a decrease in strength and hardness.

Table 3.
Tensile properties and Vickers hardness of the experimental steel.
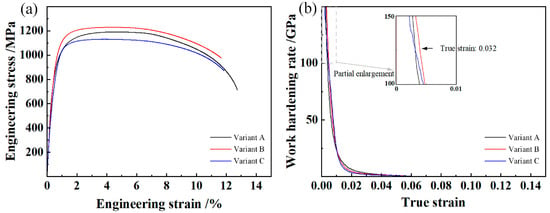
Figure 6.
Engineering stress–strain curves (a) and work-hardening rate–true strain curves (b) of the experimental steel.
SEM micrographs of tensile fractures are shown in Figure 7, in which zone A represents the rapid crack propagation zone and zone B represents the plastic deformation concentration zone. In variant A, zone A was composed of flat dimples and some deep dimples, and zone B consisted of deep dimples, indicative of the void-mode fracture characteristic (Figure 7b,c). In variant B, the small dimples and quasi-cleavage surfaces were found in zone A, while the size of dimples in zone B was decreased (Figure 7e,f). The deep dimples were expected to absorb more energy than the small dimples and quasi-cleavage surfaces, resulting in decreased plasticity in variant B [30]. The reason is that C-supersaturated martensite was obtained in variant B due to the quick cooling rate and insufficient diffusion time. For variant A, the partial carbon atom diffused from the preferentially transformed martensite and precipitated as cementite in the chemical form, thereby reducing lattice distortion and improving plasticity. In variant C, the small elongated dimples and quasi-cleavage surfaces were observed in zone A, and the morphology in zone B was similar to variant B (Figure 7h,i). The plasticity of variant B (TEL of 11.6%) under a higher stress state was similar to that of variant C (TEL of 11.8%), which depended on the effect of grain refinement.
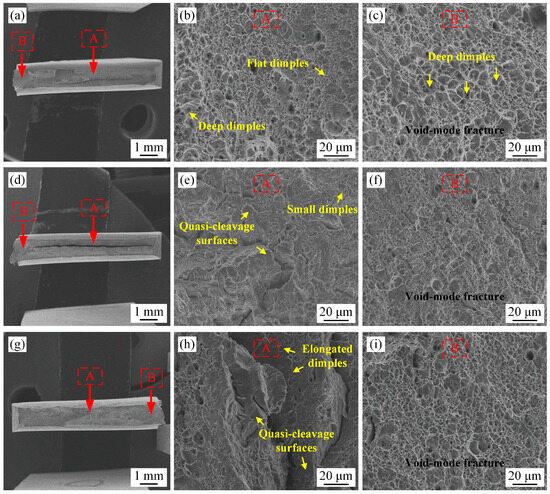
Figure 7.
Tensile fracture morphology of experimental steel (a–c) Variant A; (d–f) variant B; (g–i) variant C; (a,d,g) facture macrostructure; (b,e,h) zone A; (c,f,i) zone B.
4. Conclusions
Austenite formation on heating (α → γ through reverse phase transformation) is a relatively fast reaction. However, the transformation of undercooled prior austenite to ferrite (γ → α) during the cooling process, above the Ms temperature, is difficult to achieve. The medium Mn composition design broadens the processing window for producing high-strength martensite. In contrast with the Q&P and DQ&P processes (which need quenching to temperatures between Ms and martensite finish temperatures), high-strength martensite can be obtained over a wide range of cooling rates. This broader processing range is expected to solve the industrial challenge of precisely controlling the quenching strategy. Prior austenite can be directly cooled to PT, followed by dynamic carbon partitioning to obtain an appropriate amount of retained austenite. This strategy provides the theoretical basis for the preparation (depending on the undercooled austenite transformation behavior) of high-strength medium Mn steels.
In this study, the phase transformation mechanism and deformation behavior of a 1.2 GPa high-strength medium Mn steel were investigated. The main conclusions are summarized as follows:
(1) The austenite formation upon heating (α → γ) is a relatively fast reaction. It takes ~14 s from the starting temperature (591 °C) to complete austenitization (733 °C) at a linear heating rate of 10 °C/s.
(2) The processing window of high-strength martensite is broadened by medium Mn composition design. The undercooled prior austenite transformation above Ms temperature (γ → α) is challenging due to the high thermal stability of undercooled prior austenite. No phase transformation occurred in the air-cooling stage after austenitization and isothermal heat treatment at 360 °C for 120 h, and only martensite was formed during the final cooling process.
(3) The growth of martensite lath is limited by the boundaries of the prior austenite grain and martensite packet. HAGs are mainly distributed on the boundaries of the grain, martensite packet, and martensite block. The refined martensite and higher proportion of HAGs are obtained in the variants with a relatively lower austenitization temperature.
(4) The high-strength tensile properties with a combination of YS of 955 MPa, UTS of 1228 MPa, and TEL of 11.6% are obtained via the synergistic hardening effect of grain refinement, high-density lattice distortion, and long boundary length.
Author Contributions
Conceptualization, Y.D. (Ying Dong) and T.L.; data curation, L.M. and J.C.; formal analysis, Y.D. (Ying Dong), J.X., Q.M., H.C. and T.L.; funding acquisition, Y.D. (Ying Dong) and C.Z.; investigation, Y.D. (Ying Dong), J.C. and Q.F.; methodology, J.X., L.M. and Y.D. (Yu Du); project administration, Y.D. (Ying Dong); resources, Y.D. (Ying Dong) and C.Z.; software, H.C., T.L. and Q.F.; supervision, Y.D. (Yu Du), T.L. and C.Z.; writing—original draft, Y.D. (Ying Dong) and J.X.; validation, Y.D. (Ying Dong), Q.M. and C.Z.; visualization, Y.D. (Yu Du) and Q.F.; writing—review and editing, Y.D. (Ying Dong). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Henan Province Science and Technology Research Project (242102231033) and Key Research and Development Special Project of the Science and Technology Program of Nanyang (24ZDYF018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Lingming Meng was employed by the company Nanjing Iron and Steel Co., Ltd. Author Qinghao Miao weas employed by the company Wolong Electric Nanyang Explosion Protection Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Das, A. Understanding ductile fracture characteristics of an advanced high strength steel. Mater. Lett. 2025, 387, 138214. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Patra, P.K.; Jha, R. AHSS applications in Industry 4.0: Determination of optimum processing parameters during coiling process through unsupervised machine learning approach. Mater. Today Commun. 2022, 31, 103625. [Google Scholar] [CrossRef]
- Sadeghpour, S.; Aliabad, R.S.; Wang, S.; Javaheri, V.; Singh, H.; Somani, M.; Suh, D.W.; Karjalainen, P.; Komi, J. Significant austenite decomposition during slow heating of cold-rolled medium Mn steel with a high fraction of pre-existing austenite. Scr. Mater. 2025, 262, 116636. [Google Scholar] [CrossRef]
- Sun, B.; Alisson, K.D.S.; Wu, Y.; Ma, Y.; Chen, H.; Scott, C.; Ponge, D.; Raabe, D. Physical metallurgy of medium-Mn advanced high-strength steels. Int. Mater. Rev. 2023, 68, 786–824. [Google Scholar] [CrossRef]
- Jiang, J.l.; Liu, Y.; Yu, C.S.; Kang, J.; Li, X.L.; Li, Y.J.; Yuan, G.; Wang, G.D. In situ study of deformation behavior and strain partitioning in low-density medium Mn steel. Mater. Des. 2025, 113893. [Google Scholar] [CrossRef]
- Chen, P.C.; Peng, T.T.; Chan, Y.C.; Chen, J.M.; Chang, C.P. The effect of deformation temperature on the deformation mechanism of a medium-Mn advanced high-strength steel (AHSS). Crystals 2023, 13, 328. [Google Scholar] [CrossRef]
- Dong, Y.; Xiang, L.Y.; Zhu, C.J.; Du, Y.; Xiong, Y.; Zhang, X.Y.; Du, L.X. Analysis of phase transformation thermodynamics and kinetics and its relationship to structure-mechanical properties in a medium-Mn high strength steel. J. Mater. Res. Technol. 2023, 27, 5411–5423. [Google Scholar] [CrossRef]
- Dai, Z.B.; Chen, H.; Ding, R.; Lu, Q.; Zhang, C.; Yang, Z.G.; Zwaag, S.V.D. Fundamentals and application of solid-state phase transformations for advanced high strength steels containing metastable retained austenite. Mater. Sci. Eng. R Rep. 2021, 143, 100590. [Google Scholar] [CrossRef]
- Hu, B.J.; Zheng, Q.Y.; Lu, Y.; Jia, C.N.; Liang, T.; Zheng, C.W.; Li, D.Z. Stabilizing austenite via intercritical Mn partitioning in a medium Mn steel. Scr. Mater. 2023, 225, 115162. [Google Scholar] [CrossRef]
- Liu, R.D.; Hu, Z.P.; Lin, C.Q.; Yang, D.P.; Gu, X.L.; Xu, X.; Guo, J.Y. A novel design to eliminate Lüders band in medium-Mn steel and its microstructure-property relationship. Crystals 2023, 13, 936. [Google Scholar] [CrossRef]
- Kumar, D.; Sanyal, S.; Yadav, M.K.; Sen, I.; Bandyopadhyay, T.K. Understanding the TRIP effect in hot- and cold-rolled Al-added medium-Mn steels: Insights into austenite stability and martensitic transformation kinetics. Mater. Today Commun. 2025, 44, 111955. [Google Scholar] [CrossRef]
- Kantanen, P.; Anttila, S.; Karjalainen, P.; Latypova, R.; Somani, M.; Kaijalainen, A.; Kömi, J. Microstructures and mechanical properties of three medium-Mn steels processed via quenching and partitioning as well as austenite reversion heat treatments. Mater. Sci. Eng. A-Struct. 2022, 847, 143341. [Google Scholar] [CrossRef]
- Bharti, K.; Sharma, N.K. Factors influencing austenite stability during intercritical annealing and effect on mechanical properties of low-density medium Mn steels. Mater. Chem. Phys. 2025, 341, 130877. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.Y.; Gong, W.; Rong, X.Q.; Harjo, S.; Wu, W.H.; Lu, Q.; Nakada, N.; Yang, Z.G.; Chen, H. On the role of austenite stability in yielding behavior of a medium Mn steel with a duplex austenite-martensite microstructure. Acta Mater. 2025, 288, 120840. [Google Scholar] [CrossRef]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, M.H.; Gu, G.; Lee, J.S.; Suh, D.W. Quenching and partitioning (Q&P) processed medium Mn steel starting from heterogeneous microstructure. Materialia 2020, 12, 100757. [Google Scholar]
- Seo, E.J.; Cho, L.; De Cooman, B.C. Application of quenching and partitioning processing to medium Mn steel. Metall. Mater. Trans. A 2015, 46, 27–31. [Google Scholar] [CrossRef]
- Bai, P.F.; Ren, J.Z.; Luan, Z.W.; Yin, L.T.; Li, D.J.; Xiong, Y.; Ren, F.Z. Microstructure and properties of Mn 7 medium-Mn steel at different partitioning temperatures. Mater. Sci. Eng. A-Struct. 2025, 922, 147675. [Google Scholar] [CrossRef]
- Somani, M.C.; Porter, D.A.; Karjalainen, L.P.; Suikkanen, P.P.; Misra, R.D.K. Process design for tough ductile martensitic steels through direct quenching and partitioning. Mater. Today Proc. 2015, 2, 631–634. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, D.; Liu, D.; Kang, J.; Yuan, G.; Mao, Q.J.; Misra, R.D.K.; Wang, G.D. Combined thermo-mechanical controlled processing and dynamic carbon partitioning of low carbon Si/Al-Mn steels. Mater. Sci. Eng. A-Struct. 2018, 732, 298–310. [Google Scholar] [CrossRef]
- Ghosh, S.; Kaikkonen, P.; Javaheri, V.; Kaijalainen, A.; Miettunen, I.; Somani, M.; Kömi, J.; Pallaspuro, S. Design of tough, ductile direct quenched and partitioned advanced high-strength steel with tailored silicon content. J. Mater. Res. Technol. 2022, 17, 1390–1407. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.X.; Liu, H.; Sun, G.S.; Xie, H.; Yi, H.L.; Misra, R.D.K. Structure-mechanical property relationship in a low-C medium-Mn ultrahigh strength heavy plate steel with austenite-martensite submicro-laminate. Mater. Sci. Eng. A-Struct. 2015, 647, 144–151. [Google Scholar] [CrossRef]
- Kwok, T.; Dye, D. A review of the processing, microstructure and property relationships in medium Mn steels. Int. Mater. Rev. 2023, 68, 1098–1134. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y.N.; Zhu, C.J.; Du, Y.; Xiong, Y.; Zhu, L.; Du, L.X. Decomposition mechanism of prior austenite in low-temperature zone and its relationship to fracture behavior of high-strength medium-Mn steel. Steel Res. Int. 2023, 94, 2300234. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Q.Z.; Yan, Y. Processing, microstructure, mechanical properties, and hydrogen embrittlement of medium-Mn steels: A review. J. Mater. Res. Technol. 2024, 201, 47–57. [Google Scholar] [CrossRef]
- Strife, J.R.; Carr, M.J.; Ansell, G.S. The effect of austenite prestrain above the Md temperature on the martensitic transformation in Fe-Ni-Cr-C alloys. Metall. Trans. A 1977, 8, 1471–1484. [Google Scholar] [CrossRef]
- Duan, R.H.; Xie, G.M.; Luo, Z.A.; Xue, P.; Wang, C.; Misra, R.D.K.; Wang, G.D. Microstructure, crystallography, and toughness in nugget zone of friction stir welded high-strength pipeline steel. Mater. Sci. Eng. A-Struct. 2020, 791, 139620. [Google Scholar] [CrossRef]
- Yin, W.; Briffod, F.; Hu, H.; Yamazaki, K.; Shiraiwa, T.; Enoki, M. Role of prior austenite grain boundary and retained austenite in strain localization of medium-carbon high-strength steels. Acta Mater. 2024, 281, 120422. [Google Scholar] [CrossRef]
- Fleischhauer, Y.A.S.A.; Eduardo, B.P.; Guzela, L.R.; Ribeiro, R.M.; Mendes, M.C.; Araujo, L.S. Effect of thermomechanical processing on the grain boundary character distribution and mechanical properties of biomedical stainless steels. J. Mater. Res. Technol. 2025, 36, 3094–3103. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.Y.; Meng, Q.W.; Wang, L.Y.; Li, Y.Z.; Xu, W. Tailoring retained austenite and mechanical property improvement in Al-Si-V containing medium Mn steel via direct intercritical rolling. Mater. Sci. Eng. A-Struct. 2022, 855, 143904. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).