Abstract
The development of efficient scintillators emitting in the red and/or infrared spectral range represents an important scientific challenge, as such materials could find numerous practical applications. This work studies newly grown CsI:Yb,Sm and CsI:Eu,Sm single crystals demonstrating red and infrared luminescence. We measured luminescence spectra in the visible and near-IR range, excitation spectra across visible, UV, VUV, and X-ray ranges, Raman spectra, and thermoluminescence spectra. The results show that divalent europium and ytterbium ions can efficiently transfer excitation to samarium ions. The light output of the obtained crystals under X-ray excitation was roughly estimated from the X-ray luminescence spectra, with expected values of 37,000 photons/MeV for CsI:Yb,Sm and 40,000 photons/MeV for CsI:Eu,Sm.
Keywords:
cesium iodide; scintillator; europium; ytterbium; samarium; luminescence; single crystal; crystal growth 1. Introduction
Red-emitting scintillators represent a crucial class of materials capable of converting ionizing radiation energy into red and near-infrared luminescence. These materials are of significant interest for radiation monitoring, medical imaging, and high-energy physics, as their spectral characteristics closely match the sensitivity range of semiconductor photodetectors. Ultra-compact yet highly sensitive scintillator–photodetector assemblies could be particularly valuable for real-time dosimetry monitoring during radiation therapy [1,2,3,4]. Traditional scintillator materials often rely on trivalent rare-earth ions as activators; however, divalent samarium () has emerged as a promising alternative for NIR emission [5,6]. exhibits characteristic 5d→4f transitions that result in strong emission in the red and NIR regions, making it suitable for applications requiring efficient light conversion at these wavelengths. Additionally, divalent samarium exhibits low self-absorption, which contributes to high light yield even at high activator concentrations [7]. However, the practical application of -activated scintillators can be limited by the need for efficient energy transfer mechanisms [8,9,10]. To address these limitations, sensitization strategies involving co-doping with other rare-earth ions such as ytterbium () [6,11] or europium () have been explored [9,12]. and can act as efficient sensitizers, absorbing energy from the host lattice and transferring it to via energy transfer processes, thereby enhancing the overall scintillation efficiency. Our previous studies [13,14] of activated CsI single crystals have revealed that while samarium ions exhibit efficient red emission through transitions, their practical application is limited by poor spectral overlap between the host exciton emission band and absorption. Therefore, the luminescence of exhibits low intensity under X-ray excitation. However, co-doping with suitable sensitizers can enhance energy transfer and increase X-ray-excited luminescence. The optimal impurities for this co-doping strategy are and , as the energy transfer processes involving these activators are relatively efficient [13,15]. This article focuses on the development and characterization of novel scintillator materials based on CsI crystals activated with divalent samarium () and sensitized with ytterbium () or europium (). We explore the luminescence and scintillation properties of these materials under visible, near ultraviolet, vacuum ultraviolet, and X-ray excitation.
2. Materials and Methods
2.1. Crystal Growth
The growth setup comprised a spherical water-cooled stainless steel chamber. A crystal pulling rod, thermostatically water-cooled and driven by two stepper motors, provided simultaneous rotation and vertical displacement of the growing crystal. A graphite adapter fitted with a quartz capillary was mounted at the rod’s extremity (Figure 1). The furnace was equipped with a cylindrical resistive heater surrounded by pyrolytic graphite thermal insulation. Temperature regulation was achieved using a K-type thermocouple attached to the crucible base. Granulated CsI with a purity of 99.998% (with respect to metal impurities), produced by Lanhit, was used as the starting material for crystal growth. The activators consisted of and powders, as well as granulated , all with a purity of 99.99% (Lanhit). The initial mixture contained 150 g of CsI and 2.6 g of each activator (approximately 1 mol%). The materials were loaded into a vitreous carbon crucible inside the furnace and heated under vacuum at 500 °C for six hours to remove adsorbed oxygen and moisture. By the end of the drying process, the residual vapor pressure in the furnace was below 0.01 Pa. To suppress melt evaporation, both melting and crystal growth were conducted in an argon atmosphere maintained at 110 kPa.
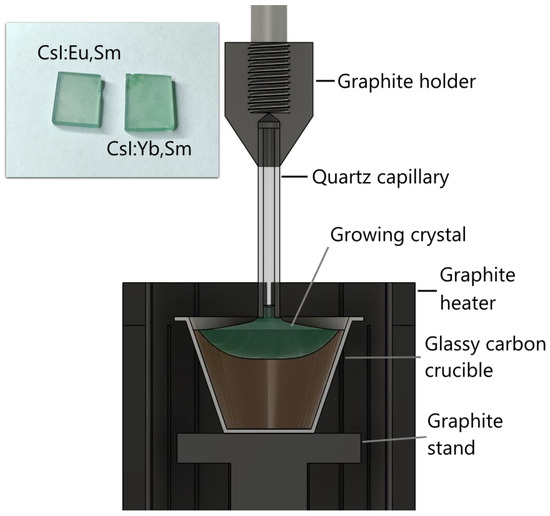
Figure 1.
Schematic of the crystal growth setup and photographs of samples prepared for spectroscopic studies.
Crystal growth was initiated by seeding onto a quartz capillary. The Kyropoulos method was employed to grow the colored crystal, as it enables higher incorporation of dopant ions into the crystal lattice compared to the Czochralski technique. The crystal was grown at a cooling rate of 0.3 °C/h. Although the Kyropoulos method typically involves crystal pulling and rotation from the melt, neither pulling nor rotation was utilized in this case to maximize dopant ion concentration in the as-grown crystal. After passing the crystallization point (determined by the crucible bottom temperature), the crystal was annealed by maintaining it near the melting point for 5 h, followed by controlled cooling over a 10 h period. As a result, a single crystal approximately 35 mm in diameter and 8 mm thick was obtained. It grew as a layer on the remaining part of the boule, where the melt began to solidify non-uniformly, exhibiting off-stoichiometry and inclusions of dendritic structures with unclear composition. This approach enables higher dopant concentrations but limits the growth of large, homogeneous crystals. Nevertheless, samples of sufficient quality for spectroscopic studies can still be cut from the resulting crystals. For comparative analysis, the spectra of CsI:Eu, CsI:Sm, and CsI:Yb crystals will be presented. These crystals were grown under identical conditions with similar activator concentrations.
2.2. Spectroscopic Measurement Setup
The luminescence and excitation spectra were recorded using a spectrometer based on SDL-1 and MDR-2 grating monochromators (LOMO, Saint Petersburg, Russia) equipped with a grating of 1200 and 600 lines per mm. A Hamamatsu H6780-04 photomultiplier module was used as a photodetector. Excitation was carried out using a high-pressure 150 W xenon arc lamp DKSH-150 through an MDR-2 monochromator with a 1200 lines/mm diffraction grating. Additional excitation sources included 405 and 450 nm lasers. Luminescence decay kinetics under nitrogen laser excitation ( = 337 nm, 8 ns pulse duration) were recorded using an MDR-2 monochromator, Hamamatsu H6780-04 photomultiplier, and RIGOL DS1202Z oscilloscope (RIGOL Technologies, Beijing, China). A closed-cycle helium cryostat was used for studies in the temperature range of 7-300 K. The X-ray luminescence spectra were measured using an MDR-2 monochromator coupled with a cooled FEU-83 photomultiplier tube (S-1 photocathode type), under excitation by a 10L-01 X-ray tube operated at 50 kV voltage and 1 mA current. A cooled photomultiplier tube with a silver-oxygen-cesium (S-1) photocathode in pulse-counting mode was used for X-ray excited luminescence measurements. For light output evaluation, samples with uniform geometric dimensions (tolerance: 0.2 mm) were prepared and secured in a dedicated holder to ensure accurate alignment with the X-ray emission source. Light output estimations were performed by comparison of the integral intensities of corrected X-ray excited luminescence spectra.
The luminescence experiments under VUV excitations were carried out using synchrotron radiation from the 1.5 GeV storage ring of MAX IV synchrotron facility (Lund, Sweden). The luminescence experiments under synchrotron radiation excitations are a powerful tool for the study of scintillators [16,17]. The experiments were performed at the photoluminescence endstation of the FinEstBeAMS beamline. The parameters of the beamline and the experimental setup are given in [18]. Before measurements, the plates of single crystal samples were polished in a dry glovebox under an inert atmosphere. They were then transferred to a vacuum-sealed cryostat to prevent the formation of a surface oxide layer. The luminescence spectra were processed using the ArDI web-application (https://ardi.fmm.ru (accessed on 20 April 2025)).
Raman spectra were measured using a WITec alpha300R confocal Raman spectrometer coupled with a 532 nm Nd:YAG laser, calibrated on crystalline silicon. Spectra were recorded with a diffraction grating of 1800 lines per millimeter and a spectral resolution of . The laser beam had an output power of 10 mW, and the focal spot diameter sample was between 5 and 10 μm. The backscattered Raman signal was collected by using a Zeiss 50x/NA 0.55 objective in a UHTS300 spectrometer equipped with a Peltier-cooled, front-illuminated CCD camera. Spectral scan durations were 20 s, with signals averaging over 5 scans.
3. Results
The undoped crystal does not exhibit Raman scattering as a result of its NaCl-type crystal lattice. However, Raman signals were detected in CsI crystals doped with 1 mol% of and , as well as in those doped with 1 mol% of and 1 mol% of . The Raman spectra of these doped crystals are presented in Figure 2. The bands observed at 80, 94, 112, 136, and 164 cm−1 in the doped crystals can be attributed to “(Ln2+I8)6−-vacancy” clusters [19].
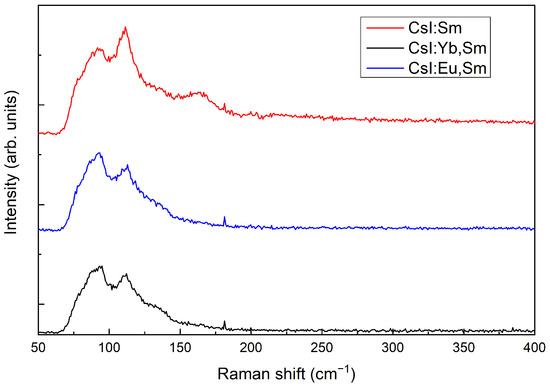
Figure 2.
Raman spectra of CsI:Sm (1); CsI:Yb,Sm (2); and CsI:Eu,Sm (3).
The luminescence spectra of CsI:Yb,Sm shown in Figure 3 were measured at temperatures of 298 K and 7 K. At room temperature, a band corresponding to the 4f55d1→4f6 luminescence of divalent samarium [20] is observed in the red and infrared spectral regions, with a maximum intensity in the range of 1.4–1.5 eV (≈827–886 nm) under 405 nm laser excitation. In the blue spectral region under 3.94 eV (≈315 nm) Xe-lamp excitation at room temperature, two bands (high-spin and low-spin) of 4f135d1→4f14 luminescence from divalent ytterbium ions are visible [21]. However, the emission intensity in these bands is two orders of magnitude lower than that of the divalent samarium band. In the long-wavelength region, the edge of the 4f55d1→4f6 emission band is truncated due to the declining sensitivity of the detector used. The excitation wavelength of 315 nm indicated in the figure was specifically selected to measure the short-wavelength edge of the ytterbium bands. When using 405 nm laser excitation, the spectral shape of the samarium bands remains unchanged.
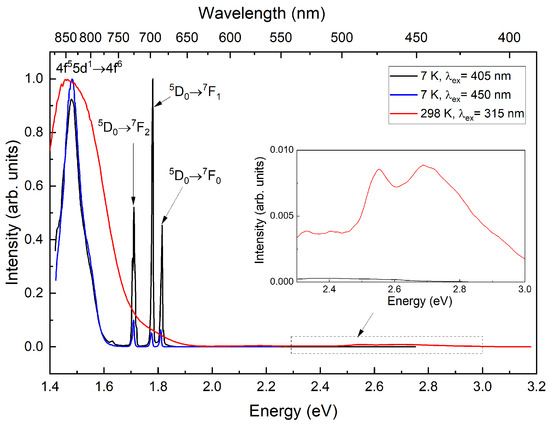
Figure 3.
Luminescence spectra of CsI:Yb,Sm measured at 298 K and 7 K.
Upon cooling, the characteristic Yb2+ emission band nearly completely loses its intensity, while the 4f55d1→4f6 emission band narrows, revealing a shoulder at 1.55 eV (≈800 nm). Additionally, 4f6→4f6 luminescence bands characteristic of divalent samarium ions appear [22,23], with observed transitions 5, 5 and 5 showing maxima at 1.81 eV (≈685 nm), 1.77 eV (≈700 nm), and 1.71 eV (≈725 nm), respectively. When excited at 450 nm within the ytterbium emission band, the intensity of the emission increases significantly, relative to the transitions.
The excitation spectrum (Figure 4) consists of transitions of Yb and Sm ions. In the region of transitions of samarium, the main excitation bands show good agreement with the excitation bands of the CsI:Yb reference sample [13]. In the emission band of samarium ions, the excitation spectrum exhibits some anticorrelation compared to the excitation spectrum of luminescence, suggesting different types of samarium emission centers responsible for and luminescence.
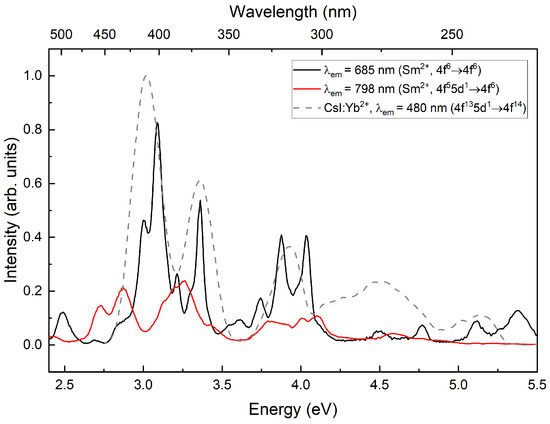
Figure 4.
Excitation spectra of CsI:Yb,Sm measured at 7 K. The dashed line represents the excitation spectrum of the CsI:Yb crystal measured at 298 K.
The luminescence of the CsI:Eu,Sm sample (Figure 5) at room temperature consists of a broadened emission band, compared to CsI:Yb,Sm. Both CsI:Yb,Sm and CsI:Eu,Sm exhibit nearly identical luminescence band profiles, indicating that the centers responsible for this emission experience similar crystal field environments. The emission band, peaking at 2.67 eV (≈464 nm) [24], also exhibits low intensity. Under 2.75 eV (450 nm laser) excitation, the samarium emission band () broadens and develops a vibrational structure. The energy spacing between these vibrational maxima falls within the 210–280 range, which corresponds to the doubled Raman frequency of divalent rare earth-iodine complexes (Figure 2). Upon cooling, four bands emerge in this spectral region, with maxima at 2.58 eV (≈481 nm), 2.66 eV (≈466 nm), 2.76 eV (≈449 nm), and 2.85 eV (≈435 nm).
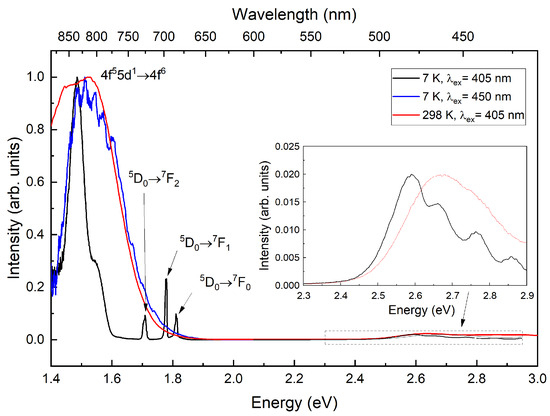
Figure 5.
Luminescence spectra of CsI:Eu,Sm measured at 298 K and 7 K.
The excitation spectrum (Figure 6) of CsI:Eu,Sm in the luminescence band is completely identical to that of CsI:Yb,Sm in the band. When excited within the luminescence band of Sm ions, a correlation with the excitation spectrum of ions is observed. From the luminescence spectra, it is evident that both Eu and Yb act as sensitizers for ions.
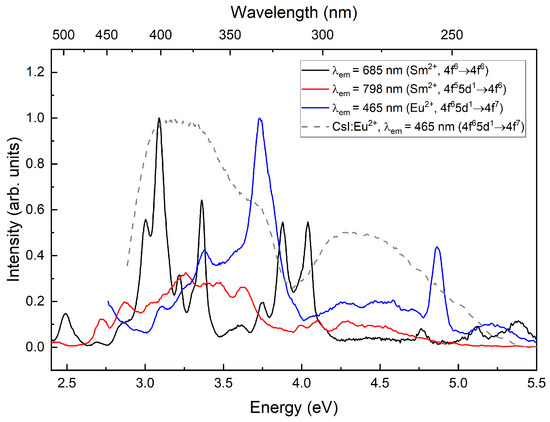
Figure 6.
Excitation spectra in the luminescence bands at 7 K.
The excitation spectrum of CsI:Eu,Sm in the vacuum ultraviolet (VUV) region was measured in the and emission bands at 7 K (Figure 7, curves (1) and (2)). A correlation is observed between the high-energy excitation spectra. In the 5–5.9 eV (≈248–210 nm) range, the excitation spectrum exhibits two bands with maxima at 5.3 eV (≈234 nm) and 5.6 eV (≈221 nm), showing good correlation with the excitation spectrum of europium emission. In contrast, the band displays a broad continuum without characteristic dip. Notably, these excitation bands are absent in the excitation spectrum of CsI:Sm (Figure 7, curve (5)). In the STE excitation region, the and crystals are not found intensive bands. However, STE-like excitation band structures can be observed (Figure 7, curves (2) and (3)). In contrast, CsI doped with only is not excited in the STE region (Figure 7, curve (5)). The excitation spectrum in the band of CsI:Eu (Figure 8) correlates with the STE excitation spectrum, indicating an efficient energy transfer mechanism from self-trapped excitons to ions.
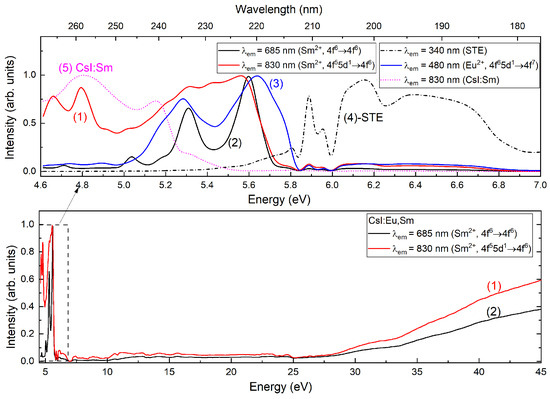
Figure 7.
Excitation spectra of CsI:Eu,Sm and CsI:Sm (dot line) samples in VUV range.
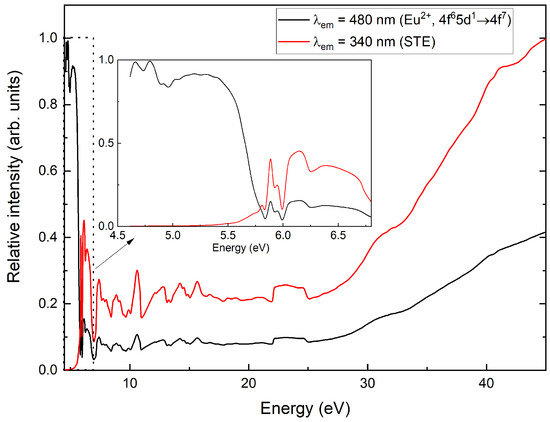
Figure 8.
Excitation spectra of CsI:Eu in VUV range.
For a more detailed study of the divalent europium luminescence band, we measured the luminescence spectra of CsI:Eu crystals at 7 K under excitations at 5.6 eV (≈221 nm), 5.7 eV (≈218 nm), 5.3 eV (≈234 nm), and 3.7 eV (≈335 nm) (Figure 9). The Gaussian fitting analysis of the emission band demonstrated that the spectrum consisted of five overlapping components with maxima at 2.69 eV (≈461 nm), 2.73 eV (≈454 nm), 2.78 eV (≈446 nm), 3.03 eV (≈409 nm), and 3.17 eV (≈391 nm). The relative intensities of these bands showed significant variation with increasing excitation energy. Furthermore, the spectrum contained an additional luminescence peak at 3.66 eV (≈339 nm) corresponding to self-trapped exciton emission.
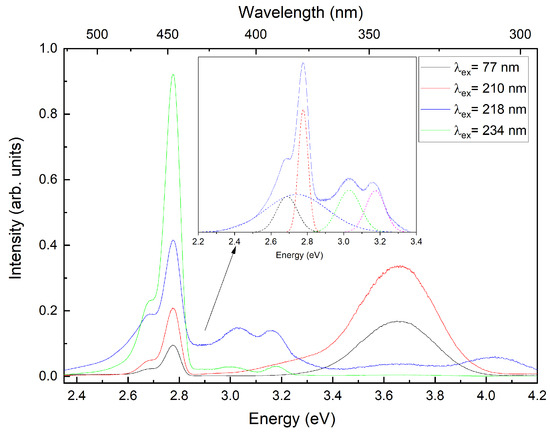
Figure 9.
Luminescence spectra of CsI:Eu under VUV excitation at 7 K. The inset shows the band approximation using Gaussian functions (dotted lines).
Light output measurement using pulse-height spectra was not performed. The rough estimation of light output could be obtained by comparing integral intensities of X-ray excited luminescence with correction for spectral sensitivity of the detection system. This method could be used for comparison of similar hosts doped with different impurities due to their identical crystal matrix - CsI:Tl, CsI:Yb,Sm and CsI:Eu,Sm exhibit equivalent X-ray absorption efficiency. Therefore, using a reference crystal CsI:Tl with known light output, we could estimate the light output of CsI:Yb,Sm and CsI:Eu,Sm. The recorded X-ray excited luminescence spectra are shown in (Figure 10). The differences in the band shapes observed in the long-wavelength spectral region compared to Figure 3 and Figure 5 are attributed to the specific characteristics of our X-ray luminescence measurement setup. This instrument exhibits enhanced sensitivity in the infrared spectral range, which enabled us to reliably detect and measure the long-wavelength edge of the emission band.
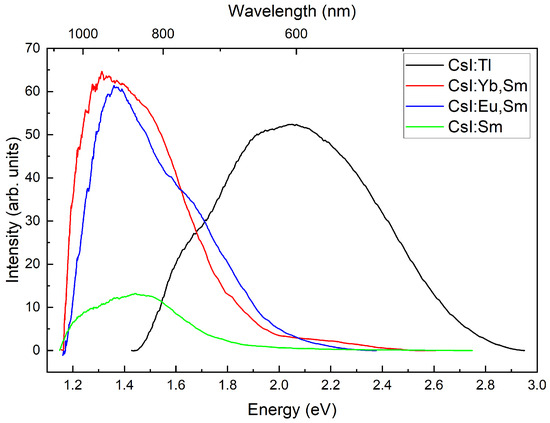
Figure 10.
Luminescence spectra of CsI:Tl; CsI:Yb,Sm; CsI:Eu,Sm; and CsI:Yb,Sm crystals under X-ray excitation.
To verify that Eu and Yb ions act as effective sensitizers in the scintillation process, we additionally measured the luminescence spectrum of a CsI:Sm crystal grown under the same conditions as the studied samples. The results of the comparison of integrated luminescence intensities under X-ray excitation are given in Table 1.

Table 1.
Relative XRL intensity and estimated light output.
We note that these values were obtained from the first experimental samples of such crystals. We believe that optimizing activator concentrations and improving crystal growth technology to enhance crystal quality could lead to a significant increase in the light yield of these crystals.
The luminescence decay curves in the band under excitation by a nitrogen laser at 337 nm (3.68 eV) are presented in Figure 11. The decay curves exhibit similar kinetics for both CsI:Yb,Sm and CsI:Eu,Sm samples: a rapid initial decay due to the short-lived component (), followed by a slow exponential decay associated with the long-lived component ().
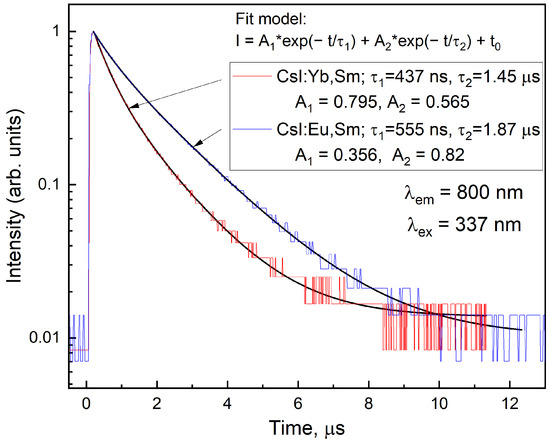
Figure 11.
Luminescence decay curves of CsI:Yb,Sm and CsI:Eu,Sm in the emission band under 3.68 eV pulsed laser excitation at room temperature. The black lines represent fitting with a bi-exponential model.
For the CsI:Yb,Sm sample faster decay times are observed ( ns, ms), and the amplitude ratio (, ) indicates the dominance of the faster component. In contrast, CsI:Eu,Sm exhibits longer decay times ( ns, ms) with a predominance of the slow component (, ).
The observed biexponential decay kinetics can be explained by two distinct excitation mechanisms: (1) direct intracenter excitation of ions (fast component) and (2) sensitizer-mediated excitation (slow component). In the first case, ions are excited directly by laser radiation. In the second mechanism, excitation occurs through a sensitizer ion followed by energy transfer to .
Ytterbium ions () exhibit weak absorption at the laser wavelength, resulting in a dominant contribution from the fast decay component associated with direct excitation. In contrast, europium () possesses a strong excitation band near the laser wavelength, leading to a more pronounced contribution of the slow decay component in CsI:Eu,Sm crystals.
The thermoluminescence glow curves are shown in Figure 12. Weak glow peaks at 125 and 140 K and intense glow peaks at 200, 255, and 275 K were observed in CsI:Yb,Sm crystals. In the CsI:Eu,Sm crystals, peaks at 205 and 290 K were observed.
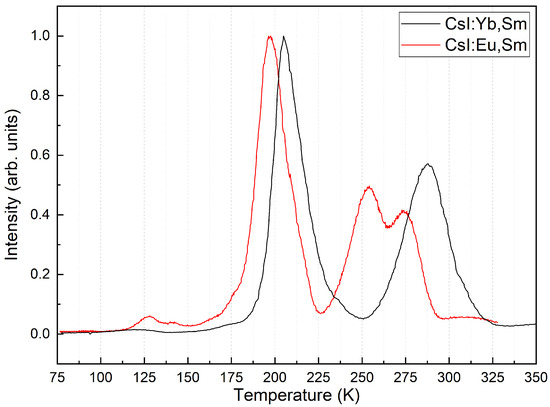
Figure 12.
Thermoluminescence spectra of CsI:Yb,Sm and CsI:Eu,Sm.
4. Discussion
The most effective scintillators are materials in which energy transfer from intrinsic excitations to activator ions occurs via excitons [26,27,28,29]. In crystals activated by , this process also takes place, making them quite promising as scintillators [13].
In CsI-Eu crystals, the exciton mechanism is similarly observed [30,31], with the formation of near-impurity excitons also observed in these crystals. In the emission spectrum of in CsI crystals, excitation near the edge of the forbidden band reveals several bands. The broad band around 2.75 eV corresponds to the emission of a near-impurity exciton localized near sodium [32]. This band is also observed in unactivated, nominally impurity-free crystals. In europium-activated crystals, four additional bands appear at 2.7 eV, 2.77 eV, 3.00 eV, and 3.17 eV. Their excitation spectra differ from each other. The bands at 2.7 eV and 2.77 eV exhibit excitation bands in the 3 to 5 eV range, associated with intracenter excitation of 4f-5d transitions of [15,33]. Higher energy bands are excited only within the energy range close to interband transitions of 5–5.6 eV. In the energy region where electron multiplications are observed, the luminescence bands at 2.7 eV and 2.77 eV are excited much more efficiently. Thus, these bands are associated with intracenter 5d-4f transitions in europium centers of different types [30]. Such centers are most likely compensated by cation vacancies in nearest-neighbor (nn) and next-nearest-neighbor (nnn) positions. The nature of the higher-energy bands is likely associated with the decay of a near-impurity exciton near two types of centers. In this case, excitation transfer from these excitons to europium ions occurs, as indicated by an intense band in the 5–5.7 eV region observed in the excitation spectrum of 5d-4f europium luminescence (bands at 2.7 eV and 2.77 eV).
From the excitation spectra of crystals with double activation (Figure 6 and Figure 7), it is evident that excitation transfer from the divalent ions of the sensitizers or to occurs. In particular, in crystals activated by europium, bands appear in the 5d-4f excitation spectrum in the 5.3 and 5.6 eV regions, associated with the excitation of a near-impurity exciton near europium [15] and weak bands in the region associated with self-trapped excitons at 5.9–6.0 eV. A similar pattern is observed in CsI-Yb,Sm crystals.
The energy transfer process is associated with the overlap of the emission bands of (Figure 9) and [13] and the absorption characteristics of [14]. When excited in the 4f-5d bands of or , intense luminescence related to is observed, causing the entire volume of the crystal to glow. The intensity of 5d-4f luminescence from increases with rising concentrations of divalent lanthanide ions, as decreasing distances between ions and sensitizer ions ( or ) facilitate energy transfer. Thus, dipole–dipole nonradiative energy transfer from sensitizer ions or to ions presumably occurs (Figure 13).
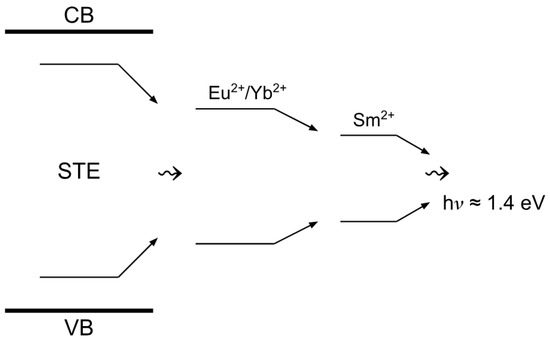
Figure 13.
Schematic model of energy transfer process.
In the excitation spectrum of 5d-4f luminescence, particularly within the region of interband transitions, coactivation of crystals with or ions reveals the occurrence of electron multiplications. This phenomenon suggests the establishment of an excitation transfer channel from the crystal lattice to ions, facilitated by the presence of and ions. Notably, a distinct band emerges in the 5d-4f excitation spectrum of luminescence, corresponding to a near-impurity exciton localized on either or . The precise origin of these excitons remains uncertain; however, it may be attributed to the deep energy states of and within the bandgap of CsI crystals, which could provide stability to the impurity exciton. Additionally, it is plausible that the decay of cationic excitons occurs at these impurity sites, as the core levels of and are situated within the energy range conducive to the formation of cationic excitons [34,35,36].
The thermoluminescence (TSL) bands in CsI-Yb,Sm and CsI-Eu,Sm crystals are associated with hole centers near the divalent lanthanide ions. Since these bands differ between crystals with various sensitizers, they likely result from the nonradiative decay of a near-impurity exciton close to and ions. The TSL peaks in CsI-Eu, Sm crystals occur below room temperature, which mean these crystals do not exhibit significant afterglow, unlike CsI-Tl crystals. In contrast, CsI-Yb, Sm crystals show a slight afterglow; however, it is several orders of magnitude less than that observed in CsI-Tl. Similarly, in CsI-Tl crystals coactivated with divalent lanthanides ions (, or ), the afterglow intensity diminishes [37,38,39,40]. The light yield of the double-activated crystals was assessed using X-ray luminescence spectra, revealing a notable enhancement compared to crystals activated solely by . Specifically, CsI-Yb,Sm and particularly CsI-Eu,Sm crystals demonstrate great potential as scintillators emitting in the near-infrared region. Their light yield surpasses that of previously studied crystals [41] and may approach that of CsI-Tl.
5. Conclusions
For the first time, CsI crystals doubly activated with rare-earth ion pairs (Eu,Sm and Yb,Sm) have been successfully grown. Our studies demonstrate that divalent europium and ytterbium ions serve as efficient sensitizers for samarium ions () in CsI crystals, where exhibits a broad 5d-4f emission band in the red and near-infrared spectral region. The light output of the obtained crystals under X-ray excitation was roughly estimated from the X-ray luminescence spectra, with expected values of 37,000 photons/MeV for CsI:Yb,Sm and 40,000 photons/MeV for CsI:Eu,Sm. Further optimization of crystal growth techniques could significantly enhance the light output, potentially leading to the development of cost-effective infrared scintillators. These findings open new possibilities for creating efficient scintillation materials with emission in the near-infrared range.
Author Contributions
Conceptualization, D.S. and R.S.; methodology, D.S., R.S., V.P. (Vladimir Pankratov) and V.P. (Viktorija Pankratova); validation, D.S., E.K. and V.P. (Vladimir Pankratov); formal analysis, V.P. (Viktorija Pankratova); investigation, D.S., R.S., V.G., V.P. (Vladimir Pankratov), V.P. (Viktorija Pankratova) and E.K.; resources, D.S.; writing—original draft preparation, D.S. and R.S.; writing—review and editing, V.P. (Vladimir Pankratov); visualization, D.S. and R.S.; supervision, D.S.; project administration, D.S. and V.P. (Vladimir Pankratov); funding acquisition, D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work (crystal growth and spectroscopy) was funded by the Russian Science Foundation (project No. 23-72-01097).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The Raman absorption spectra are available in the ArDI (Advanced spectRa Deconvolution Instrument) database—A web application designed for searching, processing, and analyzing vibrational spectra (https://ardi.fmm.ru/ (accessed on 20 April 2025)).
Acknowledgments
The authors are grateful to Kirill Chernenko for his assistance with the measurements at the MAX IV synchrotron facility. The synchrotron studies of CsI:Eu were conducted within the framework of State Assignment No. 0350-2016-0024 “Crystalline and Amorphous Functional Materials with Predictable Properties”. The crystal growth facility utilizes equipment from the Isotope-Geochemical Research Shared Equipment Center at the Vinogradov Institute of Geochemistry (IGC SB RAS). Raman spectra were measured in Center for Geodynamics and Geochronology of Institute of Earth Crust SB RAS. V.P. (Vladimir Pankratov) acknowledges LZP grant lzp-2023/1-0063. V.P. (Viktorija Pankratova) is supported by LZP grant 2022/1-0611.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| NIR | Near-Infrared Light |
| VUV | Vacuum ultraviolet |
| UV | Ultraviolet |
| STE | Self-trapped exciton |
| XRL | X-ray excited luminescence |
References
- Kim, J.; Park, J.; Park, B.; Kim, Y.; Park, B.; Park, S.H. Compact and Real-Time Radiation Dosimeter Using Silicon Photomultipliers for In Vivo Dosimetry in Radiation Therapy. Sensors 2025, 25, 857. [Google Scholar] [CrossRef] [PubMed]
- Strigari, L.; Marconi, R.; Solfaroli-Camillocci, E. Evolution of Portable Sensors for In-Vivo Dose and Time-Activity Curve Monitoring as Tools for Personalized Dosimetry in Molecular Radiotherapy. Sensors 2023, 23, 2599. [Google Scholar] [CrossRef]
- Kodama, S.; Kurosawa, S.; Morishita, Y.; Usami, H.; Torii, T.; Hayashi, M.; Sasano, M.; Azuma, T.; Tanaka, H.; Kochurikhin, V.; et al. Growth and Scintillation Properties of a New Red-Emitting Scintillator Rb2HfI6 for the Fiber-Reading Radiation Monitor. IEEE Trans. Nucl. Sci. 2020, 67, 1055–1062. [Google Scholar] [CrossRef]
- Bartram, R.H.; Kappers, L.A.; Hamilton, D.S.; Brecher, C.; Ovechkina, E.E.; Miller, S.R.; Nagarkar, V.V. Multiple Thermoluminescence Glow Peaks and Afterglow Suppression in CsI:Tl Co-Doped with Eu2+ or Yb2+, shorttitle = Multiple Thermoluminescence Glow Peaks and Afterglow Suppression in CsI. IOP Conf. Ser. Mater. Sci. Eng. 2015, 80, 012003. [Google Scholar] [CrossRef]
- van Aarle, C.; Krämer, K.W.; Dorenbos, P. Lengthening of the Sm2+ 4f55d → 4f6 Decay Time through Interplay with the 4f6[5D0] Level and Its Analogy to Eu2+ and Pr3+. J. Lumin. 2024, 266, 120329. [Google Scholar] [CrossRef]
- Wen, X.; Kucerkova, R.; Babin, V.; Prusa, P.; Kotykova, M.; Nikl, M.; Li, W.; Wang, Q.; Kurosawa, S.; Wu, Y. Scintillator-Oriented near-Infrared Emitting Cs4SrI6:Yb2+, Sm2+ Single Crystals via Sensitization Strategy. J. Am. Ceram. Soc. 2023, 106, 6762–6768. [Google Scholar] [CrossRef]
- Alekhin, M.S.; Awater, R.H.P.; Biner, D.A.; Krämer, K.W.; de Haas, J.T.M.; Dorenbos, P. Luminescence and Spectroscopic Properties of Sm2+ and Er3+ Doped SrI2. J. Lumin. 2015, 167, 347–351. [Google Scholar] [CrossRef]
- Dixie, L.C.; Edgar, A.; Bartle, M.C. Spectroscopic and Radioluminescence Properties of Two Bright X-ray Phosphors: Strontium Barium Chloride Doped with Eu2+ or Sm2+ Ions. J. Lumin. 2014, 149, 91–98. [Google Scholar] [CrossRef]
- Wolszczak, W.; Krämer, K.W.; Dorenbos, P. CsBa2I5:Eu2+,Sm2+—The First High-Energy Resolution Black Scintillator for γ-Ray Spectroscopy. Phys. Status Solidi (RRL) Rapid Res. Lett. 2019, 13, 1900158. [Google Scholar] [CrossRef]
- Wolszczak, W.; Krämer, K.W.; Dorenbos, P. Engineering Near-Infrared Emitting Scintillators with Efficient Eu2+ → Sm2+ Energy Transfer. J. Lumin. 2020, 222, 117101. [Google Scholar] [CrossRef]
- van Aarle, C.; Krämer, K.W.; Dorenbos, P. The Role of Yb2+ as a Scintillation Sensitiser in the Near-Infrared Scintillator CsBa2I5:Sm2+. J. Lumin. 2021, 238, 118257. [Google Scholar] [CrossRef]
- Awater, R.H.P.; Alekhin, M.S.; Biner, D.A.; Krämer, K.W.; Dorenbos, P. Converting SrI2:Eu2+ into a near Infrared Scintillator by Sm2+ Co-Doping. J. Lumin. 2019, 212, 1–4. [Google Scholar] [CrossRef]
- Sofich, D.; Myasnikova, A.; Bogdanov, A.; Pankratova, V.; Pankratov, V.; Kaneva, E.; Shendrik, R. Crystal Growth and Spectroscopy of Yb2+-Doped CsI Single Crystal. Crystals 2024, 14, 500. [Google Scholar] [CrossRef]
- Sofich, D.O.; Bogdanov, A.I.; Shendrik, R.Y. Spectroscopic and Vibrational Properties of CsI Single Crystals Doped with Divalent Samarium. Opt. Mater. 2025, 162, 116958. [Google Scholar] [CrossRef]
- Gektin, A.; Shiran, N.; Belsky, A.; Vasyukov, S. Luminescence Properties of CsI:Eu Crystals. Opt. Mater. 2012, 34, 2017–2020. [Google Scholar] [CrossRef]
- Pankratova, V.; Kozlova, A.P.; Buzanov, O.A.; Chernenko, K.; Shendrik, R.; Šarakovskis, A.; Pankratov, V. Time-Resolved Luminescence and Excitation Spectroscopy of Co-Doped Gd3Ga3Al2O12 Scintillating Crystals. Sci. Rep. 2020, 10, 20388. [Google Scholar] [CrossRef]
- Pankratov, V.; Kotlov, A. Luminescence Spectroscopy under Synchrotron Radiation: From SUPERLUMI to FINESTLUMI. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2020, 474, 35–40. [Google Scholar] [CrossRef]
- Chernenko, K.; Kivimäki, A.; Pärna, R.; Wang, W.; Sankari, R.; Leandersson, M.; Tarawneh, H.; Pankratov, V.; Kook, M.; Kukk, E.; et al. Performance and Characterization of the FinEstBeAMS Beamline at the MAX IV Laboratory. J. Synchrotron Radiat. 2021, 28, 1620–1630. [Google Scholar] [CrossRef]
- Rodriguez-Betancourtt, V.M.; Nattland, D. Raman Spectroscopic Study of Mixed Valence Neodymium and Cerium Chloride Solutions in Eutectic LiCl–KCl Melts. Phys. Chem. Chem. Phys. 2005, 7, 173–179. [Google Scholar] [CrossRef]
- Radzhabov, E.A. Spectroscopy of Divalent Samarium in Alkaline-Earth Fluorides. Opt. Mater. 2018, 85, 127–132. [Google Scholar] [CrossRef]
- Suta, M.; Urland, W.; Daul, C.; Wickleder, C. Photoluminescence Properties of Yb2+ Ions Doped in the Perovskites CsCaX3 and CsSrX3 (X = Cl, Br, and I) – a Comparative Study. Phys. Chem. Chem. Phys. 2016, 18, 13196–13208. [Google Scholar] [CrossRef]
- Zeng, P.; Cao, Z.; Chen, Y.; Yin, M. Investigation of the Temperature Characteristic in SrB4O7:Sm2+ Phosphor-in-Glass by Analyzing the Lifetime of 684 Nm. J. Rare Earths 2017, 35, 783–786. [Google Scholar] [CrossRef]
- Guzzi, M.; Baldini, G. Luminescence and Energy Levels of Sm2+ in Alkali Halides. J. Lumin. 1973, 6, 270–284. [Google Scholar] [CrossRef]
- Shiran, N.; Gektin, A.; Boyarintseva, Y.; Vasyukov, S.; Boyarintsev, A.; Pedash, V.; Tkachenko, S.; Zelenskaya, O.; Zosim, D. Modification of NaI Crystal Scintillation Properties by Eu-doping. Opt. Mater. 2010, 32, 1345–1348. [Google Scholar] [CrossRef]
- Shendrik, R.; Radzhabov, E. Absolute Light Yield Measurements on SrF2 and BaF2 Doped With Rare Earth Ions. IEEE Trans. Nucl. Sci. 2014, 61, 406–410. [Google Scholar] [CrossRef]
- Ucer, K.B.; Bizarri, G.; Burger, A.; Gektin, A.; Trefilova, L.; Williams, R.T. Electron Thermalization and Trapping Rates in Pure and Doped Alkali and Alkaline-Earth Iodide Crystals Studied by Picosecond Optical Absorption. Phys. Rev. B 2014, 89, 165112. [Google Scholar] [CrossRef]
- Shendrik, R.; Radzhabov, E. Energy Transfer Mechanism in Pr-Doped SrF2 Crystals. IEEE Trans. Nucl. Sci. 2012, 59, 2089–2094. [Google Scholar] [CrossRef]
- Khanin, V.; Venevtsev, I.; Chernenko, K.; Pankratov, V.; Klementiev, K.; van Swieten, T.; van Bunningen, A.J.; Vrubel, I.; Shendrik, R.; Ronda, C.; et al. Exciton Interaction with Ce3+ and Ce4+ Ions in (LuGd)3(Ga,Al)5O12 Ceramics. J. Lumin. 2021, 237, 118150. [Google Scholar] [CrossRef]
- Li, P.; Gridin, S.; Ucer, K.B.; Williams, R.T.; Del Ben, M.; Canning, A.; Moretti, F.; Bourret, E. Picosecond Absorption Spectroscopy of Excited States in BaBrCl with and without Eu Dopant and Au Codopant. Phys. Rev. Appl. 2019, 12, 014035. [Google Scholar] [CrossRef]
- Gektin, A.; Shiran, N.; Vasyukov, S.; Belsky, A.; Sofronov, D. Europium Emission Centers in CsI:Eu Crystal. Opt. Mater. 2013, 35, 2613–2617. [Google Scholar] [CrossRef]
- Yakovlev, V.; Trefilova, L.; Karnaukhova, A.; Ovcharenko, N. Energy Transfer Mechanism in CsI:Eu Crystal. J. Lumin. 2014, 148, 274–276. [Google Scholar] [CrossRef]
- Hsu, O.L.; Bates, C.W. Excitonic Emission from CsI(Na). Phys. Rev. B 1977, 15, 5821–5833. [Google Scholar] [CrossRef]
- Yakovlev, V.; Trefilova, L.; Meleshko, A.; Ovcharenko, N. Luminescence of Eu2+–Vc- Dipoles and Their Associates in CsI:Eu Crystals. J. Lumin. 2012, 132, 2476–2478. [Google Scholar] [CrossRef]
- Lushchik, C.; Lushchik, A. Evolution of Anion and Cation Excitons in Alkali Halide Crystals. Phys. Solid State 2018, 60, 1487–1505. [Google Scholar] [CrossRef]
- Shalaev, A.A.; Shendrik, R.; Myasnikova, A.S.; Bogdanov, A.; Rusakov, A.; Vasilkovskyi, A. Luminescence of BaBrI and SrBrI Single Crystals Doped with Eu2+. Opt. Mater. 2018, 79, 84–89. [Google Scholar] [CrossRef]
- Lushchik, A.; Feldbach, E.; Kink, R.; Lushchik, C.; Kirm, M.; Martinson, I. Secondary Excitons in Alkali Halide Crystals. Phys. Rev. B 1996, 53, 5379–5387. [Google Scholar] [CrossRef] [PubMed]
- Sisodiya, D.S.; Singh, S.G.; Chandrakumar, K.R.S.; Patra, G.D.; Ghosh, M.; Pitale, S.; Sen, S. Optimizing the Scintillation Kinetics of CsI Scintillator Single Crystals by Divalent Cation Doping: Insights from Electronic Structure Analysis and Luminescence Studies. J. Phys. Chem. C 2024, 128, 197–209. [Google Scholar] [CrossRef]
- Brecher, C.; Lempicki, A.; Miller, S.R.; Glodo, J.; Ovechkina, E.E.; Gaysinskiy, V.; Nagarkar, V.V.; Bartram, R.H. Suppression of Afterglow in CsI:Tl by Codoping with Eu2+—I: Experimental. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 558, 450–457. [Google Scholar] [CrossRef]
- Bartram, R.H.; Kappers, L.A.; Hamilton, D.S.; Lempicki, A.; Brecher, C.; Glodo, J.; Gaysinskiy, V.; Ovechkina, E.E. Suppression of Afterglow in CsI:Tl by Codoping with Eu2+—II: Theoretical Model. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 558, 458–467. [Google Scholar] [CrossRef]
- Shahmaleki, S.; Rahmani, F. Scintillation Properties of CsI(Tl) Co-Doped with Tm2+. Radiat. Phys. Eng. 2021, 2, 13–19. [Google Scholar] [CrossRef]
- Takase, S.; Miyazaki, K.; Nakauchi, D.; Kato, T.; Kawaguchi, N.; Yanagida, T. Development of Nd-doped CsI Single Crystal Scintillators Emitting near-Infrared Light. J. Lumin. 2024, 267, 120400. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).