Abstract
The mineralized cover of chiton (Polyplacophora) soft tissue consists of aragonite, developed as shell-plates, girdle-scales, and girdle-spicules. This study characterizes crystallographic aspects of the girdle-spicules of the species Ischnochiton rissoi, Rhyssoplax olivacea, Acanthopleura vaillantii, and Acanthopleura spinosa. Spicule crystal arrangements and texture variations are described. Different misorientations between the spicule crystals are shown and are discussed with respect to the physical properties of the biomaterial. Characterization was performed with electron backscattered diffraction (EBSD), as well as with laser confocal and Field emission scanning electron microscopy (FE-SEM) imaging. All investigated species had porous spicules and distinct structural characteristics. Spicule crystal co-orientation strength was strongly increased for R. olivacea and I. rissoi, and it was almost random for A. vaillantii. R. olivacea, I. rissoi. A. spinosa spicule crystal texture was axial, whereas A. vaillantii spicule crystals were almost untextured. For all species investigated, spicule aragonite was twinned, as demonstrated with the strong 63°/64° peak in the misorientation angle distribution diagram, indicating a {110}-twin relationship. R. olivacea and I. rissoi spicules consisted of few twinned crystals and twin boundaries; A. vaillantii and A. spinosa spicules showed an abundance of twinned crystals and twin boundaries. We observed a difference in spicule dimension, morphology, arrangement on the girdle, and crystal organization for the investigated species, but always the generation of twinned aragonite.
1. Introduction
Polyplacophora, or chitons, are dorsoventrally flattened marine mollusks [1,2,3,4,5,6,7,8,9,10,11] that are distributed worldwide. Although some species live on deep ocean floors in depths of about 7000 m, most Polyplacophora live in intertidal habitats. Chitons colonize rocky surfaces, are grazers, and feed on algae, coralline algae, or angiosperms (Ponder et al. 2020 [9] and references therein). Polyplacophora mollusks cling to hard substrate with their muscular foot, the ventral surface of their muscular girdle, and a mucous secretion produced from the sole (e.g., [7,8,9,12]).
The soft tissue of the mollusks is covered by eight overlapping plates that consist of aragonite [8,9]. These are surrounded by a thick, flexible, soft tissue girdle that is covered with aragonitic scales and/or spicules (e.g., [7,8,9,12,13,14] and references therein). The mineralized plates, scales, and spicules provide a protective surface for the soft tissue. The formation of segmented mineral elements allows flexibility of movement and locomotion at concomitant protection, such as gliding on uneven surfaces or even rolling into a ball-like conformation upon a predatory attack (e.g., [15,16]).
Polyplacophora anatomy (e.g., [8,9,13,17,18,19]) and histology of the organs, the cuticle, and other soft tissues are well characterized by now (e.g., [1,8,9,11,20,21,22]). Significantly less studied are the mineralized elements that cover the Polyplacophora soft tissue, namely, the shell plates, girdle-scales, and the girdle-spicules.
The work of Haas et al. (1979, 1980, 1981) [23,24,25], Fischer et al. (1988) [26], Leise (1988) [18], Treves et al. (2003) [27], Connors et al. (2012) [28], Connors (2014) [29], Connors et al. (2019) [30], and Kingston et al. (2018) [31] form exceptions. Haas et al. (1979, 1980, 1981) [23,24,25] and Treves et al. (2003) [27] suggest models for chiton plate, spicule, and scale biomineralization. Connors et al. (2012) [28] and Connors et al. (2019) [30] assessed functional principles of the plates and the scales. Connors et al. (2012) [28] and Connors et al. (2019) [30] provide these morphological, mechanical, and morphometric results and highlight how the plates and scales render protection at simultaneous flexibility at movement.
Aragonite crystal orientation organization of Polyplacophora plates, girdle-scales, and girdle-spicules have not been investigated yet. Diffraction techniques, such as X-ray diffraction and electron backscattered diffraction (EBSD), are ideal analytical tools for the determination of structural information of a crystallized material. The EBSD technique renders the measurement of all crystallographic axes of a crystal and, with this, the acquisition of crystal orientation. This enables the determination of the spatial orientation distribution of the crystals in a crystalline material and thus the measurement of its microstructure and texture.
The presented study focuses on Polyplacophora spicules. It describes their arrangement and distribution on the girdle, as well as their external and internal structure. It also characterizes the mode of spicule crystal orientation and organization. We selected species of the Chitonidae and Ischnochitonidae and investigated the spicules of Rhyssoplax olivacea (Sprengler, 1797), Acanthopleura vaillantii (Rochebrune, 1882), Acanthopleura spinosa (Bruguiere, 1792) (Chitonidae), and Ischnochiton rissoi (Payraudeau, 1826) (Ischnochitonidae). Structural characterization was performed with laser confocal microscopy, optical microscopy, and FE-SEM imaging. Crystal microstructure and texture were measured with electron backscatter diffraction (EBSD). The phase composition was determined with EBSD.
All four investigated Polyplacophora species live on rocky substrates. R. olivacea and I. rissoi live at very shallow water depths (up to 2 m), while A. vaillantii and A. spinosa species dwell in intertidal marine environments. R. olivacea, A. vaillantii, and A. spinosa are herbivores that scrap off microalgae from their surroundings [32,33,34,35]. Feeding information for I. rissoi has not been reported yet. However, I. rissoi lives in the same habitat as Lepidochitona cinerea (Linnaeus, 1767). This chiton has the same feeding behavior as the previously described chiton species [35]. It can therefore be assumed that I. rissoi also has this feeding behavior.
For our study, we chose Polyplacophora species that form girdle-spicules that differ significantly in size, morphology, color, and arrangement. The girdles of R. olivacea and I. rissoi are fully covered by either scales or spicules on their top and bottom surfaces. For these species, the spicules have a strongly patterned arrangement. In contrast, A. vaillantii and A. spinosa cover only the top surface of their girdle using spicules only. The arrangement of the spicules on the A. vaillantii and A. spinosa girdle lacks any structuring.
Our results demonstrate a marked difference in spicule arrangement, aragonite microstructure, and aragonite texture, not only between the Rhyssoplax/Ischnochiton and the Acanthopleura species, but also for the spicules of the two Acanthopleura species. The strong structural difference of the spicules of A. vaillantii and A. spinosa is striking, as despite similarity in habitat and genus, not only aragonite texture and crystal co-orientation strength of individual spicules differs. Spicule dimension, form, assembly density on the girdle, and the mode of spicule organization are also very different.
Furthermore, in this contribution, we concentrate not only on spicule microstructure and texture, but also on whether spicule aragonite is twinned or not. Crystal organization and crystal twinning are important hard tissue characteristics and affect the physical properties of the structural biomaterial. We show that the crystals of the spicules of R. olivacea and I. rissoi are strongly twinned, while the spicules of A. vaillantii and A. spinosa are significantly less twinned. Hence, we demonstrate that there is a difference in the extent of the spicule twinning signal for the investigated Polyplacophora species.
2. Materials and Methods
2.1. Materials
We investigated the spicules of the following Polyplacophora mollusks: Rhyssoplax olivacea (Sprengler, 1797) (Chitonidae) and Ischnochiton rissoi (Payraudeau, 1826) (Ischnochitonidae), sampled between 2014 and 2018, and Acanthopleura vaillantii (Rochebrune, 1882) and Acanthopleura spinosa (Bruguiere, 1792) (Chitonidae), sampled between 2016 and 2018. R. olivacea and I. rissoi were collected from the Mediterranean Sea, at Benalmadena coast, and littoral of Mijas, (Málaga province, Spain), respectively. A. spinosa and A. vaillantii were sampled at Los Negros Escalante, the Philippines. We investigated both entire shells as well as cut pieces of these, selected at well-defined sections covering the girdle and the spicules. Prior to embedding in resin, the samples were rinsed twice in distilled water. Subsequently, the samples were air-dried and then embedded in EPON resin or Patex superglue. The taxonomy of the studied species follows WoRMS [36] (accessed on 28 June 2024).
2.2. Methods
2.2.1. Sample Preparation for Electron Backscattered Diffraction (EBSD) Measurements
Embedded samples were trimmed, cut, and polished in an ultramicrotome with trimming, glass, and diamond knives, following the procedure described in Castro-Claros et al. (2024) [37] and Yin et al. (2024) [38]. For EBSD measurements, the samples were coated with 6 nm of carbon. Sample surfaces were not coated for light microscopy and laser confocal imaging.
Measurements were carried out on a Hitachi SU5000 field emission SEM, equipped with an Oxford Instruments Nordlys II EBSD detector. EBSD measurements were performed with step increments of 300 nm. During measurements, the SEM was operated at 20 kV. Data were collected and evaluated with Oxford Instruments AZTEC and CHANNEL 5 HKL and/or Aztec Crystal EBSD software (https://nano.oxinst.com/azteccrystal, accessed on 11 May 2025). To index the aragonite EBSD patterns, we used the following unit cell setting: a = 4.9614(3) Å, b = 7.9671(4) Å, c = 5.7404(4) Å, given by de Villiers (1971) [39].
Overviews of the investigated samples were taken either with a light microscope or a Keyence 3D laser scanning confocal microscope (VK-X1000 series, Keyence, Tokyo, Japan).
2.2.2. Terminology
Microstructures are presented with grey-scaled EBSD band contrast measurement maps as well as with color-coded EBSD orientation maps. When crystal orientation is shown, then the used color code is given in the figure. In crystal orientation maps, similar or different colors indicate similar or different crystallite orientations, respectively.
Band contrast measurement images depict the EBSD Kikuchi signal strength of each measurement point. A high signal strength corresponds to light grey colors and indicates strong diffraction at the crystal lattice. Dark colors are indicative of non-diffracting substances, e.g., polymers, or an overlap of minute crystallites that cannot be indexed automatically with the EBSD software.
The texture of a crystallized material or its crystallographic preferred orientation is presented with pole figures. These give either orientation data points or their density distributions. For the density distributions, we use the lowest possible setting for half width and cluster size, a half width of five and a cluster size of three degrees. The half width controls the extent of the spread of the poles over the surface of the projection sphere. A cluster comprises orientation data with similar orientation. An axial texture is given when the c-axes show clustering in the pole figure around a single direction, while the corresponding a- and b-axes vary in orientation on a great circle, perpendicular to the texture axis direction.
Crystal co-orientation strength is given with MUD values and is derived from the density distributions of the measured EBSD data. The MUD, multiple of uniform distribution, value is calculated with the Oxford Instruments CHANNEL 5 or the Aztec Crystal EBSD software. A high MUD indicates high crystal co-orientation strength, while low MUD values reflect a low to absent strength of crystallite co-orientation. An MUD value of 1 indicates random distribution and no preferred orientation, an MUD value of 700 and above, at a half width of five and a cluster size of three degrees, documents almost perfect crystal co-orientation, a single-crystal-like co-orientation of crystallites [40,41].
We use the terms crystallites, crystals, and assemblies of crystallites and of crystals. The idealized concept of a crystal refers to a structure in which matter is arranged in a perfectly regular, periodically repeating spatial pattern. The latter is the crystal lattice, which, for the crystals discussed in this study, is three-dimensional. Real crystals develop imperfections during their growth and adopt dislocations. Thus, real crystals, e.g., polycrystals, implement small-angle grain boundaries, where the crystal lattice takes a slightly different orientation. Accordingly, real crystals consist of subunits, mosaic blocks, or mosaic domains. These are tilted to each other by a small angle, up to 10°.
Twinned crystals are entities in which adjacent crystals of the same phase are intergrown in a regularly recurring orientational relationship. These orientational states are the twin domains of a twin crystal. The orientational relationship of twin domains is defined by the twin law. Twin laws are characteristic for the mineral in question and are crystallographically defined symmetry operations. These symmetry operations determine the orientational relationship between the twin domains of a twinned crystal. Twin laws include reflection, rotation, and inversion operations. If a twinned crystal contains domains of two orientational states that alternate in succession, then the twinned crystal is called a polysynthetic twin. In contrast to synthetic inorganic materials, in biological materials, crystal twins do not show perfectly planar and parallel composition/twin planes.
We prove the presence of twinned aragonite with the relevant misorientation boundary for the twin law. For twinned aragonite, the misorientation at the twin boundary is around 63°/64°. We show the characteristic peak in the relative frequency–misorientation angle distribution diagram for these misorientation angles.
3. Results
The presented study concentrates on the structural–crystallographic investigation of Polyplacophora spicule aragonite. The results section of the present article is structured as follows: Spicule and scale organization are shown in Figure 1, Figure 2 and Figure S1. Spicule Ca-carbonate phase is shown in Figure S2. Spicule aragonite microstructure is given in Figure 3 and Figure 4. The twinned nature of spicule aragonite is demonstrated in Figure 5 and Figure 6. The different texture patterns of spicule aragonite are highlighted in Figure 7. Figure 7 and Figure 8 summarize the observed novel structural characteristics for the spicules of the investigated species. Supplementary images visualize spicule porosity (Figure S3); the banded nature of A. spinosa spicules (Figure S4); and the difference in crystal c-axis orientation, relative to the surface of an A. spinosa and a A. vaillantii spicule (Figure S5).
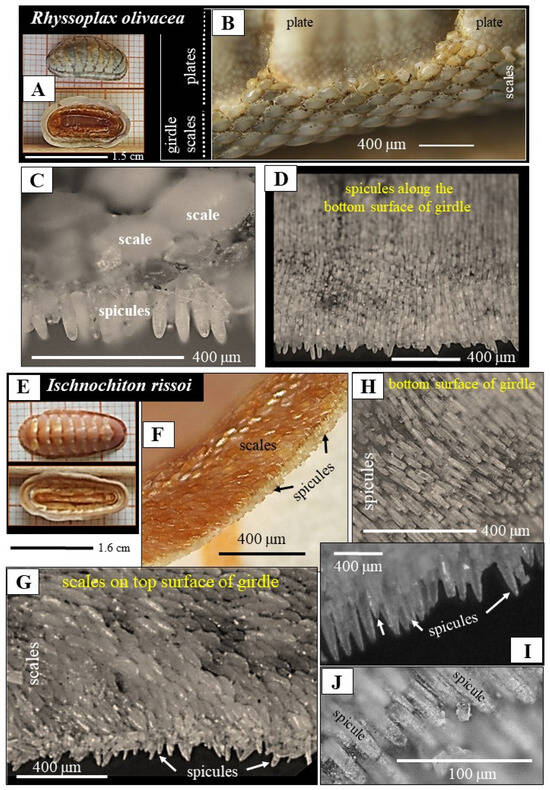
Figure 1.
The mineralized skeletal elements of the Polyplacophoran mollusk species Rhyssoplax olivacea (A–D) and Ischnochiton rissoi (E–J). Laser confocal microscopy images depict the two surfaces of the shell (A,E) together with the arrangements of the girdle-scales (B,C,F,G) and the girdle-spicules (C,D,H–J). The girdle of the mollusk is covered on its top surface by scales, on its bottom surface by spicules. Spicules are arranged in parallel and form layers. Consecutive layers of spicules overlap and form an imbricated and tessellated arrangement of the spicules. When viewed from the top, only one row of spicules seams the outermost rim of the girdle (C,G). The scales are graded in size, from proximal to distal, (C) and have a patterned structural organization, occurring also with a tessellated mode of arrangement.
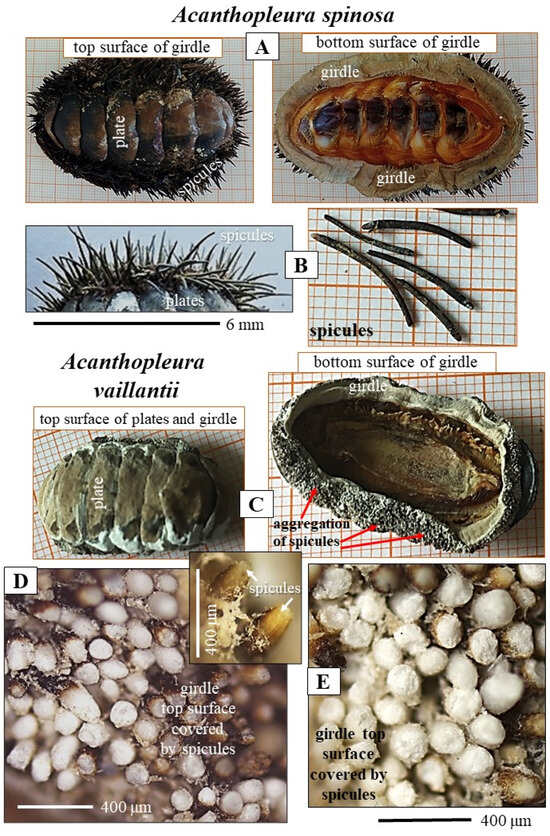
Figure 2.
The mineralized skeletal elements of the Polyplacophoran mollusk species Acanthopleura spinosa (A,B) and Acanthopleura vaillantii (C–E). Light microscopy (A–C) and laser confocal microscopy (D,E) images of Acanthopleura spinosa (A,B) and Acanthopleura vaillantii (C–E) spicules and plates. (A,C) show the top and the bottom side of Acanthopleura spinosa and Acanthopleura vaillantii girdle, respectively. The girdle is not covered by scales in any of its surfaces. For both species, adjacent to the plates is an assembly of spicules. For both species, the bottom surface of the girdle is not covered by mineralized tissue (right-hand-side images in (A,C)). Acanthopleura spinosa and Acanthopleura vaillantii spicule arrangement is not patterned (A,D,E). Spicule morphology, size, and density of accumulation differ significantly for the two investigated Acanthopleura species. The spicules of Acanthopleura spinosa are thin, long, slightly bent, and are not densely packed (A,B). The spicules of Acanthopleura vaillantii are thick, short, sturdy, straight, and densely packed (D,E). The spicules of Acanthopleura vaillantii break easily (D,E), in contrast to the spicules of Acanthopleura spinosa (B) that are highly fracture-resistant.
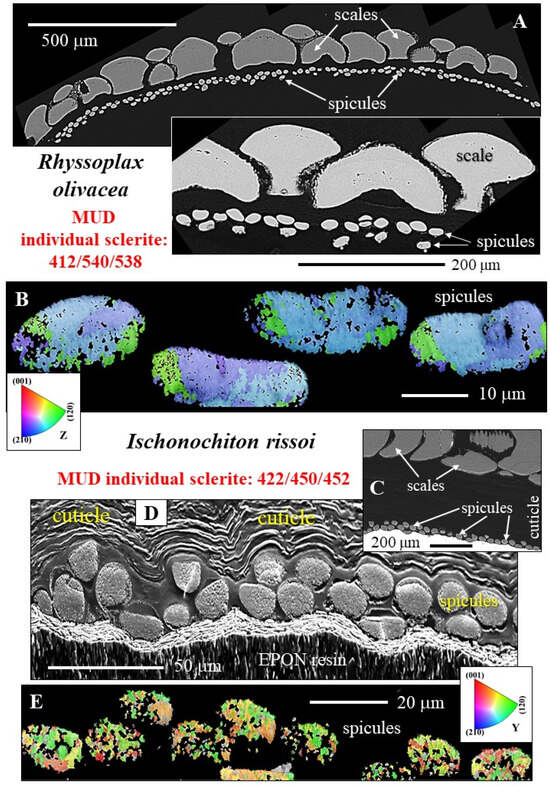
Figure 3.
The microstructure of spicule aragonite of Rhyssoplax olivacea and Ischnochiton rissoi. When the girdle is cut in cross-section, we observe up to two rows of spicules below the scales (A,C,D). Individual spicules consist of very few crystals. A spicule of R. olivacea is formed of one to maximum three differently oriented crystals (B). A spicule of I. rissoi consists of more differently oriented crystals, up to six (E). Crystals within the spicules are highly co-oriented. MUD values for R. olivacea individual spicules are above 500; for I. rissoi, MUD values for individual spicules scatter around 450. (A,C) BSE contrast, (D) SE contrast, (B,E) color-coded EBSD maps highlighting crystal orientation. Note the tessellated arrangement of the scales of R. olivacea (A).
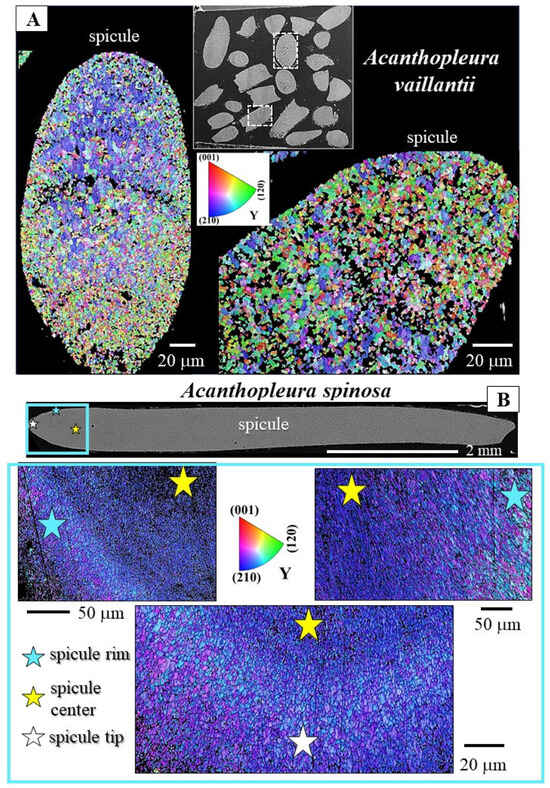
Figure 4.
The microstructure of Acanthopleura vaillantii (A) and Acanthopleura spinosa (B) spicules shown with color-coded EBSD maps. Different colors indicate different crystallite orientations; similar colors show similar crystallite orientations. There is a significant difference in mode of crystal orientation relative to the spicules of R. olivacea and I. rissoi (Figure 3) and between the spicules of the two Acanthopleura species (Figure 4A,B). A multitude of differently oriented crystals compose the spicule of A. vaillantii (A), while a multitude of rather similarly oriented crystals compose the spicule of A. spinosa (B). We observe a variation in grain size for the spicules of A. spinosa (B). An accumulation of larger grains seams the outer portion (rim and tip) of the spicule, while the center of the spicule comprises small and minute crystallites (B). The latter is not observed for the spicules of A. vaillantii (A). (A) BSE micrograph indicating with dashed rectangles the spicules that were scanned with EBSD; crystal orientation for these is shown in (A). (B) BSE image of a longitudinally sectioned spicule of A. spinosa; differently colored stars in the BSE image and EBSD maps indicate different parts of the spicule (rim, center, tip) and the corresponding positions in the EBSD scans. Well visible in the EBSD scans is the belt of larger crystals that seams outer spicule regions.
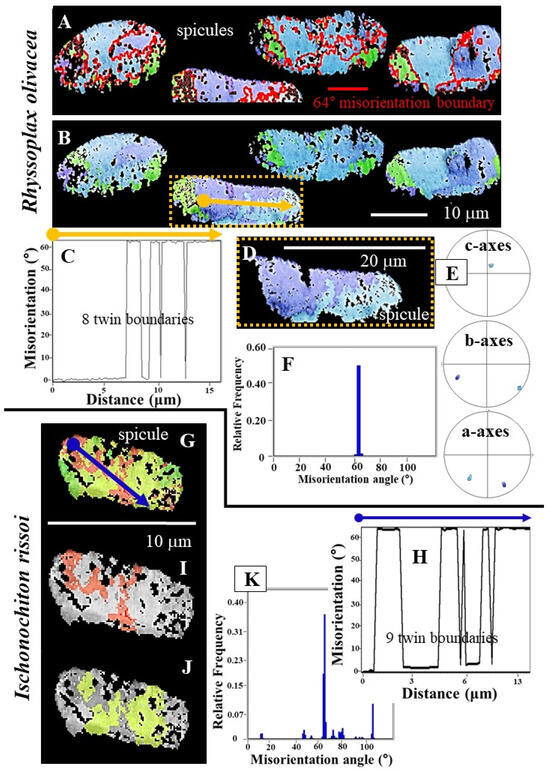
Figure 5.
The twinned nature of Rhyssoplax olivacea and Ischnochiton rissoi spicule aragonite. Most crystals that form a spicule are twinned, as demonstrated with the marked peak at 63°/64° in the relative frequency–misorientation angle diagrams (F,K). Individual spicules of R. olivacea consist often of one twinned crystal (D–F), the latter comprising only the two twin domains (D). In addition to the systematic misorientation of twinned aragonite, we also find other misorientations between crystals of I. rissoi (K). The extent of misorientation ranges from 10° to more than 100° (K). The strongly developed peak at 63°/64° misorientation (F,K), the alternation in misorientation angle between 0° and 64° degrees in the misorientation-distance diagrams (C,H), and the presence of the 64° misorientation boundary within the spicules (A) document that R. olivacea and I. rissoi spicule aragonite is formed of {110} aragonite twins. The orange arrow in (B) gives the used profile in the distance versus misorientation angle diagram shown in (C); the blue arrow in (G) indicates the used profile in the distance versus misorientation angle diagram in (H). (I,J) depict the two twin domains of the twinned crystal shown in (G). (D,E) EBSD scan and corresponding pole figure for one spicule of R. olivacea; note that the spicule consists solely of the two twin domains (shown in light and dark blue in (D)) of this twinned crystal. Accordingly, this spicule is an almost single-crystalline aragonite twin. (F) Misorientation angle diagram for the spicule is shown in (D); note there is only one peak, and this is at 63°/64°, at the twin misorientation.
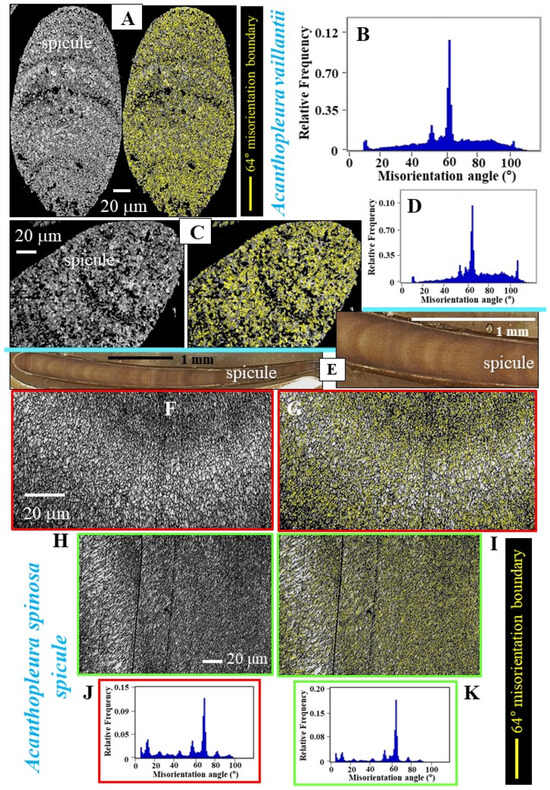
Figure 6.
The twin misorientation and range of further misorientations between crystallites of A. vaillantii and A. spinosa spicule aragonite. We show two EBSD band contrast measurement images per species. Superimposed on the band contrast is the distribution of the 63°/64° grain boundaries (yellow lines in (A,C,G,I)). (B,D,J,K) relative frequency–misorientation angle diagrams for the measurements shown in (A,C,F,H). Well-visible in the latter is the prominent peak at 63°/64°, indicating the presence of twinned aragonite. In contrast to R. olivacea and I. rissoi spicules, for A. vaillantii and A. spinosa spicules, we observe many other misorientations (B,D,J,K), in addition to the twin misorientation. Well-visible for A. spinosa spicules (imaged in (E)) is the belt of larger-sized crystals close to the surface of the spicule (F,H).
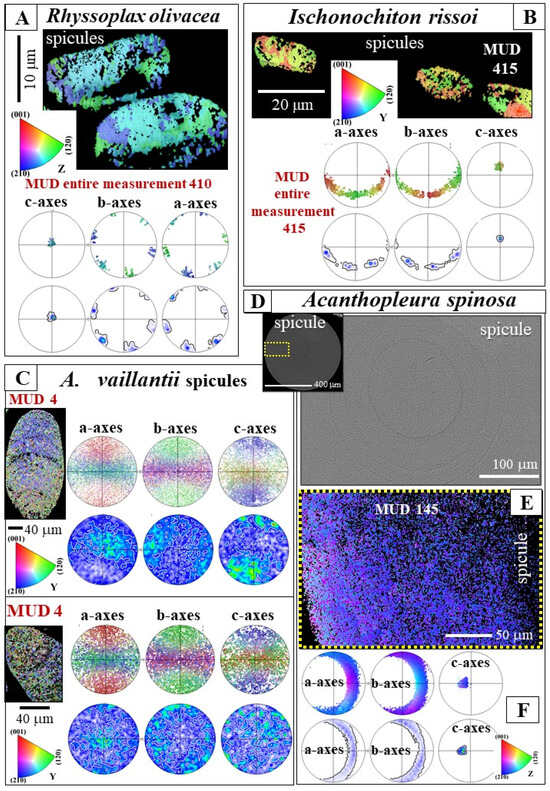
Figure 7.
The texture pattern of the aragonite that comprises the spicules of Rhyssoplax olivacea, Ischnochiton rissoi, Acanthopleura vaillantii, and Acanthopleura spinosa. See the pole figures in (A–C,F) and the corresponding EBSD scans and MUD values. Location of (E) is indicated in (D). MUD values for R. olivacea and I. rissoi are calculated for EBSD scans that cover two (A) and three (B) spicules, respectively. MUD values for the latter species are high and indicate that both spicule alignment as well as crystal alignment within individual spicules are high. MUD values of individual spicules of R. olivacea and I. rissoi are given in (C,E). We observe a marked difference in texture and crystal orientation strength (MUD) between the investigated chitonid and the Acanthopleura species. There is a striking difference in the mode of crystal assembly and crystal assembly strength (MUD) for spicule aragonite of A. vaillantii and A. spinosa.
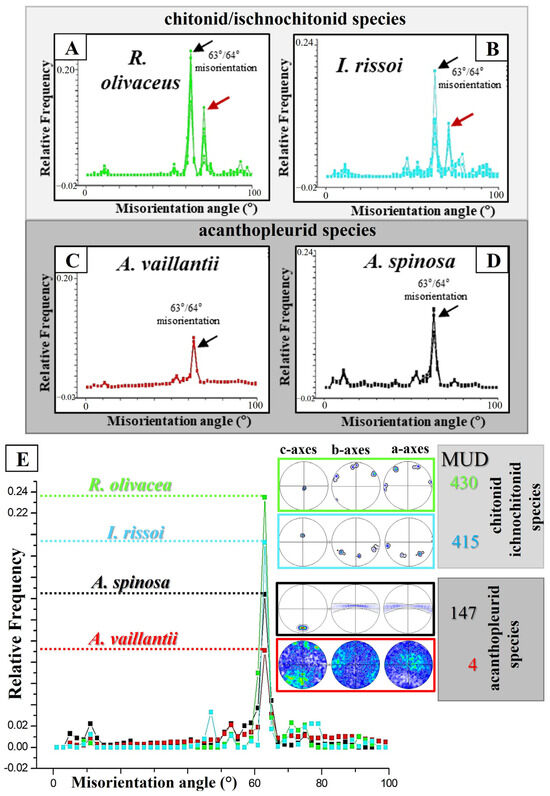
Figure 8.
Summary of structural characteristics that we find for spicule aragonite of Rhyssoplax olivacea, Ischnochiton rissoi, Acanthopleura spinosa, and Acanthopleura vaillantii. The peak at 63°/64° in the relative frequency–misorientation angle diagram shows that the spicules of all investigated species are twinned (black arrows in (A–D)). However, for the chitonid species, there is a further marked misorientation at 71°/72° (red arrows in (A,B)), which is not seen for the Acanthopleura species (C,D). Furthermore, the spicules of R. olivacea are the most twinned. For this species, we find the highest 63°/64° misorientation peak (E). The least twinned is spicule aragonite of A. vaillantii; for this species, we find the smallest 63°/64° misorientation peak (E). When based on microstructure, texture, crystal co-orientation strength, and degree of twinning, the spicules differ from each other significantly in (1) the amount of crystals composing individual spicules, (2) degree of twinning (A–E), (3) crystal co-orientation strength (MUD value (E)), and (4) mode of crystal organization (pole figures in (E)). The most twinned spicules are single-crystalline; the slightly twinned spicules are mostly polycrystalline (E). The spicules of R. olivacea and I. rissoi are twinned and single-crystal-like. The spicules of A. spinosa and A. vaillantii are twinned, granular polycrystals. There is a significant structural difference for the spicules of the investigated Acanthopleura species. The spicules of A. spinosa are textured polycrystals, while the spicules of A. vaillantii are untextured polycrystals, without any obvious mode of crystal assembly pattern.
Figure 1, Figure 2 and Figures S1–S3 show spicule shape, size, Ca-carbonate phase, porosity, and spicule arrangement on the top or on the bottom surface of the girdle. The size, form, and constitution of R. olivacea and I. rissoi spicules are comparable (Figure 1 and Figures S1 and S3). R. olivacea and I. rissoi spicule cross-sections are elongated and are in the range of about 20 µm × 10 µm and 16 µm × 12 µm, respectively. A. vaillantii spicule cross-sections are also elongated. They are, however, in the 300 µm × 160 µm range. In contrast, the cross-section of A. spinosa spicules has a round shape, with a diameter of about 600 µm (Figure 2 and Figures S1 and S3).
The spicules of R. olivacea and I. rissoi cover the bottom surface of the girdle and seam its distal rim (Figure 1). They are well aligned to each other and well arranged in parallel to form layers. These are positioned, slightly offset, on top of each other. The top surface of the girdle of R. olivacea and I. rissoi is covered by a tessellated arrangement of aragonite scales (Figure 1 and Figure S1). The girdle of R. olivacea and I. rissoi was sectioned transversely, from distal to proximal and at different positions (Figure 3). Accordingly, in cross-section and starting from the top surface of the girdle, the cut went through the scales first, then through the cuticle and other soft tissue of the girdle and, lastly, through the very few layers of spicules that covered the bottom surface of the girdle (Figure 3A,C,D). The arrangement of spicules along the bottom surface of the girdle of R. olivacea and of I. rissoi is comparable to the arrangement of tiles that cover the roof of a house. Two to three layers of spicules, with the latter being arranged in parallel in the layer, overlap and generate a cover consisting of an imbricated assembly of spicules (Figure 1D,H).
It should be kept in mind that the girdle of R. olivacea and I. rissoi is covered on both sides by a strongly patterned assembly of mineralized structural elements: (1) a dorsal layer of scales with a tessellated mode of organization and (2) two to three ventral layers of spicules with an imbricated/tiled arrangement pattern (Figure 1).
In contrast, the girdles of A. spinosa and A. vaillantii are covered by mineralized tissue only along their top surface. The bottom surface of the girdles lacks entirely mineralized hard tissue (Figure 2 and Figure S1).
The cover of spicules starts directly adjacent to the plates (Figure S1C,D). Contrasting strongly with R. olivacea and I. rissoi, the A. spinosa and A. vaillantii spicule arrangement on the girdle is random, being entirely unstructured (Figure 2 and Figure S1). The spicules of A. spinosa are long and thin, slightly bent, and do not touch each other (Figure 2). The spicules of A. vaillantii are thick and sturdy and, relative to the spicules of A. spinosa, are densely packed on the girdle and touch each other (Figure 2). The tip of A. spinosa spicules was very rarely broken off, while the tip of A. vaillantii spicules was, in general, damaged or broken off (Figure 2D,E). The spicules of R. olivacea and I. rissoi are transparent, while the spicules of A. spinosa are dark. For A. vaillantii, only the surface of the spicules is dark, and their inner portion is white to opaque (Figure 2D,E and Figure S1).
Irrespective of the above-described structural differences, the spicules of all four investigated Polyplacophora species are porous (Figure S3). Most porous and with the largest pores are the spicules of A. vaillantii (Figure S3F), relative to the spicules of the other investigated species.
Microstructure patterns of R. olivacea, I. rissoi, A. spinosa, and A. vaillantii spicules are shown in Figure 3 and Figure 4. Individual spicules of R. olivacea consisted of very few crystals, in general, less than three (Figure 3B). The aragonite of individual R. olivacea spicules was very co-oriented; MUD values (for definition, see the Terminology of the Materials and Methods section) for individual spicules were above 500. They do not exceed, however, an MUD value of 600 (Figure 3). Hence, individual R. olivacea spicules are almost single-crystal-like (see the definition of the latter in Terminology of the Materials and Methods section). MUD values of clusters of spicules are also high and scattered at an MUD value of 400. This indicates that not only the aragonite within individual spicules but also the spicules of spicule clusters are well aligned. Individual spicules of I. rissoi consist of few crystals, in general, of up to six crystals (Figure 3B,E). MUD values for I. rissoi individual spicules are high and scattered at an MUD of 450.
In contrast to R. olivacea and I. rissoi spicules, the spicules of A. vaillantii and A. spinosa are formed of a countless number of crystallites (Figure 4). These have, relative to what we observed for the crystals that comprised R. olivacea and I. rissoi spicules, a decreased (A. spinosa) to very low (A. vaillantii) crystal co-orientation strength. For individual spicules of A. spinosa, MUD values scatter at an MUD of 140, while A. vaillantii spicules have MUD values of 4 and below (Figure 7C,E and Figure 8E). Crystal co-orientation strength of A. vaillantii spicule aragonite is exceptionally low; the mode of crystal assembly in these spicules is very close to random. To our knowledge, this is the lowest MUD value detected so far for a structural Ca-carbonate biomaterial.
The spicules of A. spinosa have a banded internal structure (Figures S2G, S4 and S5A). These might be growth bands and thus might indicate successive growth stages. We checked the microscopically visible bands of A. spinosa spicules for structural variations but did not detect any structural difference between darker and lighter bands (Figure S4). Nonetheless, for A. spinosa spicules, we observe that crystal granule size is structured. We find an accumulation of larger grains close to the outer surface of the spicule (Figure 4B and Figure 6F,H). The incorporation of larger grains, circumferential for the entire spicule, reinforces the structure of the spicule. We detected formation of growth bands for A. vaillantii spicules as well, while for of R. olivacea and I. rissoi spicules, growth band formation was not observed.
Irrespective of the above-described structural differences (Figure 1, Figure 2, Figure 3 and Figure 4), our EBSD measurements showed that spicule aragonite of all investigated Polyplacophora species is twinned (Figure 5 and Figure 6). As R. olivacea spicules consist of very few twinned crystals, we detect very few twin boundaries (twin boundaries are shown with red lines in Figure 5A) in the spicules of this species (Figure 5A–F). We even repeatedly observe that an individual spicule of R. olivacea is one twinned aragonite crystal, consisting only of the two twin domains of the twinned crystal (Figure 5D–F). The latter is very remarkable and has not yet been observed for other Ca-carbonate biological structural materials. Thus, individual spicules of R. olivacea are single-crystalline, twinned biomaterials—a very specific type of Ca-carbonate biomaterial.
Comparable structural characteristics are present in the spicules of I. rissoi. These are also formed of very few twinned crystals (Figure 5G–J). Hence, in individual I. rissoi spicule aragonite, there are only very few twin boundaries. However, in contrast to R. olivacea, individual I. rissoi spicules consist of a few twinned aragonite crystals. The 63°/64° peak in the relative frequency–misorientation angle diagram is very prominent (see the large peak at 63°/64° in Figure 5K). Nonetheless, for individual I. rissoi spicules, we observe not only the systematic twin misorientation, but other misorientations as well (see the small but well observable peaks in the relative frequency–misorientation angle diagram of Figure 5K).
As A. vaillantii and A. spinosa spicules consist of a multitude of twinned crystals, we found many twin boundaries in individual spicules of the investigated Acanthopleura species (indicated with yellow lines in Figure 6A,C,G,I). As shown in the relative frequency–misorientation angle diagrams (Figure 6B,D,J,K), the 63°/64° misorientation peak, indicating twinned aragonite, is very prominent for A. vaillantii and A. spinosa spicules. Despite this, the spicules also comprise a substantial amount of misoriented, not twinned aragonite crystallites (see the relative frequency–misorientation angle diagrams in Figure 6B,D,J,K and compare them to the diagrams shown in Figure 5F,K). For the Acanthopleura species, we detect a wide range of misorientations between crystallites, ranging from less than 5° to more than 100° (Figure 6B,D,J,K). This was not observed for the spicules of R. olivacea and I. rissoi (Figure 5F,K).
Aragonite texture (the crystallographic preferred orientation of aragonite crystallites) is shown with pole figures (Figure 7 and Figure 8E). We measured significant differences in the type of texture and a considerable range in texture patterns for the spicules of the investigated Polyplacophora species (Figure 7 and Figure 8). The variation in texture ranges from a single-crystal-like texture in R. olivacea and I. rissoi spicules (Figure 7A,B) to an axial texture in A. spinosa (Figure 7F) and to an almost complete lack of preferred orientation of aragonite crystallites in the spicules of A. vaillantii (Figure 7C). This immense variety in modes of crystal assembly patterns is unexpected; in particular, the difference in texture between A. vaillantii and A. spinosa spicule aragonite, as the latter two species belong to the same chiton genus.
Differences in aragonite assembly patterns are reflected by MUD values of individual spicules (Figure 3 and Figure 7C,D) and of clusters of spicules (e.g., Figure 7A,B). R. olivacea and I. rissoi have high MUD values (up to 600, Figure 3 and Figure 7). Individual spicules of A. spinosa show increased MUD values (up to 150, Figure 7E), while MUD values of spicules of A. vaillantii are exceptionally low (MUD below 4, Figure 7C).
In essence, we observed marked differences in many structural characteristics for the spicules of the investigated Polyplacophora species (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8).
These are as follows:
- (1)
- morphology and dimension of the spicules;
- (2)
- position of the spicules on the girdle; on the top or on the bottom surface of the girdle;
- (3)
- the geometric arrangement of the spicules on the girdle;
- (4)
- the extent of structuring of the arrangement of the spicules on the girdle;
- (5)
- the number of crystals that comprise individual spicules;
- (6)
- the range of misorientations between crystallites of individual spicules;
- (7)
- spicule aragonite microstructure;
- (8)
- spicule aragonite texture;
- (9)
- aragonite crystal co-orientation strength.
The only structural similarities that the spicules of the investigated Polyplacophora species share are
- (1)
- the Ca-carbonate polymorph;
- (2)
- the porous nature of the aragonite;
- (3)
- the utilization of twinned aragonite for spicule formation.
Most surprising is
- (1)
- the more or less single-crystalline nature of individual R. olivacea and I. rissoi spicules;
- (2)
- the strongly patterned arrangement of R. olivacea and I. rissoi spicules on the girdle;
- (3)
- the marked difference in internal structure, microstructure, texture, and strength of crystal co-orientation between the spicules of A. spinosa and of A. vaillantii.
4. Discussion
4.1. The Mineralized Cover of Polyplacophora Mollusks
An important and defining characteristic of biomaterials is their ability to achieve functionality. This is startling, as organisms have only a limited range of components available for building functional soft and hard tissues (e.g., [42]). Hence, additional resources have to be added for generating biomaterial functionality [43,44]. The geologic record shows that structural solutions, based on clever geometric arrangements, are appropriate strategies to functionalize biologically secreted soft and hard materials [42,43,45]. The advantage of material functionalization is that trade-offs in material properties, such as stiffness, strength, flexibility, and weight, are avoided with the concomitant generation of the appropriate fracture resistance (e.g., [43,44,46,47,48,49,50,51]). Fracture resistance is linked to the geometric arrangement of the components of the biological composite [43,44,52,53,54,55]. In man-made materials, it can be generated by dislocation movement. For biological structural materials, the latter has not been found to be the case [43,56].
The mineralized cover of Polyplacophora soft tissue is of particular interest, as it consists of a variety of structural hard tissue elements. The polyplacophoran scleritome is a segmented mineral cover and consists of the following mineralized elements: the plates, the scales, and the spicules (e.g., Ponder et al. (2019) [57] and references therein). These have distinct sizes, morphologies, distributions on the cuticle, and modes of arrangement on the surface of the cuticle [57]. Due to Polyplacophora behavior and lifestyle preferences, their mineralized cover needs to be not only protective, but also flexible. It must allow motion and flexion at the same time. To achieve flexibility at concomitant protection, the Polyplacophora developed specific structural material design strategies and combine (1) morphological properties of the mineralized elements with (2) geometric arrangement patterns [28,29,30], complemented by (3) an evolved fabric and constitution of cuticular matrices [22] and, (4) as our study demonstrates, various arrangement patterns and specific structural characteristics of the constituting crystallites.
4.2. The Geometrical Arrangements of the Spicules
The investigated Polyplacophora mollusks live in shallow marine environments. R. olivacea and I. rissoi are subtidal [26], while A. vaillantii and A. spinosa are intertidal [58]. The organs and the soft tissue of the mollusks are encased by a cuticle that is covered, partially or fully, by mineralized structural elements.
Our crystallographic study demonstrates the marked difference in spicule arrangement, structure, and crystal organization for the investigated species. We found that four main structural characteristics are varied: (1) the overall arrangement of the spicules on the girdle, (2) the microstructure of the aragonite, (3) the amount of twinned aragonite, and (4) the texture of the aragonite.
We detected three modes of spicule organization for the investigated Polyplacophora species.
- 1.
- For R. olivacea and I. rissoi, we find a highly patterned arrangement of the spicules on the girdle. Individual spicules are aligned in parallel and form layers (Figure 1D,H). Successive layers of spicules overlap and create an imbricated/tiled arrangement. The spicules of these species cover the bottom surface of the girdle. The top surface of the girdle is coated with mineralized tissue as well, with scales (Figure 1C,G). The latter show also a highly structured arrangement, occurring, however, in a tessellated mode of organization. In addition, we observed a gradation in size for the scales, such that individual scales increase in size from distal to the proximal. Accordingly, the two surfaces of the R. olivacea and I. rissoi girdle are covered by strongly structured mineralized elements, with the latter having distinct morphologies.
- 2.
- A. vaillantii covers the top surface of its girdle with closely packed spicules. These touch one and another (Figure 2C,D,E). There was no macroscopic arrangement pattern for the spicules on the girdle. The bottom surface of the A. vaillantii girdle is not covered by mineralized tissue (Figure 2C). Thus, the spicules have specific morphologies, but are, however, not structured in arrangement or any other structural characteristic, nor are they graded in size.
- 3.
- A. spinosa secretes thin, long, slightly bent spicules (Figure 2B), significantly different in morphology from the spicules of A. vaillantii (Figure 2D,E). The spicules of A. spinosa are quite spaciously positioned on the girdle. Their arrangement is very different from the close-packed arrangement of A. vaillantii spicules. Nonetheless, as it is the case for A. vaillantii, the spicules of A. spinosa also cover only the top surface of the girdle. There is no mineralized hard tissue present on the bottom surface of the girdle (Figure 2A). Hence, for A. spinosa, there is a lack of both a dense cover of the girdle with mineralized elements and the formation of any type of arrangement pattern of the spicules and of the scales.
In essence, even though the investigated Polyplacophora species live in comparable habitats, we find a considerable variation in spicule morphology, spicule attachment onto the one or the other surface of the girdle, and in degree of structuring of the mineralized elements that cover the girdle.
Most interesting are the patterned arrangements of the girdle spicules and of the girdle scales of R. olivacea and I. rissoi. The spicules show an imbricated/tiled mode of organization, while the scales show a tessellated mode of organization. Imbrication and tessellation are structural motifs that involve that soft and hard elements are arranged periodically and in series. This mode of mineralized and soft tissue organization is also described for shark and ray skeletons [49,59], for fish [51,60,61], and for chiton scales [28,30]. The major effect of imbrication/tiling and tessellation is that orientation-dependent stiffness becomes generated [61], without any loss of flexibility. Hence, the toughness of the composite material is maximized, with little reduction in stiffness and strength [43]. Jayasankar et al. (2017) [49] showed that, irrespective of tile and tesserae shapes, the stiffness of the composite material increases with the decreasing width of the organic joints between the mineralized elements and not with the number of organic joints. Furthermore, a tessellated tissue organization ensures good protection, as few gaps become available for predators to get to the soft parts of the organism.
A. vaillantii and A. spinosa mineralized elements did not have imbricated, tiled, or tessellated modes of arrangement, nor a full coverage with mineralized elements of both sides of the girdle. This was apparently not needed for the lifestyle and habitat requirements of these mollusks. A. vaillantii and A. spinosa live in intertidal environments. The mollusks need to retain water on the surface and in their girdle during low tide. To have the ventral girdle surface covered only with soft tissue might facilitate better absorption of water from rocky substrates, in contrast to a girdle surface that is covered with arrays of mineralized elements.
4.3. The Aragonite Crystal Microstructures and Textures
We find three aragonite microstructures and three corresponding textures for the girdle spicules of the investigated chitons:
- (1)
- R. olivacea and I. rissoi spicules consist of very few crystallites, strongly co-oriented to each other with the aragonite c-axis being parallel to the morphological, long axis of the spicule (Figure 7A,B). The texture pattern of the spicules can be addressed as being single-crystal-like (Figure 7A,B and Figure 8E). A single-crystal texture is present when MUD values are or exceed an MUD value of 700, at a half width of 5 and a cluster size of 3. The latter is the case when carbonate crystals precipitate from solution [40,41]. As the MUD values of individual R. olivacea and I. rissoi spicules do not exceed a MUD of 600, individual spicules cannot be considered as single crystals, just as being single-crystal-like.
- (2)
- In strong contrast is the microstructure and texture of A. vaillantii spicules (Figure 4A and Figure 7C). A multitude of randomly oriented, granular, aragonite crystals comprise the spicules (Figure 4A). With an MUD value of 4 (Figure 7C), for individual spicules, there is virtually no co-orientation between the crystals. Aragonite c-axes orientation is random within the spicule, as well as relative to the surface of the spicule (Figure S4B). The aragonite of A. vaillantii spicules is an untextured Ca-carbonate biomaterial.
- (3)
- Even though they belong to the same genus and live in comparable marine environments, the morphology; dimension; constitution; and, especially, texture of aragonite crystals of A. spinosa spicules differ significantly from the spicules of A. vaillantii. The spicules of A. spinosa are polycrystalline as well (Figure 4B), like the spicules of A. vaillantii, but are textured (see pole figures in Figure 7E and Figure S5A). Crystal co-orientation strength in A. spinosa spicules is not very high; it is, however, increased, relative to an untextured crystalline material. MUD values are variable and range up to 140 (Figure 7E). We can consider the aragonite crystals of A. spinosa spicules as textured polycrystals. In contrast to A. vaillantii spicules (Figure S5B), aragonite c-axis orientation of A. spinosa spicules is, more or less, perpendicular to the surface of the spicule and follows its curvature (Figure S5A).
Hence, based on the texture pattern and the co-orientation strength of the aragonite, we observe for the spicules of R. olivacea, I. rissoi, and A. vaillantii two extremes in extent and mode of crystal assembly: an almost fully ordered (R. olivacea, I. rissoi) and an almost fully disordered (A. vaillantii) crystal assembly pattern (compare pole figures in Figure 7A–C). The spicules of the investigated Polyplacophora species are not the only examples of Ca-carbonate biological hard tissues, where we observe a large range of crystal ordering strength (e.g., Griesshaber et al. (2017) [62] and references therein). It has been shown by now that both a high order as well as a high disorder in crystal organization can be advantageous for the organism in different circumstances (e.g., Huber et al. (2015) [63]).
EBSD measurements of polyplacophoran plates, scales, and spicules have shown that individual mollusk species are able to vary aragonite texture and crystal co-orientation strength of their skeletal elements. We show this characteristic for A. vaillantii. It forms its plates and spicules of granular aragonite, organized in an axial texture in the plates (see the pole figures in Figure 9A,B) and with an almost random texture in the spicules (see the pole figures in Figure 7C). It should be kept in mind that the microstructure of the mineralized elements is not varied (Figure 4A and Figure 9A,B). It is granular for the spicules (Figure 4A) and is also granular for large parts of the plates (Figure 9A,B). The texture pattern is different for A. vaillantii plates and spicules and, as will be discussed subsequently, the extent of crystal twin formation. The extent of twin formation varies, within the same mineralized cover for the different mineralized elements, from slightly to significantly twinned aragonite (Figure 9C, Figure 10 and Figure S6).
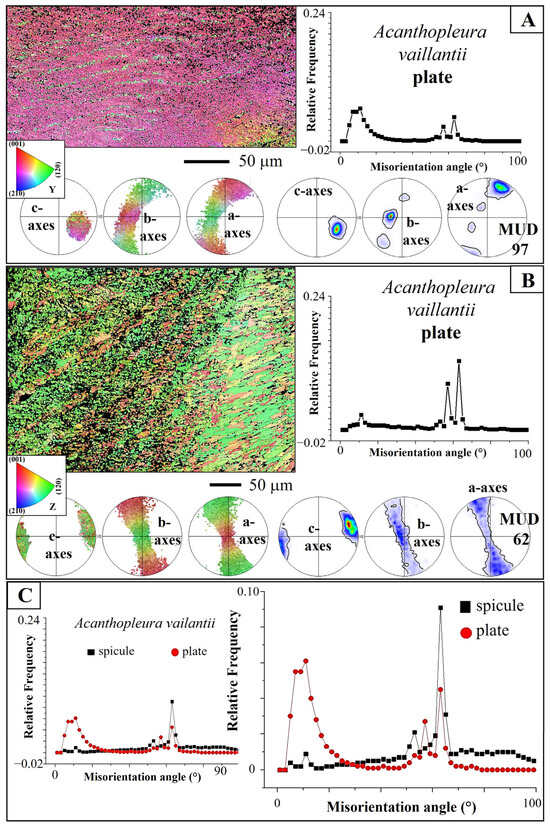
Figure 9.
Microstructure and texture of Acanthopleura vaillantii plates; difference in the degree of twinning for plate and spicule aragonite. (A,B) EBSD scans, corresponding pole figures, MUD values, and relative frequency–misorientation angle diagrams for two measurements taken on the plates. Well visible is the axial texture of plate aragonite and the corresponding increased MUD value. This contrasts with A. vaillantii spicule aragonite that has an entirely different texture and crystal co-orientation strength (see also Figure 10E). The aragonite of A. vaillantii plates is twinned as well, however, to a lesser degree, when compared to spicule aragonite (B,C).
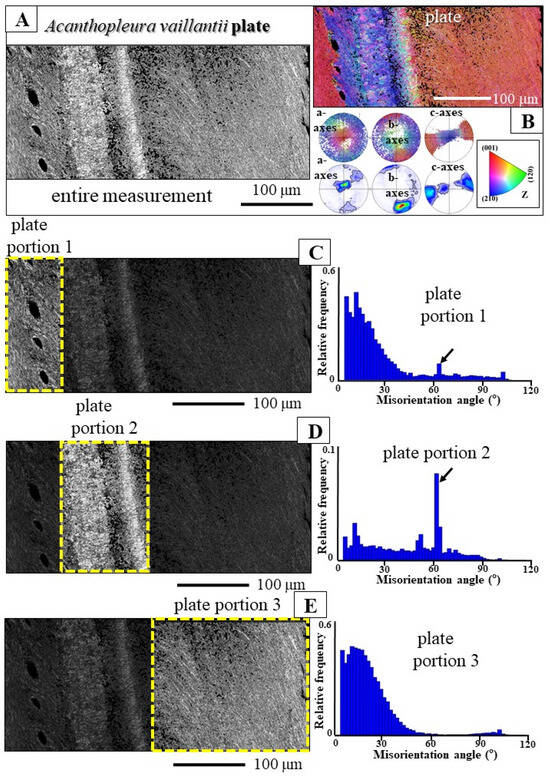
Figure 10.
Variation in degree of twinning within an individual plate of Acanthopleura vaillantii. The EBSD scan (A,B) covers the entire cross-section of this particular plate. (A) EBSD band contrast measurement image, (B) color-coded EBSD map and pole figure visualizing crystal orientation. We find within the same scan different microstructures, grain sizes (band contrast measurement image in (A)) and degrees of twinning (relative frequency–misorientation angle diagrams in (C,D) and c-axes orientations (B)). Twinned aragonite (D) is present in mainly one part of the plate; for other plate sections, the aragonite is not twinned, just misoriented (C,E). For a better visualization of these structural characteristics, see enlargements of the EBSD scan given in (A,B) in the supplementary Figure S6.
4.4. The Twinned Nature of Spicule Aragonite Crystals
There is one structural characteristic that the spicules of all investigated Polyplacophora mollusk species share: formation of twinned aragonite (Figure 5, Figure 6 and Figure 8). Most twinned are the spicules of R. olivacea and I. rissoi; slightly less twinned are the spicules of A. spinosa, least twinned are the spicules of A. vaillantii (Figure 8A–D). It is surprising to find, at least for the spicules of the investigated Polyplacophora mollusk species, that there is some relationship between the degree of twinning and crystal co-orientation strength and the mode of texture (Figure 8E). Aragonite crystals assembled in the spicule with a high crystal co-orientation strength and an almost single-crystalline texture are strongly twinned (Figure 8E). In the spicule formed of aragonite with an almost random crystal co-orientation strength and an almost random texture, twin formation is decreased (Figure 8E).
It should be noted that for R. olivacea and I. rissoi spicules, we find two marked peaks in the relative frequency–misorientation angle diagram (Figure 8A,B). One is the 63°/64° peak, indicating the {110}-twin of the aragonite. For R. olivacea and I. rissoi, there is a further, distinct peak of misorientation: at 70° (Figure 8A,B). The latter peak is not detected for A. vaillantii and A. spinosa (Figure 8C,D).
The relative frequency–misorientation angle diagrams of Figure 9 highlight that the plates of A. vaillantii are twinned as well (Figure 9A,B). In general, the strength of the twinning of the aragonite decreases for the plates, relative to the degree of twinning of the spicules (Figure 9C). Twinned aragonite in A. vaillantii plates might even be entirely absent (Figure 10E and Figure S6) or be strongly reduced (Figure 10C and Figure S6). Despite this, we observed portions with a marked accumulation of twinned aragonite for the plates of A. vaillantii (Figure 10D).
These differences in strength of twinning within the same shell are not yet reported for other Ca-carbonate biological hard tissues. With this study, it is demonstrated that A. vaillantii is able to influence the amount of twinned aragonite within its hard tissue, as we find that the spicules contain more twinned aragonite, relative to the plates. We observe that even within one particular skeletal element, a plate in this case, there is a strong variation in the degree of twinning for the different regions of the skeletal element.
4.5. What Is the Effect of Crystal Twinning?
Formation of crystal twins can take place at growth, deformation, or phase transition (e.g., [64,65,66]). For biologically secreted Ca-carbonate hard tissues, growth or deformation twins are reported. Growth twins are intrinsic for the material in question and are generated at hard tissue formation [67,68,69,70,71,72,73,74,75,76,77]. Deformation twins derive from external impact [66,78,79,80,81].
Growth twins are the result of an accidental departure from the condition of minimal energy of an un-twinned crystal individual [64,82]. As at nucleation and growth, often high supersaturation conditions and, thus, high nucleation rates prevail, and a group of atoms can take a subminimum energy position [83,84]. In this case, a second crystal individual forms, which is in a twin-relationship to the original crystal. Before the second crystal individual moves to a minimum energy position, corresponding to that of the un-twinned crystal, it starts to grow in an alternative position and with a different orientation. In this case, the original crystal and the second crystal individual become the twin domains of the twinned crystal [64,82].
When a crystal twin forms, two or more domains of the same species with similar chemical composition intergrow and are related to each other in a symmetrical fashion. The twin domains share lattice points. These form an interface, termed as the twin plane of a twin crystal. The shared lattice points give the junction between the twin domains a much greater strength than the boundary between randomly oriented grains [85]. Accordingly, the twin domains of a twin crystal cannot easily be separated. Twin planes are unique structures. They can be used for identifying the relevant twin law and for enhancement of material properties of the material in question. As twin planes are the result of the structural relationship between adjacent twin domains, the orientation of twin domains at the twin plane is inherent for a specific twin and is described by the twin law [85]. For inorganic aragonite, only one twin law is reported. The twin plane in twinned aragonite is a glide plane parallel to (110), and adjacent twin domains are misoriented to each other by 63°/64° [69,86].
Work over the last decades demonstrates that the incorporation of defects and boundaries into the crystal lattice change, in general, improve material properties [85,87,88]. The advantage of the latter enhancement process is that the chemical composition of the crystalline material remains unchanged. It is well known by now that grain boundaries act as barriers against dislocation motion [88]. This causes strengthening of the material and is known as the Hall–Petch hardening effect [85,87]. Accordingly, the strength of a structural hard tissue increases with decreasing grain size, as with a decrease in grain size, additional grain boundaries become incorporated into the structural hard tissue.
Twin planes are also boundaries, but they are, however, special boundaries. Twin boundaries are low energy, highly coherent, and stable boundaries. For man-made materials, twin boundaries are straight; for biological carbonates, twin boundaries are curved [73,74,75,76,77]. As is the case for grain boundaries, twin boundaries are efficient barriers for dislocation motion. They block crack propagation and thus initiate an increase in fracture toughness and stabilize the nanostructure of the material [54,81,87,89,90,91].
We observe that R. olivacea and I. rissoi spicules are strongly twinned (Figure 8E). Individual spicules contain few twin boundaries due to the large size of the twin domains that form spicules (Figure 5A). In contrast, A. vaillantii and A. spinosa spicules are less twinned (Figure 8E). However, due to the multitude of grains that form a spicule, there are many twin boundaries present in the spicule (Figure 6A,C,G,I). As discussed above, twin boundary formation is a gain for the material and increases its material properties.
The large number of twin boundaries within A. vaillantii and A. spinosa spicules appears to be enough to generate the required and appropriate material properties. The possibly less marked improvement of material properties of R. olivacea and I. rissoi spicules, due to formation of fewer twin boundaries, is compensated for by gains obtained from the highly patterned organization of the spicules on the surface of the girdle. It has been proven that twinned aragonite structures such as nacre are resistant to impact [92]. This is particularly useful to protect the species from predatory attacks or impacts against tidal change [93]. This might be a possible reason for the twinned nature of the spicules, in particular, for the larger amount of twin boundaries in the spicules of the species that live in intertidal environments.
5. Conclusions
The cover of mineralized skeletal elements of Polyplacophora mollusks provides protection from external attack and concomitant flexibility for locomotion on rough surfaces. This versatility in function is made possible, in addition to chemical and structural characteristics of the cuticle, by the segmented nature of the polyplacophoran shell and formation of distinct mineralized elements, such as the plates, the scales, and the spicules.
In the present study, we highlight and discuss structure, microstructure, and texture concepts of girdle spicules for Rhyssoplax olivacea, Ischnochiton rissoi, Acanthopleura vaillantii, and Acanthopleura spinosa.
The following conclusions can be drawn for our study:
- (1)
- Girdle-spicules cover the bottom surface of the girdle for R. olivacea and I. rissoi. For A. spinosa and A. vaillantii, the spicules encase the top surface of the girdle. We observe a highly patterned arrangement of the spicules for R. olivacea and I. rissoi, in contrast to A. spinosa and A. vaillantii, where a structuring of spicule arrangement is absent.
- (2)
- The spicules of all investigated Polyplacophora species are porous.
- (3)
- Spicule aragonite of all investigated Polyplacophora species is twinned. Crystal twinning corresponds to a {110}-twin relationship.
- (4)
- The spicules of R. olivacea and I. rissoi are comparable in size, morphology, and internal constitution. Aragonite crystallites within individual spicules are very co-oriented. Crystal organization within individual spicules is almost single-crystalline; all three aragonite crystallographic axes show an almost perfect 3D-orientational coherence.
- (5)
- The spicules of A. vaillantii and A. spinosa differ significantly in size, morphology, microstructure, texture, crystal co-orientation strength, and mode of arrangement on the girdle from the spicules of R. olivacea and I. rissoi.
- (6)
- The spicules of A. vaillantii are thick and sturdy and form a dense surface cover of the cuticle. The aragonite of individual spicules is very little textured; crystal co-orientation strength is exceptionally low. A. vaillantii spicules can be regarded as untextured polycrystals.
- (7)
- The spicules of A. spinosa are rather long, thin, bent, loosely packed, and randomly arranged on the surface of the cuticle. The spicules consist of a multitude of granular crystallites and can also be addressed as polycrystals. However, the aragonite of individual A. spinosa spicules is textured and has increased crystal co-orientation strength. The spicules of Acanthopleura spinosa can be regarded as textured polycrystals.
- (8)
- Our structural–crystallographic study highlights the strong difference in spicule arrangement and spicule aragonite crystal assembly pattern for R. olivacea and I. rissoi species, on the one hand, and for the Acanthopleura species, on the other.
- (9)
- While structural characteristics of the spicules and of the aragonite are similar for R. olivacea and I. rissoi, we observed significant structural differences for the two investigated Acanthopleura species. This characteristic is most surprising, as the latter two Polyplacophora species belong to the same genus and live in similar habitats.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst15050466/s1. Figure S1: Laser confocal microscopy micrographs depicting the top surface of the girdle of the investigated Polyplacophora species. When viewed from the top, we find for Rhyssoplax olivacea and Ischnochiton rissoi that scales are next to the plates and that spicules seam only the outermost rim of the girdle (A,B). In contrast, for Acanthopleura vaillantii and Acanthopleura spinosa, the cuticle adjacent to the plates is covered by spicules only (C,D). Figure S2: The Ca-carbonate phase that forms the spicules of R. olivacea, I. rissoi, A. vailantii, and A. spinosa. Red: aragonite; blue: calcite. Figure S3: The porous nature of Rhyssoplax olivacea, Ischnochiton rissoi, Acanthopleura vaillantii, and Acanthopleura spinosa spicules. Laser confocal (A,C,E) and SE images (B,D,F,H) visualizing internal structural features of the spicules. Even though being strongly mineralized, the spicules are porous (B,D,F,H), in particular, the spicules of Acanthopleura vaillantii. (G) Light microscopy image of a spicule of Acanthopleura spinosa; well visible is a banded appearance of the spicule, possibly indicating growth lines and different episodes of growth. Figure S4: The banded nature of Acanthopleura vaillantii spicules (A). EBSD scans (B,C,D,E,F) do not show any structural or texture difference for a scan performed on a darker or lighter spicule region (B,E,F). The blue rectangle in (A) indicates the spicule portion shown in (B). The yellow rectangles in (B,C) indicate the positions of the two EBSD measurements conducted on the spicule. White arrows in (D) point to the scanned regions; the micrograph shown in (D) was taken subsequent to the EBSD measurement. Measurement 2 scans over lighter and darker bands; measurement 1 scans predominantly over a lighter, not banded, spicule portion. (A,B) are laser confocal microscopy, (C,D) are BSE images. (E,F) show EBSD band contrast measurement images together with pole figures, with the latter depicting aragonite crystallographic axes orientations of measurements 1 and 2 (B). We do not see any structural difference for the observed bands (A,B), or for lighter or darker spicule regions. Figure S5: Difference in mode of crystal organization and aragonite c-axis orientation between Acanthopleura spinosa (A) and Acanthopleura vaillantii (B) spicules. The spicules are cut longitudinally. Crystal orientation in Acanthopleura vaillantii spicules is random; hence, aragonite c-axes orientation within the spicules is almost random (B). In contrast, the crystals that form Acanthopleura spinosa spicules are assembled with an axial texture, and aragonite c-axes orientations are directional. However, aragonite c-axes are always at an angle to the outer surface of the spicule and rotate with its curvature. (A) Laser confocal microscopy image. (B) EBSD measurement depicting crystal orientation in color; the used color-code is given in Figure 7C. Figure S6: EBSD scan, taken over the entire cross-section of a plate of Acanthopleura vaillantii. Enlargement of the map shown in Figure 10A,B. (A,C) EBSD band contrast measurement image, (B) crystal orientation given color-coded; the used color-code is shown in Figure 10B. Numbers 1 to 3 indicate different regions of the plate: 1 equals plate position 1 in Figure 10C; 2 equals plate position 2 in Figure 10D; 3 equals plate position 3 in Figure 10E. The different regions of the plate show different crystal sizes, orientations, degrees of twinning, and presence of other misorientations (see also Figure 10).
Author Contributions
J.D.C.-C. collected and selected the samples and performed first sample preparation. X.Y. embedded the samples in EPON and cut and polished them in a microtome. X.Y. and E.G. conducted the EBSD measurements. A.S.V. and E.G. performed EBSD data evaluation and the imaging of the spicules and sclerites. E.G., C.S., A.G.C. and W.W.S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
C.S., J.-D.C.-C., and A.G.C. were funded by projects PID2023-146392NB-I00 of the Spanish Ministerio de Ciencia e Innovación (MCIN/AEI/10.13039/501100011033/) and PCM 00092 (Junta de Andalucía). J-D.C.-C. (FPU18/00547) was supported by a pre-doctoral FPU grant (Ministry of Science, Innovation and Universities). W.W.S., E.G., X.Y., and A.S.V. were funded by the German Research Council Programmes GR 9/1234, SCHM 930/11-2.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank H. Gensler and M. Heß, Systematic Zoology, LMU Munich, Munich, Germany, for the possibility to embed the mollusk samples into EPON in their laboratories. We thank S. Gofas, Departamento de Biología Animal, Facultad de Ciencias, Universidad de Málaga, Málaga, Spain, for Rhyssoplax olivacea and Ischnochiton rissoi specimens. A.G.C. acknowledges the Research Group RNM363 (Consejería de Economía, Innovación, Ciencia y Empleo, Junta de Andalucía), the Unidad Científica de Excelencia UCE-PP2016-05 (University of Granada) and the grant PCIN-2017–098 (Junta de Andalucía).
Conflicts of Interest
Author Xiaofei Yin was employed by the company Bruker Scientific Technology Co., Ltd., The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kaas, P.; van Belle, R. Monograph of Living Chitons (Mollusca: Polyplacophora), Volume 1. Order Neoloricata: Lepidopleurina; Lyneborg, L., Ed.; E. J. Brill Publishers: Leiden, The Netherlands; New York, NY, USA; Koln, Germany; Kobenhavn, Denmark, 1985; ISBN 978-90-04-43167-6. [Google Scholar]
- Kaas, P.; van Belle, R. Monograph of Living Chitons (Mollusca: Polyplacophora), Volume 2. Suborder Ischnochitonina. Ischnochitonidae: Schizoplacinae, Callochitoninae and Lepidochitoninae; Lyneborg, L., Ed.; E. J. Brill Publishers: Leiden, The Netherlands; New York, NY, USA; Koln, Germany; Kobenhavn, Denmark, 1985; ISBN 978-90-04-43167-6. [Google Scholar]
- Kaas, P.; van Belle, R. Monograph of Living Chitons (Mollusca: Polyplacophora), Volume 3. Suborder Ischnochitonina Ischnochitonidae: Chaetopleurinae, & Ischnochitoninae (Pars) Additions to Vols. 1 and 2; Lyneborg, L., Ed.; E. J. Brill Publishers: Leiden, The Netherlands; New York, NY, USA; Koln, Germany; Kobenhavn, Denmark, 1987. [Google Scholar]
- Kaas, P.; van Belle, R. Monograph of Living Chitons (Mollusca: Polyplacophora). Volume 4. Suborder Ischnochitonina: Ischnochitonidae: Ischnochitonina (Continued). Callistoplacidae; Mopaliidae. Additions to Vols. 1–3; Lyneborg, L., Ed.; E. J. Brill Publishers: Leiden, The Netherlands; New York, NY, USA; Koln, Germany; Kobenhavn, Denmark, 1990. [Google Scholar]
- Kaas, P.; van Belle, R. Monograph of Living Chitons (Mollusca: Polyplacophora). Volume 5. Suborder Ischnochitonina: Ischnochitonidae: Ischnochitonina (Concluded). Callistoplacidae; Mopaliidae. Additions to Vols. 1–4; Lyneborg, L., Ed.; E. J. Brill Publishers: Leiden, The Netherlands; New York, NY, USA; Koln, Germany; Kobenhavn, Denmark, 1994. [Google Scholar]
- Schwabe, E. A Catalogue of Recent and Fossil Chitons (Mollusca: Polyplacophora) Addenda. Novapex 2005, 6, 89–105. [Google Scholar]
- Todt, C.; Okusu, A.; Schänder, C.; Schwabe, E. 4. Solenogastres, Caudofoveata, and Polyplacophora. In Phylogeny and Evolution of the Mollusca; Winston, P., Lindberg, D.R.R., Eds.; University of California Press: Berkeley, CA, USA, 2008; pp. 71–96. ISBN 978-0-520-93370-5. [Google Scholar]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Polyplacophora, Monoplacophora, and Aplacophorans. In Biology and Evolution of the Mollusca, Volume 2; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-351-11525-4. [Google Scholar]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Shell, Body, and Muscles. In Biology and Evolution of the Mollusca, Volume 1; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-351-11566-7. [Google Scholar]
- Irisarri, I.; Uribe, J.E.; Eernisse, D.J.; Zardoya, R. A Mitogenomic Phylogeny of Chitons (Mollusca: Polyplacophora). BMC Evol. Biol. 2020, 20, 22. [Google Scholar] [CrossRef]
- Sigwart, J.; Sumner-Rooney, L. Mollusca: Caudofoveata, Monoplacophora, Polyplacophora, Scaphopoda, Solenogastres. In Structure and Evolution of Invertebrate Nervous Systems; Schmidt-Rhaesa, A., Harzsch, S., Purschke, G., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 172–189. [Google Scholar]
- Schwabe, E. Illustrated Summary of Chiton Terminology (Mollusca: Polyplacophora). Spixiana 2010, 33, 171–194. [Google Scholar]
- Eernisse, D.; Reynolds, P. Polyplacophora. In Microscopic Anatomy of Invertebrates: Mollusca 1; Wiley-Liss: Hoboken, NJ, USA, 1994; Volume 5, pp. 55–110. [Google Scholar]
- Steiner, G.; Salvini-Plawen, L. Acaenoplax—Polychaete or Mollusc? Nature 2001, 414, 601–602. [Google Scholar] [CrossRef]
- Bullock, R.C. The Genus Chiton in the New World (Polyplacophora: Chitonidae). Veliger 1988, 31, 141–191. [Google Scholar]
- Sigwart, J.D.; Vermeij, G.J.; Hoyer, P. Why Do Chitons Curl into a Ball? Biol. Lett. 2019, 15, 20190429. [Google Scholar] [CrossRef]
- Hyman, L.H. Mollusca I: Aplacophora, Polyplacophora, Monoplacophora, Gastropoda; The Invertebrates; McGraw-Hill: New York, NY, USA, 1967; Volume 6. [Google Scholar]
- Leise, E. Sensory Organs in the Hairy Girdles of Some Mopaliid Chitons. Am. Malacol. Bull. 1988, 6, 141–151. [Google Scholar]
- Ponder, W.; Lindberg, D.R. (Eds.) Phylogeny and Evolution of the Mollusca; University of California Press: Berkeley, CA, USA, 2008; ISBN 978-0-520-25092-5. [Google Scholar]
- Sirenko, B.I. Position in the System and the Origin of Deep-Water Chitons of the Family Ferreiraellidae (Mollusca: Polyplacophora). Ruthenica 1997, 7, 77–89. [Google Scholar]
- Sirenko, B. New Outlook on the System of Chitons (Mollusca: Polyplacophora)(<Special Number>the 2nd International Chiton Symposium). Venus J. Malacol. Soc. Jpn. 2006, 65, 27–49. [Google Scholar] [CrossRef]
- Checa, A.G.; Vendrasco, M.J.; Salas, C. Cuticle of Polyplacophora: Structure, Secretion, and Homology with the Periostracum of Conchiferans. Mar. Biol. 2017, 164, 64. [Google Scholar] [CrossRef]
- Haas, W.; Kriesten, K.; Watabe, N. Notes on the Shell Formation in the Larvae of the Placophora (Mollusca). Biominer. Res. Rep. 1979, 10, 1–8. [Google Scholar]
- Haas, W.; Lamotte, M.; Meier Brook, C. Evolution of Calcareous Hardparts in Primitive Molluscs. Malacologia 1981, 21, 403–418. [Google Scholar]
- Haas, W.; Kriesten, K.; Watabe, N. Preliminary Note on the Calcification of the Shell Plates in Chiton Larvae. In The mechanisms of Biomineralization in Animals and Plants; Omori, M., Watabe, N., Eds.; Tokai University Press: Tokyo, Japan, 1980; pp. 67–72. [Google Scholar]
- Fischer, F.P.; Eisensamer, B.; Miltz, C.; Singer, I. Sense Organs in the Girdle of Chiton olivaceus (Molusca: Polyplacophora). Am. Malacol. Bull. 1988, 6, 121–131. [Google Scholar]
- Treves, K.; Traub, W.; Weiner, S.; Addadi, L. Aragonite Formation in the Chiton (Mollusca) Girdle. Helv. Chim. Acta 2003, 86, 1101–1112. [Google Scholar] [CrossRef]
- Connors, M.J.; Ehrlich, H.; Hog, M.; Godeffroy, C.; Araya, S.; Kallai, I.; Gazit, D.; Boyce, M.; Ortiz, C. Three-Dimensional Structure of the Shell Plate Assembly of the Chiton Tonicella marmorea and Its Biomechanical Consequences. J. Struct. Biol. 2012, 177, 314–328. [Google Scholar] [CrossRef]
- Connors, M.J. Design of a Multifunctional Biomineralized Armor System: The Shell of Chitons. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2014. [Google Scholar]
- Connors, M.; Yang, T.; Hosny, A.; Deng, Z.; Yazdandoost, F.; Massaadi, H.; Eernisse, D.; Mirzaeifar, R.; Dean, M.N.; Weaver, J.C.; et al. Bioinspired Design of Flexible Armor Based on Chiton Scales. Nat. Commun. 2019, 10, 5413. [Google Scholar] [CrossRef]
- Kingston, A.C.N.; Chappell, D.R.; Speiser, D.I. Evidence for Spatial Vision in Chiton tuberculatus, a Chiton with Eyespots. J. Exp. Biol. 2018, 221, jeb183632. [Google Scholar] [CrossRef]
- O’Connor, S.; Ulm, S.; Fallon, S.J.; Barham, A.; Loch, I. Pre-Bomb Marine Reservoir Variability in the Kimberley Region, Western Australia. Radiocarbon 2010, 52, 1158–1165. [Google Scholar] [CrossRef]
- Gomez, J.E.; Calle, W. Moluscos: Guía de Especies Marinas. Available online: https://wastemagazine.es/rhyssoplax-olivacea.htm (accessed on 5 May 2025).
- Acanthopleura Vaillantii. Available online: https://www.reeflex.net/tiere/16150_Acanthopleura_vaillantii.htm (accessed on 5 May 2025).
- Rueda, J.L.; Gofas, S.; Urra, J.; Salas, C. A Highly Diverse Molluscan Assemblage Associated with Eelgrass Beds (Zostera Marina L.) in the Alboran Sea: Micro-Habitat Preference, Feeding Guilds and Biogeographical Distribution. Sci. Mar. 2009, 73, 679–700. [Google Scholar] [CrossRef]
- WoRMS—World Register of Marine Species. Available online: https://www.marinespecies.org/ (accessed on 5 April 2025).
- Castro-Claros, J.D.; Yin, X.; Salas, C.; Griesshaber, E.; Hörl, S.; Checa, A.G.; Schmahl, W.W. Biomineral Crystallographic Preferred Orientation in Solenogastres Molluscs (Aplacophora) Is Controlled by Organic Templating. Sci. Rep. 2024, 14, 10309. [Google Scholar] [CrossRef]
- Yin, X.; Castro-Claros, J.D.; Griesshaber, E.; Salas, C.; Sancho Vaquer, A.; Checa, A.G.; Schmahl, W.W. Molluscs Generate Preferred Crystallographic Orientation of Biominerals by Organic Templates, the Texture and Microstructure of Caudofoveata (Aplacophora) Shells. Sci. Rep. 2024, 14, 13469. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, J.P.R. Crystal Structures of Aragonite, Strontianite, and Witherite. Am. Mineral. 1971, 56, 758–767. [Google Scholar]
- Greiner, M.; Yin, X.; Fernández-Díaz, L.; Griesshaber, E.; Weitzel, F.; Ziegler, A.; Veintemillas-Verdaguer, S.; Schmahl, W.W. Combined Influence of Reagent Concentrations and Agar Hydrogel Strength on the Formation of Biomimetic Hydrogel–Calcite Composites. Cryst. Growth Des. 2018, 18, 1401–1414. [Google Scholar] [CrossRef]
- Yin, X.; Griesshaber, E.; Fernández-Díaz, L.; Ziegler, A.; García-García, F.J.; Schmahl, W.W. Influence of Gelatin–Agarose Composites and Mg on Hydrogel-Carbonate Aggregate Formation and Architecture. Cryst. Growth Des. 2019, 19, 5696–5715. [Google Scholar] [CrossRef]
- Eder, M.; Amini, S.; Fratzl, P. Biological Composites—Complex Structures for Functional Diversity. Science 2018, 362, 543–547. [Google Scholar] [CrossRef]
- Fratzl, P.; Kolednik, O.; Fischer, F.D.; Dean, M.N. The Mechanics of Tessellations—Bioinspired Strategies for Fracture Resistance. Chem. Soc. Rev. 2016, 45, 252–267. [Google Scholar] [CrossRef]
- Deng, Z.; Jia, Z.; Li, L. Biomineralized Materials as Model Systems for Structural Composites: Intracrystalline Structural Features and Their Strengthening and Toughening Mechanisms. Adv. Sci. 2022, 9, 2103524. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s Hierarchical Materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Currey, J.D.; Sheppard, P.M. Mechanical Properties of Mother of Pearl in Tension. Proc. R. Soc. London. Ser. B. Biol. Sci. 1997, 196, 443–463. [Google Scholar] [CrossRef]
- Berman, A.; Hanson, J.; Leiserowitz, L.; Koetzle, T.F.; Weiner, S.; Addadi, L. Biological Control of Crystal Texture: A Widespread Strategy for Adapting Crystal Properties to Function. Science 1993, 259, 776–779. [Google Scholar] [CrossRef]
- Ritchie, R.O. How Does Human Bone Resist Fracture? Ann. N. Y. Acad. Sci. 2010, 1192, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Jayasankar, A.K.; Seidel, R.; Naumann, J.; Guiducci, L.; Hosny, A.; Fratzl, P.; Weaver, J.C.; Dunlop, J.W.C.; Dean, M.N. Mechanical Behavior of Idealized, Stingray-Skeleton-Inspired Tiled Composites as a Function of Geometry and Material Properties. J. Mech. Behav. Biomed. Mater. 2017, 73, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Hazell, P.J.; Escobedo, J.P.; Wang, H. Biomimetic Armour Design Strategies for Additive Manufacturing: A Review. Mater. Des. 2021, 205, 109730. [Google Scholar] [CrossRef]
- Rawat, P.; Zhu, D.; Rahman, M.Z.; Barthelat, F. Structural and Mechanical Properties of Fish Scales for the Bio-Inspired Design of Flexible Body Armors: A Review. Acta Biomater. 2021, 121, 41–67. [Google Scholar] [CrossRef]
- Barthelat, F.; Li, C.-M.; Comi, C.; Espinosa, H.D. Mechanical Properties of Nacre Constituents and Their Impact on Mechanical Performance. J. Mater. Res. 2006, 21, 1977–1986. [Google Scholar] [CrossRef]
- Dunlop, J.W.C.; Fratzl, P. Biological Composites. Annu. Rev. Mater. Sci. 2010, 40, 1–24. [Google Scholar] [CrossRef]
- Kunitake, M.E.; Mangano, L.M.; Peloquin, J.M.; Baker, S.P.; Estroff, L.A. Evaluation of Strengthening Mechanisms in Calcite Single Crystals from Mollusk Shells. Acta Biomater. 2013, 9, 5353–5359. [Google Scholar] [CrossRef]
- Pasquini, L.; Molinari, A.; Fantazzini, P.; Dauphen, Y.; Cuif, J.-P.; Levy, O.; Dubinsky, Z.; Caroselli, E.; Prada, F.; Goffredo, S.; et al. Isotropic Microscale Mechanical Properties of Coral Skeletons. J. R. Soc. Interface 2015, 12, 20150168. [Google Scholar] [CrossRef]
- Ritchie, R.O. The Conflicts between Strength and Toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Biology and Evolution of the Mollusca; CRC Press: Boca Ratón, FL, USA, 2019; Volume 1. [Google Scholar]
- Sadeghi, P.; Loghmani, M. First Record of Acanthopleura vaillantii (Mollusca: Polyplacophora) from Iran—Chabahar Bay in the Oman Sea. Mar. Biodivers. Rec. 2010, 3, e7. [Google Scholar] [CrossRef]
- Seidel, R.; Blumer, M.; Chaumel, J.; Amini, S.; Dean, M.N. Endoskeletal Mineralization in Chimaera and a Comparative Guide to Tessellated Cartilage in Chondrichthyan Fishes (Sharks, Rays and Chimaera). J. R. Soc. Interface 2020, 17, 20200474. [Google Scholar] [CrossRef] [PubMed]
- Browning, A.; Ortiz, C.; Boyce, M.C. Mechanics of Composite Elasmoid Fish Scale Assemblies and Their Bioinspired Analogues. J. Mech. Behav. Biomed. Mater. 2013, 19, 75–86. [Google Scholar] [CrossRef]
- Zolotovsky, K.; Varshney, S.; Reichert, S.; Arndt, E.M.; Dao, M.; Boyce, M.C.; Ortiz, C. Fish-Inspired Flexible Protective Material Systems with Anisotropic Bending Stiffness. Commun. Mater. 2021, 2, 35. [Google Scholar] [CrossRef]
- Griesshaber, E.; Yin, X.; Ziegler, A.; Kelm, K.; Checa, A.; Eisenhauer, A.; Schmahl, W.W. Patterns of Mineral Organization in Carbonate Biological Hard Materials. In Highlights in Applied Mineralogy; Heuss-Aßbichler, S., Amthauer, G., John, M., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2017; pp. 245–272. ISBN 978-3-11-049734-2. [Google Scholar]
- Huber, J.; Griesshaber, E.; Nindiyasari, F.; Schmahl, W.W.; Ziegler, A. Functionalization of Biomineral Reinforcement in Crustacean Cuticle: Calcite Orientation in the Partes Incisivae of the Mandibles of Porcellio Scaber and the Supralittoral Species Tylos europaeus (Oniscidea, Isopoda). J. Struct. Biol. 2015, 190, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Buerger, M.J. The Genesis of Twin Crystals. Am. Mineral. 1945, 30, 469–482. [Google Scholar]
- Hahn, T.; Klapper, H. Twinning of Crystals. In International Tables for Crystallography Volume D: Physical Properties of Crystals; Authier, A., Ed.; International Tables for Crystallography; Springer: Dordrecht, The Netherlands, 2003; pp. 393–448. ISBN 978-1-4020-5409-9. [Google Scholar]
- Lacombe, O.; Parlangeau, C.; Beaudoin, N.E.; Amrouch, K. Calcite Twin Formation, Measurement and Use as Stress–Strain Indicators: A Review of Progress over the Last Decade. Geosciences 2021, 11, 445. [Google Scholar] [CrossRef]
- Kobayashi, I.; Akai, J. Twinned Aragonite Crystals Found in the Bivalvian Crossed Lamellar Shell Structure. J. Geol. Soc. Jpn. 1994, 100, 177–180. [Google Scholar] [CrossRef]
- Suzuki, M.; Kim, H.; Mukai, H.; Nagasawa, H.; Kogure, T. Quantitative XRD Analysis of {1 1 0} Twin Density in Biotic Aragonites. J. Struct. Biol. 2012, 180, 458–468. [Google Scholar] [CrossRef]
- Griesshaber, E.; Schmahl, W.W.; Ubhi, H.S.; Huber, J.; Nindiyasari, F.; Maier, B.; Ziegler, A. Homoepitaxial Meso- and Microscale Crystal Co-Orientation and Organic Matrix Network Structure in Mytilus edulis Nacre and Calcite. Acta Biomater. 2013, 9, 9492–9502. [Google Scholar] [CrossRef]
- Kogure, T.; Suzuki, M.; Kim, H.; Mukai, H.; Checa, A.G.; Sasaki, T.; Nagasawa, H. Twin Density of Aragonite in Molluscan Shells Characterized Using X-Ray Diffraction and Transmission Electron Microscopy. J. Cryst. Growth 2014, 397, 39–46. [Google Scholar] [CrossRef]
- Almagro, I.; Drzymała, P.; Berent, K.; Sainz-Díaz, C.I.; Willinger, M.G.; Bonarski, J.; Checa, A.G. New Crystallographic Relationships in Biogenic Aragonite: The Crossed-Lamellar Microstructures of Mollusks. Cryst. Growth Des. 2016, 16, 2083–2093. [Google Scholar] [CrossRef]
- Crippa, G.; Griesshaber, E.; Checa, A.G.; Harper, E.M.; Simonet Roda, M.; Schmahl, W.W. Orientation Patterns of Aragonitic Crossed-Lamellar, Fibrous Prismatic and Myostracal Microstructures of Modern Glycymeris Shells. J. Struct. Biol. 2020, 212, 107653. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Griesshaber, E.; Checa, A.G.; Nindiyasari-Behal, F.; Sánchez-Almazo, I.; Ziegler, A.; Schmahl, W.W. Calcite Crystal Orientation Patterns in the Bilayers of Laminated Shells of Benthic Rotaliid Foraminifera. J. Struct. Biol. 2021, 231, 107707. [Google Scholar] [CrossRef] [PubMed]
- Lastam, J.; Griesshaber, E.; Yin, X.; Rupp, U.; Sánchez-Almazo, I.; Heß, M.; Walther, P.; Checa, A.; Schmahl, W.W. The Unique Fibrilar to Platy Nano- and Microstructure of Twinned Rotaliid Foraminiferal Shell Calcite. Sci. Rep. 2023, 13, 2189. [Google Scholar] [CrossRef]
- Lastam, J.; Griesshaber, E.; Yin, X.; Rupp, U.; Sánchez-Almazo, I.; Heß, M.; Walther, P.; Checa, A.; Schmahl, W.W. Patterns of Crystal Organization and Calcite Twin Formation in Planktonic, Rotaliid, Foraminifera Shells and Spines. J. Struct. Biol. 2023, 215, 107898. [Google Scholar] [CrossRef]
- Hoerl, S.; Le Moine, T.; Peter, N.J.; Amini, S.; Griesshaber, E.; Wang, J.; Harper, E.M.; Salas, C.; Checa, A.G.; Schwaiger, R.; et al. Crystal Organisation and Material Properties of Chama and Glycymeris Myostraca and Shells. Materialia 2024, 36, 102149. [Google Scholar] [CrossRef]
- Hoerl, S.; Griesshaber, E.; Checa, A.G.; Schmahl, W.W. The Biological Crystals in Chamid Bivalve Shells: Diversity in Morphology and Crystal Arrangement Pattern. Crystals 2024, 14, 649. [Google Scholar] [CrossRef]
- Burkhard, M. Calcite Twins, Their Geometry, Appearance and Significance as Stress-Strain Markers and Indicators of Tectonic Regime: A Review. J. Struct. Geol. 1993, 15, 351–368. [Google Scholar] [CrossRef]
- Schedl, A. Applications of Twin Analysis to Studying Meteorite Impact Structures. Earth Planet. Sci. Lett. 2006, 244, 530–540. [Google Scholar] [CrossRef]
- Kelly, A.; Knowles, K.M. Crystallography and Crystal Defects; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-0-470-75014-8. [Google Scholar]
- Li, L.; Ortiz, C. Pervasive Nanoscale Deformation Twinning as a Catalyst for Efficient Energy Dissipation in a Bioceramic Armour. Nat. Mater. 2014, 13, 501–507. [Google Scholar] [CrossRef]
- Nespolo, M.; Ferraris, G. The Oriented Attachment Mechanism in the Formation of Twins—A Survey. Eur. J. Mineral. 2004, 16, 401–406. [Google Scholar] [CrossRef]
- Hartman, P.; Perdok, W.G. On the Relations between Structure and Morphology of Crystals. I. Acta Crystallogr. 1955, 8, 49–52. [Google Scholar] [CrossRef]
- Hartman, P.; Perdok, W.G. On the Relations between Structure and Morphology of Crystals. II. Acta Crystallogr. 1955, 8, 521–524. [Google Scholar] [CrossRef]
- Uttam, P.; Kumar, V.; Kim, K.-H.; Deep, A. Nanotwinning: Generation, Properties, and Application. Mater. Des. 2020, 192, 108752. [Google Scholar] [CrossRef]
- Wooster, W.A. Atomic Arrangements on the Twin Boundaries of Crystals of Calcite and Aragonite. Mineral. Mag. 1982, 46, 265–268. [Google Scholar] [CrossRef]
- Lu, K. Stabilizing Nanostructures in Metals Using Grain and Twin Boundary Architectures. Nat. Rev. Mater. 2016, 1, 16019. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, Y.; Chen, J.; Xu, J.; Ma, Y.; Wang, X. Effect of Twin Boundaries on the Microstructure and Mechanical Properties of Inconel 625 Alloy. Mater. Sci. Eng. A 2019, 767, 138361. [Google Scholar] [CrossRef]
- Wolf, D.; Yamakov, V.; Phillpot, S.R.; Mukherjee, A.; Gleiter, H. Deformation of Nanocrystalline Materials by Molecular-Dynamics Simulation: Relationship to Experiments? Acta Mater. 2005, 53, 1–40. [Google Scholar] [CrossRef]
- Zhu, T.; Gao, H. Plastic Deformation Mechanism in Nanotwinned Metals: An Insight from Molecular Dynamics and Mechanistic Modeling. Scr. Mater. 2012, 66, 843–848. [Google Scholar] [CrossRef]
- Côté, A.S.; Darkins, R.; Duffy, D.M. Deformation Twinning and the Role of Amino Acids and Magnesium in Calcite Hardness from Molecular Simulation. Phys. Chem. Chem. Phys. 2015, 17, 20178–20184. [Google Scholar] [CrossRef]
- Huang, Z.; Li, H.; Pan, Z.; Wei, Q.; Chao, Y.J.; Li, X. Uncovering High-Strain Rate Protection Mechanism in Nacre. Sci. Rep. 2011, 1, 148. [Google Scholar] [CrossRef]
- Barthelat, F.; Tang, H.; Zavattieri, P.D.; Li, C.-M.; Espinosa, H.D. On the Mechanics of Mother-of-Pearl: A Key Feature in the Material Hierarchical Structure. J. Mech. Phys. Solids 2007, 55, 306–337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).