Research Progress on the Application of Carbon-Based Materials in Electrocatalytic CO2 Reduction Reaction
Abstract
1. Introduction
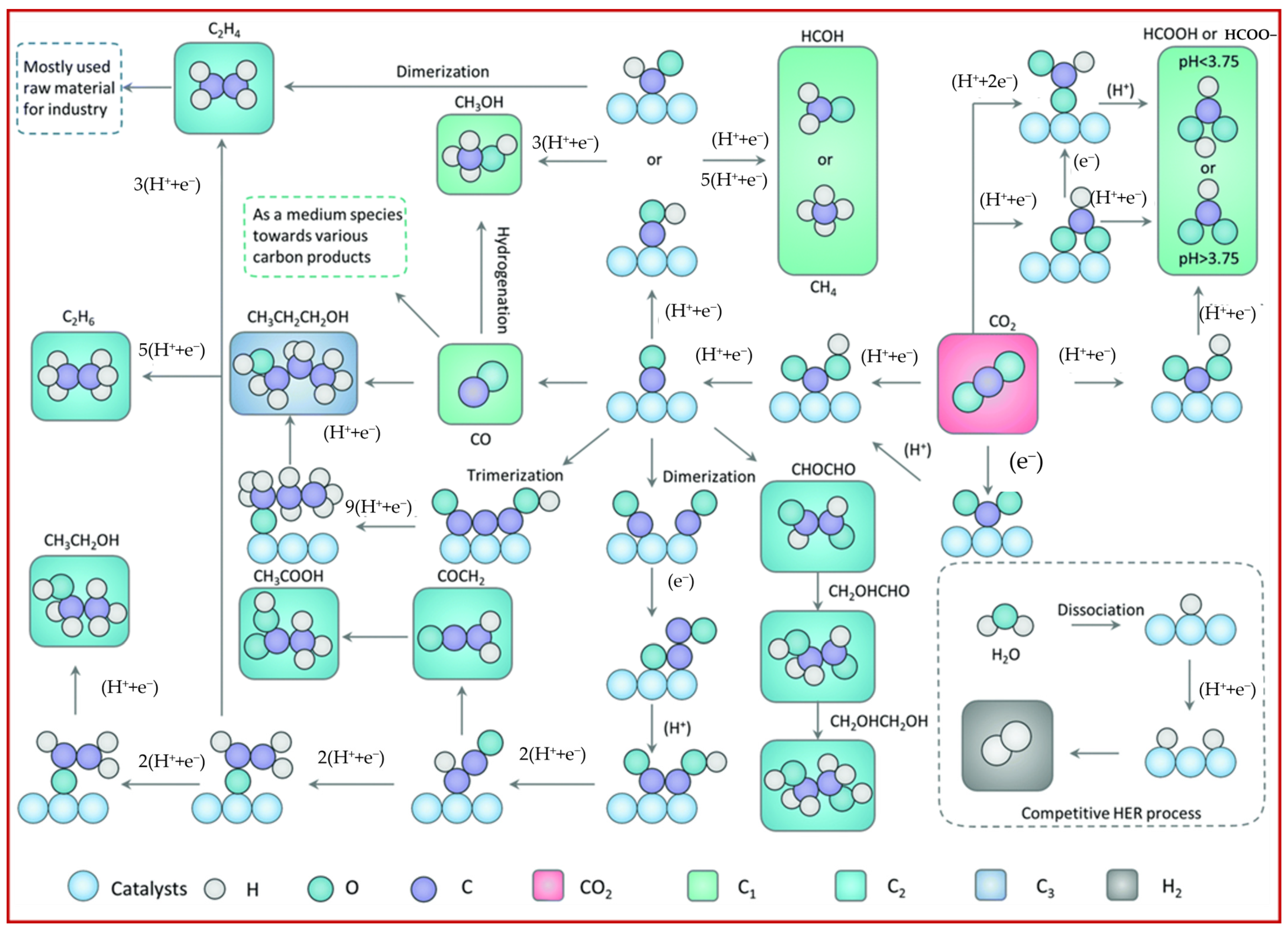
2. Non-Metallic Carbon Materials as Catalysts for Catalyzing the CO2 Reduction Reaction
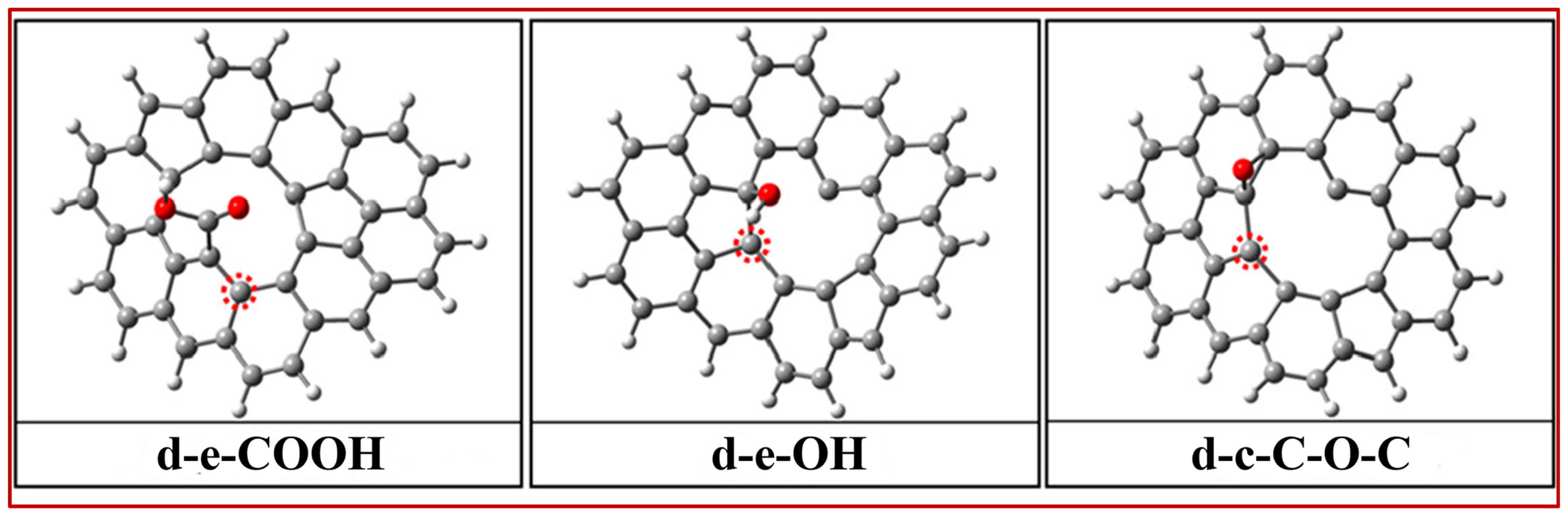
2.1. Carbon Material Catalyst
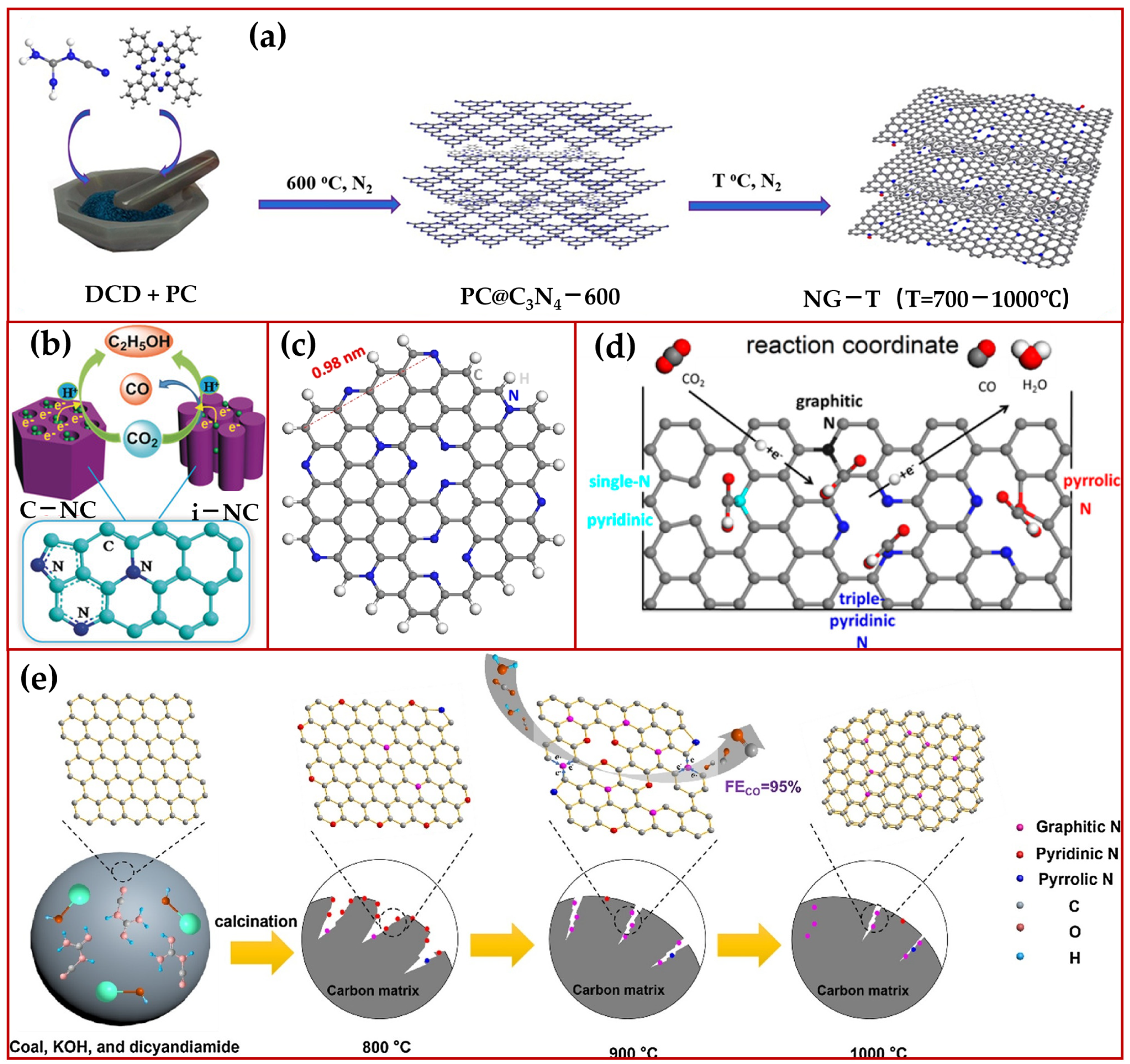
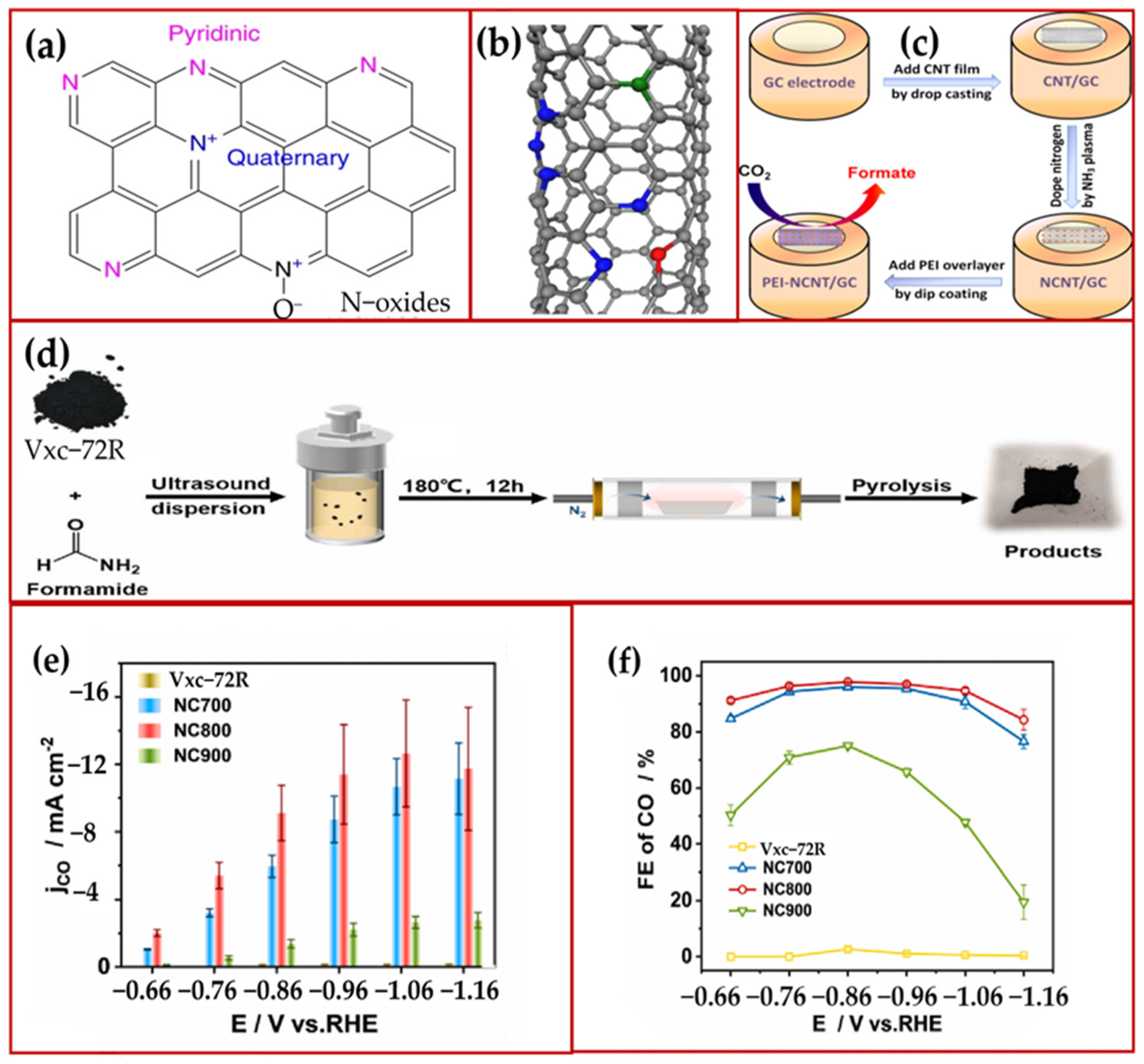
2.2. N-Doped Carbon Material Catalysts
2.3. N Co-Doped Catalysts with Non-Metallic Elements Such as S, P, and F
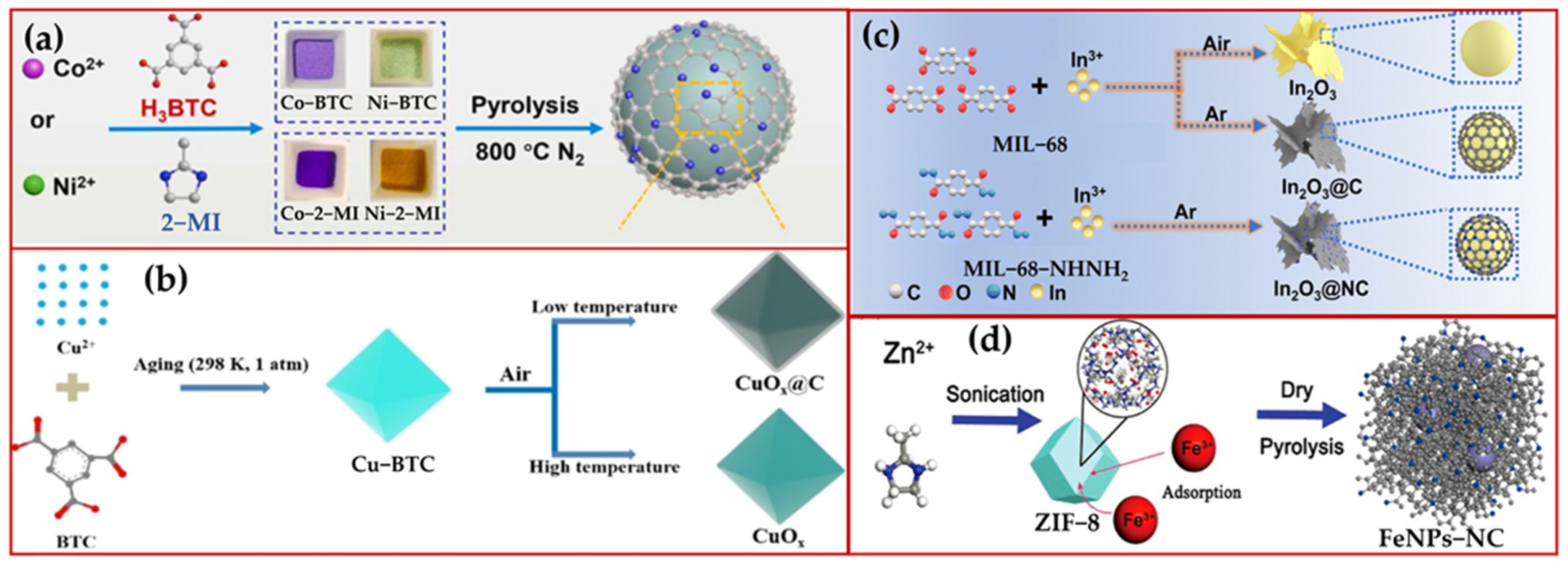
3. Carbon as a Metal Coating
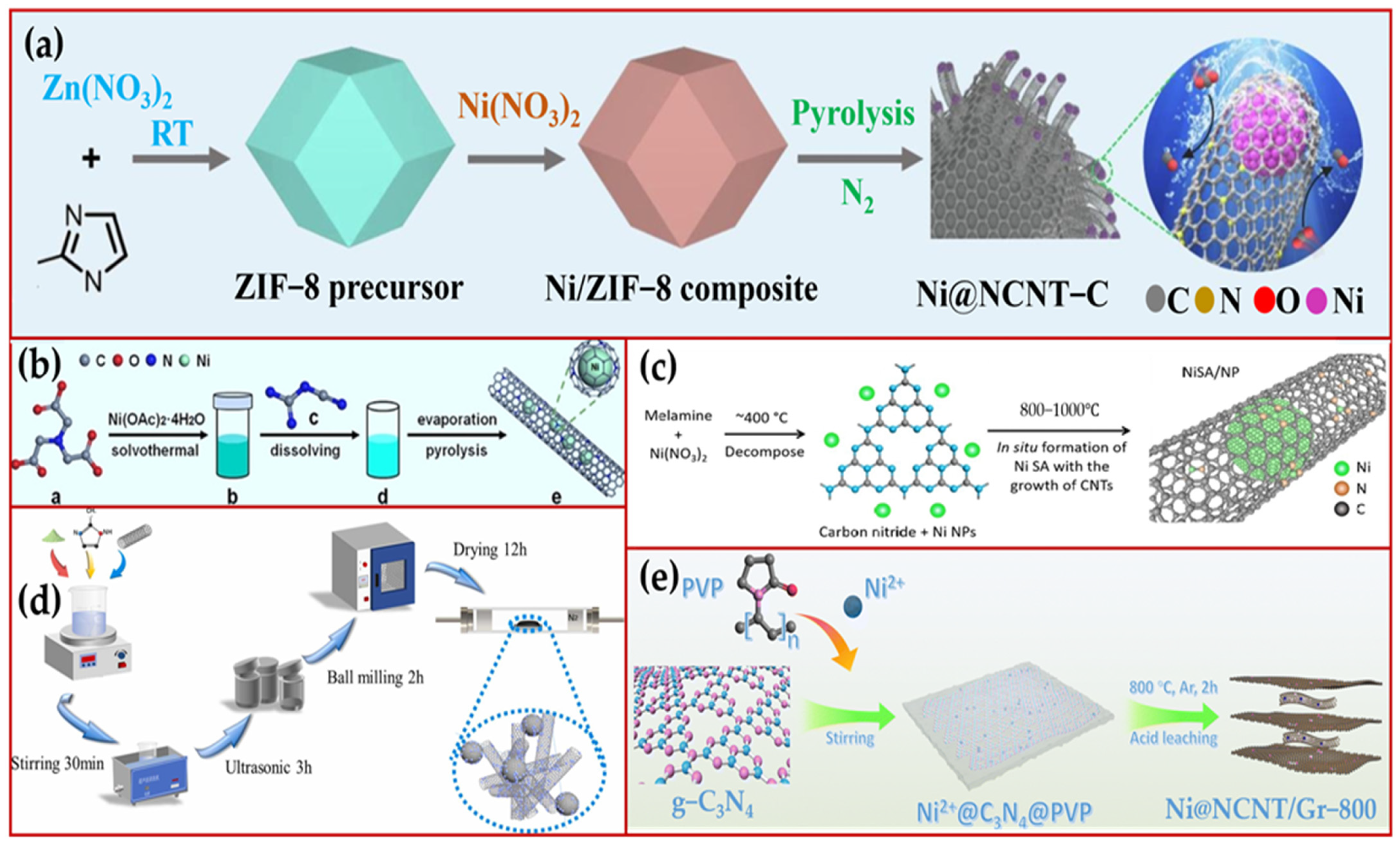
3.1. Pyrolysis of MOF Materials
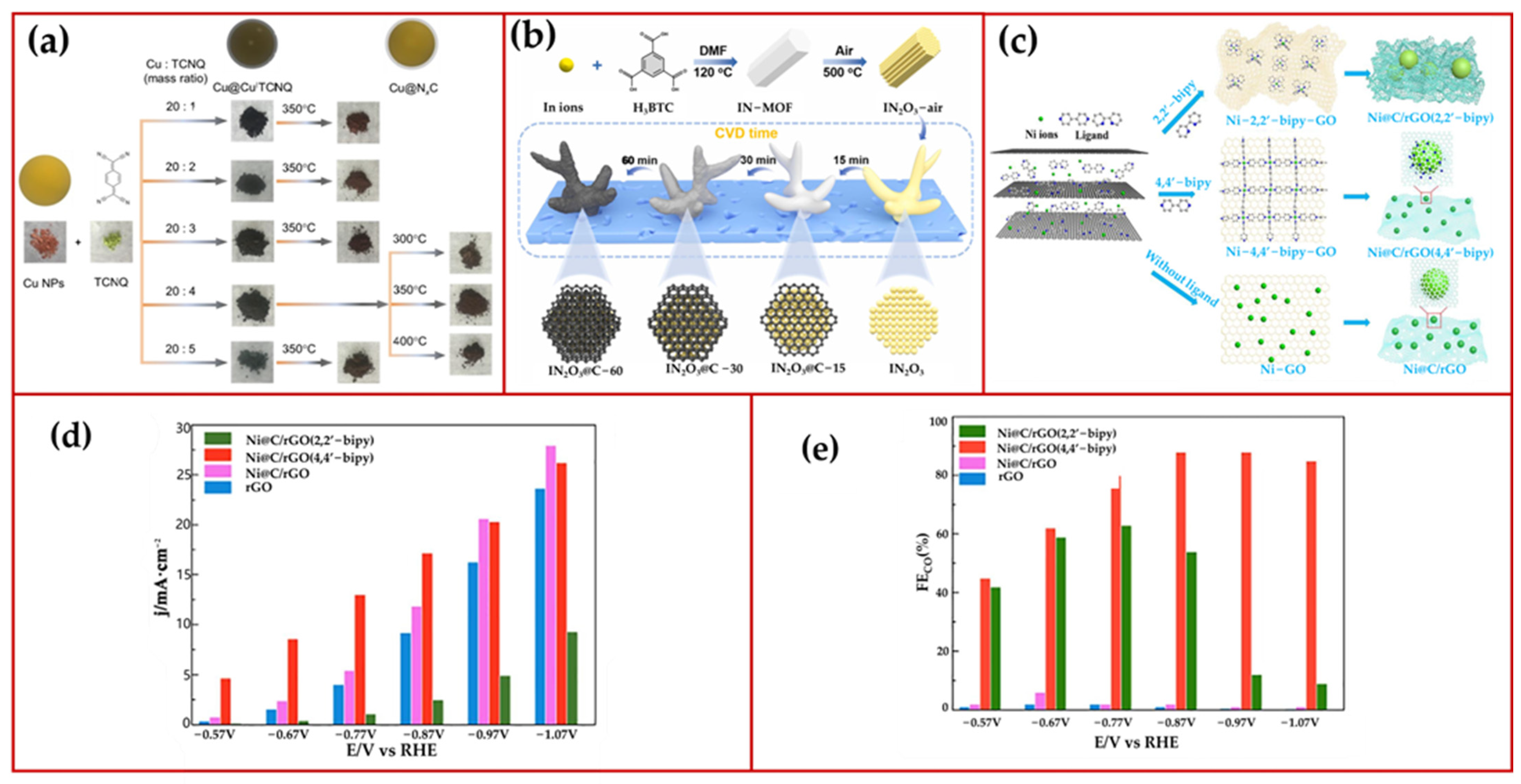
3.2. Metals with N- and C-Sources
3.3. Chemical Vapor Deposition (CVD) Method
3.4. Carbon Nanotubes (CNTs) as a Coating
4. Carbon Materials as Catalyst-Based
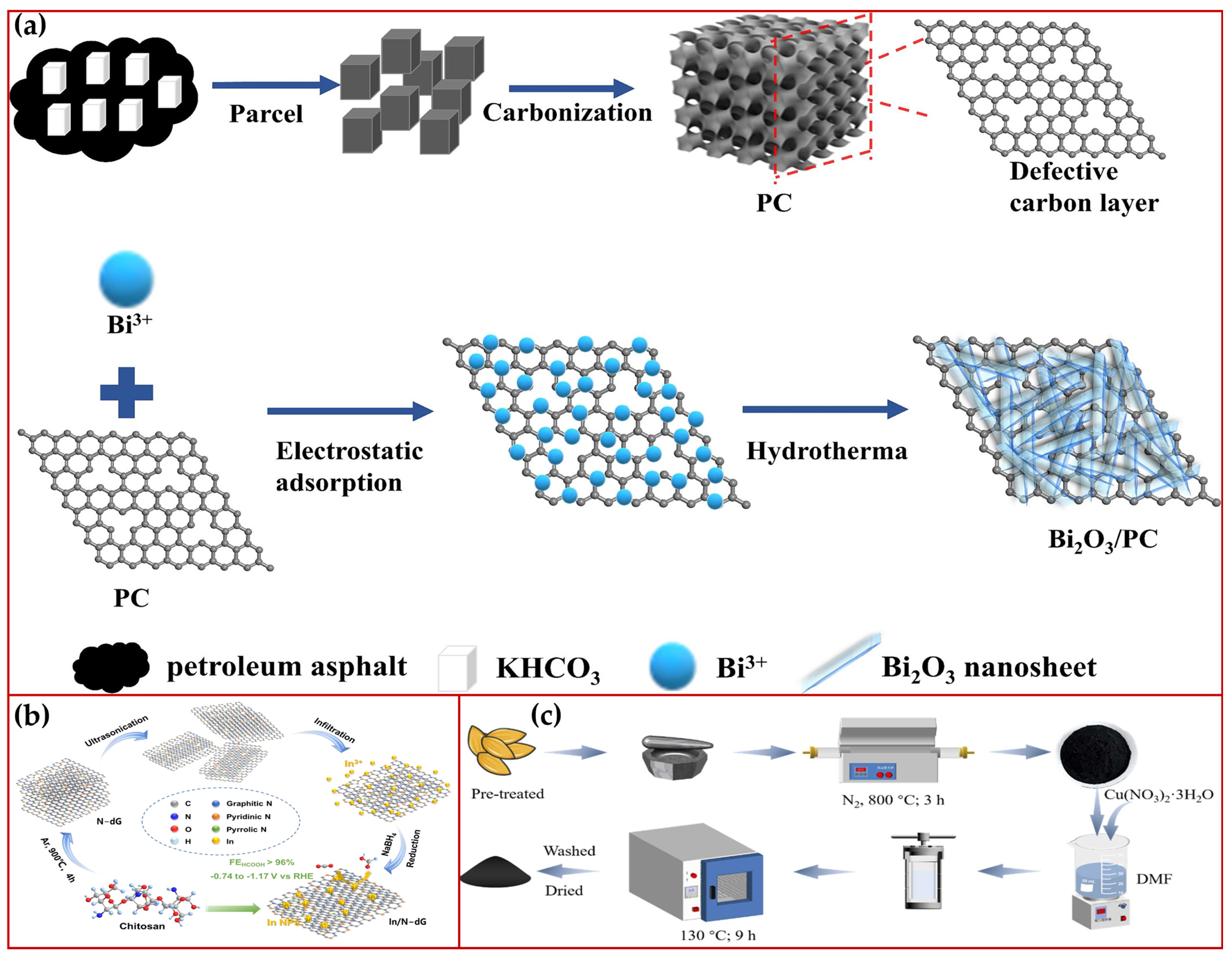
4.1. Self-Assembled Precursor Pyrolytic Carbon-Based
4.2. MOF Material Pyrolyzed Carbon-Based
4.3. Petroleum Asphalt-Based Carbon Material Based
4.4. Bio-Based Carbon-Based
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, W.; Wang, P.; Fan, L.; Chen, K.; Yi, L.; Huang, J.; Cai, P.; Liu, X.; Chen, Q.; Wang, G.; et al. Cu–Ni Alloy Decorating N-Doped Carbon Nanosheets toward High-Performance Electrocatalysis of Mildly Acidic CO2 Reduction. Inorg. Chem. Front. 2023, 10, 2276–2284. [Google Scholar] [CrossRef]
- Dai, X.; Qi, K.; Liu, C.; Lu, X.; Qi, W. Cooperative Multifunctional Nanocarbon as Efficient Electro-Catalysts for CO2 Fixation to Value-Added Cyclic Carbonates Under Mild Conditions. Carbon 2023, 202, 51–58. [Google Scholar] [CrossRef]
- Wu, S.; Yi, F.; Ping, D.; Huang, S.; Zhang, Y.; Han, L.; Wang, S.; Wang, H.; Yang, X.; Guo, D.; et al. Constructing Single-Atomic Nickel Sites in Carbon Nanotubes for Efficient CO2 Electroreduction. Carbon 2022, 196, 1–9. [Google Scholar] [CrossRef]
- Dabsamut, K.; Takahashi, K. Controlling C–C Coupling Reactivity through Pore Shape Engineering of B-Doped Graphyne Family. Carbon 2024, 218, 118672. [Google Scholar] [CrossRef]
- Wang, G.; Chen, J.; Ding, Y.; Cai, P.; Yi, L.; Li, Y.; Tu, C.; Hou, Y.; Wen, Z.; Dai, L. Electrocatalysis for CO2 Conversion: From Fundamentals to Value-Added Products. Chem. Soc. Rev. 2021, 50, 4993–5061. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Sun, Y.; Xie, Y. Rational Design of Electrocatalytic Carbon Dioxide Reduction for a Zero-Carbon Network. Chem. Soc. Rev. 2022, 51, 1234–1252. [Google Scholar] [CrossRef]
- Kong, X.; Zhao, J.; Xu, Z.; Wang, Z.; Wu, Y.; Shi, Y.; Li, H.; Ma, C.; Zeng, J.; Geng, Z. Dynamic Metal–Ligand Coordination Boosts CO2 Electroreduction. J. Am. Chem. Soc. 2023, 145, 14903–14911. [Google Scholar] [CrossRef]
- Huang, D.-S.; Zhu, H.-L.; Zhao, Z.-H.; Huang, J.-R.; Liao, P.-Q.; Chen, X.-M. A Stable and Low-Cost Metal-Azolate Framework with Cyclic Tricopper Active Sites for Highly Selective CO2 Electroreduction to C2+ Products. ACS Catal. 2022, 12, 8444–8450. [Google Scholar] [CrossRef]
- Dai, L. Metal-Free Carbon Electrocatalysts: Recent Advances and Challenges Ahead. Adv. Mater. 2019, 31, 1900973. [Google Scholar] [CrossRef]
- Fu, S.; Li, M.; De Jong, W.; Kortlever, R. Tuning the Properties of N-Doped Biochar for Selective CO2 Electroreduction to CO. ACS Catal. 2023, 13, 10309–10323. [Google Scholar] [CrossRef]
- Sun, W.; Liu, S.; Sun, H.; Hu, H.; Li, J.; Wei, L.; Tian, Z.; Chen, Q.; Su, J.; Chen, L. Low-Coordinated Ni Single Atom Catalyst with Carbon Coordination for Efficient CO2 Electroreduction. Adv. Energy Mater. 2025, 15, 2500283. [Google Scholar] [CrossRef]
- Dutta, A.; Rahaman, M.; Luedi, N.C.; Mohos, M.; Broekmann, P. Morphology Matters: Tuning the Product Distribution of CO2 Electroreduction on Oxide-Derived Cu Foam Catalysts. ACS Catal. 2016, 6, 3804–3814. [Google Scholar] [CrossRef]
- Ye, K.; Zhou, Z.; Shao, J.; Lin, L.; Gao, D.; Ta, N.; Si, R.; Wang, G.; Bao, X. In Situ Reconstruction of a Hierarchical Sn-Cu/SnOx Core/Shell Catalyst for High-Performance CO2 Electroreduction. Angew. Chem. Int. Ed. 2020, 59, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, J.; Song, Y.; Yang, Y.; Li, M.; Zhao, Y.; Teng, Y.; Han, B.; Chen, Z. Cu2O Nano-Homojunction for High-Efficiency Electrocatalytic CO2-to-Ethylene Conversion. Angew. Chem. Int. Ed. 2025, 64, e202501554. [Google Scholar] [CrossRef]
- Yang, F.; Ma, X.; Cai, W.-B.; Song, P.; Xu, W. Nature of Oxygen-Containing Groups on Carbon for High-Efficiency Electrocatalytic CO2 Reduction Reaction. J. Am. Chem. Soc. 2019, 141, 20451–20459. [Google Scholar] [CrossRef]
- Wang, W.; Shang, L.; Chang, G.; Yan, C.; Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Yang, D.; Zhang, T. Intrinsic Carbon-Defect-Driven Electrocatalytic Reduction of Carbon Dioxide. Adv. Mater. 2019, 31, 1808276. [Google Scholar] [CrossRef]
- Song, Y.; Chen, W.; Zhao, C.; Li, S.; Wei, W.; Sun, Y. Metal-Free Nitrogen-Doped Mesoporous Carbon for Electroreduction of CO2 to Ethanol. Angew. Chem. Int. Ed. 2017, 56, 10840–10844. [Google Scholar] [CrossRef]
- Li, J.; Zan, W.-Y.; Kang, H.; Dong, Z.; Zhang, X.; Lin, Y.; Mu, Y.-W.; Zhang, F.; Zhang, X.-M.; Gu, J. Graphitic-N Highly Doped Graphene-like Carbon: A Superior Metal-Free Catalyst for Efficient Reduction of CO2. Appl. Catal. B Environ. 2021, 298, 120510. [Google Scholar] [CrossRef]
- Wu, J.; Ma, S.; Sun, J.; Gold, J.I.; Tiwary, C.; Kim, B.; Zhu, L.; Chopra, N.; Odeh, I.N.; Vajtai, R.; et al. A Metal-Free Electrocatalyst for Carbon Dioxide Reduction to Multi-Carbon Hydrocarbons and Oxygenates. Nat. Commun. 2016, 7, 13869. [Google Scholar] [CrossRef]
- Wu, J.; Liu, M.; Sharma, P.P.; Yadav, R.M.; Ma, L.; Yang, Y.; Zou, X.; Zhou, X.-D.; Vajtai, R.; Yakobson, B.I.; et al. Incorporation of Nitrogen Defects for Efficient Reduction of CO2 via Two-Electron Pathway on Three-Dimensional Graphene Foam. Nano Lett. 2016, 16, 466–470. [Google Scholar] [CrossRef]
- Liu, W.; Qi, J.; Bai, P.; Zhang, W.; Xu, L. Utilizing Spatial Confinement Effect of N Atoms in Micropores of Coal-Based Metal-Free Material for Efficiently Electrochemical Reduction of Carbon Dioxide. Appl. Catal. B Environ. 2020, 272, 118974. [Google Scholar] [CrossRef]
- Wu, J.; Yadav, R.M.; Liu, M.; Sharma, P.P.; Tiwary, C.S.; Ma, L.; Zou, X.; Zhou, X.-D.; Yakobson, B.I.; Lou, J.; et al. Achieving Highly Efficient, Selective, and Stable CO2 Reduction on Nitrogen-Doped Carbon Nanotubes. ACS Nano 2015, 9, 5364–5371. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Ubnoske, S.; Brennaman, M.K.; Song, N.; House, R.L.; Glass, J.T.; Meyer, T.J. Polyethylenimine-Enhanced Electrocatalytic Reduction of CO2 to Formate at Nitrogen-Doped Carbon Nanomaterials. J. Am. Chem. Soc. 2014, 136, 7845–7848. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Tan, T.H.; Ng, Y.H.; Amal, R. Highly Selective and Stable Reduction of CO2 to CO by a Graphitic Carbon Nitride/Carbon Nanotube Composite Electrocatalyst. Chem. A Eur. J. 2016, 22, 11991–11996. [Google Scholar] [CrossRef]
- Daiyan, R.; Tan, X.; Chen, R.; Saputera, W.H.; Tahini, H.A.; Lovell, E.; Ng, Y.H.; Smith, S.C.; Dai, L.; Lu, X.; et al. Electroreduction of CO2 to CO on a Mesoporous Carbon Catalyst with Progressively Removed Nitrogen Moieties. ACS Energy Lett. 2018, 3, 2292–2298. [Google Scholar] [CrossRef]
- Zeng, Q.; Yang, G.; Chen, J.; Zhang, Q.; Liu, Z.; Qin, B.; Peng, F. Effects of Nitrogen and Oxygen on Electrochemical Reduction of CO2 in Nitrogen-Doped Carbon Black. Carbon 2023, 202, 1–11. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, H.; Qian, X.; Lu, Q.; Wang, L.; Hu, H.; Chen, Y. Municipal Sludge-Derived Carbon Dots-Decorated, N-Doped Hierarchical Biocarbon for the Electrochemical Reduction of Carbon Dioxide. Resour. Conserv. Recycl. 2022, 177, 105980. [Google Scholar] [CrossRef]
- Takada, R.; Okada, H.; Narimatsu, K.; Miyake, K.; Uchida, Y.; Tsuji, E.; Nishiyama, N. Metal-Free N, P-Codoped Carbon for Syngas Production with Tunable Composition via CO2 Electrolysis: Addressing the Competition Between CO2 Reduction and H2 Evolution. ChemSusChem 2025, 18, e202402249. [Google Scholar] [CrossRef]
- Kumar, B.; Asadi, M.; Pisasale, D.; Sinha-Ray, S.; Rosen, B.A.; Haasch, R.; Abiade, J.; Yarin, A.L.; Salehi-Khojin, A. Renewable and Metal-Free Carbon Nanofibre Catalysts for Carbon Dioxide Reduction. Nat. Commun. 2013, 4, 2819. [Google Scholar] [CrossRef]
- Li, R.; Liu, F.; Zhang, Y.; Guo, M.; Liu, D. Nitrogen, Sulfur Co-Doped Hierarchically Porous Carbon as a Metal-Free Electrocatalyst for Oxygen Reduction and Carbon Dioxide Reduction Reaction. ACS Appl. Mater. Interfaces 2020, 12, 44578–44587. [Google Scholar] [CrossRef]
- Pan, F.; Li, B.; Xiang, X.; Wang, G.; Li, Y. Efficient CO2 Electroreduction by Highly Dense and Active Pyridinic Nitrogen on Holey Carbon Layers with Fluorine Engineering. ACS Catal. 2019, 9, 2124–2133. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Lin, Q.; Fan, L.; Chai, X.; Zhang, Q.; Liu, J.; He, C.; Lin, Z. Composition Tailoring via N and S Co-doping and Structure Tuning by Constructing Hierarchical Pores: Metal-Free Catalysts for High-Performance Electrochemical Reduction of CO2. Angew. Chem. Int. Ed. 2018, 57, 15476–15480. [Google Scholar] [CrossRef]
- Han, H.; Park, S.; Jang, D.; Lee, S.; Kim, W.B. Electrochemical Reduction of CO2 to CO by N,S Dual-Doped Carbon Nanoweb Catalysts. ChemSusChem 2020, 13, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ghausi, M.A.; Wang, J.; Wang, X.; Wang, W.; Yang, R.; Wu, M.; Zhang, Q.; Wang, Y. Low-Energy CO2 Reduction on a Metal-Free Carbon Material. ChemElectroChem 2020, 7, 2145–2150. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H.; Bi, X.; Zhao, Y.; Wu, M. Efficient Electrocatalytic Reduction of CO2 to CO Enhanced by the Synergistic Effect of N,P on Carbon Aerogel. Chem. Commun. 2024, 60, 6439–6442. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, X.; Yan, X.; Wu, Y.; Liu, H.; Zhu, Q.; Bediako, B.B.A.; Han, B. Boosting CO2 Electroreduction on N,P-Co-doped Carbon Aerogels. Angew. Chem. Int. Ed. 2020, 59, 11123–11129. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, W.; Ma, C.; Zhang, C.; Li, C.; Hong, Y.; Sun, Y.; Niu, J.; Guo, S.; Yao, S. Nitrogen/Phosphorus/Fluorine Heteroatoms Codoped Carbon Nanotube Networks as Free-Standing Cathode for Rechargeable Li-CO2 Batteries. ACS Appl. Nano Mater. 2025, 8, 1499–1507. [Google Scholar] [CrossRef]
- Yan, Q.; Wen, W.; Qiang, L.; Liu, C.; Gao, Y.; Ma, J.; Zhao, P.; Xiao, H.; Wu, J.; Zhao, M.; et al. Advanced Progress in Constructing Carbon-Coated Metal Materials for Electrocatalytic CO2 Reduction. J. Alloys Compd. 2024, 1003, 175705. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Yin, Z.; Wei, X.; Peng, H.; Lyu, K.; Wei, F.; Xiao, L.; Wang, G.; Abruña, H.D.; et al. Interface-Enhanced Catalytic Selectivity on the C2 Products of CO2 Electroreduction. ACS Catal. 2021, 11, 2473–2482. [Google Scholar] [CrossRef]
- Liang, M.; Liu, Y.; Zhang, J.; Wang, F.; Miao, Z.; Diao, L.; Mu, J.; Zhou, J.; Zhuo, S. Understanding the Role of Metal and N Species in M@NC Catalysts for Electrochemical CO2 Reduction Reaction. Appl. Catal. B Environ. 2022, 306, 121115. [Google Scholar] [CrossRef]
- Lin, W.; Chen, H.; Li, Z.; Sasaki, K.; Yao, S.; Zhang, Z.; Li, J.; Fu, J. A Cu2 O-derived Polymeric Carbon Nitride Heterostructured Catalyst for the Electrochemical Reduction of Carbon Dioxide to Ethylene. ChemSusChem 2021, 14, 3190–3197. [Google Scholar] [CrossRef]
- Meng, J.; Liang, M.; Mu, J.; Miao, Z.; Huang, H.; Qi, R.; Diao, L.; Zhuo, S.; Zhou, J. Regulating P-Orbital Electronic Configuration of In2O3 by Thickness-Controlled Carbon Layer for Efficient Electrocatalytic CO2 Reduction to HCOOH. Appl. Catal. B Environ. Energy 2025, 361, 124596. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, M.; Xu, H.; Huang, H.; Meng, J.; Mu, J.; Miao, Z.; Zhou, J. A N-Doped Carbon-Supported In2 O3 Catalyst for Highly Efficient CO2 Electroreduction to HCOOH. Chem. Commun. 2024, 60, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Bao, G.; Chen, X.; Meng, Y.; Zhang, S.; Ma, X. Iron Nanoparticles Tuned to Catalyze CO2 Electroreduction in Acidic Solutions through Chemical Microenvironment Engineering. ACS Catal. 2022, 12, 7517–7523. [Google Scholar] [CrossRef]
- Wang, F.; Miao, Z.; Mu, J.; Zhao, Y.; Liang, M.; Meng, J.; Wu, X.; Zhou, P.; Zhao, J.; Zhuo, S.; et al. A Ni Nanoparticles Encapsulated in N-Doped Carbon Catalyst for Efficient Electroreduction CO2: Identification of Active Sites for Adsorption and Activation of CO2 Molecules. Chem. Eng. J. 2022, 428, 131323. [Google Scholar] [CrossRef]
- Liang, M.; Liu, Y.; Huang, H.; Diao, L.; Mu, J.; Miao, Z.; Zhou, J.; Zhuo, S. A Robust Ni@NCNT-C Catalyst for Highly Efficient Electrochemical CO2 Reduction to CO over a Wide Potential Range. Chem. Eng. J. 2022, 450, 137962. [Google Scholar] [CrossRef]
- Jia, M.; Choi, C.; Wu, T.-S.; Ma, C.; Kang, P.; Tao, H.; Fan, Q.; Hong, S.; Liu, S.; Soo, Y.-L.; et al. Carbon-Supported Ni Nanoparticles for Efficient CO2 Electroreduction. Chem. Sci. 2018, 9, 8775–8780. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, C.; Wang, Y.; Fang, D.; Zhang, L.; Wang, C. Confining Chainmail-Bearing Ni Nanoparticles in N-doped Carbon Nanotubes for Robust and Efficient Electroreduction of CO2. ChemSusChem 2021, 14, 1140–1154. [Google Scholar] [CrossRef]
- Ren, W.; Tan, X.; Jia, C.; Krammer, A.; Sun, Q.; Qu, J.; Smith, S.C.; Schueler, A.; Hu, X.; Zhao, C. Electronic Regulation of Nickel Single Atoms by Confined Nickel Nanoparticles for Energy-Efficient CO2 Electroreduction. Angew. Chem. Int. Ed. 2022, 61, e202203335. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, J.; Niu, Q.; Zhang, X.; Qian, J.; Chu, C.; He, Y. Blending of Nitrogen-Doped Carbon Nanotubes with Transition Metal: Effect of Type and Amount of Metal Seed on CO2 Electroreduction to CO. J. Environ. Chem. Eng. 2025, 13, 115159. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, J.; Wang, Z.; Lu, Z.; Das, S.; Cool, P. Design of a New Ni@NCNT/Graphene Hybrid Structured Catalyst for High-Performance Electrochemical CO2 Reduction: Unravelling the Roles of N-Doping. Chem. Sci. 2025, 16, 2850–2860. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, Y.; Yang, F.; Liu, Y.; Yu, H.; Han, X.; Chen, J.; Chen, S.; Zhou, S.; Deng, S.; et al. Selective CO2 Electroreduction to Ethanol on Encapsulated Nickel Nanoparticles by N-Doped Carbon Nanotubes. Carbon 2023, 201, 460–466. [Google Scholar] [CrossRef]
- Song, Y.; Mao, J.; Zhu, C.; Li, S.; Li, G.; Dong, X.; Jiang, Z.; Chen, W.; Wei, W. Ni Nanoclusters Anchored on Ni–N–C Sites for CO2 Electroreduction at High Current Densities. ACS Appl. Mater. Interfaces 2023, 15, 10785–10794. [Google Scholar] [CrossRef]
- Zang, Y.; Liu, T.; Wei, P.; Li, H.; Wang, Q.; Wang, G.; Bao, X. Selective CO2 Electroreduction to Ethanol over a Carbon-Coated CuOx Catalyst. Angew. Chem. Int. Ed. 2022, 61, e202209629. [Google Scholar] [CrossRef]
- Han, J.; Ma, J.; Zhou, J.; Chen, X.; Wan, Z.; Zhao, Y. Insight into the Effect of Surface Coverage of Carbon Support on Selective CO2 Electroreduction to C2H4 over Copper-Based Catalyst. Appl. Surf. Sci. 2023, 609, 155394. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, Y.; Niu, D.; Han, X.; Ma, L.; Xu, J. Polyethylene Oxide-Engineered Graphene with Rich Mesopores Anchoring Bi2O3 Nanoparticles for Boosting CO2 Electroreduction to Formate. Electrochim. Acta 2022, 433, 141256. [Google Scholar] [CrossRef]
- Wu, Z.; Jing, H.; Zhao, Y.; Lu, K.; Liu, B.; Yu, J.; Xia, X.; Lei, W.; Hao, Q. Grain Boundary and Interface Interaction Co-Regulation Promotes SnO2 Quantum Dots for Efficient CO2 Reduction. Chem. Eng. J. 2023, 451, 138477. [Google Scholar] [CrossRef]
- Lyu, H.; Ma, C.; Zhao, J.; Shen, B.; Tang, J. A Novel One-Step Calcination Tailored Single-Atom Iron and Nitrogen Co-Doped Carbon Material Catalyst for the Selective Reduction of CO2 to CO. Sep. Purif. Technol. 2022, 303, 122221. [Google Scholar] [CrossRef]
- Sun, M.; Guan, W.; Chen, C.; Wu, C.; Liu, X.; Meng, B.; Chen, T.; Han, Y.; Wang, J.; Xi, S.; et al. Mechanistic Insight into the Synergy Between Nickel Single Atoms and Nanoparticles on N-Doped Carbon for Electroreduction of CO2. J. Energy Chem. 2025, 100, 327–336. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, S.; Yuan, Q.; Wang, A.; Sun, K.; Wang, Z.; Fan, M.; Jiang, J. Copper Cluster Regulated by N, B Atoms for Enhanced CO2 Electroreduction to Formate. J. Colloid. Interface Sci. 2025, 678, 456–464. [Google Scholar] [CrossRef]
- Yue, T.; Sui, H.; Jia, J.; Chang, Y.; Guo, S.; Su, Y.; Jia, M. ZIF-8-Derived Carbon Substrates Embedded with Atomically Dispersed FeN2O2 Active Sites as Bifunctional Catalysts for Electrochemical Carbon Dioxide and Oxygen Reduction. Appl. Catal. B Environ. Energy 2025, 361, 124618. [Google Scholar] [CrossRef]
- Liang, S.; Huang, L.; Gao, Y.; Wang, Q.; Liu, B. Electrochemical Reduction of CO2 to CO over Transition Metal/N-Doped Carbon Catalysts: The Active Sites and Reaction Mechanism. Adv. Sci. 2021, 8, 2102886. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, Z.; Wang, M.; Song, D.; Zhou, R.; Wang, H.; Wang, S.; Zheng, B.; Wang, X.; Ning, H.; et al. Bismuth Oxide Nanoflakes Grown on Defective Microporous Carbon Endows High-Efficient CO2 Reduction at Ampere Level. J. Colloid Interface Sci. 2025, 678, 309–316. [Google Scholar] [CrossRef]
- Shen, T.; Shen, Y.; Ma, Z.; Zhu, C.; Yan, F.; Ma, X.; Xu, J.; Chen, Y. Electronic Interactions between SnO2 Crystals and Porous N-Doped Carbon Nanoflowers Accelerate Electrochemical Reduction of CO2 to Formate. J. Colloid Interface Sci. 2025, 682, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Li, P.; Liu, J.; Wang, Y.; Song, X.; Kang, X.; Sun, X.; Zhu, Q.; Han, B. High-Rate CO2 Electrolysis to Formic Acid over a Wide Potential Window: An Electrocatalyst Comprised of Indium Nanoparticles on Chitosan-Derived Graphene. Angew. Chem. Int. Ed. 2023, 62, e202307612. [Google Scholar] [CrossRef]
- Shao, X.; Bian, Z.; Li, B.; Zhan, F.; Cheng, X.; Shen, Y.; Li, Z.; Zhou, Q.; Cai, R.; Feng, C. Enhanced Mass Transport on Single-Atom Ni-N-C Catalysts with Hierarchical Pore Structures for Efficient CO2 Electroreduction. Sep. Purif. Technol. 2025, 359, 130576. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, R.; Liu, C.; Wu, F.; Li, R. Hollow Porous Carbon Nanocubes with High-Loading Accessible Iron Atomic Sites for CO2 Electroreduction. ACS Appl. Nano Mater. 2025, 8, 1111–1118. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Qi, Y.; Guo, T. CuxO Coupled with In-Situ Nitrogen-Doped Biomass Carbon Electrocatalysts for Accelerated CO2 Electroreduction to Ethylene. J. Environ. Chem. Eng. 2025, 13, 115187. [Google Scholar] [CrossRef]
- Zhang, W.; Mehmood, A.; Ali, G.; Liu, H.; Chai, L.; Wu, J.; Liu, M. Nickel Nanocluster-Stabilized Unsaturated Ni–N3 Atomic Sites for Efficient CO2-to-CO Electrolysis at Industrial-Level Current. Angew. Chem. Int. Ed. 2025, 64, e202424552. [Google Scholar] [CrossRef]
- Fang, Z.; Zhai, Y.; Guo, W.; Sun, Z.; Jiao, L.; Zhu, Z.; Lu, X.; Tang, J. Highly Selective Electroreduction of CO2 to CO with ZnO QDs/N-Doped Porous Carbon Catalysts. Chem. Commun. 2024, 60, 3575–3578. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, J.; Pan, X.; Li, L.; Yu, Z.; Wang, X.; Ma, T.; Lin, S.; Lin, J. Tuning the Inter-Metal Interaction between Ni and Fe Atoms in Dual-Atom Catalysts to Boost CO2 Electroreduction. Angew. Chem. Int. Ed. 2024, 63, e202411543. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Kao, Y.-L.; Ni, L.; Ehnert, R.; Herrmann-Geppert, I.; Van De Krol, R.; Stark, R.W.; Jaegermann, W.; Kramm, U.I.; Bogdanoff, P. Influence of the Metal Center in M–N–C Catalysts on the CO2 Reduction Reaction on Gas Diffusion Electrodes. ACS Catal. 2021, 11, 5850–5864. [Google Scholar] [CrossRef]
- Cai, Y.; Fu, J.; Zhou, Y.; Chang, Y.-C.; Min, Q.; Zhu, J.-J.; Lin, Y.; Zhu, W. Insights on Forming N,O-Coordinated Cu Single-Atom Catalysts for Electrochemical Reduction CO2 to Methane. Nat. Commun. 2021, 12, 586. [Google Scholar] [CrossRef]
- Yin, S.; Zhao, J.; Wu, S.; Wang, X.; Quan, Y.; Ren, J. Electrochemical Reduction of CO2 to CO on Bimetallic CoCu-N-C Catalyst. J. Clean. Prod. 2022, 371, 133569. [Google Scholar] [CrossRef]
- Song, Q.; Kong, F.; Liu, B.-F.; Song, X.; Ren, H.-Y. Biochar-Based Composites for Removing Chlorinated Organic Pollutants: Applications, Mechanisms, and Perspectives. Environ. Sci. Ecotechnol. 2024, 21, 100420. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Y.; Geng, S.; Li, R.; Chen, K. Boosting Electrocatalytic CO2 Reduction to Multi-Carbon Products via Modulated Asymmetric Cu Sites. Adv. Funct. Mater. 2025, 35, 2503497. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Yang, S.; Shao, T.; Li, X.; Cao, R.; Cao, M. Nanoscale Cu–Ag Heterostructures for CO2 Reduction to C2+ Products. ACS Appl. Nano Mater. 2025, 8, 1893–1902. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Y.; Liang, X.; Chen, B.; Shen, Y.; Chen, Y.; Yang, J.; Yu, Y.; Huang, F.; He, H.; et al. Highly Selective Electroreduction of CO2 to CH4 on Cu–Pd Alloy Catalyst: The Role of Palladium-Adsorbed Hydrogen Species and Blocking Effect. Adv. Sci. 2025, 12, 2417247. [Google Scholar] [CrossRef]
- Ren, W.; Tan, X.; Qu, J.; Li, S.; Li, J.; Liu, X.; Ringer, S.P.; Cairney, J.M.; Wang, K.; Smith, S.C.; et al. Isolated Copper–Tin Atomic Interfaces Tuning Electrocatalytic CO2 Conversion. Nat. Commun. 2021, 12, 1449. [Google Scholar] [CrossRef]
- Jiang, J.; Lian, J.; Xu, Z.; Wang, Y.; Dong, Y.; Yan, J.; Li, D.; Liu, S. Steering CO2 Electroreduction to Methane Production over Copper via Polymer-Regulated Hydrolysis. Adv. Funct. Mater. 2025, 35, 2420881. [Google Scholar] [CrossRef]




| Catalysts | Primary Product | Faraday Efficiency (FE) (%) | Stability (h) | Optimal Potential (V) | Current Density at the Optimal Potential (mA∙cm−2) | Electrolyzer | Ref. |
|---|---|---|---|---|---|---|---|
| c-NC | C2H5OH | 77.00% | 24 | −0.56 | \ | H-type cell | [17] |
| NG-T | CO | 95.00% | \ | −0.72 | 9.07 | flow cell | [18] |
| NGQDs | C2+ | 90.00% | \ | −0.86 | 23.00 | flow cell | [19] |
| N-doped 3D graphene foams | CO | 85.00% | 5 | −0.58 | −1.80 | H-type cell | [20] |
| NPC | CO | 95.00% | 10 | –0.67 | −4.80 | H-type cell | [21] |
| NCNTs | CO | 80.00% | 10 | −0.26 | −2.25 | flow cell | [22] |
| PEI-NCNTs/GC | HCOO− | 87.00% | 20 | −1.80 | 9.50 | H-type cell | [23] |
| g-C3N4/MWCNTs | CO | 60.00% | 50 | −0.95 | 2.50 | H-type cell | [24] |
| NRMC | CO | 80.00% | \ | −0.60 | −2.90 | H-type cell | [25] |
| NC800 | CO | 97.80% | 12 | −0.86 | 6.70 | H-type cell | [26] |
| ANBC800 | CO | 89.30% | 80 | −0.82 | −1.59 | flow cell | [10] |
| MSC-HA | C2H5OH | 90.14% | 30 | −0.71 | 24.23 | H-type cell | [27] |
| N-doped carbon foams | CO | 95.00% | \ | −0.50 | \ | H-type cell | [28] |
| Catalysts | Primary Product | Faraday Efficiency (FE) (%) | Stability (h) | Optimal Potential (V) | Current Density at the Optimal Potential (mA∙cm−2) | Electrolyzer | Ref. |
|---|---|---|---|---|---|---|---|
| NSHPC | CO | 87.8% | 8 | 0.45 | 5.49 | H-type cell | [30] |
| NSHCF] | CO | 94.0% | 36 | −0.70 | −103 | H-type cell | [32] |
| NSCNW | CO | 93.4% | 20 | −0.49 | −5.93 | H-type cell | [33] |
| NF-C | CO | 90.0% | 10 | −0.49 | 1.9 | H-type cell | [31] |
| NPC | CO | 70.4% | 10 | −0.80 | 0.36 | H-type cell | [28] |
| NPC | CO | 86.0% | 12 | −0.45 | 0.81 | H-type cell | [34] |
| NPCA | CO | 91.4% | 35 | −1.80 | −22.86 | H-type cell | [35] |
| NPCA | CO | 99.1% | 24 | −2.40 | −143.6 | flow cell. | [36] |
| NPF-CNTs | CO | \ | \ | 1.16 | 0.10 | H-type cell | [37] |
| Catalysts | Primary Product | Faraday Efficiency (FE) (%) | Stability (h) | Optimal Potential (V) | Current Density at the Optimal Potential (mA∙cm−2) | Electrolyzer | Ref. |
|---|---|---|---|---|---|---|---|
| Cu-NxC | C2H4, CH3CH2OH | 80.00 | \ | −1.1 | \ | \ | [39] |
| Ni@NC | CO | 98.00 | 100 | −0.87 | 220.0 | H-type cell | [40] |
| CuxO/CN-10 | C2H4 | 42.20 | 10 | −1.20 | 25.0 | H-type cell | [41] |
| In2O3@C | HCOOH | 97.00 | 10 | −1.27 | 144.2 | flow cell | [42] |
| In2O3@NC | HCOOH | 97.10 | 60 | −1.70 | 190.0 | flow cell | [43] |
| CuxO@C | CH3CH2OH | 46.00 | 50 | −1.00 | −166.0 | flow cell | [38] |
| FeNPs-C | CO | 90.00 | \ | \ | \ | flow cell | [44] |
| Ni@N-C/rGO | CO | 88.00 | 10 | −0.97 | 20.0 | H-type cell | [45] |
| Ni@NCNT-C | CO | 100.00 | 40 | −0.27 | 230.0 | H-type cell | [46] |
| Ni-NC-ATPA@C | CO | 93.70 | 24 | −1.10 | 22.7 | H-type cell | [47] |
| Ni@NC@NCNT | CO | 94.10 | 43 | −1.10 | 48.0 | H-type cell | [48] |
| NiSA/NP | CO | 98.00 | 10 | −2.30 | 310.0 | flow cell | [49] |
| Ni-N-C/CNT | CO | 97.00 | 22.5 | −1.22 | −60.0 | H-type cell | [50] |
| Ni@NCNT/Gr | CO | 90.00 | 24 | −0.71 | −37.4 | H-type cell | [51] |
| Ni@NCNT-700 | CH3CH2OH | 38.50 | 22 | −0.50 | 128.0 | flow cell | [52] |
| NiNCNT | CO | 99.30 | 40 | −0.35 | −300 | flow cell | [53] |
| Ni@NCNT/Gr | CO | >90 | 20 | −0.50 | −9.20 | H-type cell | [51] |
| Primary Product | Faraday Efficiency (FE) (%) | Stability (h) | Optimal Potential (V) | Current Density at the Optimal Potential (mA∙cm−2) | Electrolyzer | Ref. | |
|---|---|---|---|---|---|---|---|
| Cu-10/NC | C2H4 | 37.0 | 7 | −1.20 | \ | H-type cell | [55] |
| Bi2O3/p-rGO | HCOOH | 94.3 | 39 | −1.09 | −16.8 | H-type cell | [56] |
| SnO2/NC | HCOOH | 87.6 | 20 | −1.13 | 33.6 | H-type cell | [57] |
| Fe-N-C(SACs) | CO | 73.0 | 9 | −0.60 | \ | H-type cell | [58] |
| Ni-NC-T | CO | 98.0 | 20 | −1.17 | 58.0 | H-type cell | [59] |
| Cu/BN-C | HCOOH | 70.0 | 12 | −1.00 | 20.8 | H-type cell | [60] |
| Fe-N2O2/NC | CO | 95.5 | 12 | −1.20 | 22.19 | H-type cell | [61] |
| CoCu-N-C | CO | 76.5 | \ | \ | \ | \ | [62] |
| Bi2O3/PC | HCOOH | 91.5 | 16 | −1.10 | 150.0 | H-type cell | [63] |
| SnO2@N-GPC | HCOO− | 96.3 | 10 | −1.20 | 104.7 | flow cell | [64] |
| In/N-dG | HCOOH | 100.0 | 14 | −1.17 | 700.0 | flow cell | [65] |
| Ni-N-C(P)-8 | CO | 99.0 | 10 | −0.90 | −20.0 | H-type cell | [66] |
| H-Fe-NC SACs | CO | 94.6 | 10 | −0.98 | −23.5 | H-type cell | [67] |
| CuxO@NC | C2H4 | 48.4 | 19 | −0.98 | −8.32 | H-type cell | [68] |
| Ni6@Ni-N3 | CO | 99.7 | 10 | −1.15 | 500 | flow cell | [69] |
| ZnOQDs/P-NC | CO | 95.3 | 24 | 2.2 | −21.6 | H-type cell | [70] |
| NiFe-N bridge | CO | 83 | 20 | −0.5 | −9.2 | H-type cell | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Gong, G.; Yin, A.; Han, R.; Yao, J.; Liu, Z.; Ming, H.; Wu, M. Research Progress on the Application of Carbon-Based Materials in Electrocatalytic CO2 Reduction Reaction. Crystals 2025, 15, 467. https://doi.org/10.3390/cryst15050467
Yang X, Gong G, Yin A, Han R, Yao J, Liu Z, Ming H, Wu M. Research Progress on the Application of Carbon-Based Materials in Electrocatalytic CO2 Reduction Reaction. Crystals. 2025; 15(5):467. https://doi.org/10.3390/cryst15050467
Chicago/Turabian StyleYang, Xinyuan, Guifan Gong, Aoxiang Yin, Runyao Han, Jirong Yao, Zimeng Liu, Hui Ming, and Mei Wu. 2025. "Research Progress on the Application of Carbon-Based Materials in Electrocatalytic CO2 Reduction Reaction" Crystals 15, no. 5: 467. https://doi.org/10.3390/cryst15050467
APA StyleYang, X., Gong, G., Yin, A., Han, R., Yao, J., Liu, Z., Ming, H., & Wu, M. (2025). Research Progress on the Application of Carbon-Based Materials in Electrocatalytic CO2 Reduction Reaction. Crystals, 15(5), 467. https://doi.org/10.3390/cryst15050467






