Synthesis and Characterization of MgO-ZrO2 Heterostructure: Optical, Mechanical and Electrical Properties
Abstract
1. Introduction
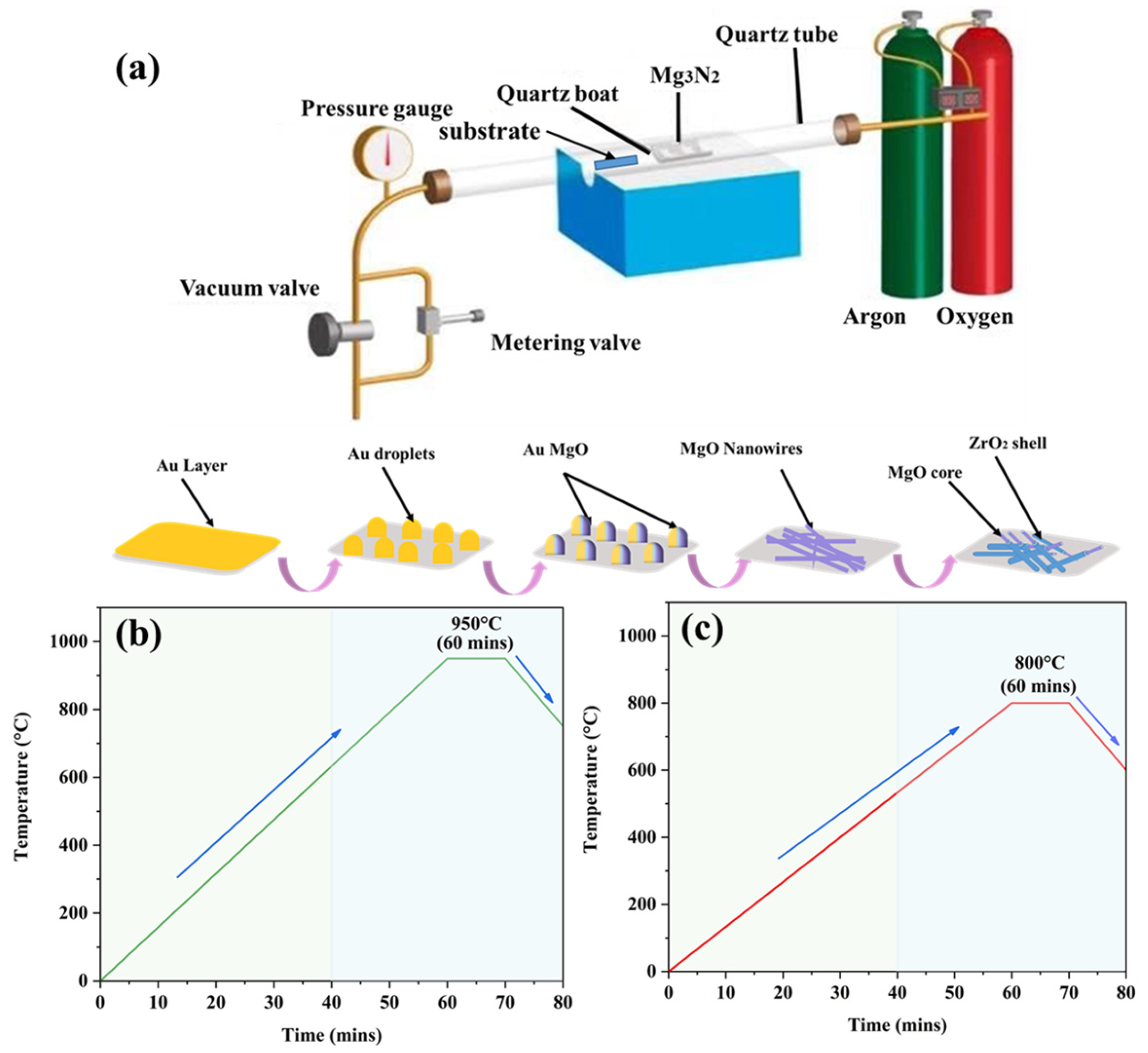
2. Materials and Methods
Sample Preparation
3. Results and Discussion
3.1. XRD Analysis and Phase Identification
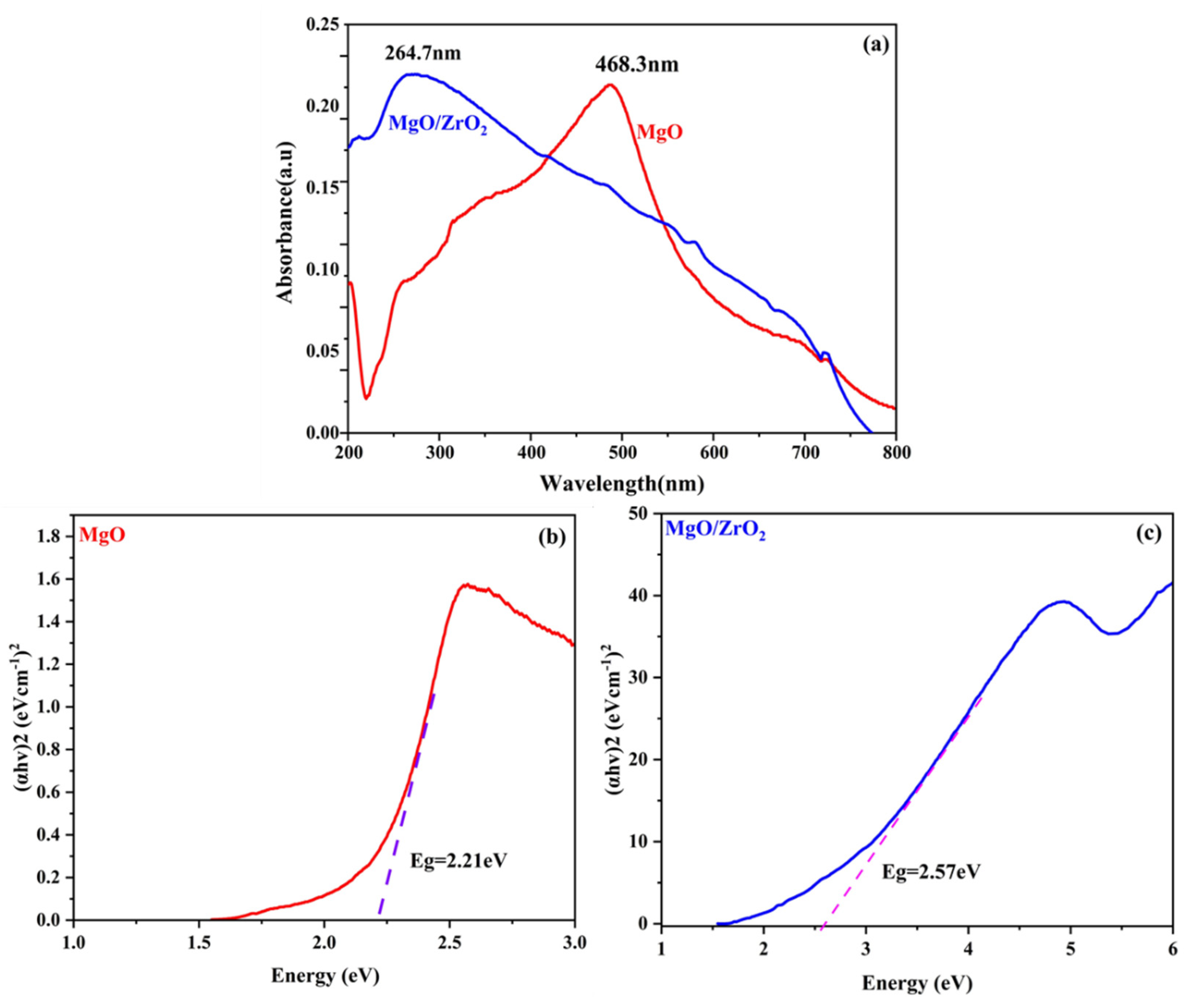
3.2. Optical Properties
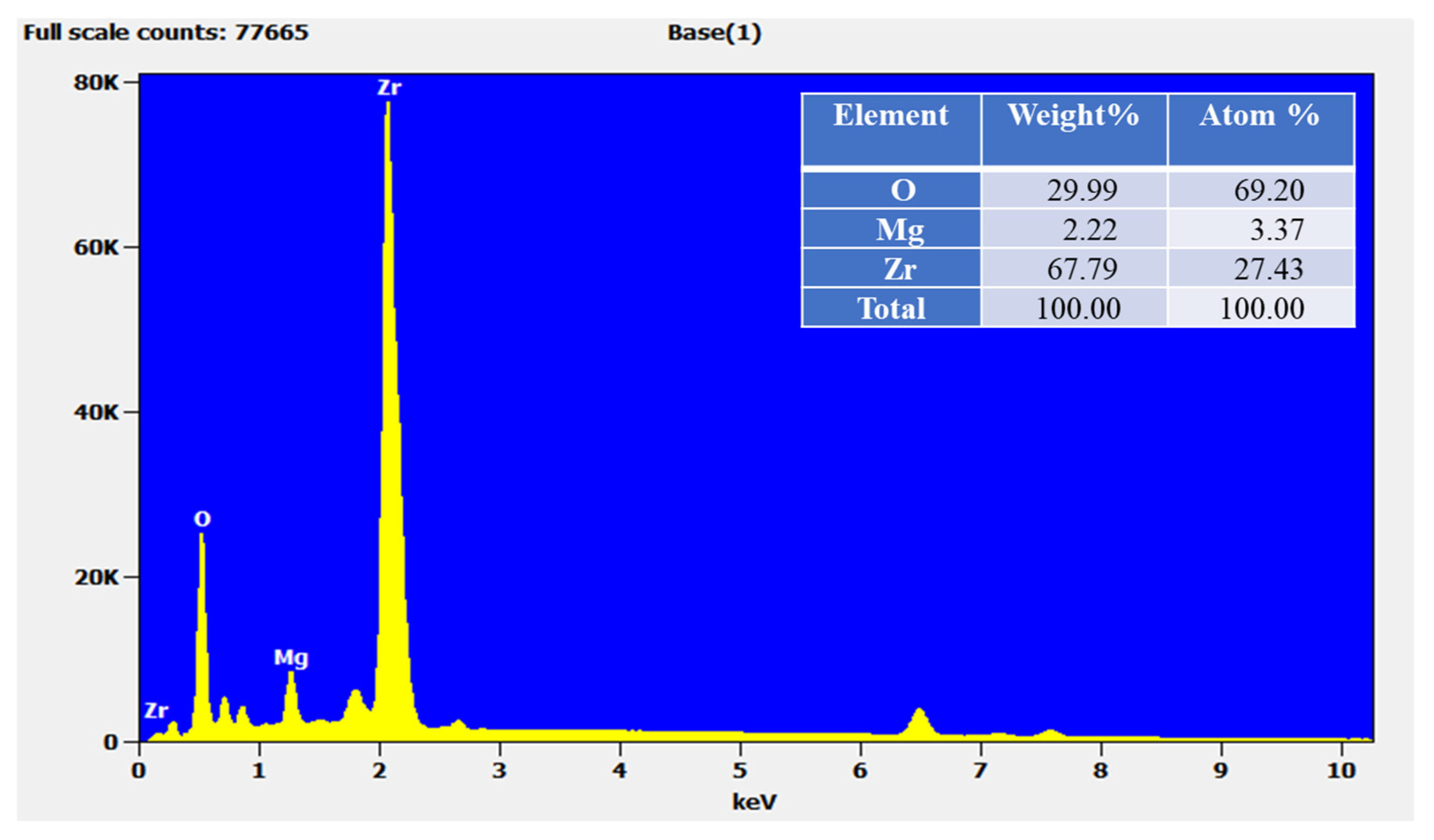
3.3. EDS Analysis
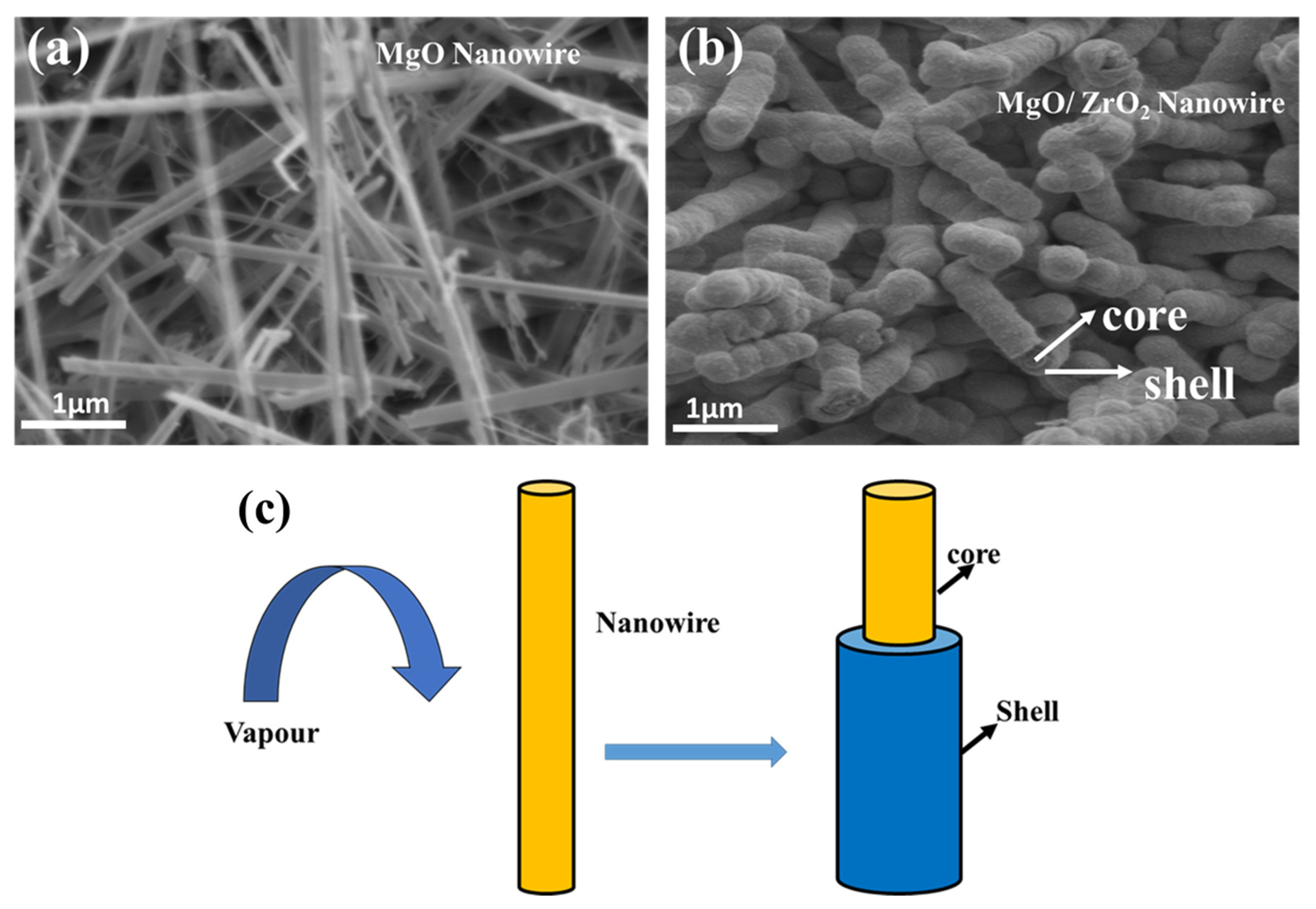
3.4. Morphological and Compositional Analysis
Transmission Electron Microscopes (TEM)
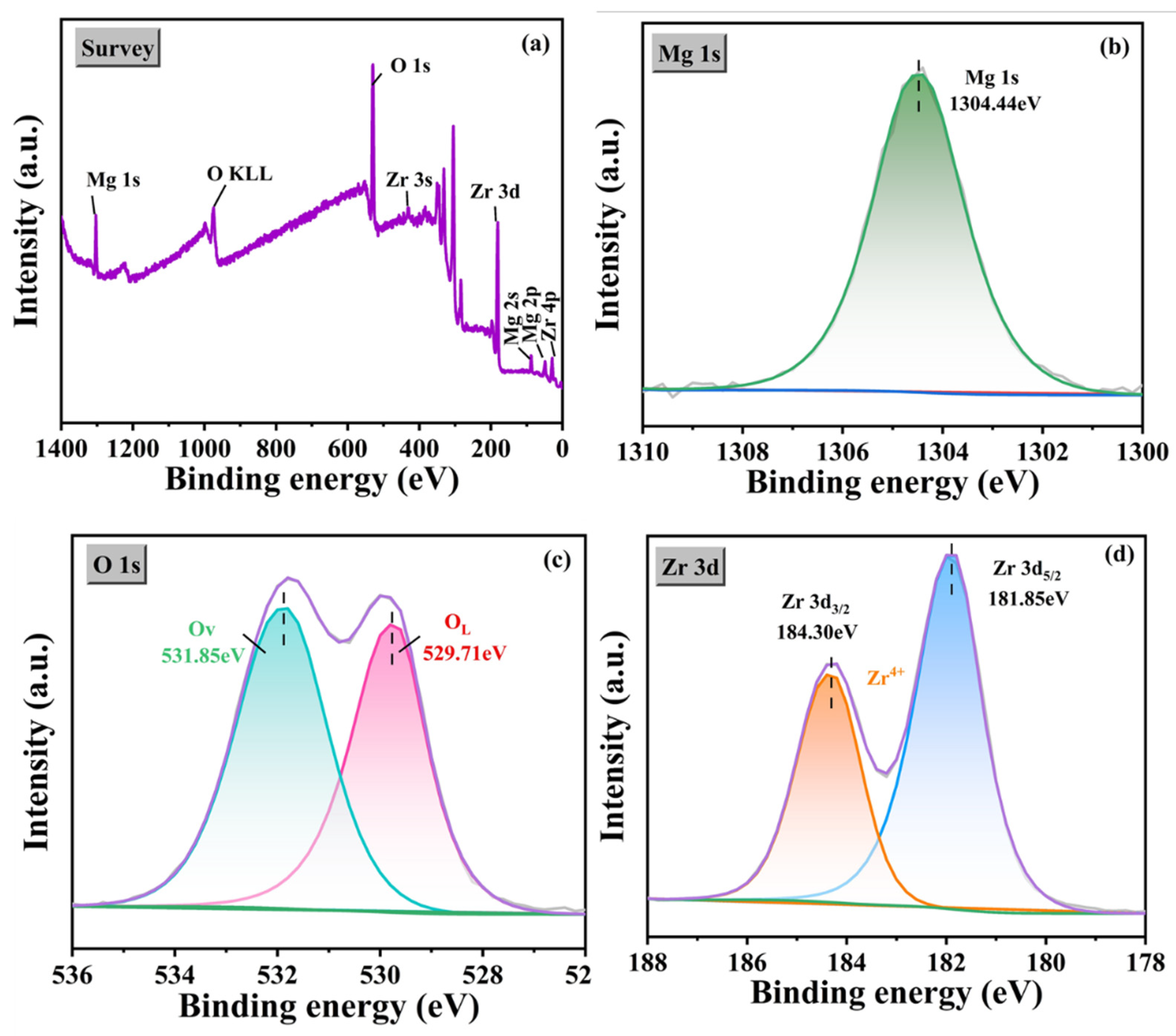
3.5. Chemical State Analysis
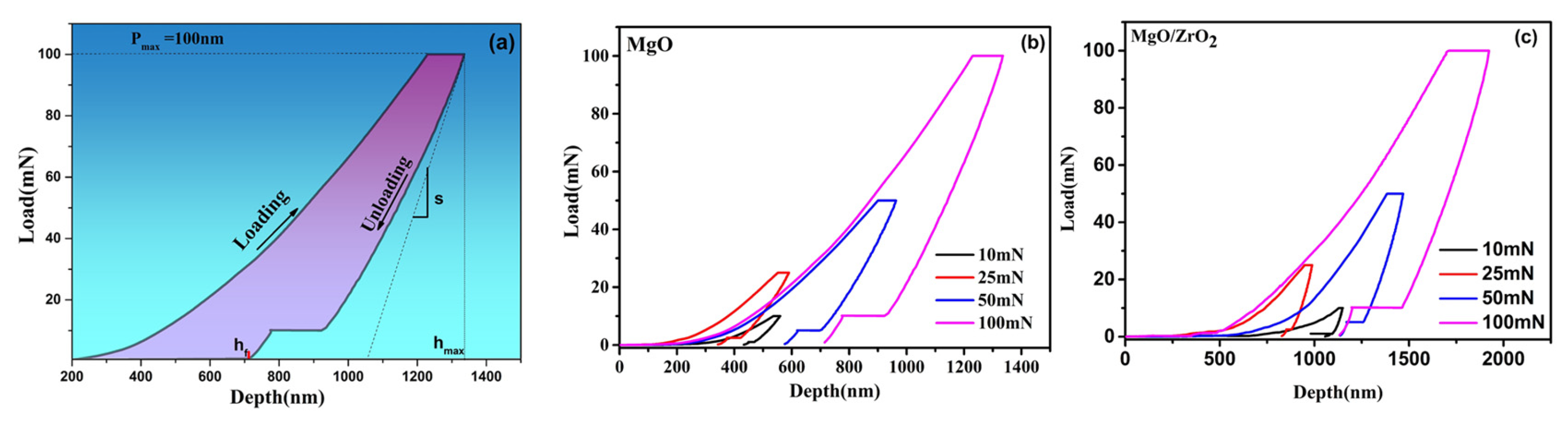
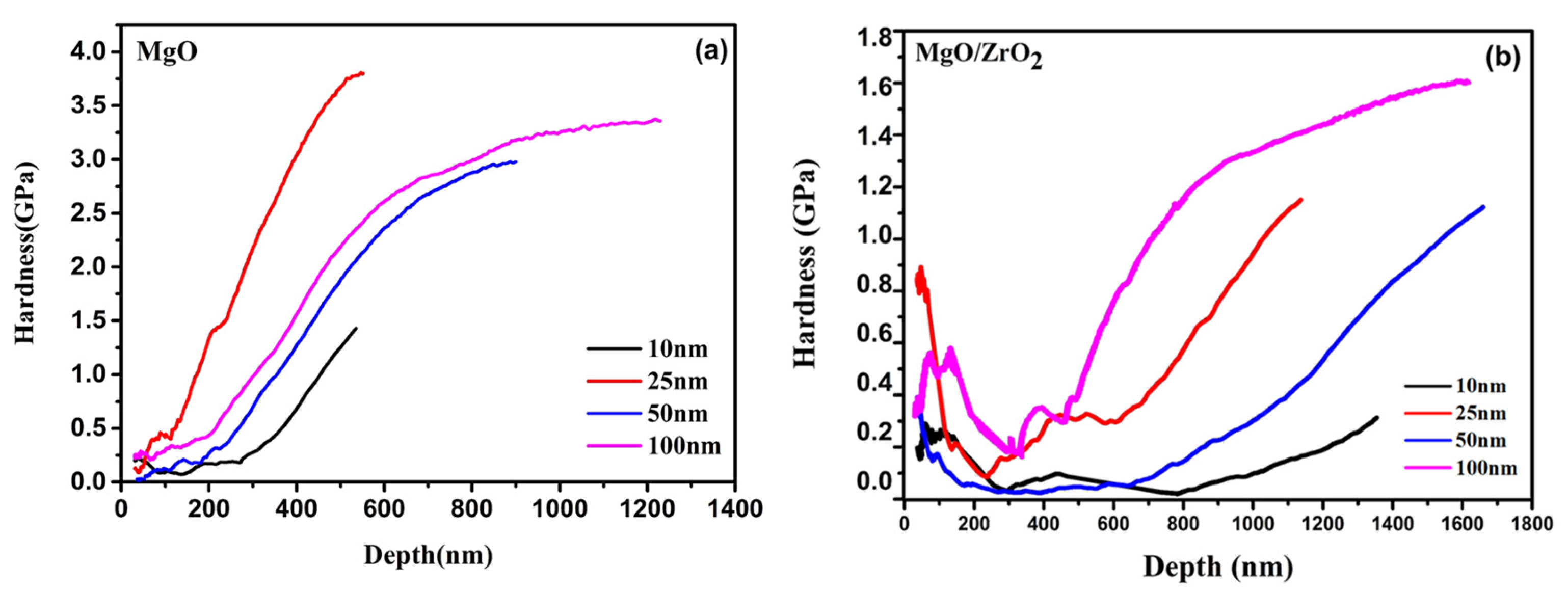
3.6. Mechanical Properties
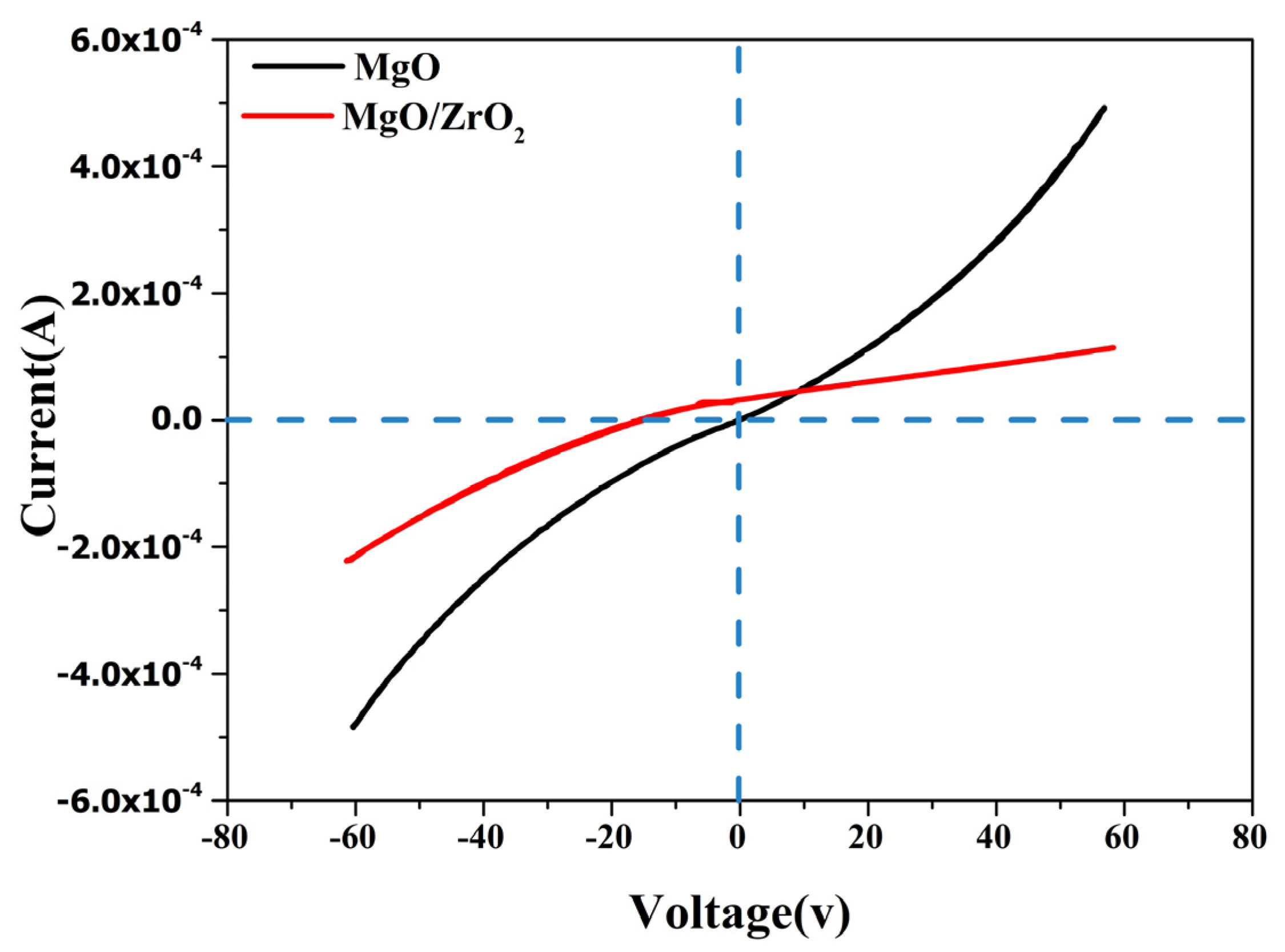
3.7. I–V Characteristics of the MgO-ZrO2 Heterostructure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Min, Y.; Yan, T.; Lee, C.-Y.; Miao, Y.; Chen, C.; Luo, M.; Ye, N.J. Epitaxial growth of aligned MgO nanowire arrays on a single crystalline substrate. Dalton Trans. 2022, 51, 3740–3746. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Shon, I.-J. Mechanochemical synthesis and fast low-temperature consolidation of nanostructured Ni-ZrO2 composite and its mechanical properties. Mater. Trans. 2015, 56, 171–174. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Peng, J.; Koppala, S.; Omran, M.; Chen, G. One-step preparation of CaO-doped partially stabilized zirconia from fused zirconia. Ceram. Int. 2020, 46, 6484–6490. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, F.; Zoogman, P.; Fabbri, J.; Chan, S.W.; Zhu, Y.; Brus, L.E.; Steigerwald, M.L. Martensitic phase transformation of isolated HfO2, ZrO2, and HfxZr1–xO2 (0 < x < 1) nanocrystals. Adv. Funct. Mater. 2005, 15, 1595–1602. [Google Scholar]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- Fernández-Garcia, M.; Rodgriguez, J. Metal Oxide Nanoparticles; Brookhaven National Lab. (BNL): Upton, NY, USA, 2007. [Google Scholar]
- Deng, W.; Li, Y. High-temperature electrical properties of polycrystalline MgO-doped ZrO2. Mater. Res. Bull. 2019, 113, 182–189. [Google Scholar] [CrossRef]
- Lu, N.; He, G.; Yang, Z.; Yang, X.; Li, Y.; Li, J. Fabrication and reaction mechanism of MgO-stabilized ZrO2 powders by combustion synthesis. Ceram. Int. 2022, 48, 7261–7264. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, Y.; Li, Y.; Li, Z.; Nath, M.; Sang, S.; Wang, Q.; Zhu, T.; Liao, N.; Liang, X. Role of ZrO2 in sintering and mechanical properties of CaO containing magnesia from cryptocrystalline magnesite. Ceram. Int. 2022, 48, 6236–6244. [Google Scholar] [CrossRef]
- Muccillo, R.; Muccillo, E.; Saito, N. Thermal shock behavior of ZrO2: MgO solid electrolytes. Mater. Lett. 1998, 34, 128–132. [Google Scholar] [CrossRef]
- Dongare, M.; Sinha, A. Low temperature preparation of stabilized zirconia. J. Mater. Sci. 1984, 19, 49–56. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic applications of metal oxides for sustainable environmental remediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Sierra, J.; Hernández-Ramírez, A.; Hinojosa-Reyes, L.; Guzmán-Mar, J. A review on the development of visible light-responsive WO3-based photocatalysts for environmental applications. Chem. Eng. J. Adv. 2021, 5, 100070. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Sun, Q.; Li, F.; Huang, B.; Dai, Y. Two-dimensional germanium monochalcogenides for photocatalytic water splitting with high carrier mobility. Appl. Catal. B: Environ. 2017, 217, 275–284. [Google Scholar] [CrossRef]
- Ibrahim, M. Photocatalytic activity of nanostructured ZnO–ZrO2 binary oxide using fluorometric method. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2015, 145, 487–492. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Zhou, Q.; Liang, X.; Liu, D. Facile synthesis of novel gully-like double-sized mesoporous structural Sr-doped ZrO2–TiO2 composites with improved photocatalytic efficiency. J. Solid State Chem. 2019, 269, 375–385. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Parghi, K.; Shrikhande, J.J.; Pandey, R.K.; Ghumman, C.A.A.; Ghumman, C.A.A.; Bundaleski, N.; Teodoro, O.M.N.D.; Jayaram, R.V. Synthesis and characterization of versatile MgO–ZrO2 mixed metal oxide nanoparticles and their applications. Catal. Sci. Technol. 2011, 1, 1653–1664. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.K.; Branco, P.S.; Potewar, T.; Velhinho, A.; Nogueira, I.D.; Tolstogouzov, A.; Ghumman, C.A.A.; Teodoro, O.M. Nano-MgO–ZrO2 mixed metal oxides: Characterization by SIMS and application in the reduction of carbonyl compounds and in multicomponent reactions. RSC Adv. 2013, 3, 3611–3617. [Google Scholar] [CrossRef]
- Prasad, G.; Kumar, J.P.; Pandey, L.; Singh, B. Nanoporous materials for the remediation of industrial toxic chemicals. Adv. Porous Mater. 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Makhanya, N.P. Solvent-Free Knoevenagel Condensation over Supported Mixed Metal Oxides Catalysts. Ph.D. Thesis, Durban University of Technology, Durban, South Africa, 2017. [Google Scholar]
- Liu, Z.; Yin, Z.; Cox, C.; Bosman, M.; Qian, X.; Li, N.; Zhao, H.; Du, Y.; Li, J.; Nocera, D.G. Room temperature stable CO x-free H2 production from methanol with magnesium oxide nanophotocatalysts. Sci. Adv. 2016, 2, e1501425. [Google Scholar] [CrossRef]
- Sugiyama, S.; Sato, K.; Yamasaki, S.; Kawashiro, K.; Hayashi, H. Ketones from carboxylic acids over supported magnesium oxide and related catalysts. Catal. Lett. 1992, 14, 127–133. [Google Scholar] [CrossRef]
- Yang, J.; Chen, A.; Liu, F.; Gu, L.; Xie, X.; Ding, Z. Hybrid coating of polydimethylsiloxane with nano-ZrO2 on magnesium alloy for superior corrosion resistance. Ceram. Int. 2022, 48, 35280–35289. [Google Scholar] [CrossRef]
- Cubillos, G.; Bethencourt, M.; Olaya, J.; Alfonso, J.; Marco, J. The influence of deposition temperature on microstructure and corrosion resistance of ZrOxNy/ZrO2 coatings deposited using RF sputtering. Appl. Surf. Sci. 2014, 309, 181–187. [Google Scholar] [CrossRef]
- Qing, W.; Liu, F.; Yao, H.; Sun, S.; Chen, C.; Zhang, W. Functional catalytic membrane development: A review of catalyst coating techniques. Adv. Colloid Interface Sci. 2020, 282, 102207. [Google Scholar] [CrossRef]
- Luo, J.; Zhao, K.; Zhao, J.; Li, J.; Liu, Y.; Chen, D.; Xu, Q.; Chen, M. Functional ceramic support as an independent catalyst layer for direct liquid fuel solid oxide fuel cells. J. Adv. Ceram. 2023, 12, 474–486. [Google Scholar] [CrossRef]
- Khan, M.E.; Mohammad, A.; Ali, W.; Imran, M.; Bashiri, A.H.; Zakri, W. Properties of metal and metal oxides nanocomposites. In Nanocomposites-Advanced Materials for Energy and Environmental Aspects; Elsevier: Amsterdam, The Netherlands, 2023; pp. 23–39. [Google Scholar]
- Sanchis-Gual, R.; Coronado-Puchau, M.; Mallah, T.; Coronado, E. Hybrid nanostructures based on gold nanoparticles and functional coordination polymers: Chemistry, physics and applications in biomedicine, catalysis and magnetism. Coord. Chem. Rev. 2023, 480, 215025. [Google Scholar] [CrossRef]
- Cheng, F.; Nie, F.; Zhao, C.; Li, W.; Pan, J. Efficient and stable removal of phosphate from aqueous solutions by hollow microspheres of MgO/ZrO2 composite oxide. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 635, 128095. [Google Scholar] [CrossRef]
- Sajjadi, S.M.; Haghighi, M.; Rahmani, F. Sol–gel synthesis of Ni–Co/Al2O3–MgO–ZrO2 nanocatalyst used in hydrogen production via reforming of CH4/CO2 greenhouse gases. J. Nat. Gas Sci. Eng. 2015, 22, 9–21. [Google Scholar] [CrossRef]
- Keerthana, L.; Sakthivel, C.; Prabha, I. MgO-ZrO2 mixed nanocomposites: Fabrication methods and applications. Mater. Today Sustain. 2019, 3, 100007. [Google Scholar] [CrossRef]
- Śnieżek, E.; Szczerba, J.; Stoch, P.; Prorok, R.; Jastrzębska, I.; Bodnar, W.; Burkel, E. Structural properties of MgO–ZrO2 ceramics obtained by conventional sintering, arc melting and field assisted sintering technique. Mater. Des. 2016, 99, 412–420. [Google Scholar] [CrossRef]
- Huma, T.; Hakimi, N.; Younis, M.; Huma, T.; Ge, Z.; Feng, J.J.N. MgO heterostructures: From synthesis to applications. Nanomaterials 2022, 12, 2668. [Google Scholar] [CrossRef]
- Palacio, M.L.; Bhushan, B.J. Depth-sensing indentation of nanomaterials and nanostructures. Mater. Charact. 2013, 78, 1–20. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.G.; Choi, D.; Park, H.S. Nanoindentation of annealed Naf ion/sulfonated graphene oxide nanocomposite membranes for the measurement of mechanical properties. J. Membr. Sci. 2014, 451, 40–45. [Google Scholar] [CrossRef]
- Jian, S.-R.; Chen, G.-J.; Hsu, W.-M. Mechanical properties of Cu2O thin films by nanoindentation. Materials 2013, 6, 4505–4513. [Google Scholar] [CrossRef]
- Jian, S.-R.; Lin, Y.-Y.; Ke, W.-C. Effects of thermal annealing on the structural, electrical and mechanical properties of Al-doped ZnO thin films deposited by radio-frequency magnetron sputtering. Sci. Adv. Mater. 2013, 5, 7–13. [Google Scholar] [CrossRef]
- Jian, S.-R.; Lee, Y.-H. Nanoindentation-induced interfacial fracture of ZnO thin films deposited on Si (1 1 1) substrates by atomic layer deposition. J. Alloys Compd. 2014, 587, 313–317. [Google Scholar] [CrossRef]
- Shi, Z.-F.; Zhang, Y.-T.; Cui, X.-J.; Zhuang, S.-W.; Wu, B.; Chu, X.-W.; Dong, X.; Zhang, B.-L.; Du, G.-T. Photoluminescence performance enhancement of ZnO/MgO heterostructured nanowires and their applications in ultraviolet laser diodes. Phys. Chem. Chem. Phys. 2015, 17, 13813–13820. [Google Scholar] [CrossRef]
- Kim, G.; Martens, R.L.; Thompson, G.B.; Kim, B.C.; Gupta, A. Selective area synthesis of magnesium oxide nanowires. J. Appl. Phys. 2003, 370, 770–773. [Google Scholar]
- Yin, Y.; Zhang, G.; Xia, Y. Synthesis and characterization of MgO nanowires through a vapor-phase precursor method. Adv. Funct. Mater. 2002, 12, 293–298. [Google Scholar] [CrossRef]
- Ma, R.; Bando, Y.J. Uniform MgO nanobelts formed from in situ Mg3N2 precursor. Chem. Phys. Lett. 2003, 370, 770–773. [Google Scholar] [CrossRef]
- Ko, J.B.; Kim, S.M. Preparation and electric characteristics of MgO films deposited by plasma-enhanced chemical vapor deposition. J. Ceram. Process. Res. 2009, 10, 643–646. [Google Scholar]
- Wu, Y.; Wu, W.; Zou, X.; Xu, L.; Li, J.J. Growth and great UV emission improvement of highly crystalline quality core–shell ZnO/MgO nanowires. Mater. Lett. 2012, 84, 147–150. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.S.; Lee, C.; Kebede, M.A.; Yoo, K.-H.; Kim, D.Y. MgO-core/ZnO-shell nanocables sheathed by using the sputtering technique. J. Korean Phys. Soc. 2009, 55, 1887–1891. [Google Scholar] [CrossRef]
- Haycock, D.E.; Kasrai, M.; Nicholls, C.J.; Urch, D.S. The electronic structure of magnesium hydroxide (brucite) using X-ray emission, X-ray photoelectron, and auger spectroscopy. J. Chem. Soc. Dalton Trans. 1978, 12, 1791–1796. [Google Scholar] [CrossRef]
- García, L.; Mendivil, M.; Roy, T.D.; Castillo, G.; Shaji, S. Laser sintering of magnesia with nanoparticles of iron oxide and aluminum oxide. Appl. Surf. Sci. 2015, 336, 59–66. [Google Scholar] [CrossRef]
- Wan, Y.; Samundsett, C.; Bullock, J.; Hettick, M.; Allen, T.; Yan, D.; Peng, J.; Wu, Y.; Cui, J.; Javey, A. Conductive and stable magnesium oxide electron-selective contacts for efficient silicon solar cells. Adv. Energy Mater. 2017, 7, 1601863. [Google Scholar] [CrossRef]
- Koirala, R.; Gunugunuri, K.R.; Pratsinis, S.E.; Smirniotis, P.G. Effect of zirconia doping on the structure and stability of CaO-based sorbents for CO2 capture during extended operating cycles. J. Phys. Chem. C 2011, 115, 24804–24812. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Huang, A.; Chu, P.K. Structure and properties of zirconia (ZrO2) films fabricated by plasma-assisted cathodic arc deposition. J. Phys. D Appl. Phys. 2007, 40, 2293. [Google Scholar] [CrossRef]
- Rameshan, C.; Li, H.; Anic, K.; Roiaz, M.; Pramhaas, V.; Rameshan, R.; Blume, R.; Hävecker, M.; Knudsen, J.; Knop-Gericke, A. In situ NAP-XPS spectroscopy during methane dry reforming on ZrO2/Pt (1 1 1) inverse model catalyst. J. Phys. Condens. Matter 2018, 30, 264007. [Google Scholar] [CrossRef]
- Bumajdad, A.; Nazeer, A.A.; Al Sagheer, F.; Nahar, S.; Zaki, M.I. Controlled synthesis of ZrO2 nanoparticles with tailored size, morphology and crystal phases via organic/inorganic hybrid films. Sci. Rep. 2018, 8, 3695. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Nanowire Growth |
|---|---|
| Substrate | Si (n-type) (100) |
| Precursor | Mg3N2, ZrCl4 |
| Gas Flow | Argon, Nitrogen, Oxygen |
| Growth Temperature | 800 °C, 950 °C |
| Growth rate | 10 °C/min |
| Growth Pressure | 160SCCM, 100SCCM, 6SCCM |
| Precursor Used | Substrate | Carrier Gas | Time | Nanostructure | Ref |
|---|---|---|---|---|---|
| ZnO/MgO (800 °C) | c-Al2O3 | O2 | 30 min | NWs | [40] |
| Mg3N2 (650 °C) | Si | N2 + O2 | 60 min | NWs | [41] |
| MgB4 (700 °C) | Si | Ar | 2 h | NWs | [42] |
| Mg3N2 (650 °C, 800 °C) | Al | N2 + O2 | 2 h | Nanobelts | [43] |
| Mg(thd)2 (150–400 °C) | Si (100) | Ar + O2 | 10 min | Films | [44] |
| Zn (500 °C) | Si | Ar + O2 | 60 min | NWs | [45] |
| MgB2 (900 °C) | Si | Ar + O2 | 8 min | NWs | [46] |
| Mg 1s eV | Zr 3d eV | ||
|---|---|---|---|
| Samples | MgO | ZrO2 3d3/2 | ZrO2 3d5/2 |
| Binding energy | 1304.44 eV | 184.30 eV | 181.85 eV |
| From the literature | 1303.8 [47], 1303.9 [47,48], 1303.4 [49] | 183 [50], 184.9 [51] | 181.1 [52], 182 [53] |
| Pmax (mN) | hmax (nm) | S (mN/nm) | hc (nm) | H (GPa) | E (GPa) | Er (GPa) |
|---|---|---|---|---|---|---|
| 9.9858 | 1596.3 | 0.18 | 1554.6 | 1.402 | 58.37 | 59.17 |
| 24.978 | 1293.9 | 0.23 | 1212.4 | 2.826 | 73.48 | 73.52 |
| 49.96 | 2134.8 | 0.14 | 1867.2 | 1.758 | 54.15 | 54.94 |
| 99.89 | 1281.1 | 0.66 | 1167.6 | 3.284 | 90.11 | 88.73 |
| Pmax (mN) | hmax (nm) | S (mN/nm) | hc (nm) | H (GPa) | E (GPa) | Er (GPa) |
|---|---|---|---|---|---|---|
| 9.988 | 1650.5 | 0.22 | 1616.4 | 0.38 | 44.97 | 46.08 |
| 18.757 | 1991.4 | 0.43 | 1958.6 | 1.00 | 59.32 | 59.9 |
| 49.96 | 1616.9 | 0.23 | 1454 | 0.759 | 60.25 | 60.97 |
| 99.87 | 1944.6 | 0.77 | 1847.3 | 1.241 | 68.36 | 68.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huma, T.; Hakimi, N.; Anwar ul haq, M.; Huma, T.; Xu, L.; Zhu, X. Synthesis and Characterization of MgO-ZrO2 Heterostructure: Optical, Mechanical and Electrical Properties. Crystals 2025, 15, 465. https://doi.org/10.3390/cryst15050465
Huma T, Hakimi N, Anwar ul haq M, Huma T, Xu L, Zhu X. Synthesis and Characterization of MgO-ZrO2 Heterostructure: Optical, Mechanical and Electrical Properties. Crystals. 2025; 15(5):465. https://doi.org/10.3390/cryst15050465
Chicago/Turabian StyleHuma, Tabasum, Nadimullah Hakimi, Muhammad Anwar ul haq, Tanzeel Huma, Lei Xu, and Xinkun Zhu. 2025. "Synthesis and Characterization of MgO-ZrO2 Heterostructure: Optical, Mechanical and Electrical Properties" Crystals 15, no. 5: 465. https://doi.org/10.3390/cryst15050465
APA StyleHuma, T., Hakimi, N., Anwar ul haq, M., Huma, T., Xu, L., & Zhu, X. (2025). Synthesis and Characterization of MgO-ZrO2 Heterostructure: Optical, Mechanical and Electrical Properties. Crystals, 15(5), 465. https://doi.org/10.3390/cryst15050465








