Abstract
This study investigates the impact of sulphuric acid concentration (40% vs. 60%) on the extraction of cellulose nanocrystals (CNCs) from alkali-treated sugarcane bagasse (SCB) and their reinforcement in poly(furfuryl) alcohol (PFA) composites. Probing into the physicochemical changes through scanning electron microscopy (SEM) displayed drastic morphological changes, alkali removal of noncellulosic components followed by sulphuric acid hydrolysis further refined cellulose to nanoscale morphologies. The X-ray diffraction (XRD) study showed that after alkali treatment, the crystallinity was significantly higher (65%), and the crystallinity index of CNCs prepared from 40% H2SO4 was greater than the CNCs prepared from 60% H2SO4 (61%). Fourier transform infrared spectral and thermogravimetric analysis (TGA) suggested that improving the polymeric performance by the incorporation of a CNC resulted in a decrease in the thermal stability of the modified polyelectrolyte, which was largely attributed to higher sulphate esterification achieved at higher acid concentrations. It is possible to use CNCs to achieve higher mechanical performance while also indicating that optimizing thermal properties and mechanical performance of high-performance materials will require an improved understanding of the microstructural parameters governing the polymer–filler interface. This work demonstrates that acid concentration critically balances CNC crystallinity and thermal performance, offering insights for optimizing sustainable nanocomposites.
1. Introduction
Nanocellulose is an effective reinforcement of polymer matrices and offers valuable improvements in the physical, mechanical, thermal, and thermo-mechanical properties of polymer composites. Nanocellulose, which is harvested from renewable lignocellulosic sources, provides a variety of beneficial attributes, including high tensile strength, low density, and biodegradability, which makes it a suitable substitute for synthetic reinforcements [1,2]. In recent years, several investigations producing poly(furfural)alcohol (PFA) biocomposites reinforced with cellulose nanowhiskers extracted from different natural sources have been reported [3,4,5,6,7]. These findings suggest that boosting the acid concentration during hydrolysis improves the thermal stability of PFA composites and gives rise to strong interfacial interactions between the fibres and polymer matrix [8]. Poly(furfuryl) alcohol (PFA), a bio-based thermosetting resin derived from furfuryl alcohol, was selected for its compatibility with cellulose and potential for green composites. In addition, the results of dynamic mechanical analysis (DMA) have demonstrated a decreased tan delta peak, indicating decreased energy dissipation and, therefore, an increase in the elasticity while also reflecting a higher storage modulus.
In addition to cellulose nanowhiskers, other research efforts have investigated the use of cellulose nanocrystals (CNCs) and nanoclay reinforcements in PFA biocomposites. Kasyapi et al. and Morelli et al. explored in situ polymerization methods to fabricate solvent-free PFA biocomposites reinforced with CNCs and montmorillonite (MMT) nanoclay [9,10]. Their findings revealed that CNCs containing sulphate groups, introduced through acid hydrolysis, facilitated polymerization, as confirmed by Fourier transform infrared spectroscopy (FTIR). The presence of cross-linking within the polymer matrix was indicated by significant increases in absorption band intensities at key wavenumbers, demonstrating the formation of strong intermolecular interactions. Similarly, Ahmad et al. reinforced PFA with sisal whisker nanocrystals using in situ polymerization, reporting slight improvements in thermal properties [11,12]. However, FTIR analysis suggested no direct chemical interactions between PFA and the whiskers. Despite this, well-dispersed nanocrystals contributed to strong interfacial adhesion, as evidenced by the reduction in the tan delta peak, which further supports the improvement of elasticity in the composite material. PFA was chosen due to its renewable origin, cross-linking capability, and reported compatibility with lignocellulosic fillers. Its furan ring structure facilitates interfacial interactions with cellulose, enhancing mechanical properties in composites [8,11].
With growing concerns regarding the environmental impact of synthetic materials, there is an increasing shift towards sustainable and biodegradable alternatives. Conventional synthetic polymers, widely used in composite applications, pose challenges related to their non-renewability and persistence in the environment. Consequently, researchers are exploring naturally derived polymers and nanofillers as potential replacements. Lignocellulosic fibres such as cotton, sugarcane bagasse, maize stalks, wheat straws, and rice straws have emerged as promising reinforcement materials due to their abundance, renewability, biodegradability, and superior mechanical properties. These fibres have found applications in diverse fields, including papermaking, composite fabrication, and the production of nanocellulose-based materials [13,14,15,16,17,18,19]. The ability of lignocellulosic fibres to be chemically modified and incorporated into polymer matrices further enhances their suitability for high-performance biocomposites.
Given these advancements, this study aims to investigate the effect of acid concentration on the in situ polymerization of PFA reinforced with nanocellulose extracted from alkali-treated sugarcane bagasse. Sugarcane bagasse is an attractive source of cellulose due to its widespread availability as an agricultural byproduct, making it a cost-effective and sustainable material for composite reinforcement. This work aims to assess the effects of varying acid concentrations on the mechanical, thermal, and structural characteristics of the resultant PFA-based nanocomposite materials. The characteristics of both untreated and chemically treated sugarcane bagasse will be analysed using a variety of characterization techniques, such as Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and thermogravimetric analysis (TGA). To ascertain the ideal circumstances for improved polymerization and reinforcing, the composites’ mechanical performance, interfacial interactions, and thermal stability will also be evaluated.
We hypothesize that acid concentration during hydrolysis governs CNC crystallinity, surface chemistry, and dispersion in PFA, ultimately determining composite performance. The goal is to correlate acid concentration (40% vs. 60% H2SO4) with CNC properties and evaluate their reinforcement efficacy in PFA composites.
This study advances the creation of high-performance, sustainable biocomposites with improved mechanical and thermal properties by methodically investigating how acid concentration affects polymerization and composite performance. The results of this investigation should encourage the use of nanocellulose-reinforced PFA composites in biodegradable and ecologically friendly materials by offering important insights into their optimization.
This work aligns with the growing global efforts to reduce reliance on synthetic polymers and transition towards renewable and sustainable materials for advanced engineering applications.
2. Materials and Methods
2.1. Materials
Sugarcane bagasse (SCB) was supplied by a sugar company in Empangeni, South Africa. It is a useful fibre which comes out as a waste residue after drawing out sugar juice from sugarcane stalks. The SCB is essentially used for energy supply in sugar and ethanol mills.
2.2. Chemicals
Sodium hydroxide (NaOH) and sulphuric acid (H2SO4) of both 98%, glacial acetic acid (CH3COOH), p-toluene sulphonic acid and furfural alcohol (FA) minimum assay of 98% purity were obtained from Laboratory consumables, South Africa. Commercial sodium hypochlorite (3.5% M/V) was purchased from a local supermarket in Empangeni, South Africa.
2.3. Alkali Treatment
The most popular technique used to purify natural fibres is alkali treatment. By removing significant quantities of noncellulosic components including lignin, hemicellulose, and extractives that enclose the fibres’ cell wall’s surface and bind cellulose fibres, sodium hydroxide has been used extensively to purify natural fibres [3]. In order to increase the adhesion between a reinforcing fibre and matrix in the composites, this technique has also been utilized to modify the surface of natural fibres. Fibres become rough when noncellulosic components are removed, which improves their interfacial adhesion with the polymer matrix [4]. This treatment also enhances and reduces the diameters of the individual fibres [5]. The raw sugarcane bagasse, as shown in Figure 1, was treated with 2 wt% (0.508 M) of sodium hydroxide solution to remove noncellulosic constituents.

Figure 1.
Sugarcane bagasse and treated fibres (2 wt% (0.508 M) NaOH).
After four hours of washing at 100 °C to get rid of dust, sugarcane bagasse was allowed to idle at room temperature overnight. Hemicellulose and cemented lignin in SCB were eliminated by alkali treatment. A 2 wt% (0.508 M) sodium hydroxide (NaOH) solution was applied to the dry SCB, and it was left at 100 °C for an hour. Until a neutral solution was achieved, the treated SCB was filtered and repeatedly cleaned using tap water and then deionized water. The process was carried out four times. The treated fibre was left overnight in the oven at 30 °C. This step is frequently followed by the bleaching of treated fibres, as shown in Figure 2, to remove excessive lignin and to whiten the fibres to yield purified cellulose.

Figure 2.
Bleaching treatment under mechanical stirring.
Cellulose and nanocellulose extraction are often impeded by lignin and hemicellulose. Therefore, eliminating these undesirable noncellulosic components is convenient. A concentration of alkaline solutions and oxidizing agents, such as hydrogen peroxide and NaOCl, were necessary for the extraction of cellulose. Additionally, lignin can be dissolved by acidified NaOCl, a common oxidizing agent [6]. The treatment was performed according to Lu et al. [7].
Using sodium hypochlorite, SCB treated with 2 wt% (0.508 M) NaOH was bleached. In addition to an acetate buffer (27 g of sodium hydroxide (NaOH) and 75 mL glacial acetic acid, diluted in a 1 L volumetric flask using deionized water), the bleaching solution employed contains 1.7 weight percent sodium hypochlorite (NaOCl) in water. The procedure was carried out four times with filtering and an hourly rinse with deionized water while being mechanically stirred. After that, the fibre was dried in an oven set to 30 °C for 24 h. The dried cellulose fibres were then ground into a finer powder by crushing or grinding them again.
2.4. Extraction of Nanocellulose
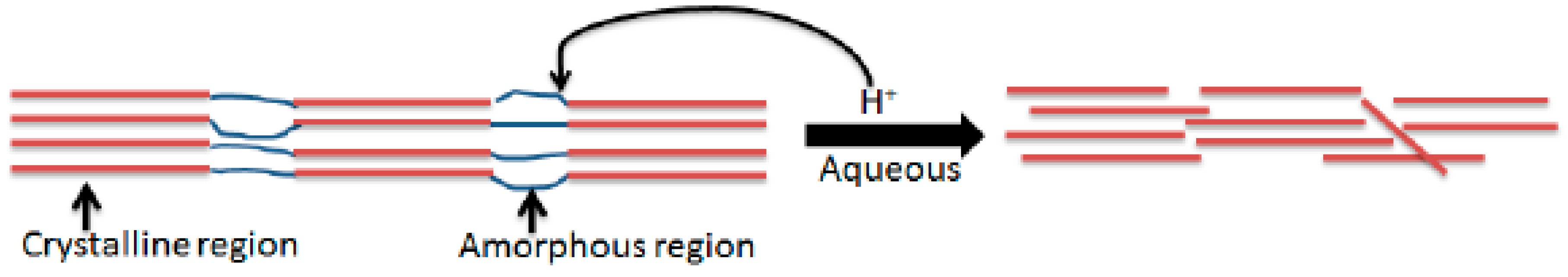
According to Neto et al., cellulose nanocrystals were produced by hydrolysing sulphuric acid since sulphuric acid produces stable CNCs [16]. A more stable suspension containing CNCs is produced when fibres are treated with H2SO4 to produce sulphate group charges on the surface of CNCs through an esterification process. So, 40% and 60% H2SO4 were selected based on preliminary studies showing 40% optimally preserves crystallinity, while 60% maximizes sulphation for colloidal stability [16,19]. Extreme concentrations (>60%) risk excessive degradation [2]. As seen schematically in Figure 3, the hydronium ions of the acid cleave the glycosidic bonds by penetrating the amorphous portion of the cellulose fibres.
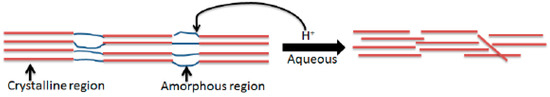
Figure 3.
Acid hydrolysis of cellulosic fibres.
Cellulose was acid hydrolysed in a fume hood using 40 and 60% acid (H2SO4) for 50 min while being stirred chemically. The chemical reaction was exothermic. During chemical treatments, the cellulose fibre was between 5 and 6 wt.%. The suspension was prepared using icy cold water to prevent spontaneous continuous reaction. It was then progressively cleaned by centrifugation at 5000 rpm for 30 min. The free acid was then removed from the suspension by subsequent dialysis against distilled water for five days. A sonication stage employing a Cole Parmer Ultrasonic Processor for 10 min at a rated frequency of 50–60 KHz produced complete dispersion of the nanocellulose. The suspension obtained was stored in a refrigerator at 4 °C to avoid fungal growth.
2.5. Preparation of PFA/Cellulose Nanocomposites
PFA/cellulose nanocomposites were prepared by p-toluene sulphonic acid monohydrate according to the literature method. The catalyst was added dropwise to 200 mL of furfuryl alcohol (FA) while being constantly stirred after the acids had been dissolved in 10 mL of deionized water. Two millilitres of the suspension was then left in the oven to dry. The volume fraction of the suspension needed to achieve 2% nanocellulose in PFA bioresin was calculated using the amount of solid that was achieved. Following a gradual addition of acid-catalysed FA to the nanocellulose, the mixture was then left at room temperature for the entire night. After that, the viscous material was kept for 5 days at 50 °C without any disturbance to allow the FA to polymerize. This was followed by 4 h of additional drying at 100 °C. To reach the required level of polymerization, the temperature was then raised to 160 °C for 30 min. After that, the composites were allowed to cool to room temperature.
2.6. Characterization Techniques
Crystallinity
The crystallinity of untreated, chemically treated SCB, PFA, and nanocomposite samples was ascertained by X-ray diffraction studies. It is a practical method for figuring out crystal structures. Powder X-ray diffraction spectroscopy (Bruker AXS Advance D8 diffractometer) was used to produce the XRD profiles at a rate of one second per step in the 2θ range of 5° to 90°. Amorphous and crystalline peaks are often confirmed by XRD.
2.7. Fourier Transform Infrared Spectroscopy (FT-IR)
A Perkin Elmer attenuated total reflection FTIR spectrometer (Perkin Elmer UATR Two, Johannesburg, South Africa) was used to analyse the samples using Fourier transform infrared spectroscopy (FTIR) in diffuse reflectance mode. The investigation’s spectral range was 4000–500 cm−1.
2.8. Thermal Stabilities
Using nitrogen as purge gas and a heating rate of 10 °C·min−1, thermogravimetric analysis (TGA) was performed using a Perkin Elmer Pyris1. The range of samples analysed was 10–15 mg. Under a nitrogen flow, the TGA is configured to raise the temperature linearly from ambient temperature to 600 °C. The sample’s temperature was tracked, and the weight loss was reported as a percentage of total weight. Only after isothermal conditions were achieved did the analysis begin. The sample was left to stand at room temperature for five minutes to accomplish this. Thermal stability, pyrolysis behaviour under various conditions, and a polymer’s decomposition temperature are all commonly measured using thermal analysis techniques like TGA.
2.9. Scanning Electron Microscopy (SEM)
Samples were subjected to SEM studies using an FEI Quanta 200 electron microscope running at a 20 kV acceleration voltage. Before being examined, the samples were carbon-coated using Edward’s E306A coating system (FEI Company, Hillsboro, OR, USA).
3. Results and Discussion
3.1. Scanning Electron Microscopy (SEM) Analysis
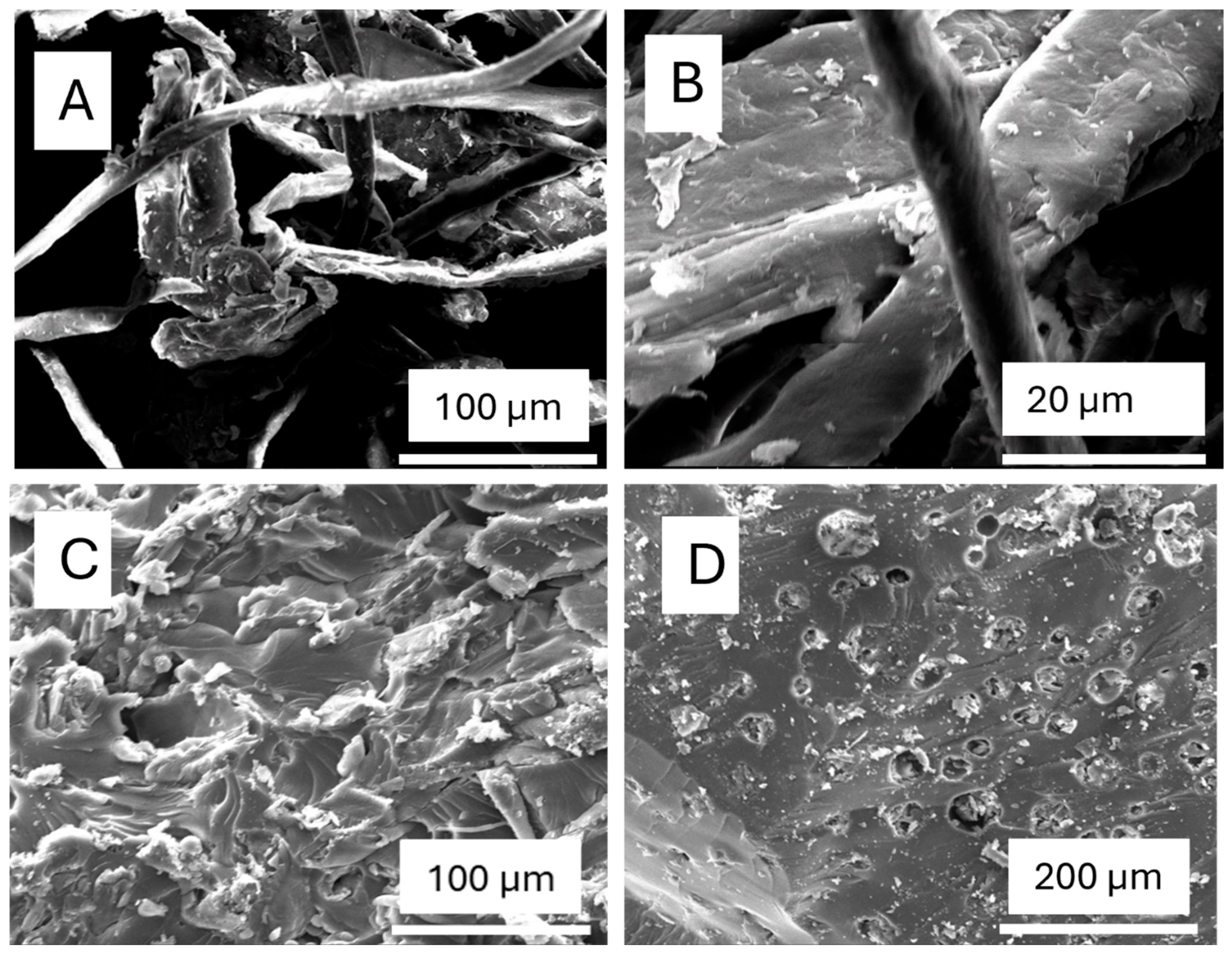
The SEM images (Figure 4A–D) provide a detailed visualization of the morphological changes in sugarcane bagasse (SCB) and its derived cellulose and nanocellulose through different treatment processes. Image (A) displays the structure of alkali-treated SCB, where some noncellulosic components, such as lignin and hemicellulose, have been removed, resulting in a rough and fibrillated surface. This structural change enhances interfacial adhesion in composite applications and reduces fibre diameters, making them more suitable for polymer reinforcement. Image (B) represents purified cellulose, showing a cleaner and more compact fibre structure compared to (A), indicating the successful removal of lignin and hemicellulose. The fibres appear smoother and denser, suggesting improved purity with cellulose fibrils still intact [20].
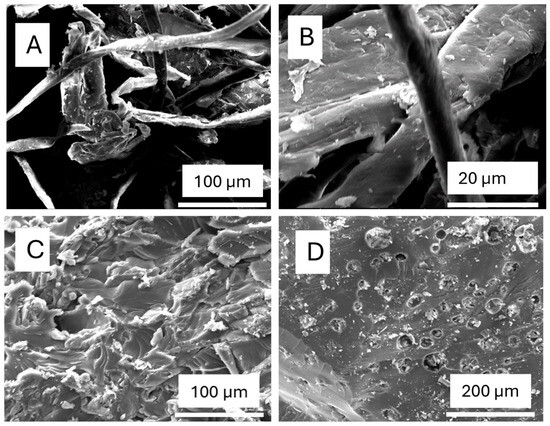
Figure 4.
SEM images of (A) alkali-treated SCB, (B) cellulose, (C) CNC-PFA (with 40% H2SO4), and (D) CNC-PFA (with 60% H2SO4).
The transformation becomes more pronounced in images (C) and (D), which depict cellulose nanocrystals (CNCs) incorporated into polyfurfuryl alcohol (PFA) composites following sulphuric acid hydrolysis. In image (C), CNCs treated with 40% H2SO4 exhibit a partially exfoliated structure with some fibrillar remnants, suggesting that while hydrolysis has broken down amorphous cellulose, the material retains some integrity [21]. In contrast, image (D) shows CNCs treated with 60% H2SO4, where the surface is highly porous and rough, indicative of increased hydrolysis leading to greater fragmentation into nanoscale particles. The higher acid concentration not only enhances sulphation, stabilizing the CNCs in suspension, but also results in significant etching and degradation of the cellulose structure. These morphological changes confirm the effectiveness of the treatments in refining SCB into cellulose and nanocellulose, influencing their structural properties and potential application in composite materials.
3.2. X-Ray Diffraction (XRD) Analysis
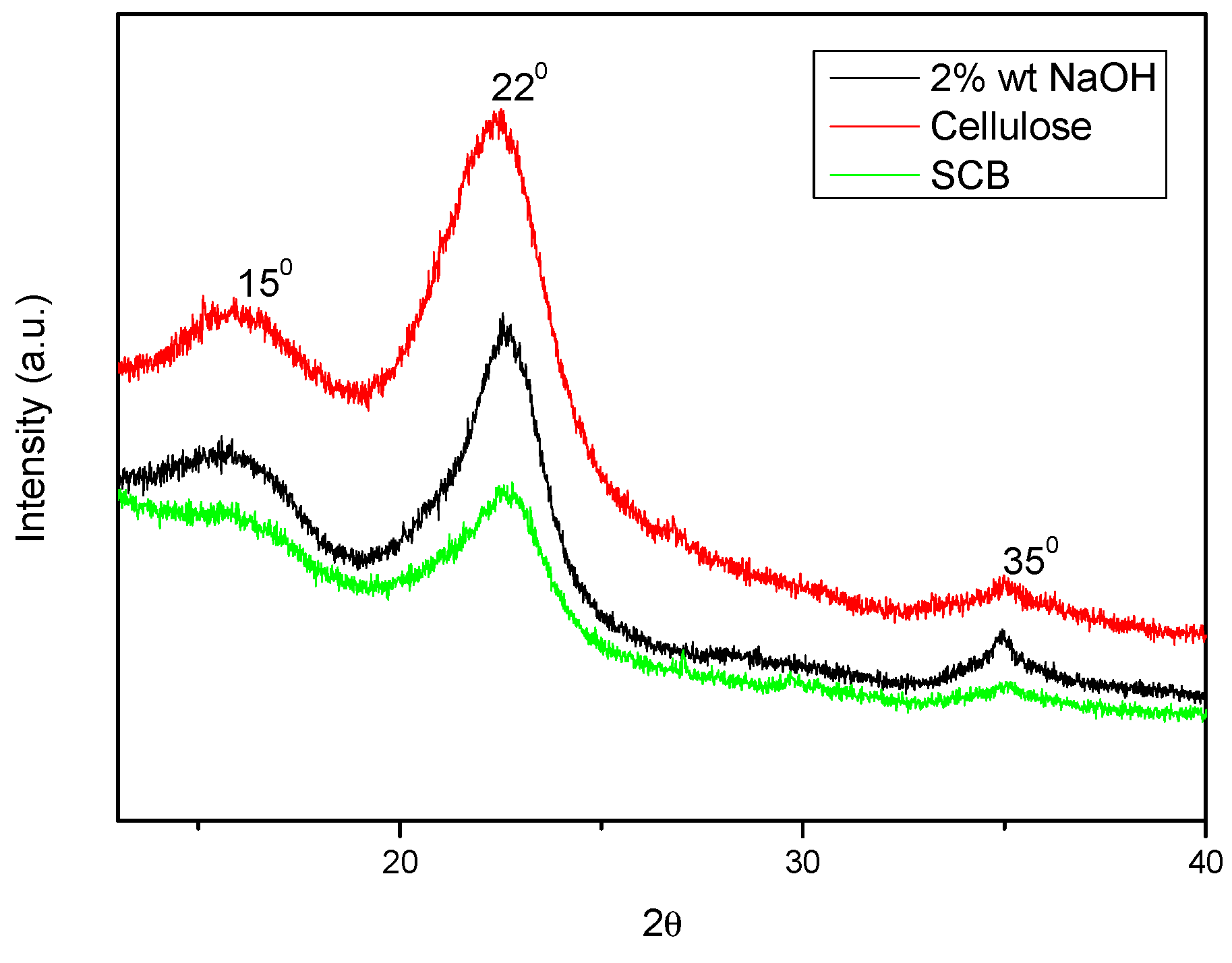
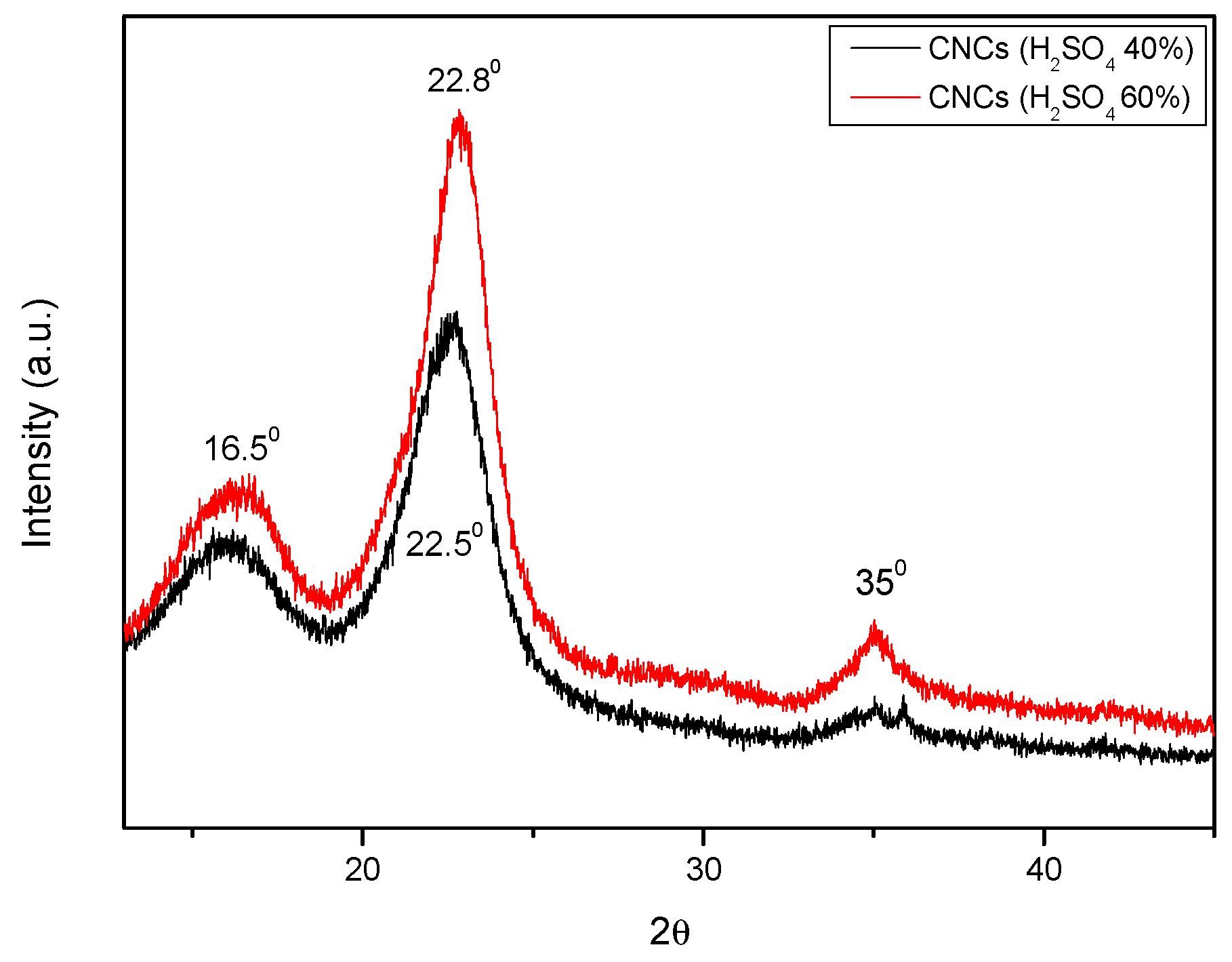
The crystallinity of the fibre was measured using the XRD technique both before and after alkali treatment. The material’s crystallinity was assessed using the peak height method [15]. Figure 5 displays the XRD profiles of cellulose, SCB, and SCB that has been alkali-treated. Additionally, Figure 6 displays the results of an X-ray diffraction examination for cellulosic material at varying sulphuric acid contents of 40% and 60%.
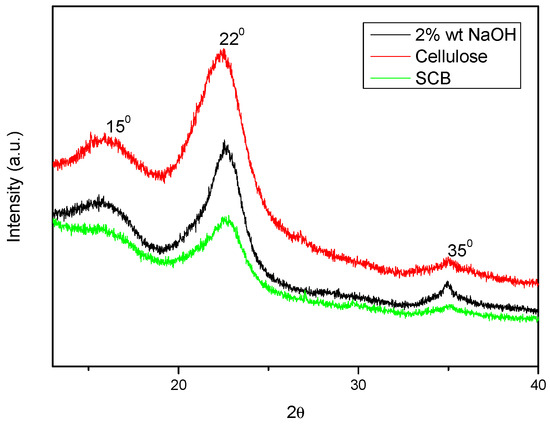
Figure 5.
XRD spectra of SCB, alkali-treated SCB, and cellulose.
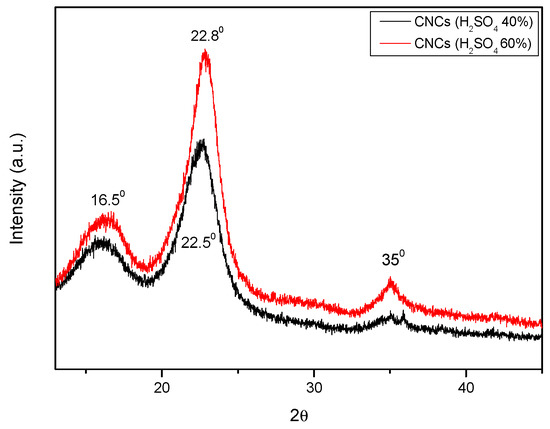
Figure 6.
XRD patterns for CNC-PFA (with 40% H2SO4) and CNC-PFA (with 60% H2SO4).
All three samples’ diffraction patterns showed comparable peaks at 2θ values of roughly 150, 220, and 350. These peaks were associated with cellulose’s crystalline structure. Furthermore, the resemblance suggested that while alkali treatment changed the crystallinity of fibres, it did not alter their structure. Because of the cellulose crystal’s axis misalignment and the higher concentrations of lignin, hemicellulose, and extractives, untreated SCB fibres were shown to have a poorer crystallinity index [22]. The crystallinity index of treated sodium hypochlorite fibres was expected to increase further but instead decreased, and this was due to the long treatment duration of the alkali treatment.
The XRD patterns above reveal two strong peaks at diffraction angles (2θ°) = 16.50 and 22.80, which represent the cellulose I lattice planes (101) and (002) [13,14]. At 2θ° = 350, another little peak was seen, which was attributed to (400) of crystalline (cellulose I) following acid hydrolysis of various H2SO4 contents. Using the peak height approach, a quantitative analysis of the XRD results was conducted, and the results are displayed in Table 1 [16].

Table 1.
Crystallinity indices of SCB fibres before and after chemical treatment.
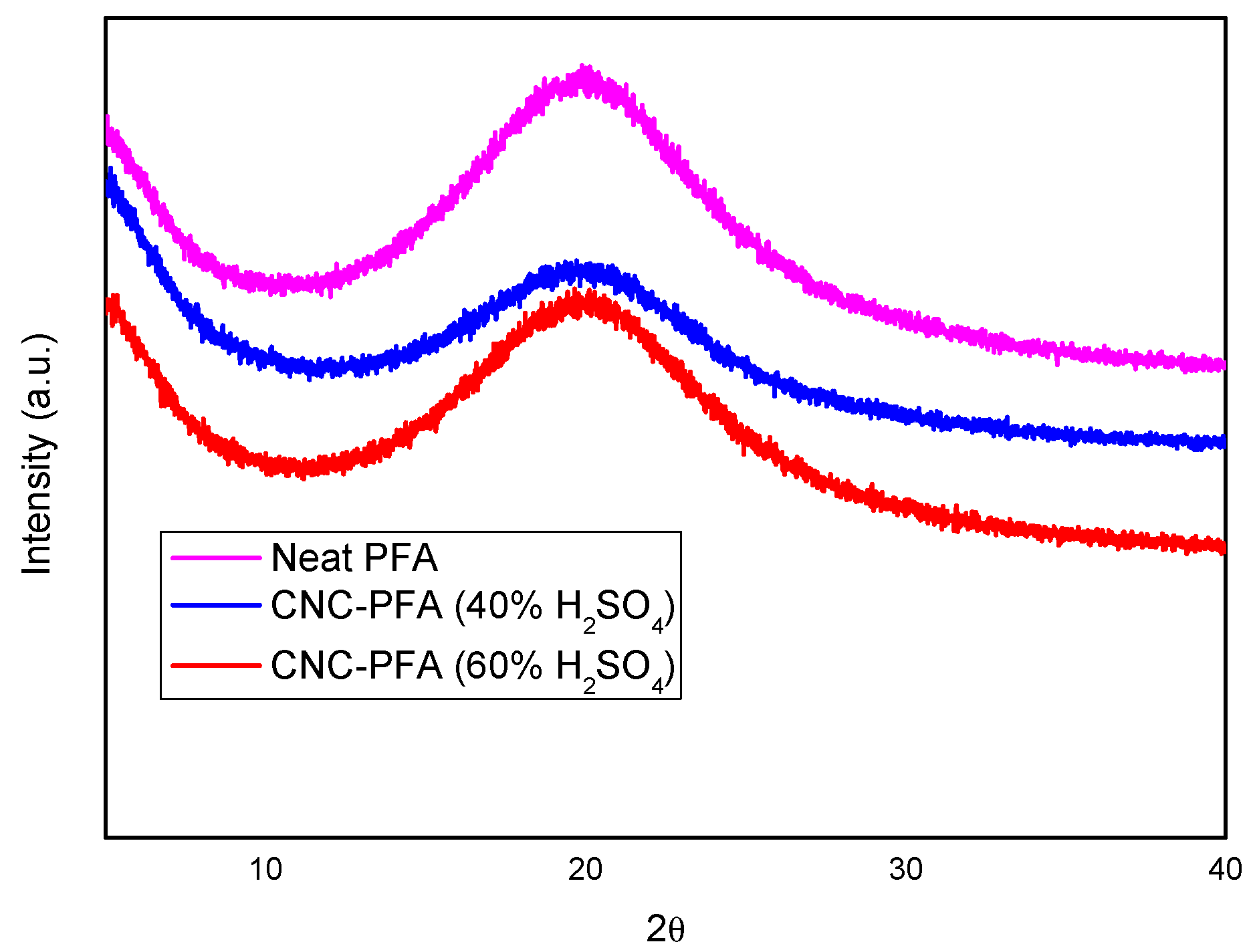
XRD analyses were performed to understand the crystalline nature of neat PFA and PFA reinforced with CNCs, and the results are shown in Figure 7.
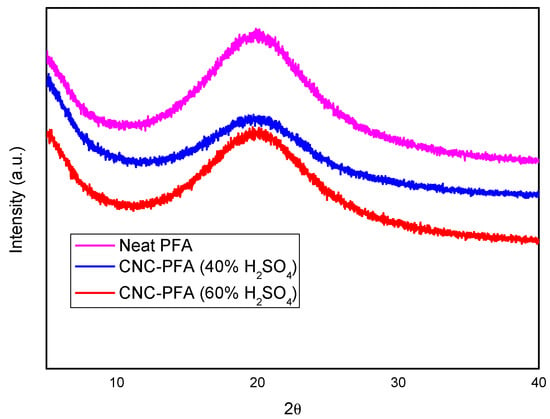
Figure 7.
XRD patterns of neat PFA and cellulose–PFA nanocomposites.
The X-ray diffraction (XRD) spectra provide insight into the crystalline properties of neat poly furfuryl alcohol (PFA) and PFA composites reinforced with cellulose nanocrystals (CNCs) derived from sulphuric acid hydrolysis at different concentrations. The diffractogram reveals broad peaks, indicating the amorphous nature of PFA, with slight variations in intensity and peak positions upon CNC incorporation.
The XRD patterns of neat PFA and CNC-PFA composites (Figure 7) exhibit a broad peak at ≈ 20°(2θ), characteristic of the amorphous nature of PFA. However, upon closer inspection, the CNC-PFA composites show subtle but critical differences.
Peak broadening and shoulder formation: The CNC-PFA (40% H2SO4) sample (blue curve) displays a slight shoulder near 22.8°, corresponding to the (002) lattice plane of cellulose I, which is obscured by the dominant amorphous PFA peak. This indicates partial retention of CNC crystallinity.
Intensity variations: The CNC-PFA (60% H2SO4) sample (red curve) shows reduced peak intensity compared to the 40% variant, suggesting higher sulphate esterification disrupts crystalline order. Prior studies note similar overlaps but confirm CNC presence via complementary techniques (FTIR/TGA) [20].
Overall, the XRD analysis suggests that CNCs derived using a higher acid concentration (60% H2SO4) contribute to a less structured and ordered composite material, whereas CNCs from 40% H2SO4 result in a comparatively higher degree of crystallinity. This difference may influence the material’s physical properties, including its rigidity, thermal stability, and mechanical strength.
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
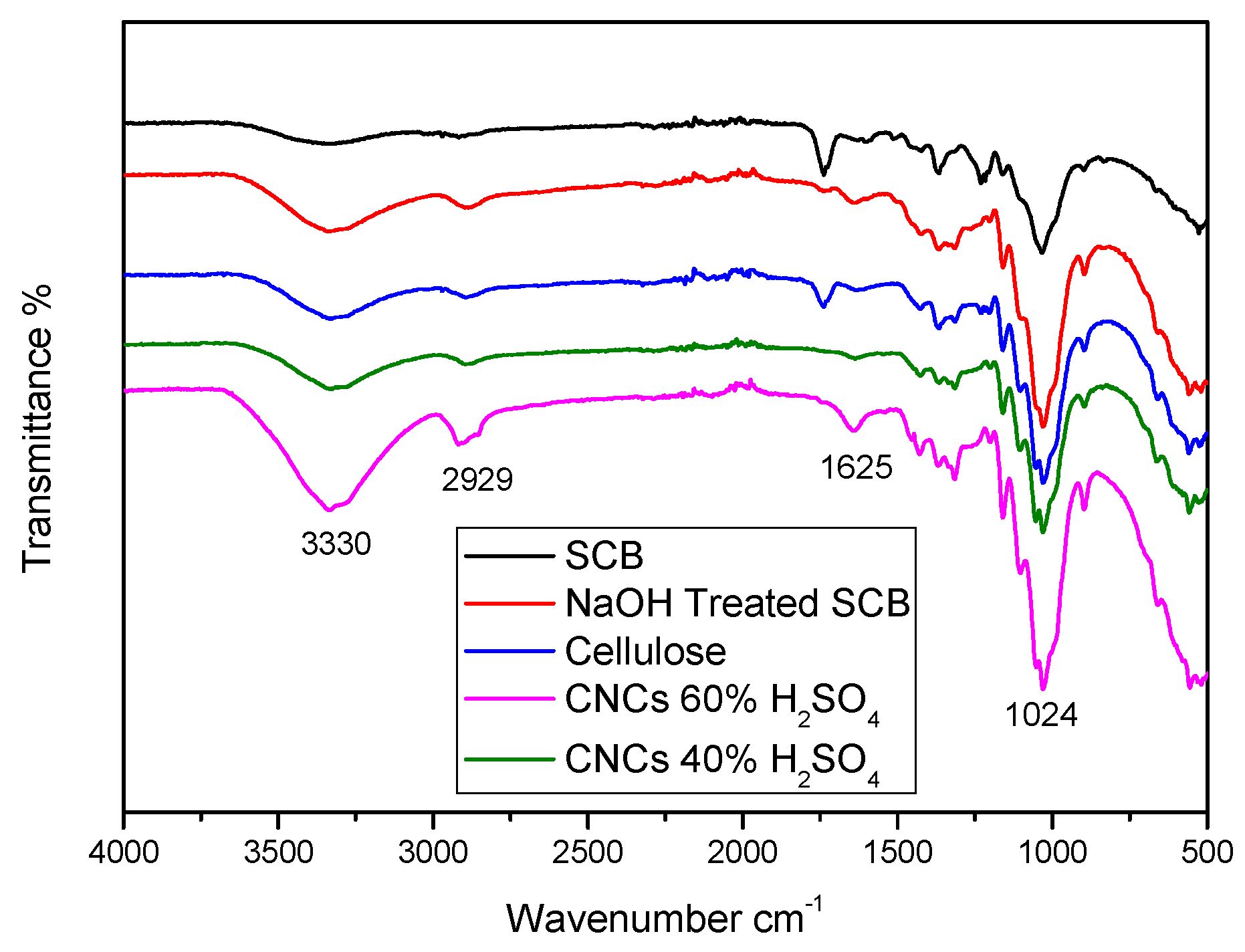
To understand the effects of chemical treatment of different sulphuric acid hydrolysis concentrations on the chemical structure of SCB fibres, FTIR tests were conducted, as illustrated in Figure 8.
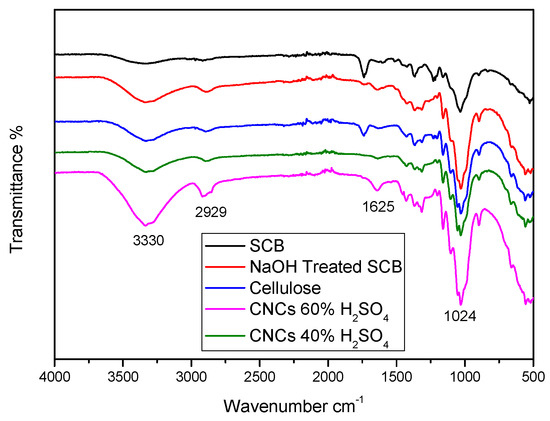
Figure 8.
FTIR spectra of untreated SCB, alkali-treated SCB, cellulose, CNC (hydrolysed with 40% H2SO4), and CNC (hydrolysed with 60% H2SO4).
Both untreated and chemically treated SCB have the following absorption bands. (i) The OH stretching of cellulose and hemicellulose was attributed to the wider absorption band at a wavenumber of 3330 cm−1. (ii) The asymmetric and symmetric C-H stretching of cellulose and hemicellulose was identified as the cause of the intensity peak absorption band at wavenumber 2929 cm−1. (iii) O-H distortion, which could be attributed to water absorption, characterized the absorption band at wavenumber 1625 cm−1. Additionally, (iv) the C-O stretching of cellulose and hemicellulose was associated with the peak at wavenumber 1024 cm−1. The intensities of all absorption bands were found to increase following chemical treatment; this could be because noncellulosic components were removed, exposing cellulose [17,23]. According to reports, chemically treated surface fibres improved the adherence of natural fibres to the polymer matrix [18]. The peak corresponded to water absorption and was governed by the hydrolysis process.
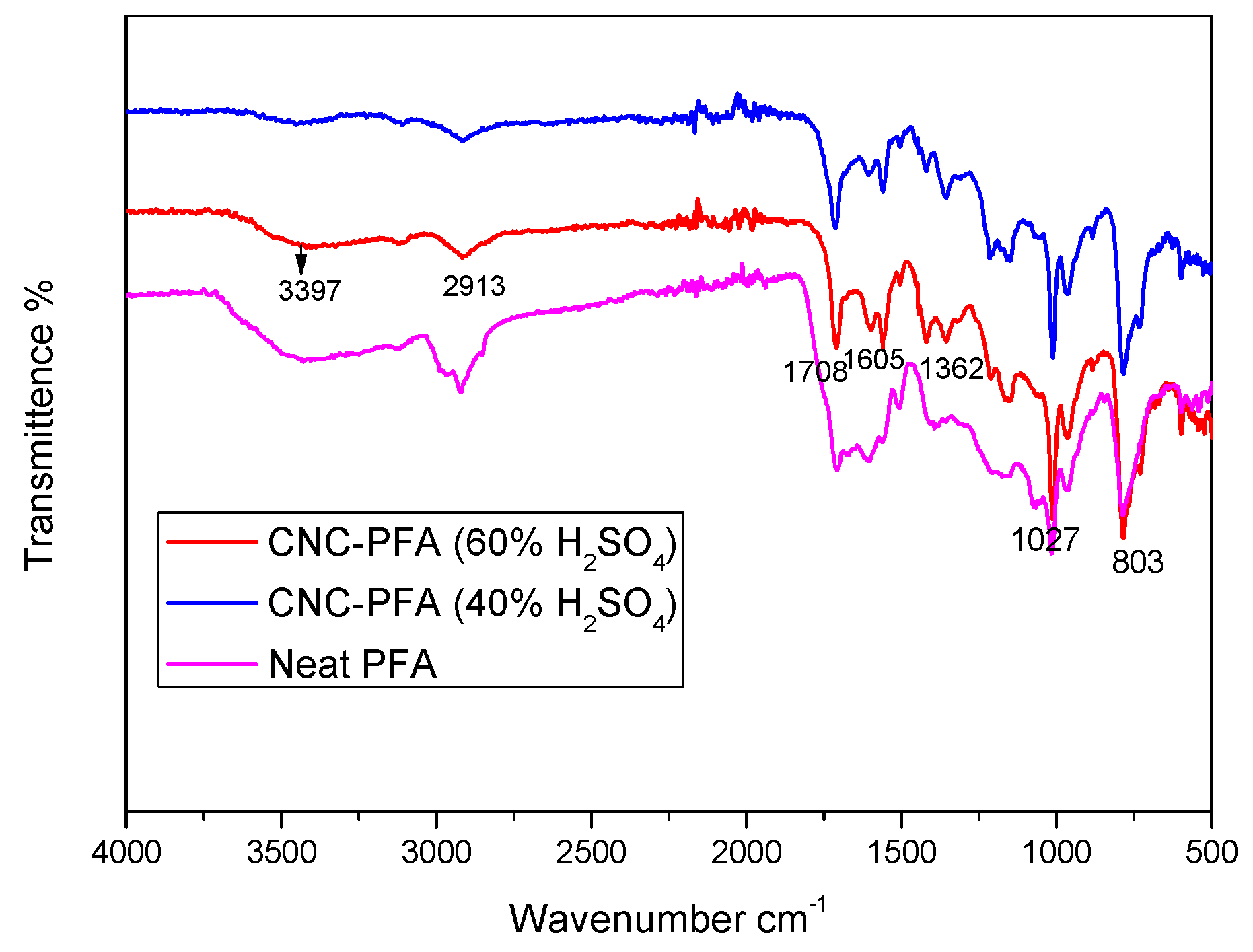
The FTIR spectra of the pure PFA and the PFA nanocomposites reinforced with 40% and 60% H2SO4 are displayed in Figure 9. Following the addition of nanocellulose as reinforcement, it is noted that no additional functional groups are present. Absorption peaks at a wavenumber of 3397 cm−1 are attributed to OH stretching, to C–H stretching at wavenumber 2913 cm−1, and C=C stretching of the aromatic ring at wavenumber 1605 cm−1 demonstrated the presence of the furan ring in all the PFA composites’ spectra [10,24]. The C–H deformation and C=O stretching were identified as the causes of the absorption bands at wavenumbers 1362 cm−1 and 1708 cm−1, which show that the PFA backbone and carboxyl group cross-linked during the acid-catalysed ring opening of the furan aromatic ring [16,25].
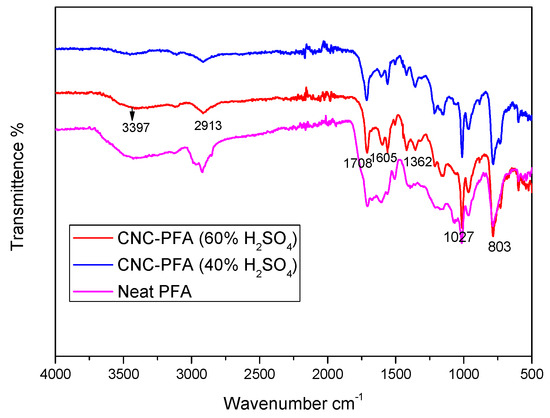
Figure 9.
FTIR spectra of the neat PFA and PFA nanocomposites reinforced with nanocellulose of 40% and 60% H2SO4.
3.4. Thermogravimetric Analysis (TGA)
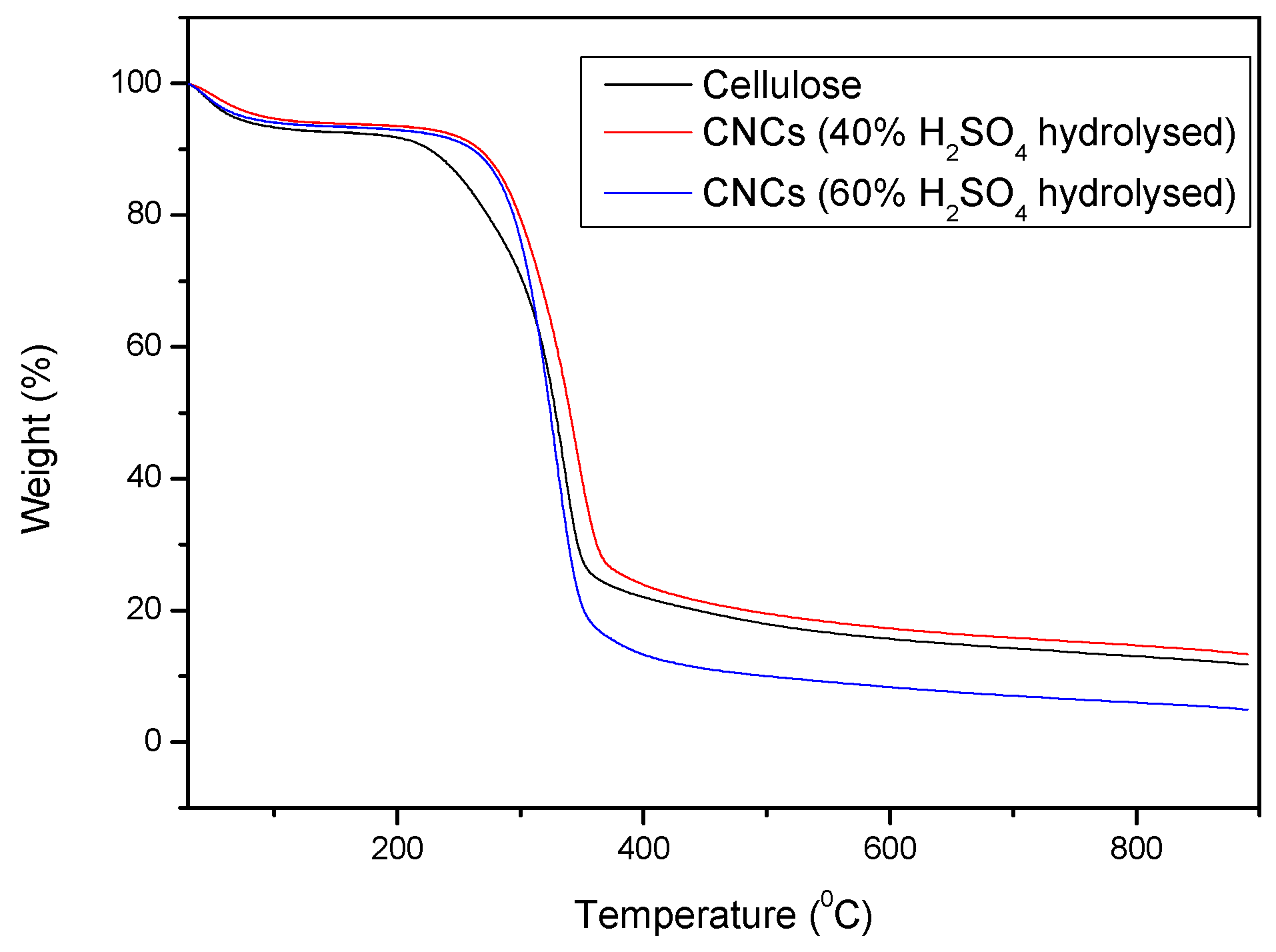
The thermogravimetric analysis (TGA) curves of cellulose, CNCs (40% H2SO4 hydrolysed), and CNCs (60% H2SO4 hydrolysed) provide critical insights into their thermal stability and decomposition behaviour (Figure 10).
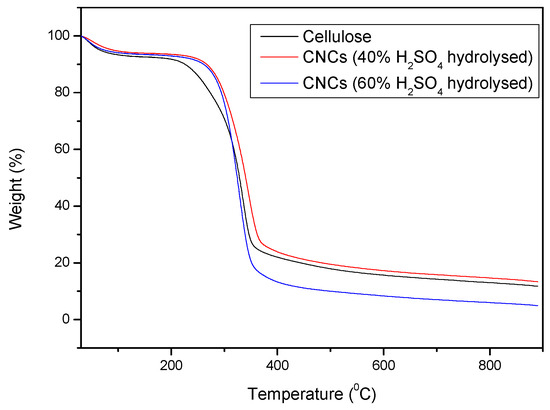
Figure 10.
TGA curves of cellulose, CNCs (40% acid hydrolysed), and CNCs (60% acid hydrolysed).
The TGA curve for cellulose shows a typical two-stage degradation profile. The initial weight loss occurs below 150 °C, attributed to moisture evaporation. The major decomposition stage starts around 200 °C and peaks at approximately 350–400 °C, where significant thermal degradation of cellulose occurs due to depolymerization and breakdown of glycosidic linkages [22].
The CNCs obtained through 40% H2SO4 hydrolysis display a similar degradation trend but with slightly reduced thermal stability compared to pure cellulose. The onset of major thermal degradation shifts slightly towards higher temperatures, indicating that sulphuric acid hydrolysis introduces sulphate groups onto the CNC surface, which stabilizes the material against degradation [26]. The residual weight at higher temperatures is lower than that of neat cellulose, indicating increased char formation.
For CNCs treated with 60% H2SO4, a further reduction in thermal stability is observed. The major degradation occurs at a lower temperature than both cellulose and CNCs (40% H2SO4), likely due to a higher degree of surface modification and sulphate esterification, which accelerates decomposition. The final residue content is the lowest among the samples, suggesting greater volatilization and reduced char formation.
Overall, the TGA results confirm that higher acid concentrations in hydrolysis lead to increased sulphate esterification, which enhances CNC dispersion but lowers thermal stability. While CNCs contribute to reinforcement in composites, their thermal degradation must be carefully considered when designing high-temperature applications.
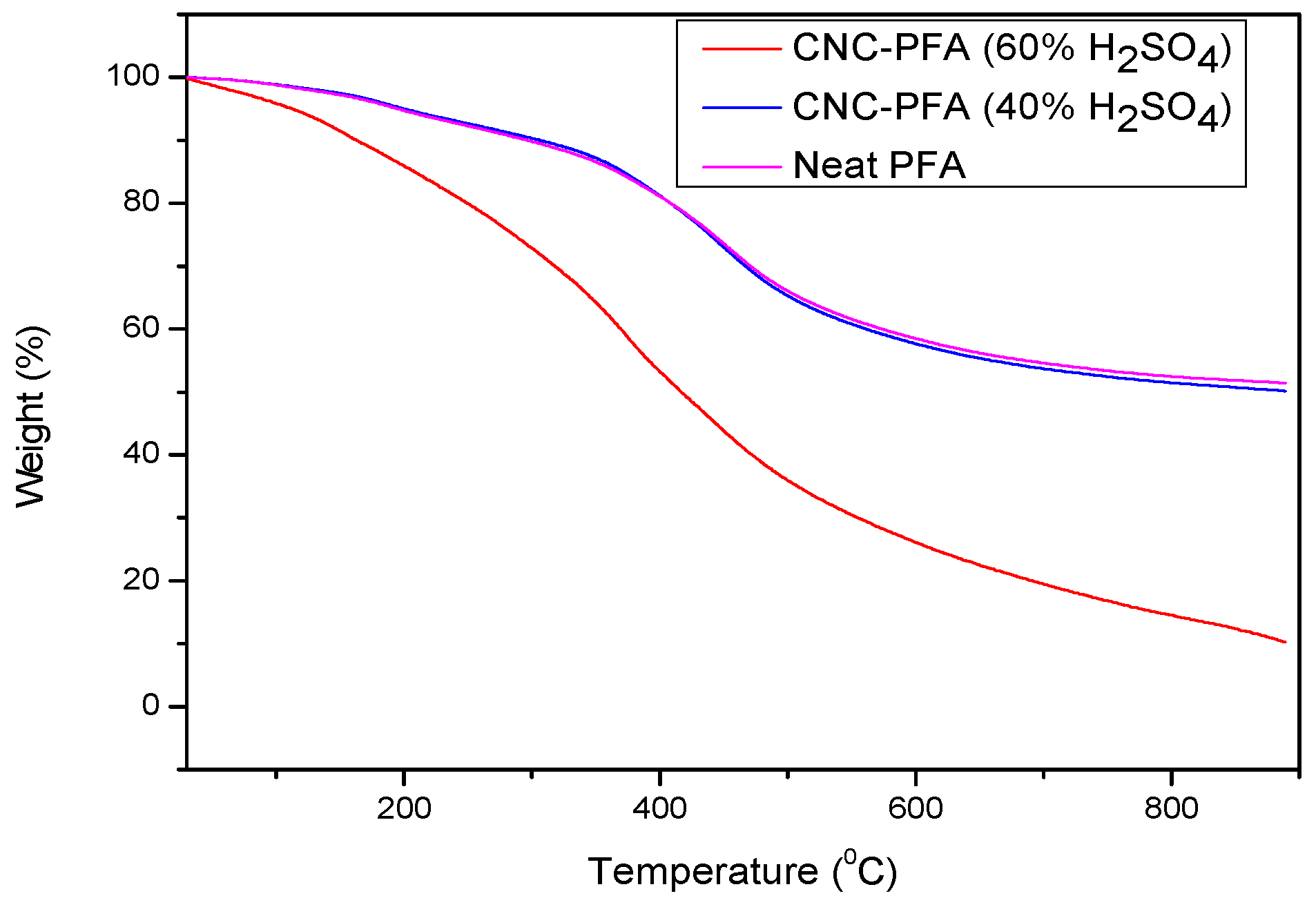
The thermogravimetric analysis (TGA) curves of neat PFA, CNC-PFA (40% H2SO4), and CNC-PFA (60% H2SO4) reveal significant differences in their thermal stability and degradation behaviour (Figure 11). Neat PFA exhibits a gradual weight loss at relatively high temperatures, demonstrating good thermal stability with a steady degradation profile and a higher final char residue. The addition of CNCs hydrolysed with 40% H2SO4 slightly reduces the thermal stability of PFA, as indicated by a lower onset degradation temperature. This is likely due to the sulphate groups introduced during CNC hydrolysis, which act as catalytic sites for degradation [11,27,28], though further quantification of sulphur with EDS, ICP-OES can provide further information. However, the degradation profile remains like that of neat PFA, and the final residue is only slightly reduced. In contrast, CNC-PFA (60% H2SO4) exhibits a more pronounced reduction in thermal stability, with a significantly lower onset degradation temperature. This suggests that a higher sulphate content accelerates thermal decomposition, leading to increased volatilization and a reduced final char residue. The results indicate that the effect on thermal stability must be carefully considered. The presence of sulphate groups in CNCs derived from higher acid concentrations appears to act as a catalyst for degradation, making CNC-PFA (60% H2SO4) less thermally stable than CNC-PFA (40% H2SO4) and neat PFA [16,29]. Therefore, the selection of CNCs for polymer reinforcement should balance both mechanical enhancements and thermal performance to ensure suitability for high-temperature applications.
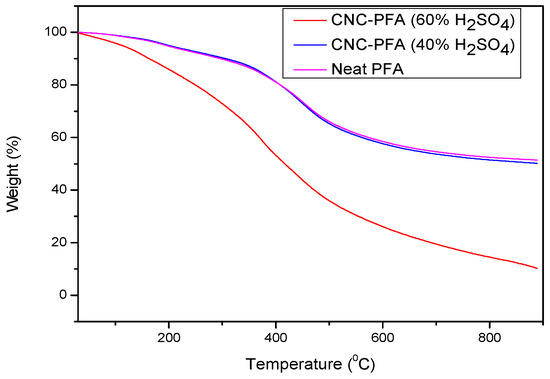
Figure 11.
TGA curves of CNCs, neat PFA, and PFA reinforced with CNCs.
3.5. Summary and Conclusions
The comprehensive characterization of sugarcane bagasse (SCB) and its derivatives through SEM, XRD, FTIR, and TGA analyses highlights the significant structural, chemical, and thermal transformations induced by different treatments. SEM imaging confirms that alkali treatment effectively removes noncellulosic components, refining the fibre structure, while sulphuric acid hydrolysis further breaks down cellulose into nanocrystals, with higher acid concentrations resulting in increased fragmentation. XRD analysis reveals that while alkali treatment does not alter the cellulose structure, it enhances crystallinity, with CNCs from 40% H2SO4 exhibiting higher crystallinity than those from 60% H2SO4, which undergo greater surface modification. FTIR spectra validate the successful removal of lignin and hemicellulose, exposing cellulose functional groups, and indicate that CNC incorporation does not introduce new chemical functionalities in PFA composites. TGA results demonstrate that CNCs contribute to polymer reinforcement but also affect thermal stability, with higher sulphate content accelerating decomposition. This work highlights the impact of sulphuric acid concentration on the structure, crystallinity, and thermal properties of CNCs extracted from sugarcane bagasse and their reinforcement effect in PFA composites. CNCs derived using 40% H2SO4 yielded higher crystallinity and better composite performance, while 60% led to more sulphate esterification and thermal degradation. These findings offer insight into tailoring CNC properties for high-performance bioresin composites, supporting the transition to sustainable, biodegradable materials in polymer engineering.
Author Contributions
Conceptualization, N.L.K., T.E.M. and S.M.M.; methodology, investigation, writing—original draft preparation, N.L.K.; visualization, T.D.M., S.V.M. and L.F.K.; supervision, T.E.M. and S.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This project received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
No conflict of interest.
References
- Motaung, T.E. Recent applications and innovations of cellulose based materials: A critical review. Cellul. Chem. Technol. 2021, 55, 1–12. [Google Scholar] [CrossRef]
- Mohomane, S.M.; Motloung, S.V.; Koao, L.F.; Motaung, T.E. Effects of acid hydrolysis on the extraction of cellulose nanocrystals (CNCs): A review. Cellul. Chem. Technol. 2022, 56, 691–703. [Google Scholar] [CrossRef]
- Pullawan, T.; Wilkinson, A.; Eichhorn, S. Discrimination of matrix–fibre interactions in all-cellulose nanocomposites. Compos. Sci. Technol. 2010, 70, 2325–2330. [Google Scholar] [CrossRef]
- Åström, B.T. Manufacturing of Polymer-Matrix Composites; Chapman & Hall: London, UK, 1997; pp. 1–6. [Google Scholar]
- Mohanty, A.; Drzal, L.; Misra, M. Engineered natural fiber reinforced polypropylene composites: Influence of surface modifications and novel powder impregnation processing. J. Adhes. Sci. Technol. 2002, 16, 999–1015. [Google Scholar] [CrossRef]
- Ridzuan, M.J.M.; Abdul Majid, M.; Afendi, M.; Aqmariah Kanafiah, S.; Nuriman, M. Effects of alkaline concentrations on the tensile properties of Napier grass fibre. Appl. Mech. Mater. 2015, 786, 23–27. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Motaung, T.E.; Linganiso, L.Z. Critical review on agrowaste cellulose applications for biopolymers. Int. J. Plast. Technol. 2018, 22, 185–216. [Google Scholar] [CrossRef]
- Kasyapi, N.; Chaudhary, V.; Bhowmick, A.K. Bionanowhiskers from jute: Preparation and characterization. Carbohydr. Polym. 2013, 92, 1116–1123. [Google Scholar] [CrossRef]
- Morelli, C.L.; Marconcini, J.M.; Pereira, F.V.; Bretas, R.E.S.; Branciforti, M.C. Extraction and characterization of cellulose nanowhiskers from balsa wood. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ahmad, E.; Luyt, A.S.; Djoković, V. Thermal and dynamic mechanical properties of bio-based poly (furfuryl alcohol)/sisal whiskers nanocomposites. Polym. Bull. 2013, 70, 1265–1276. [Google Scholar] [CrossRef]
- Khumalo, N.L.; Mohomane, S.M.; Motloung, S.V.; Koao, L.F.; Thembinkosi, M.D.; Motaung, T.E. Preparation and analysis of cellulose PFA composites: A critical review. Cellul. Chem. Technol. 2021, 55, 299–309. [Google Scholar] [CrossRef]
- Zhao, H.; Kwak, J.H.; Zhang, Z.C.; Brown, H.M.; Arey, B.W.; Holladay, J.E. Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis. Carbohydr. Polym. 2007, 68, 235–241. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Neto, W.P.F.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue–Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Effect of chemical modifications of the pineapple leaf fiber surfaces on the interfacial and mechanical properties of laminated biocomposites. Compos. Interfaces 2008, 15, 169–191. [Google Scholar] [CrossRef]
- Burket, C.L.; Rajagopalan, R.; Marencic, A.P.; Dronvajjala, K.; Foley, H.C. Genesis of porosity in polyfurfuryl alcohol derived nanoporous carbon. Carbon 2006, 44, 2957–2963. [Google Scholar] [CrossRef]
- Vryonis, O.; Anastassopoulos, D.; Vradis, A.; Psarras, G. Dielectric response and molecular dynamics in epoxy-BaSrTiO3 nanocomposites: Effect of nanofiller loading. Polymer 2016, 95, 82–90. [Google Scholar] [CrossRef]
- Khumalo, N.; Mohomane, S.; Linganiso, L.; Linganiso, E.; Songca, S.; Motaung, T. Effect of Acid Hydrolyses on Properties of Cellulosepoly Furfural Alchol Pfa Composites from Maize Stalk. Wood Res. 2023, 68, 96–111. [Google Scholar] [CrossRef]
- Khumalo, N.; Mohomane, S.; Motloung, S.V.; Koao, L.; Thembinkosi, M.D.; Motaung, T.E. Effect of H2SO4/HClO4 mixture on properties of sugarcane bagasse cellulose crystals. Wood Res. 2022, 67, 929–940. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Motaung, T.E.; Mtibe, A. Alkali treatment and cellulose nanowhiskers extracted from maize stalk residues. Mater. Sci. Appl. 2015, 6, 1022–1032. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Mochane, M.J.; Motaung, T.E.; Linganiso, L.Z.; Thekisoe, O.M.; Songca, S.P. Sugarcane bagasse and cellulose polymer composites. In Sugarcane—Technology and Research; IntechOpen: Rijeka, Croatia, 2018; pp. 225–240. [Google Scholar]
- Mzimela, Z.N.T.; Linganiso, L.Z.; Revaprasadu, N.; Motaung, T.E. Comparison of cellulose extraction from sugarcane bagasse through alkali. Mater. Res. 2018, 21, e20170750. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Effect of pre-acid-hydrolysis treatment on morphology and properties of cellulose nanowhiskers from coconut husk. Cellulose 2011, 18, 443–450. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Zavaglia, R.; Vincent, L.; Sbirrazzuoli, N. New insights on the thermal degradation pathways of neat poly (furfuryl alcohol) and poly (furfuryl alcohol)/SiO2 hybrid materials. Polym. Degrad. Stab. 2009, 94, 908–913. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Eco-friendly composite resins based on renewable biomass resources: Polyfurfuryl alcohol/lignin thermosets. Eur. Polym. J. 2010, 46, 1016–1023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).