Abstract
Anodic aluminum oxide (AAO) is a promising material for sensor applications due to its unique nanoporous structure and high surface area. This study investigates enhancing AAO’s sensing capabilities by incorporating carbyne-enriched nanomaterials. This research aimed to create a novel surface acoustic wave (SAW) sensor with improved performance characteristics. AAO films were fabricated using a two-step anodization process, followed by carbyne-enriched coating deposition via ion-assisted pulse-plasma deposition. The dielectric properties of the resulting composite material were characterized using impedance spectroscopy, while the sensing performance was evaluated by exposing the sensor to various ethanol concentrations. The results showed a significant increase in capacitance and dielectric permittivity for the carbyne-filled AAO compared to pristine AAO, along with a 5-fold improvement in sensitivity to ethanol vapor. The increased sensitivity is attributed to the synergistic combination of the AAO’s high surface area and the carbyne’s unique electrical properties. This work demonstrates the successful fabrication and characterization of a novel high-sensitivity gas sensor, highlighting the potential of carbyne-enriched AAO for advanced sensor applications.
1. Introduction
Anodic aluminum oxide (AAO) has garnered significant attention in recent years due to its unique structural properties and versatility in various applications, including nanotechnology, catalysis, energy harvesting, and sensing technologies [1,2,3]. Recent advances in nanotechnology have underscored the importance of harnessing nanostructured materials to develop high-performance sensors. The unique attributes of AAO, combined with the recent discoveries in novel one- and two-dimensional nanomaterials, have opened new frontiers in sensor technology [4]. The demand for highly sensitive and selective sensors has surged across various industries, including environmental monitoring, health care, and food safety. For instance, the increasing prevalence of airborne pollutants and the need for rapid detection of hazardous substances have accelerated research into gas sensors. Recent studies have highlighted the effectiveness of nanostructured materials in achieving lower detection limits and faster response times [5,6]. The utilization of AAO as a template not only facilitates the creation of nanoscale architectures but also enhances the integration of functional materials, thereby providing avenues for innovative sensing applications. New techniques for optimizing the anodization parameters, such as pulsed anodization and variable temperature settings, have shown promise in enhancing the structural characteristics and functional properties of AAO films (insert reference). These innovations allow for finer control over pore morphology, enabling researchers to tailor AAO to specific applications better. Furthermore, hybrid approaches combining AAO with other nanomaterials—such as metal nanoparticles or conducting polymers—have been researched, yielding composite materials with significantly enhanced spectral and sensing characteristics [7].
AAO is formed through the electrochemical anodization of aluminum, resulting in a nanoporous structure characterized by uniform pore size and interpore distance, as well as high surface area, which can be finely tuned by adjusting the anodization parameters such as electrolyte composition, voltage, and temperature [8,9]. This tunability makes AAO an ideal template for the synthesis of nanostructured materials, enabling advancements in fields ranging from energy conversion to biosensing [10,11]. Recent studies have highlighted the potential of incorporating, in particular, carbon-based nanomaterials, such as carbon nanotubes, graphene, and piezoelectric polymers, into AAO matrices to enhance their mechanical, electrical, sensing, and harvesting properties [12,13]. For this purpose, a highly ordered array of AAO nanopores is needed, requiring high-purity aluminum foil to serve as the substrate. The two-step anodization process, a well-established technique in the literature [14], should be employed to generate such structures. This method involves two sequential anodization steps, each optimized to control specific aspects of the pore formation and ordering. A comparative study has been conducted using oxalic acid, sulfuric acid, and phosphoric acid as electrolytes, allowing for a systematic evaluation of their respective influences on the AAO structure [15].
The inherent porous structure of AAO, extensively documented in the literature [16,17], provides a high surface area for analyte interaction. Simultaneously, the exceptional electronic properties of carbyne, a one-dimensional carbon allotrope [18], offer the potential for heightened sensitivity and selectivity in sensing applications. For example, it has already demonstrated its affinity to alcohol vapors [19,20]. A carbyne-based sensor using the surface acoustic wave (SAW) operational principle has been demonstrated [21]. SAW devices have been commercially employed in various industrial sectors, including communications, automotive electronics, and environmental monitoring [22]. By applying alternating current (AC) at a designated frequency to electrodes with a particular design, a piezoelectric material is stimulated, resulting in the generation of an acoustic wave that travels along its surface. When an analyte interacts with the sensing layer, whether physically or chemically, modifications occur within the system. Variations in mass and viscosity at the sensitive layer can be monitored by detecting shifts in acoustic wave characteristics, such as velocity, attenuation, resonant frequency, or time delay. This configuration has been extensively studied for sensing and fluidic applications in sophisticated lab-on-chip devices [23].
Except for imaging techniques like scanning electron microscopy or atomic force microscopy, it is possible to conclude about the pore filling by the change in capacitance of the structure that is due to the change in dielectric constant resulting from the pore filling. Several methods can be employed to extract the dielectric permittivity of the AAO [24,25]. Among them, impedance spectroscopy is preferable due to the possibility of differentiating relaxation processes in dielectric materials, which is essential for understanding the intricate behavior of nanoporous structures. It is particularly effective for analyzing the complex electrical behavior of nanostructured materials. By varying the frequency, researchers can identify specific relaxation processes within the material, enabling the evaluation of how pore structure and surface modifications influence conductivity, permittivity, and overall sensor performance [26,27]. When integrated into sensing platforms, impedance spectroscopy enables the real-time monitoring of changes in the electrical properties of the sensing layer upon exposure to target analytes. This characteristic not only allows for enhanced sensitivity in detecting small concentrations of volatile organic compounds but also provides insight into the dynamics of adsorption and desorption processes, which are critical for improving response times [28,29]. A significant advantage of impedance spectroscopy is its non-destructive nature, which allows for repeated measurements on the same sample without affecting its properties. This is especially valuable for applications in sensor development and long-term stability testing, as it facilitates the assessment of performance degradation over time [30,31]. Thus, impedance spectroscopy can address the complexities of composite materials like nanoporous AAO filled with air or other substances, providing meaningful data even in heterogeneous systems [32].
This paper investigates the synergistic combination of AAO and carbyne-enriched nanomaterials to create a novel surface acoustic wave-based sensor platform with enhanced performance characteristics. To the authors’ knowledge, AAO template-based nanostructuring of carbyne-enriched material has never been published. Moreover, an AAO nanoporous structure has not been integrated into a SAW device. We aim to introduce enhanced sensitivity due to the increased surface-to-volume ratio of the carbyne-enriched material introduced by the AAO template and to characterize the test structures by impedance spectroscopy. The integration of these materials is expected to overcome the dynamic range limitation of existing sensor technology by creating a composite material with enhanced detection capabilities for volatile organic compounds (VOCs).
2. Materials and Methods
For the fabrication of the surface acoustic wave sensor, single polished LiNbO3 wafers, which were cleaned in an ammonia-based solution, served as the substrates. These wafers were of SAW grade, specifically a 128° Y-cut of the LiNbO3 crystal, exhibiting excellent temperature stability of the electromechanical coupling factor in the range of the operational and film deposition temperatures. This stability indicates that the SAW distribution is independent of temperature. Aluminum films with a thickness of 920 nm were deposited by vacuum thermal evaporation. A standard photolithographic patterning technique, combined with wet anisotropic chemical etching, was utilized to create the three fundamental topologies of the samples—input and output interdigitated electrodes (IDTs) and the sensing zone subjected to anodization. The geometrical dimensions are as follows: finger length was 2600 µm, finger pitch was 500 µm, and the number of fingers in each transducer from the pair (input and output IDT) was symmetric and equal to 25. A photograph of the sample is shown in Figure 1a.
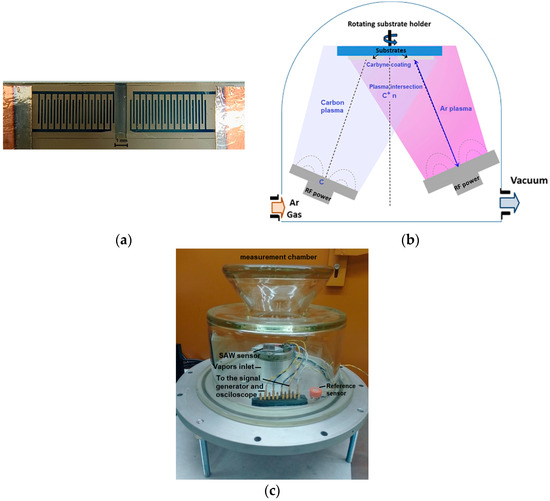
Figure 1.
Fabrication and testing of the sensor: (a) photo of the fabricated sample with AAO between the IDT electrodes, filled by carbyne-enriched material, (b) deposition chamber, and (c) measurement setup.
To remove any natural oxidation, the aluminum regions were additionally treated in a solution of 40 g/L CrO3 mixed with 90 mL/L H3PO4 at a temperature of 75 °C for 20 min. This solution is used later to open the opposite ends of the nanopores. The electrolyte temperature was maintained constant during the process by a circulating flow of liquid cooling down with a system of copper tubes, Peltier elements, and fans. AAO film was produced in an electrolyte solution of 5% H3PO4 without overheating or degradation of the substrates and oxide layer, at the process conditions that were established earlier [33]: Us = 150 V (necessary voltage for this electrolyte solution); I = 0.7 A/dm2; and temperature of the electrolyte Ta = 50 °C (±0.1 °C). The thickness of the resulting AAO film was 512 nm, and the size of the AAO spot between the IDTs was (1 × 5) mm.
The carbyne-enriched coating was deposited as a sensing layer over the prepared AAO array using ion-assisted pulse-plasma deposition. The components of the pulse-plasma deposition reactor for producing 2D-ordered linear-chain carbon nanomatrices include a vacuum chamber, a pulse-plasma carbon generator, an ion source for ionic stimulation, and a target material. Ion and plasma beams converge above and on the substrate surface (Figure 1b). The irradiation from the ion beam in the deposition area creates bends in the attached carbon chains, which helps to stabilize the growing ensemble. An electrode system directs chains of carbon atoms to strike the substrate surface, where polycondensation of the carbon chains occurs. Controlled bond breaking and sp-phase transformation can be achieved through predictive ion-assisted stimulation at specific energy levels. The optimal deposition conditions were determined to be a target-to-substrate distance of 1 m, 3000 pulses of carbon plasma, an arc discharge voltage of 300 V between the main discharge cathode (the carbon source) and the substrate anode, a main capacitor charge of 2000 µF pulsing at 5 Hz, and Ar-ion plasma power set to 150 W. The SAW containing AAO was filled by carbyne-enriched material at these conditions. Previous analyses using Raman spectroscopy and X-ray photoelectron spectroscopy have confirmed the presence of carbyne-like sp1-hybridized structures in the samples [34,35].
A laboratory-made measurement chamber was used for the characterization of the sensing device. A Petri dish with a 3 mL capacity was used inside the chamber onto a ceramic heater. A current controller, utilizing thermocouple feedback, regulated the current through the heater to achieve precise temperature rate changes, thereby ensuring a specific evaporation rate for the 96% concentrated high purity grade ethanol. This process converted the liquid into ethanol vapor, which was subsequently distributed in the chamber by controlling the vaporization duration. An additional control ethanol sensor MQ- (ME075), providing the necessary resolution, was installed near the device being tested as a reference measurement device of the concentration. The output voltage magnitude, waveform, voltage difference, and delay time between the input and output signals were recorded using a Tektronix TDS 1012B oscilloscope (Tektronix, Berkshire, UK). The effective output voltage was measured with a computer-connected multimeter Peakmeter PM8236 (Peakmeter, Guilin, China). Electrochemical Impedance Analyzer Hioki IM3590 (Hioki, Nagano, Japan) recorded the capacitance and the impedance spectra. The measurement setup is shown in Figure 1c. A cross-sectional observation of the sample was conducted by scanning electron microscope SEM LYRA/TESCAN combined with Energy dispersive X-ray (EDX) analysis.
3. Results and Discussion
The SEM image of the cross-sectional view of the sample (Figure 2a left) reveals the formation of nanowires from carbyne, well-templated by the AAO with a length of approximately 1 µm. The EDX elemental analysis (Figure 2a right) shows that the carbon element is deeply inserted inside the pores, due to the carbyne filling. The presence of N and P elements is contaminating by-products incorporated during the etching procedure for the pores’ opening. However, their content is less than 5%, which is not significant for the system behavior. This is evidence for successful nanostructuring of the carbyne material in the form of high aspect ratio nanowires. It is expected that exposure to ethanol will result in a higher sensitivity and broader dynamic range of response, as compared to the same sensor fabricated with a non-structured carbyne coating. Similarly, the same microstructural and elemental characterization was conducted for the non-coated AAO sample, and the results are presented in Figure 2b, respectively.
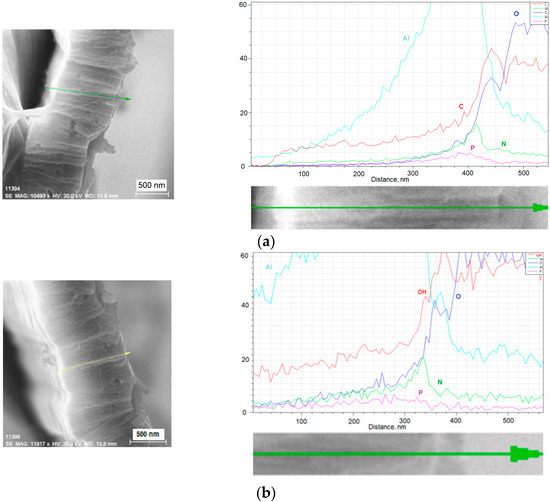
Figure 2.
SEM image and EDX analysis of (a) carbyne-filled AAO sample and (b) non-coated AAO sample.
Due to the limited resolution, the lack of carbyne in the AAO nanopores cannot be differentiated on the SEM image. However, based on the elemental analysis of the AAO samples non-coated by carbyne, the pores of the anodic aluminum oxide are filled by the hydroxyl groups from the moisture existing in the environment due to the hydrophilic property of the AAO array. In contrast, the EDX of the carbyne-filled AAO does not exhibit absorption of moisture components, thus improving the selectivity of the sensor. Moreover, although in [36] the authors have reported AAO-based chemo-capacitive sensor for ethanol gas due to diffusion of a small quantity of alcohol molecules in the pores, they highlighted that the sensor sensitivity exhibited changes with slopes at different measurement frequencies. This variation is attributed to changes in the permittivity of the dielectric material with frequency. Additionally, the signal-to-noise ratio is also strongly dependent on the measurement frequency. This problem was eliminated in the present sensor, as the frequency is fixed with the geometry of the interdigitated electrodes, relying at the same time on a noise-resistant design. Moreover, the interaction between the carbyne and the ethanol is controllable, as we previously found [21], and the sensing mechanism does not rely on a small quantity of successfully but randomly absorbed ethanol molecules in the pores. The combination of carbyne with AAO created a larger effective surface area for interaction with ethanol. Carbyne’s properties amplified this effect, allowing more ethanol molecules to be sensed at once.
The dielectric properties of the structure with a pristine AAO zone and the carbyne-filled AAO composite were characterized using impedance spectroscopy. In addition, the dielectric permittivity was determined based on the measured capacitance before and after exposure to ethanol. The measurements were performed at two frequencies—a standard frequency for measurement in electronics of 1 kHz and a higher one of 10 kHz, where the capacitance is expected to change significantly. Also, measurements were conducted at two different temperatures—operational temperature during detection (accepted to be 25 °C) and one during the sensor recovery (50 °C for evaporation of the ethanol). Specific values for capacitance, dielectric constant, and dielectric loss were obtained and presented in Table 1 for both AAO and carbyne-filled AAO under various conditions, providing quantitative data for comparison and analysis. The results are summarized in Figure 3.

Table 1.
Extracted data about the dielectric behavior of the AAO and composite carbyne-filled AAO.
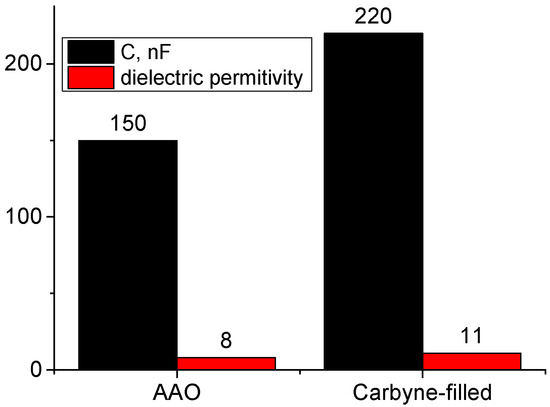
Figure 3.
Comparison between the dielectric properties (permittivity and capacitance) of AAO and carbyne-filled AAO (at f = 1 kHz).
As was expected, since the pores are filled with carbyne-enriched material instead of air, the overall dielectric permittivity of the composite system is higher than that of pristine AAO (Figure 3). The extent to which the dielectric permittivity increases also depends on the volume fraction of carbyne molecules relative to the AAO, which cannot be precisely calculated due to imperfections in the pores’ formation and uniformity of the carbyne flux. If the carbyne occupies a significant portion of the nanopores, it is expected to have a more pronounced effect on the effective dielectric constant, which is not the case. Therefore, it can be expected that the carbyne-enriched material exists only on the inner wall of the AAO nanopores, which is favorable for the space needed to penetrate ethanol vapor for detection.
The dielectric characterization of the pristine AAO and the carbyne-filled AAO revealed significant differences in their electrical behavior. The capacitance of the carbyne-filled AAO was found to be substantially higher than that of the pristine AAO across the entire frequency range tested. This increase in capacitance was attributed to the high dielectric constant of carbyne, which enhances the material’s ability to store electrical charge. The dielectric constant of the carbyne-filled AAO also exhibited a significant increase compared to the pristine AAO, further confirming the influence of carbyne on the material’s dielectric properties. The dielectric loss (tan δ), representing energy dissipation within the material, was found to be slightly higher in the carbyne-filled AAO compared to the pristine AAO (Table 1). This increase in dielectric loss is likely due to increased conductivity associated with the presence of carbyne. The temperature dependence of the dielectric properties was also investigated. Both the capacitance and dielectric constant of the carbyne-filled AAO showed a negligible temperature dependence than the pristine AAO, indicating that the presence of carbyne does not increase the system’s sensitivity to temperature variations. These results demonstrate that the incorporation of carbyne significantly alters the dielectric properties of AAO, enhancing its capacitance and increasing its sensitivity to frequency but not to temperature changes (Figure 4).
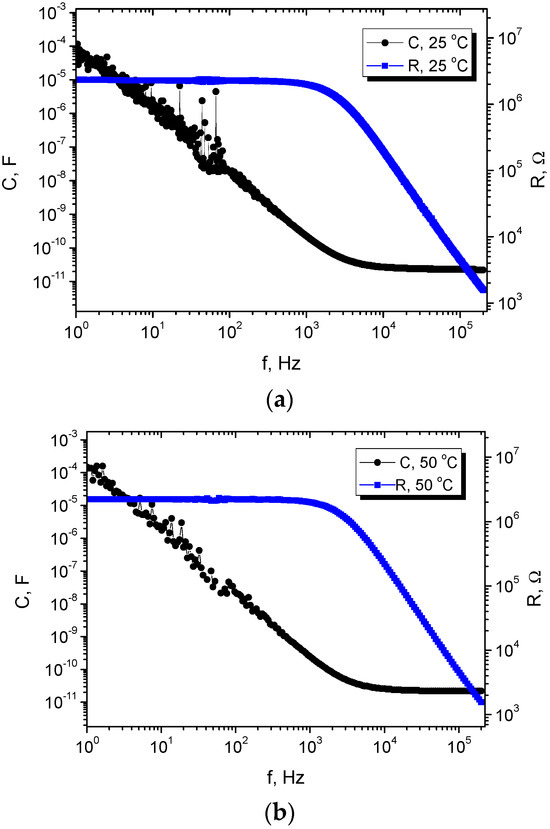
Figure 4.
Parameters of the carbyne-filled AAO, extracted from impedance measurements, showing the capacitance and resistivity of the system at different frequencies and temperatures: (a) 25 °C (considered as a room temperature) and (b) 50 °C.
The sensor’s response to various concentrations of ethanol vapor was measured, providing an assessment of its sensitivity and dynamic range. The sensitivity was quantified by measuring the change in sensor signal in response to changes in ethanol concentration. In the case of the SAW design of the sensor, the response is associated as an output voltage attenuation as compared to the input voltage of the IDT transducers. This attenuation is related to elastic wave energy dissipation due to a change in the distribution medium density, due to the mass loading with different concentrations of ethanol molecules (Table 2). Thus, the quantity of the ethanol is directly related to the piezoelectric voltage difference of the SAW device terminals. Response time, defined as the phase shift between the output and input signals of the SAW device was determined to assess the sensor’s speed and efficiency (Figure 5). The time delay of the elastic wave transformed into piezoelectric voltage is directly related to the sensor response at different absorbed quantities of ethanol molecules.

Table 2.
SAW sensor parameters (voltage attenuation in mV and phase shift in µs) at different ethanol concentrations for a control sample with AAO only and for carbyne-filled AAO.
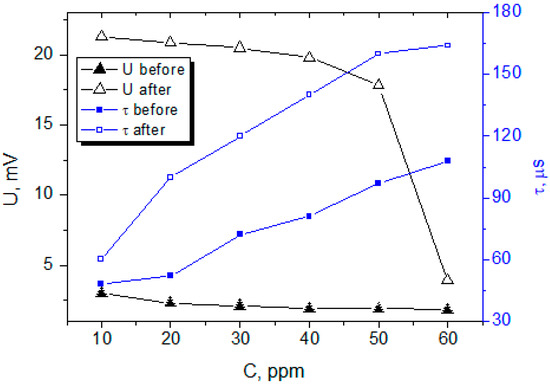
Figure 5.
Comparison of SAW sensors sensitivity and response time after using carbyne-filled AAO sensing layer and before that when using non-structured carbyne (U represents the sensitivity and τ the response time of the sensors).
The non-structured carbyne film consists of a continuous layer of carbyne-enriched material deposited on a substrate, lacking any intentional, controlled nanoscale features or organization. It is produced through a simple deposition technique, like described above, however, without the use of the template. It possesses a relatively low surface area compared to its nanostructured counterpart. The active sensing area is limited to the exposed surface of the continuous film. In contrast, the nanostructured carbyne film (AAO template-based) is composed of carbyne-enriched material organized into a controlled nanoscale architecture. The AAO template provides a mold for creating these structures. It exhibits a significantly higher surface area compared to non-structured carbyne films. The nanoscale features increase the number of active sensing sites; thus, the overall sensitivity is expected to increase. This is an additional confirmation (together with the SEM image) for filling the pores of AAO with carbyne.
The gas sensing measurements revealed an enhancement in the sensitivity of the carbyne-filled AAO sensor compared to a control sensor with non-structured carbyne, which is an average 5-fold increase for most of the measured concentrations (Figure 5). The control sensor with non-structured carbyne was explored in a previous study, and the results were published elsewhere [21]. The carbyne-filled AAO sensor exhibited a substantially higher response to ethanol vapor across a wide range of concentrations, providing approximately 18–20 mV voltage attenuation in the range 10–50 ppm. For comparison, the non-structured carbyne shows less than 5 mV attenuation for the same range. The sensitivity of the control sample in the linear part of the plot (30–60 ppm) was ~10 µV/ppm, while the sensitivity of the nanostructured carbyne-based SAW was ~117 µV/ppm for the same range of concentration, where the linearity of the sensor is sufficiently high for determination of the relative changes accurately. In addition, for the nanostructured carbyne, there is a slope in the plot between 50 ppm and 60 ppm, demonstrating much higher sensitivity and linearity—approximately 1.4 mV/ppm. The exact physical mechanism behind this behavior is still unclear, but this range is of interest for further optimization of the sensor and expanding the range of concentrations where these parameters are valid. The generally enhanced sensitivity is attributed to the high surface area of the AAO, facilitating interaction with ethanol molecules, coupled with the high sensitivity of carbyne to VOCs.
The response time of the carbyne-filled AAO sensor was not faster than that of the control sensor, indicating the additional time needed for the ethanol molecules to penetrate in the porous carbyne and affect its density. The difference becomes more pronounced at the higher concentrations as compared to the lower concentrations because of the higher capacity of the sensitive layer to absorb ethanol molecules. The recovery time, representing the time required for the sensor to return to its baseline after ethanol removal, followed a similar trend to the response time, suggesting that the carbyne nanostructuring also affects the desorption kinetics.
The enhanced dielectric properties and sensing performance observed in the carbyne-filled AAO are directly linked to the parameters of the AAO’s porous structure and carbyne’s unique electronic properties. The high surface area provided by the AAO allows for efficient interaction with analyte molecules, increasing the number of interaction sites and thus increasing the overall signal. Carbyne’s density contributes to the sensor’s enhanced sensitivity by facilitating acoustic wave re-distribution on the surface of the substrate and electrical signal attenuation upon analyte adsorption. The observed increase in capacitance and dielectric constant of the sensing zone is consistent with the high dielectric constant of carbyne. The slight increase in dielectric loss is likely due to the increased conductivity introduced by carbyne. The negligible temperature dependence of the dielectric properties in the carbyne-filled AAO suggests that the presence of carbyne does not increase the material’s sensitivity to temperature variations, which is beneficial for the simplicity of the device and the lack of necessity to provide temperature-compensated circuits. The superior sensing performance of the carbyne-filled AAO, as evidenced by its increased sensitivity, despite the slower response time and good linearity in the different ethanol ranges, highlights the effectiveness of this composite material for ethanol detection. These findings align with previous studies demonstrating the potential of carbyne-based materials for gas sensing and the use of AAO in sensor applications. The results obtained in this study surpass the performance of previously reported sensors, demonstrating the potential of this novel composite material for advanced sensing technologies.
Significantly, results for the effect of the carbyne nanostructuring on the sensor’s repeatability across the samples are presented (Figure 6). The performance stability was very good for samples having the same position in the carbyne deposition chamber. Any differences can be ascribed to the deposition process of the carbyne and its compositional and/or microstructural organization at the molecular level and not to the AAO or other sensor components’ fabrication. We noted that the AAO template application equalized the response of the sensors across samples, because it allows for the creation of well-defined nanoscale pores and structures with uniform size and spacing. This precise control over nanostructuring ensures that each sensor exhibits similar geometric and surface characteristics, which leads to consistent performance across multiple samples, in contrast to the flat-surfaced plain carbyne. This can be monitored through the sensor impedance Z as a function of the signal frequency for the studied four samples, randomly selected from the batch. In the present sensor configuration, the aspect ratio effect is dominant for the sensor performance over the carbyne microstructural or compositional specifics, and its stability is ensured by a well-controlled anodization process. As a result, the comparison showed more than a 14% variation in the impedance response from sample to sample (averaged for the working frequency) for the non-structured carbyne and an approximately 8% variation in the impedance response from sample to sample (for the same frequency) for nanostructured carbyne.
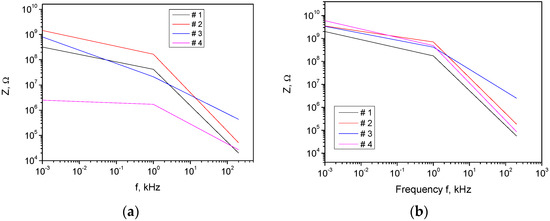
Figure 6.
Impedance response across the sensor samples (a) for a non-structured carbyne and (b) for AAO-nanostructured carbyne.
4. Conclusions
This study demonstrates the successful fabrication and characterization of a novel gas sensor based on carbyne-enriched nanostructure prepared by anodic aluminum oxide templating. The integration of carbyne significantly enhanced the dielectric properties and sensing performance of AAO, leading to a sensor with superior sensitivity, compared to sensors based on non-structured carbyne. The enhanced sensitivity is attributed to the synergistic combination of the high surface area of the fabricated nanowires and the high sensitivity of carbyne towards ethanol. The results confirm the significant potential of this composite material for advanced gas sensor applications. Future research directions include optimizing the sensor’s performance by further refining the deposition parameters, exploring different carbyne deposition methods, and investigating the sensor’s long-term stability and reliability. This study contributes to advancing novel sensor technologies based on integrating AAO and carbyne-enriched nanomaterials, paving the way for developing sensitive and selective gas sensors for various applications.
Author Contributions
Conceptualization, M.A.; methodology, M.A. and T.T.; validation, M.A. and D.N.G.; formal analysis, M.A.; investigation, M.A., T.T. and D.N.G.; resources, M.A. and D.N.G.; data curation, M.A., T.T. and D.N.G.; writing—original draft preparation, M.A.; writing—review and editing, D.N.G. and T.T.; visualization, M.A.; supervision, M.A.; project administration, D.N.G.; funding acquisition, D.N.G. All authors have read and agreed to the published version of the manuscript.
Funding
Bulgarian National Scientific Fund is gratefully acknowledged for the support of this investigation (grand KP-06-COST/15/08.08.2023) that is based upon work from COST Action COST-CA20126-Network for research, innovation and product development on
porous semiconductors and oxides (NETPORE).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We acknowledge Andrey Brigadin for the carbyne film deposition and Atanas Tsonev for the SEM/EDX measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vrublevsky, I.; Chemyakova, K.; Lushpa, N.; Tuchkovsky, A.; Tzaneva, B.; Videkov, V. Obtaining, properties and application of nanoscale films of anodic titanium dioxide on Ti-Al films for perovskite solar cells. In Proceedings of the 2021 XXX International Scientific Conference Electronics, Sozopol, Bulgaria, 15–17 September 2021; Volume 1–4. [Google Scholar]
- Aleksandrova, M.; Tsanev, T.; Gupta, A.; Singh, A.K.; Dobrikov, G.; Videkov, V. Sensing ability of ferroelectric oxide nanowires grown in templates of nanopores. Materials 2020, 13, 1777. [Google Scholar] [CrossRef] [PubMed]
- Rusev, R.P. Piezo Effect of Polyvinylidene Fluoride Layer with Rochelle Salt. In Proceedings of the 2023 XXXII International Scientific Conference Electronics (ET), Sozopol, Bulgaria, 13–15 September 2023; pp. 1–4. [Google Scholar]
- Nagaoka, S.; Yoshida, K.; Hirota, Y.; Komachi, Y.; Takafuji, M.; Ihara, H. Functionalized aluminum oxide by immobilization of totally organic aromatic polymer spherical nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128438. [Google Scholar] [CrossRef]
- Panigrahi, P.K.; Chandu, B.; Puvvada, N. Recent Advances in Nanostructured Materials for Application as Gas Sensors Pravas Kumar Panigrahi, Basavaiah Chandu, and Nagaprasad Puvvada. ACS Omega 2024, 9, 3092–3122. [Google Scholar]
- Panžić, I.; Bafti, A.; Radovanović-Perić, F.; Gašparić, D.; Shi, Z.; Borenstein, A.; Mandić, V. Advancements in Nanostructured Functional Constituent Materials for Gas Sensing Applications: A Comprehensive Review. Appl. Sci. 2025, 15, 2522. [Google Scholar] [CrossRef]
- Dong, J.; Li, C.; Wang, Y.; Fan, Y.; Han, Q.; Gao, W.; Wang, Y.; Ren, K.; Qi, J.; He, E. Fabrication of complexed nanostructure using AAO template for ultrasensitive SERS detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 312, 124044. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, B.; Milusheva, V.S.; Kolev, H.; Tzaneva, B.R. Photocatalytic activation of tio2-functionalized anodic aluminium oxide for electroless copper deposition. Catal. Sci. Technol. 2022, 12, 7027–7037. [Google Scholar] [CrossRef]
- Bruera, F.A.; Kramer, G.R.; Vera, M.L.; Ares, A.E. Low-Cost Nanostructured Coating of Anodic Aluminium Oxide Synthesized in Sulphuric Acid as Electrolyte. Coatings 2021, 11, 309. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Zhang, W. Fabrication and application of nanoporous anodic aluminum oxide: A review. Nanotechnology 2021, 32, 222001. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, M.; Zhao, H.; Lei, Y. Ordered nanostructures arrays fabricated by anodic aluminum oxide (AAO) template-directed methods for energy conversion. Nanotechnology 2021, 32, 502006. [Google Scholar] [CrossRef]
- Ryzhkov, I.I.; Kharchenko, I.A.; Mikhlina, E.V.; Minakov, A.V.; Guzei, D.V.; Nemtsev, I.V.; Volochaev, M.N.; Korobko, A.V.; Simunin, M.M. Growth of carbon nanotubes inside porous anodic alumina membranes: Simulation and experiment. Int. J. Heat Mass Transf. 2021, 176, 121414. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Chernozem, R.V.; Pariy, I.O.; Surmeneva, M.A. A review on piezo- and pyroelectric responses of flexible nano- and micropatterned polymer surfaces for biomedical sensing and energy harvesting applications. Nano Energy 2021, 79, 105442. [Google Scholar] [CrossRef]
- Chahrour, K.M.; Ooi, P.C.; Hamzah, A.A. Influence of the Voltage on Pore Diameter and Growth Rate of Thin Anodic Aluminium Oxide (AAO) Pattern on Silicon Substrate. J. Appl. Sci. Nanotechnol. 2021, 1, 10–15. [Google Scholar] [CrossRef]
- Sumtong, P.; Eiad-ua, A.; Chalapat, K. Nanoporous Anodic Aluminum Oxide (AAO) Thin Film Fabrication with Low-Grade Aluminium. Mater. Sci. Forum 2016, 872, 152–156. [Google Scholar] [CrossRef]
- Rozhdestvenska, L.; Kudelko, K.; Ogenko, V.; Chang, M. Membrane materials based on porous anodic aluminium oxide. Ukr. Chem. J. 2021, 86, 67–102. [Google Scholar] [CrossRef]
- Kynclová, H.; Smilek, J.; Sedláček, P.; Prásek, J.; Klučáková, M.; Hubálek, J. Preparation and Utilization of Alumina Oxide Membranes for Sensor Devices. Mater. Sci. Forum 2016, 851, 159–164. [Google Scholar] [CrossRef]
- Aleksandrova, M.; Kolev, G.; Tsanev, T.; Raptis, I.; Tserepi, A.; Gogolides, E.; Kolev, G. Microheater Topology for Advanced Gas Sensor Applications with Carbyne-Enriched Nanomaterials. Appl. Sci. 2024, 14, 1728. [Google Scholar] [CrossRef]
- Petrov, I.A.; Tsareva, E.R.; Smirnov, A.V.; Kokshina, A.V. Metal oxide-carbon film materials based on copper oxide and linear-chain carbon as a basis for creating gas sensors for alcohol vapors. Spec. Issue J. Phys. Educ. Univ. 2019, 25, 342–343. [Google Scholar]
- Smirnov, A.V.; Kazakov, V.A.; Platonov, P.S.; Tyunterov, E.S. Synthesis and study of the gas sensitive properties of composite thin films of copper oxide and linear chain carbon. J. Phys. Conf. Ser. 2020, 1697, 012133. [Google Scholar] [CrossRef]
- Aleksandrova, M.; Kolev, G.; Brigadin, A.; Lukin, A. Gas-Sensing Properties of a Carbyne-Enriched Nanocoating Deposited onto Surface Acoustic Wave Composite Substrates with Various Electrode Topologies. Crystals 2022, 12, 501. [Google Scholar] [CrossRef]
- Qureshi, S.; Hanif, M.; Jeoti, V.; Stojanović, G.M.; Khan, M.T. Review of fabrication of SAW sensors on flexible substrates: Challenges and future. Results Eng. 2024, 22, 102323. [Google Scholar] [CrossRef]
- Winkler, A.; Harazim, S.M.; Menzel, S.B.; Schmidt, H. SAW-based fluid atomization using mass-producible chip devices. Lab A Chip 2015, 15, 3793–3799. [Google Scholar] [CrossRef] [PubMed]
- Andreev, S.; Spasova, N. Investigation of the dielectric permittivity of anodic aluminum oxide substrates for multi-chip modules. In Proceedings of the 2017 15th International Conference on Electrical Machines, Drives and Power Systems (ELMA), Sofia, Bulgaria, 1–3 June 2017; pp. 224–227. [Google Scholar]
- Mardare, A.I.; Kaltenbrunner, M.; Sariçiftçi, N.S.; Bauer, S.; Hassel, A.W. Ultra-thin anodic alumina capacitor films for plastic electronics. Phys. Status Solidi 2012, 209, 813–818. [Google Scholar] [CrossRef]
- Dadkhah Tehrani, F.; O’Toole, M.D.; Collins, D.J. Tutorial on impedance and dielectric spectroscopy for single-cell characterisation on microfluidic platforms: Theory, practice, and recent advances. Lab A Chip 2025, 25, 837–855. [Google Scholar] [CrossRef] [PubMed]
- Cordier, A.; El Khal, H.; Siebert, E.; Steil, M.C. On the role of the pore morphology on the electrical conductivity of porous yttria-stabilized zirconia. J. Eur. Ceram. Soc. 2019, 39, 2518–2525. [Google Scholar] [CrossRef]
- Liu, Q.; Xiang, R.; Zhao, Y.; Cui, L. Exploration of the adsorption and desorption performance of volatile organic compounds by activated carbon with different shapes based on fixed-bed experiments. Chemosphere 2024, 364, 143161. [Google Scholar] [CrossRef]
- Kaur, P.; Stier, I.K.; Bagchi, S.; Pol, V.G.; Bhondekar, A.P. Impedimetric Early Sensing of Volatile Organic Compounds Released from Li-Ion Batteries at Elevated Temperatures. Batteries 2023, 9, 562. [Google Scholar] [CrossRef]
- Lvovich, V.F. Electrochemical Impedance Spectroscopy (EIS) Applications to Sensors and Diagnostics. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K.-I., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. A review of electrochemical impedance spectroscopy for bioanalytical sensors. Anal. Methods 2022, 14, 4602–4624. [Google Scholar] [CrossRef]
- Balaban, O.; Izhyk, O.; Andrushchak, A. Impedance Study of Anodic Aluminum Oxide. In Proceedings of the 2022 IEEE 41st International Conference on Electronics and Nanotechnology (ELNANO), Kyiv, Ukraine, 10–14 October 2022; pp. 302–305. [Google Scholar]
- Tsanev, T.; Aleksandrova, M.; Videkov, V. Study of nanoporous anodic aluminum oxide as a template filled with piezoelectric materials. In Proceedings of the 2019 IEEE 31st International Conference on Microelectronics (MIEL), Nis, Serbia, 16–18 September 2019; pp. 125–128. [Google Scholar]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nischak, O.Y.; Pavlikov, A.V. Multiphonon replicas in Raman spectra and conductivity properties of carbon films with different concentrations of sp1-bonds. Thin Solid Film. 2019, 671, 31–35. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nischak, O.Y.; Dvoryak, S.V. Electrical conductivity and structural properties of a-C:N films deposited by ion-assisted pulse-arc sputtering. Thin Solid Film. 2020, 701, 137948. [Google Scholar] [CrossRef]
- Lim, G.-H.; Kim, I.-Y.; Park, J.-Y.; Choa, Y.-H.; Lim, J.-H. Anodic Aluminum Oxide-Based Chemi-Capacitive Sensor for Ethanol Gas. Nanomaterials 2024, 14, 70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).