Abstract
In this work, an analysis was made of the microstructural effects derived from the incorporation of silver (Ag) at different concentrations (0.5, 1, 2, 3, and 8% wt) to obtain ZnO-Ag nanocomposites. The results show an increase in the particle size of Ag in relation to the increase in the weight percentage of the precursor. ZnO-Ag is obtained through an infusion of Origanum vulgare as a reducing agent for Ag in the first stage. Subsequently, the solid-state method was used, resulting in the formation of Zinc Oxide (ZnO) and the ZnO-Ag nanoparticles (NPs). The physicochemical characterization was carried out using X-ray Diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR), Transmission Electron Microscopy (TEM), and High-Resolution Transmission Electron Microscopy (HRTEM). The XRD results confirm the presence of Ag and ZnO. Ag shows a preferred orientation of [111] with a crystallite size ranging from 28.46 to 44.92 nm, which increases with the percentage of Ag in the system over ZnO. The wurtzite ZnO presents a preferential orientation of [101] with an increasing crystallite size from 24.9 to 29.84 nm. In the FTIR analysis, a stretching band at 682 cm−1, characteristic of the Zn-O bond, as well as a strain vibration band at 457 cm−1 of ZnO, were observed. The nanoparticle size is attributed to the phytochemical composition of Origanum vulgare, which includes secondary metabolites such as phenolic acids, flavonoids, terpenoids, and flavonoid-based reducing compounds. These compounds help reduce the agglomeration of the particles.
1. Introduction
Inorganic materials like ZnO and Ag are extensively researched due to their wide-ranging applications [1]. They are primarily utilized at the nanoscale [2], contributing to advancements in gas sensors [3], textiles [4], biomaterials [5], biocides [6], catalysts [7], and cosmetics [8]. Zinc oxide nanostructures can be synthesized using various methods such as thermal decomposition, sol-gel processing, solvothermal synthesis, and chemical vapor deposition [9,10,11].
Among the various synthesis techniques, the laser ablation method stands out due to its ability to produce high-purity ZnO and Ag nanoparticles without the need for chemical precursors or stabilizing agents. Laser ablation in liquid (LAL) is particularly advantageous because it allows precise control over particle size and distribution while avoiding contamination from by-products [12]. Furthermore, this method is environmentally friendly, as it does not require toxic solvents or surfactants, aligning with green chemistry principles [13]. Recent studies have demonstrated that ZnO nanoparticles synthesized via laser ablation exhibit enhanced photocatalytic and antibacterial properties due to their controlled morphology and high surface area [14].
Despite the variety of synthesis methods, including hydrothermal [15], wet [16,17], electrodeposition [18], sol-gel, mechanochemical [19], surfactant methods [17], and laser ablation [12], many of these approaches generate significant by-products. This underscores the necessity for sustainable synthesis methods that minimize environmental impact while maintaining the functional properties of the materials.
Sustainable chemistry offers multiple advantages, such as reduced pollutant generation, the use of renewable reducing agents, and adherence to green chemistry principles [20,21]. In this context, plant extracts serve as eco-friendly reducing and stabilizing agents, leading to safer and more biocompatible nanomaterials. However, ensuring compatibility with the human body remains crucial, requiring non-toxicity, biocompatibility, and mechanical properties suitable for biomedical applications [22].
ZnO and Ag nanoparticles (NPs) exhibit excellent antibacterial properties [6,23], surpassing conventional bactericides in physical and chemical stability [24], making them ideal for biomedical applications [2,25,26]. Recent studies have demonstrated the successful synthesis of ZnO and Ag NPs using plant extracts. For example, Pragati Jamdagni et al. (2016) synthesized ZnO NPs ranging from 12 to 32 nm using Nyctanthes arbor-tristis flower extract, showing potent antifungal activity [27]. Tu Uyen Doan Thi et al. (2020) utilized orange peel extract to produce ZnO particles of 35–60 nm with strong antibacterial activity against E. coli and S. aureus [28]. Muhammad Jamil Ahmed et al. (2015) synthesized Ag nanostructures using Skimmia laureola leaf extract, demonstrating efficacy against human pathogens [29].
To further explore the impact of silver concentration on ZnO-Ag nanostructures and their antibacterial properties, this study employed a sustainable chemical synthesis approach using Origanum vulgare extract. Ag concentrations ranging from 0.5% to 8% were investigated to assess their influence on the structural and antibacterial properties of the ZnO-Ag system. This approach offers several benefits over conventional methods, including cost-effectiveness, scalability, reduced waste production, utilization of renewable resources, and the use of safe solvents [20,21].
The results of this study demonstrate that silver concentration significantly affects the morphology, particle size, and antibacterial efficacy of ZnO-Ag nanostructures. The ZnO crystallite size and particle distribution varied with increasing Ag content, impacting their antimicrobial effectiveness. These findings contribute to the growing body of research on sustainable nanomaterials and highlight the potential of ZnO-Ag composites for biomedical and environmental applications. However, further studies are needed to optimize synthesis conditions and fully understand the interaction mechanisms between ZnO and Ag at different concentrations.
By integrating the principles of green chemistry in the synthesis of nanomaterials, this study advances the development of environmentally friendly antibacterial materials due to the fact that this plant has excellent fungicidal, bactericidal, and cytotoxic properties. In addition, it is widely used in the pharmaceutical industry for its anti-inflammatory, antiseptic, antispasmodic, expectorant, and antimicrobial properties [30], thus ensuring biocompatibility due to its phytochemical composition based on Luteolin, apigenin, carvacrol, rosmarinic acid, thymol, limonene [31], and amines [32], aligning with the broader objectives of sustainability and innovation in materials science.
2. Methodology
2.1. ZnO and ZnO-Ag System Synthesis
The synthesis of ZnO and ZnO-Ag nanostructures was carried out at different Ag concentrations from 0 (M1), 0.5 (M2), 1 (M3), 2 (M4), 3 (M5), to 8 (M6) % wt. The products were obtained by combining sustainable chemistry and solid-state methods, using 100 mL of Origanum vulgare extract, as described by Rajith Kumar et al. (2020) [33]. Origanum vulgare was purchased from a local supplier in the state of Hidalgo, Mexico. First, a selection of Origanum vulgare leaves was handled and then dried at 40 °C for 24 h. Then, grinding was performed, until powdered leaves were obtained (Figure 1a). The infusion is produced by placing 250 mL of deionized water at boiling point and adding 12.5 g of Origanum vulgare, stirring constantly for 30 min. It is then filtered and refrigerated (Figure 1b).
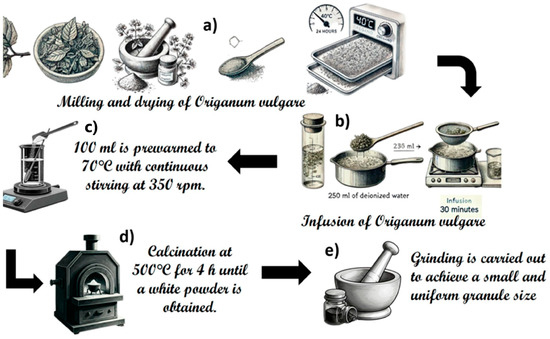
Figure 1.
Experimental procedure to produce ZnO and ZnO/Ag. (a) The leaves of Origanum vulgare are milled and dried at 40 °C for 24 h. (b) An infusion is prepared by adding 250 mL of deionized water to the dried leaves, boiling for 30 min, and then filtering. (c) 100 mL of the infusion is heated to 70 °C with continuous stirring at 350 rpm, and Zinc acetate dihydrate and silver nitrate are added in different proportions as indicated in the text. (d) The resulting material is calcinated at 500 °C for 4 h until a white powder is obtained. (e) The final product is ground to achieve a small and uniform granule size.
After obtaining the infusion, 100 mL is preheated to 70 °C with continuous stirring at 350 rpm. To this, 61.4175 mmol of zinc acetate dihydrate (Zn(CH3COO)2*(2H2O)) (99–101%, Chemsavers) is added for sample M1. Additionally, 0.2317 mmol of silver nitrate (AgNO3) (99–100%, Meyer) is added for sample M2, 0.4634 mmol of AgNO3 for sample M3, 0.9270 mmol of AgNO3 for sample M4, 1.3900 mmol of AgNO3 for sample M5, and 3.7070 mmol of AgNO3 for sample M6, as illustrated in Figure 1c and Table 1. All solutions are stirred continuously for 4 h to reduce the added silver precursor (Figure 1c). Finally, the obtained solutions are subjected to evaporation and calcination at 500 °C for 4 h until a white powder is obtained (Figure 1d). This powder is then ground to achieve a small and uniform granule size (Figure 1e).

Table 1.
Molar composition of the system ZnO/Ag.
2.2. Characterization
The synthesized samples were characterized using a JEOL INSTRUMENT 2010 FEG FASTEM model transmission electron microscope (TEM) with a working voltage of 200 kv. Subsequently, X-ray diffraction (XRD) analysis was performed using a D8 Discover Bruker system (radiation source CuKα = 1.5406 Å) operating at 40 kV and 40 mA. Additionally, the crystallite size was determined using the Modified Scherrer Equation [34].
Diffraction patterns were collected over a 10° to 80° range in 2θ, with an incremental step size of 0.03° and a step time of 0.45 s. Additionally, Fourier Transform Infrared Spectroscopy (FTIR) analysis was conducted in the 4000 to 450 cm−1 range, with a step size of 2 cm−1, using a Perkin Elmer FT-IR System Spectrum GX spectrometer with KBr pellets. Finally, a High-Resolution Transmission Electron Microscope (HRTEM), JEOL brand, 2010FEG model, with an atomic resolution of 0.19 nm, was used. For sample preparation, the powder was dispersed onto a 300-mesh Cu grid using a glass syringe.
3. Results
3.1. X-Ray Diffraction
In Figure 2, the diffractograms of the samples from M1 to M6 samples, sintered at 500 °C with different Ag concentrations ranging from 0.5 to 8%, can be observed. The diffractograms of each sample were indexed according to PDF card 05-0664 corresponding to the hexagonal wurtzite phase of ZnO, as per the International Centre of Diffraction Data (ICDD). Presents reflections (hkl) at 2θ angles of 31.73°, 34.40°, 36.22°, 47.49°, 56.57°, 62.84°, 66.38°, 67.92°, 69.03°, and 76.95°, corresponding to the (100), (002), (101), (012), (110), (013), (200), (112), (201), and (202) crystal planes, with a preferential plane (101) at 36.22° in 2θ, respectively, represented in black in the figure. However, in the diffractograms of samples M2 to M6, the fcc cubic phase of Ag was identified. These samples were indexed according to PDF card 04-0783, where diffraction peaks were identified at 2θ angles of 38.07°, 44.25°, 64.38°, and 77.34° corresponding to the (111), (200), (220), and (311) crystalline planes, with a preferential plane (111) a 38.07° in 2θ marked in red.
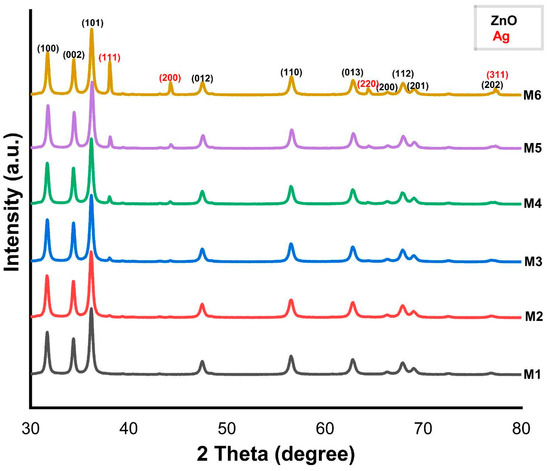
Figure 2.
XRD patterns of the M1: ZnO, M2: ZnO/0.5%Ag, M3: ZnO/1%Ag, M4: ZnO/2%Ag, M5: ZnO/3%Ag, and M6: ZnO/6%Ag to sample system.
The average crystallite size of the samples M1 to M6 was determined by means of a Debye–Scherrer approximation, obtaining the results presented in Table 2.

Table 2.
ZnO and Ag average crystallite size obtained by the Debye–Scherrer equation.
Table 2 shows a significant growth of Ag crystallite size compared to ZnO, whose growth is lower. As seen in Figure 3, as the percentage of silver increases, the crystallite size shows a lower growth rate, suggesting that after a certain point, crystallite growth slows down. Likewise, the increase in crystallite size with higher silver concentrations is attributed to the phytochemical compounds present in the Origanum vulgare extract. These compounds, such as flavonoids and phenolic acids, act as reducing and stabilizing agents, influencing the nucleation and growth process of the crystallites.
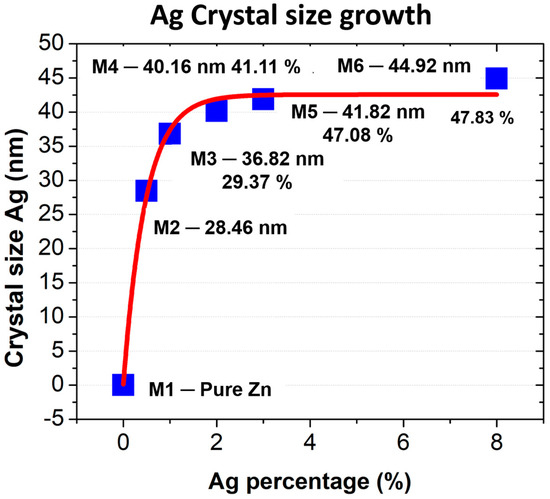
Figure 3.
Ag crystallite size growth.
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
The FTIR spectrum obtained for the Origanum vulgare extract presents a series of absorption bands (Figure 4). The absorption band between 3300 and 3500 cm−1 corresponds to the stretching of the hydroxyl group (-OH) [27]. The bands at 1729 and 1635 cm−1 indicate the presence of C=O, C-O, and O-H groups. The band at 1635 cm−1 is likely associated with C=O stretching vibrations from carbonyl groups and/or N-H bending vibrations from primary amines, secondary amines, or amides. The hydroxyl (-OH) and amino (N-H) groups in Origanum vulgare extract are primarily responsible for the reduction of Ag+ ions to Ag0. Additionally, carbonyl groups can bind strongly with the metal and act as stabilizing agents, preventing agglomeration [16]; the carbonyl group of amino acids has a strong binding capacity with the metal and acts as a stabilizing agent to prevent agglomeration in the aqueous medium.
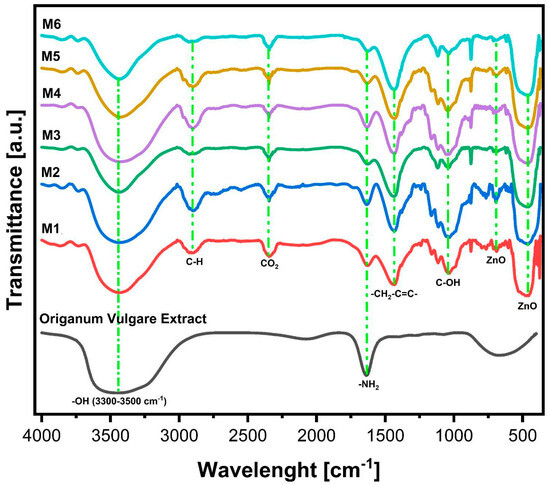
Figure 4.
FT−IR spectroscopy of Origanum vulgare infusion and M1: ZnO, M2: ZnO/0.5%Ag, M3: ZnO/1%Ag, M4: ZnO/2%Ag, M5: ZnO/3%Ag, and M6: ZnO/8%Ag.
In the FTIR analysis, characteristic bands at 3394, 2926, 1635, 1432, 1390, 682, and 457 cm−1 were identified. The broad absorption band between 3394 and 3500 cm−1 corresponds to the stretching of hydroxyl (-OH) groups, commonly associated with adsorbed water molecules and surface hydroxyl groups. Additionally, the presence of amides should exhibit N-H stretching vibrations at wavenumbers above 3000 cm−1. In this spectral region, C-H stretching bands of organic residues from the Origanum vulgare extract can also be observed. In the FTIR spectra of Origanum vulgare and the Ag M1 to M6 nanostructures, a similar band at 1635 cm−1 is observed, corresponding to the functional groups of Origanum vulgare infusion from the C=C and C=O bonds, which may be attributed to the low calcination temperature of 500 °C. Therefore, the functional groups of C=C and C=O completely disintegrate. At 682 cm−1, the characteristic stretching band of the (Zn-O) bond is present, and finally, at 457 cm−1, a tensile vibration band of the Zn-O bond is observed. The vibrational modes in the 400–600 cm−1 region indicate the presence of ZnO [35].
3.3. Transmission Electron Microscopy (TEM)
Figure 5 illustrates micrographs and size distributions of ZnO samples with varying weight percentages of Ag. In Figure 5a, spherical, quasi-spherical, and agglomerated structures are evident. Figure 5b shows larger agglomerates composed of spherical structures around 75 nm. Figure 5c displays less dispersion, consisting of spherical and quasi-spherical NPs with an average size of 80 nm. In Figure 5d, agglomerates approximately 87 nm in size are observed, formed by smaller spherical and quasi-spherical structures. These changes in morphology are attributed to increasing Ag percentage in the ZnO-Ag system from 0.5% to 8% wt, resulting in agglomerate formation. As observed, higher percentages of Ag in M4, M5, and M6 lead to larger particle sizes and increased agglomeration, reducing nanoparticle dispersion. These interactions contribute to the observed changes in morphology and properties of ZnO-Ag compounds, where higher Ag percentages result in larger particle sizes and increased agglomeration.
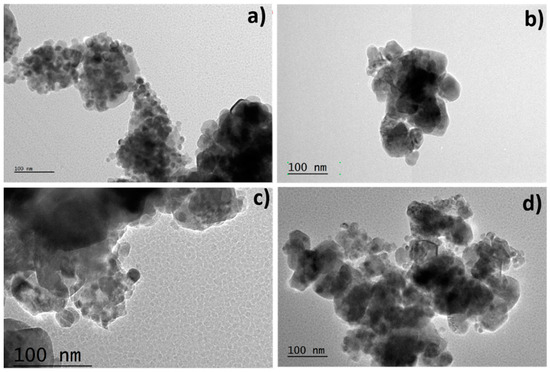
Figure 5.
Micrographs of the samples sintered at 500 °C at different weight percentages of Ag. M1 (a), M4 (b), M5 (c), M6 (d).
3.4. High-Resolution Transmission Electron Microscopy (HRTEM)
HRTEM analysis was performed to understand the behavior of the heterostructure of Ag formed by M1 to M6, in addition to ZnO. In Figure 6a, the HRTEM analysis shows a [010] zone axis, ZnO nano-spheres, and the (100) planes with an interplanar distance of 2.8 Å, and the (002) plane with an interplanar distance of 2.58 Å, along with a resultant plane (102), corresponding to the hexagonal phase of ZnO exhibiting the monocrystalline nature of M1 belonging to ZnO’s wurtzite, in the radial axis, indicating a growth direction [0002] for the ZnO nano-sphere. In the HRTEM analysis of Figure 6b, the [010] zone axis is observed, along with the (100) and (002) planes, with interplanar distances of 2.92 Å and 2.65 Å, respectively. In Figure 6c, the HRTEM and Fast Fourier Transform (FFT) analysis show the (002) and (110) planes, with an interplanar distance of 2.64 Å and 2.47 Å, respectively, and a resultant vector (112). Finally, in Figure 6d, the () and (002) planes are shown, with an interplanar distance of 2.68 Å for the (002) plane, along with a resultant plane (101), corresponding to the hexagonal cell of ZnO.
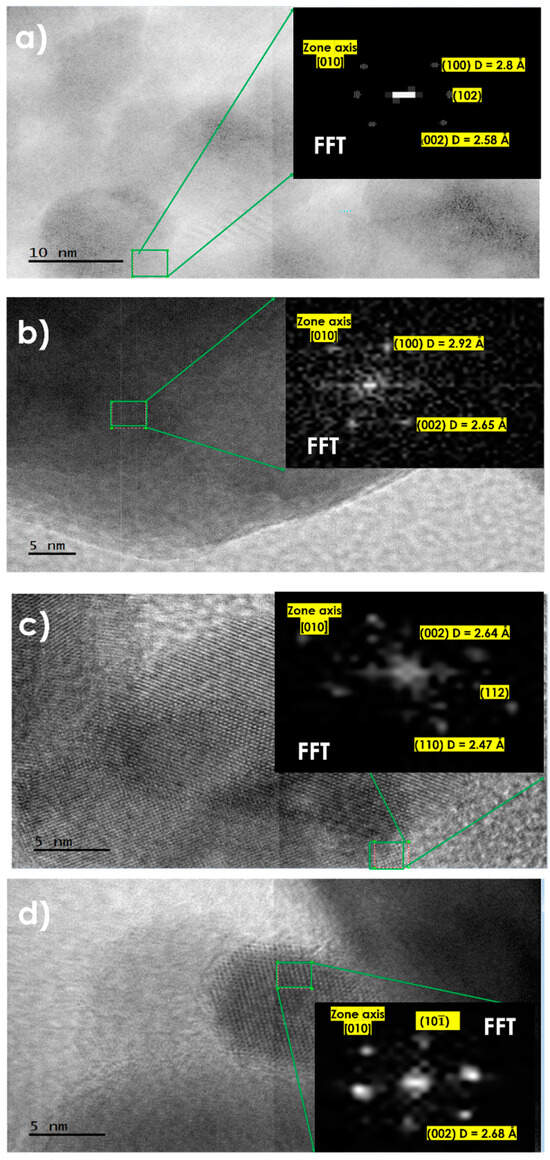
Figure 6.
HRTEM micrographs with their respective FFT of samples at different Ag concentrations: M1 (a), M4 (b), M5 (c), and M6 (d).
3.5. Reaction Mechanism
According to Tu Uyen Doan Thi and collaborators, organic components such as flavonoids and carotenoids are responsible for acting as ligand agents [19]. Based on the results obtained in this research, Figure 7 presents the reaction mechanisms for the formation of ZnO and Ag, highlighting the nucleation-growth process influenced by temperature and the composition of the extract. This process is driven by the main phytochemicals in Origanum vulgare extract, such as luteolin, apigenin, and rosmarinic acid, which consist of aromatic rings with hydroxyl groups that form ligand complexes. The precursors AgNO3 and Zn(CH3COO)2·2H2O dissolve in the Origanum vulgare extract, providing electrons through their flavonoids, playing a crucial role as reducing agents and stabilizers during the reduction of silver ions (Ag+) to metallic silver (Ag0) (Figure 7a,b). This leads to the nucleation and growth of Ag nanoparticles at 70 °C, promoting the formation of clusters or metallic Ag nanoparticles, driven by the phytochemicals in the extract with antioxidant properties that stabilize the formed nanoparticles. During the reduction of Ag+, a color change was observed in the colloid from pale yellow, indicating the formation of Ag nanoparticles. This change is attributed to the alteration of the optical properties of the nanostructures [30]. Additionally, it enhances the formation of Zn(OH)2 (Figure 7c). Subsequently, through a calcination process at 500 °C, the formation of ZnO nanoparticles is favored (Figure 7d), as the resulting solution mixture decomposes at temperatures up to 400 °C [27]. This leads to the reactions outlined in Equations (1) and (2).
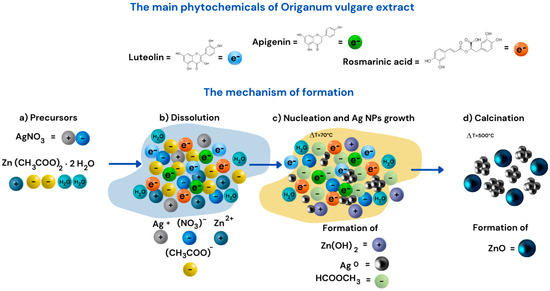
Figure 7.
Mechanism of Ag+ reduction to Ag0. (a) Addition of precursors, (b) Dissociation of AgNO3 precursor in infision, (c) Nucleation of AgNPs and Growth of reduced AgNP, (d) Calcination and formation of ZnO.
The components of the extract consist of aromatic rings, constituted by hydroxyl groups, which form complex ligands. The solution mixture is decomposed up to 400 °C, favoring the formation of ZnO NPs [24]. However, the following reactions take place.
The following reaction will take place in the presence of an Origanum vulgare infusion, composed of flavonoids such as “Luteolin” contained in the plant leaves, which act as proton donors. This is due to the hydroxyl groups in the flavonoid aromatic rings, reducing the Ag+ cations, resulting in the following:
4. Discussions
Several studies have utilized plant extracts through complex methods, employing precipitating agents and varying synthesis temperatures. Table 3 presents a comparison with similar research, highlighting that the minimum synthesis temperature in previous studies is 120 °C, while this study achieves comparable results at only 70 °C, demonstrating a more energy-efficient and sustainable approach. X-ray diffraction analysis confirmed the growth of ZnO and Ag nanocrystals, with the growth rate of ZnO crystallite size decreasing by 30 nm and Ag by 45 nm as the Ag concentration increased.

Table 3.
Comparison of synthesis methods through infusions to obtain the ZnO-Ag system.
Furthermore, FTIR analysis revealed absorption bands at 3394 and 3421 cm−1, resulting from amine (-NH) stretching, along with a stretching band indicative of the hydroxyl group (-OH) [22]. Additionally, a stretching band at 682 cm−1, characteristic of the ZnO bond, and a vibration band at 457 cm−1, indicating metallic oxides, were observed [26].
The novelty of this work lies in the sustainable synthesis of ZnO-Ag nanostructures using Origanum vulgare extract, which acts as a natural reducing and stabilizing agent, minimizing the use of hazardous chemicals. Additionally, this study provides new insights into the effect of silver concentration on particle size, morphology, and antibacterial properties, enabling a better understanding of their potential applications. The findings demonstrate that Ag concentration directly impacts the size and distribution of ZnO-Ag nanoparticles, which could be optimized for biomedical applications such as antibacterial wound dressings. This research contributes to the advancement of environmentally friendly nanomaterial synthesis and broadens the potential applications of ZnO-Ag composites in biomedicine.
Structural and Morphological Characterization
TEM analysis confirmed the presence of spherical, quasi-spherical, and agglomerated structures. As observed, the increase in the percentage of Ag in M4, M5, and M6 promotes larger agglomerates, reducing nanoparticle dispersion. The change in morphology is attributed to the increasing Ag content, which enhances particle agglomeration.
To minimize agglomeration or mitigate its impact on the material properties, various strategies can be considered, such as optimizing the Ag concentration, which is essential since higher percentages of this metal lead to increased agglomeration; therefore, it is necessary to control its content at an Optimal level that balances particle size and dispersion. The use of capping agents, such as organic molecules (e.g., flavonoids or amino acids in Origanum vulgare), helps stabilize the particles and prevent agglomeration. Additionally, controlling the synthesis process by reducing the calcination temperature or adjusting the stirring speed can decrease particle collisions and clustering. Finally, post-synthesis dispersion through sonication or mechanical stirring aids in breaking agglomerates and improving dispersion in the final material. These strategies can be specifically applied to disperse ZnO-Ag nanostructures.
The infusion of Origanum vulgare has proven effective for silver reduction and the production of the ZnO-Ag system. This synthesis approach offers environmental and economic benefits, as Origanum vulgare is widely available in Mexico, the second-largest producer worldwide. The resulting nanostructures exhibit promising properties for future applications, including bactericidal activity (see Figure 8).
Figure 8.
Importance of synthesis of ZnO-Ag nanostructures using Origanum vulgare infusion.
5. Conclusions
The use of Origanum vulgare extract in the synthesis of ZnO-Ag nanostructures represents a sustainable and cost-effective alternative aligned with green chemistry principles. This approach utilizes a plant commonly available in Mexico, which is not only economically accessible but also reduces the reliance on hazardous chemicals and solvents. Additionally, experimental data demonstrate its applicability under real-world conditions and its comparison with conventional methods. A direct correlation was observed between the concentration of the silver precursor and the size of the nanoparticles (NPs). Higher concentrations of the silver precursor resulted in larger NPs. This behavior is consistent with previous studies but highlights the use of Origanum vulgare as a natural reducing agent, allowing efficient control over the NP size [27,31,32,33,34,35].
X-ray diffraction (XRD) analysis indicated that the concentration of the silver precursor had no significant effect on the size of the ZnO crystallites, suggesting that the ZnO structure remains stable even in the presence of silver, which is important for applications where the integrity of the ZnO structure is crucial. Moreover, it was confirmed that combining varying temperatures with sustainable synthesis methods favors the formation of smaller nanoparticles, opening new possibilities for controlling the characteristics of ZnO-Ag nanostructures for specific applications. The performance analysis indicates that the synthesized material exhibits enhanced properties compared to traditional approaches, reinforcing its potential for industrial and technological applications. The use of Origanum vulgare, known for its antibacterial properties, emphasizes the potential of the ZnO-Ag nanostructures for applications in water treatment, antibacterial materials, and other environmental technologies. This sustainable synthesis approach demonstrates high efficiency in obtaining ZnO-Ag nanostructures while minimizing the environmental footprint associated with conventional synthesis techniques. The ability to control particle size and distribution through Ag concentration adjustments opens new avenues for optimizing these nanomaterials in biomedical and catalytic applications. Future research should focus on refining synthesis parameters to enhance nanoparticle stability and dispersion, ensuring their effectiveness in real-world applications.
Finally, considering that Mexico is the second-largest global producer of Origanum vulgare, this study highlights the local availability of this resource, increasing the feasibility of this approach as an economical and sustainable option for synthesizing ZnO-Ag nanostructures in both research and industrial settings.
Author Contributions
Conceptualization: V.R.L., J.A.A.A., and D.M.A.; formal analysis: M.P.M.M., D.M.A., V.R.L., and J.E.M.-P.; investigation: M.P.M.M., J.E.M.-P., V.R.L., and D.M.A.; methodology: D.M.A., V.R.L., and L.S.V.-C., project administration: V.R.L.; supervision: V.R.L., J.E.M.-P., and D.M.A.; Validation: J.A.A.A., D.M.A., and V.R.L. Writing—original draft: J.E.M.-P., M.P.M.M., V.R.L., J.A.A.A., and D.M.A.; writing—review and editing: M.P.M.M., D.M.A., J.E.M.-P., V.R.L., and L.S.V.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
The authors thank the Instituto Nacional de Investigaciones Nucleares (ININ) for allowing us to use their characterization facilities. Finally, we thank the Secretaría de Ciencias Humanidades Tecnología e Innovación (SECIHTI) for the financial support of the PostDoctoral fellowship of Dra. L.S. Villaseñor-Ceron and the CONACHCYT Frontier Project CBF 2023-2024-267.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Özgür, Ü.; Hofstetter, D.; Morkoç, H. ZnO Devices and Applications: A Review of Current Status and Future Prospects. Proc. IEEE 2010, 98, 1255–1268. [Google Scholar] [CrossRef]
- Ayan, B.; Rajesh, K.; Chittaranjan, P. Biomedical applications of zinc oxide nanoparticles. Inorg. Framew. Smart Nanomed. 2018, 6, 239–278. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Baratto, C.; Faglia, G.; Sberveglieri, G. Nanostructured ZnO chemical gas sensors. Ceram. Int. 2015, 41, 14239–14244. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; Nostro, P.L.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanoparticle Res. 2008, 10, 679–689. [Google Scholar]
- Cruz, D.M.; Mostafavi, E.; Vernet-Crua, A.; Barabadi, H.; Shah, V.; Cholula-Díaz, J.L.; Guisbiers, G.; Webster, T.J. Green nanotechnology-based zinc oxide (ZnO) nanomaterials for biomedical applications: In review. J. Phys. Mater. 2020, 3, 034005. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Hussein, M.Z.; Ibrahim, N.A. Synthesis, antibacterial and thermal studies of cellulose nanocrystal stabilized ZnO-Ag heterostructure nanoparticles. Molecules 2013, 18, 6269–6280. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2017, 81 Pt 1, 536–551. [Google Scholar] [CrossRef]
- Kuo, C.; Wang, C.; Ko, H.; Hwang, W.; Chang, K. Synthesis of zinc oxide nanocrystalline powders for cosmetic applications. Ceram. Int. 2010, 36, 693–698. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.; Xiao, Z.; Li, W.; Li, B.; Huang, X.; Liu, X.; Hu, J. Heterostructures of CuS nanoparticle/ZnO nanorod arrays on carbon fibers with improved visible and solar light photocatalytic properties. J. Mater. Chem. A 2015, 3, 7304–7313. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, L.; Zhang, W. Controlled fabrication and properties of three-dimensional ZnO@ hemimorphite nano-heterostructures. Ceram. Int. 2014, 40, 14119–14126. [Google Scholar]
- Zhang, W.L.; Sun, Y.G.; Ding, D.R.; Rao, P.H.; Cai, L.Y.; Xu, J.N. Simple synthesis and enhanced photocatalytic performance of La-modified ZnO nanosheet-assembled flower-like microstructures. Funct. Mater. Lett. 2014, 5, 1450052. [Google Scholar] [CrossRef]
- Barcikowski, S.; Compagnini, G. Advanced nanoparticle generation and excitation by lasers in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3022–3042. [Google Scholar]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gökce, B. Laser synthesis and processing of colloids: Fundamentals and applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.H.; Wei, X.W.; Hong, J.M.; Ye, Y. Hydrothermal preparation and optical properties of ZnO nanorods. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2005, 121, 42–47. [Google Scholar] [CrossRef]
- McBride, R.A.; Kelly, J.M.; McCormack, D.E. Growth of well-defined ZnO microparticles by hydroxide ion hydrolysis of zinc saltsElectronic supplementary information (ESI) available: SEM images of initial precipitate and of particles formed by Method A. J. Mater. Chem. 2003, 13, 1196–1201. [Google Scholar] [CrossRef]
- Lu, C.H.; Yeh, C.H. Emulsion precipitation of submicron zinc oxide powder. Mater. Lett. 1997, 33, 129–132. [Google Scholar] [CrossRef]
- Liu, Z.; E, L.; Ya, J.; Xin, Y. Growth of ZnO nanorods by aqueous solution method with electrodeposited ZnO seed layers. Appl. Surf. Sci. 2009, 255, 5415–6420. [Google Scholar] [CrossRef]
- Aghababazadeh, R.; Mazinani, B.; Mirhabibi, A.; Tamizifar, M. ZnO Nanoparticles Synthesised by mechanochemical processing. J. Phys. Conf. Ser. 2006, 26, 312–314. [Google Scholar] [CrossRef]
- Ivanković, A. Review of 12 Principles of Green Chemistry in Practice. Int. J. Sustain. Green. Energy 2017, 6, 39. [Google Scholar] [CrossRef]
- DeVierno Kreuder, A.; House-Knight, T.; Whitford, J.; Ponnusamy, E.; Miller, P.; Jesse, N.; Rodenborn, R.; Sayag, S.; Gebel, M.; Aped, I.; et al. A Method for Assessing Greener Alternatives between Chemical Products Following the 12 Principles of Green Chemistry. ACS Sustain. Chem. Eng. 2017, 5, 2927–2935. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Rajeswari, V.D. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar]
- Espitia, P.J.P.; Soares, N.D.F.F.; Coimbra, J.S.D.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess. Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Noohpisheh, Z.; Amiri, H.; Farhadi, S.; Mohammadi-gholami, A. Green synthesis of Ag-ZnO nanocomposites using Trigonella foenum-graecum leaf extract and their antibacterial, antifungal, antioxidant and photocatalytic properties. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 240, 118595. [Google Scholar]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud. Univ.-Sci. 2018, 30, 168–175. [Google Scholar]
- Thi, T.U.D.; Nguyen, T.T.; Thi, Y.D.; Thi, K.H.T.; Phan, B.T.; Pham, K.N. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar]
- Ahmed, M.J.; Murtaza, G.; Mehmood, A.; Bhatti, T.M. Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: Characterization and antibacterial activity. Mater. Lett. 2015, 153, 10–13. [Google Scholar]
- Foroughbakhch, R.; Alvarado-Vázquez, M.; Sánchez, J.; Guzman, M.; Hernandez-Piñero, J.; Rocha, A. Caracterización Polinologico de las especies de Oregano: De los géneros Lippia (Verbenaceae) y Poliomintha (Lamiaceae) de Nuevo León. Cienc. UANL 2014, 17, 49–56. [Google Scholar]
- Garcia-Perez, E.; Castro-Álvarez, F.; Gutiérrez-Uribe, J.; García-Lara, S. Revision of the production, phytochemical composition, and nutraceutical properties of Mexican oregano. Rev. Mex. Cienc. Agrícolas 2012, 3, 339–353. [Google Scholar]
- Pandia-Estrada, S.; Romero-Santivañez, R.; Céspedes-Chombo, R.; Solari-Godiño, A. Edible films gelatin-based obtained from mahi-mahi skin (Coryphaena hippurus) and oregano extract: Physicochemical, antimicrobial, structural and surface characteristics. Sci. Agropecu. 2021, 12, 229–237. [Google Scholar] [CrossRef]
- Rajith Kumar, C.R.; Betageri, V.S.; Nagaraju, G.; Pujar, G.H.; Onkarappa, H.S.; Latha, M.S. Synthesis of Core/Shell (ZnO/Ag) Nanoparticles Using Calotropis gigantea and Their Applications in Photocatalytic and Antibacterial Studies. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3410–3417. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O. Comparative study of crystallite size using Williamson-Hall and Debye- Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045013. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Raj, G.A. Bio-approach: Plant mediated synthesis of ZnO nanoparticles and their catalytic reduction of methylene blue and antimicrobial activity. Adv. Powder Technol. 2015, 26, 1639–1651. [Google Scholar] [CrossRef]
- Slathia, S.; Gupta, T.; Chauhan, R.P. Green synthesis of Ag–ZnO nanocomposite using Azadirachta indica leaf extract exhibiting excellent optical and electrical properties. Phys. B Condens. Matter. 2021, 621, 413287. [Google Scholar] [CrossRef]
- Fouladi-Fard, R.; Aali, R.; Mohammadi-Aghdam, S.; Mortazavi-derazkola, S. The surface modification of spherical ZnO with Ag nanoparticles: A novel agent, biogenic synthesis, catalytic and antibacterial activities. Arab. J. Chem. 2022, 15, 103658. [Google Scholar] [CrossRef]
- Khatami, M.; Varma, R.S.; Zafarnia, N.; Yaghoobi, H.; Sarani, M.; Kumar, V.G. Applications of green synthesized Ag, ZnO and Ag/ZnO nanoparticles for making clinical antimicrobial wound-healing bandages. Sustain. Chem. Pharm. 2018, 10, 9–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).