The B30.2/SPRY-Domain: A Versatile Binding Scaffold in Supramolecular Assemblies of Eukaryotes
Abstract
1. Introduction
2. The B30.2 Domain
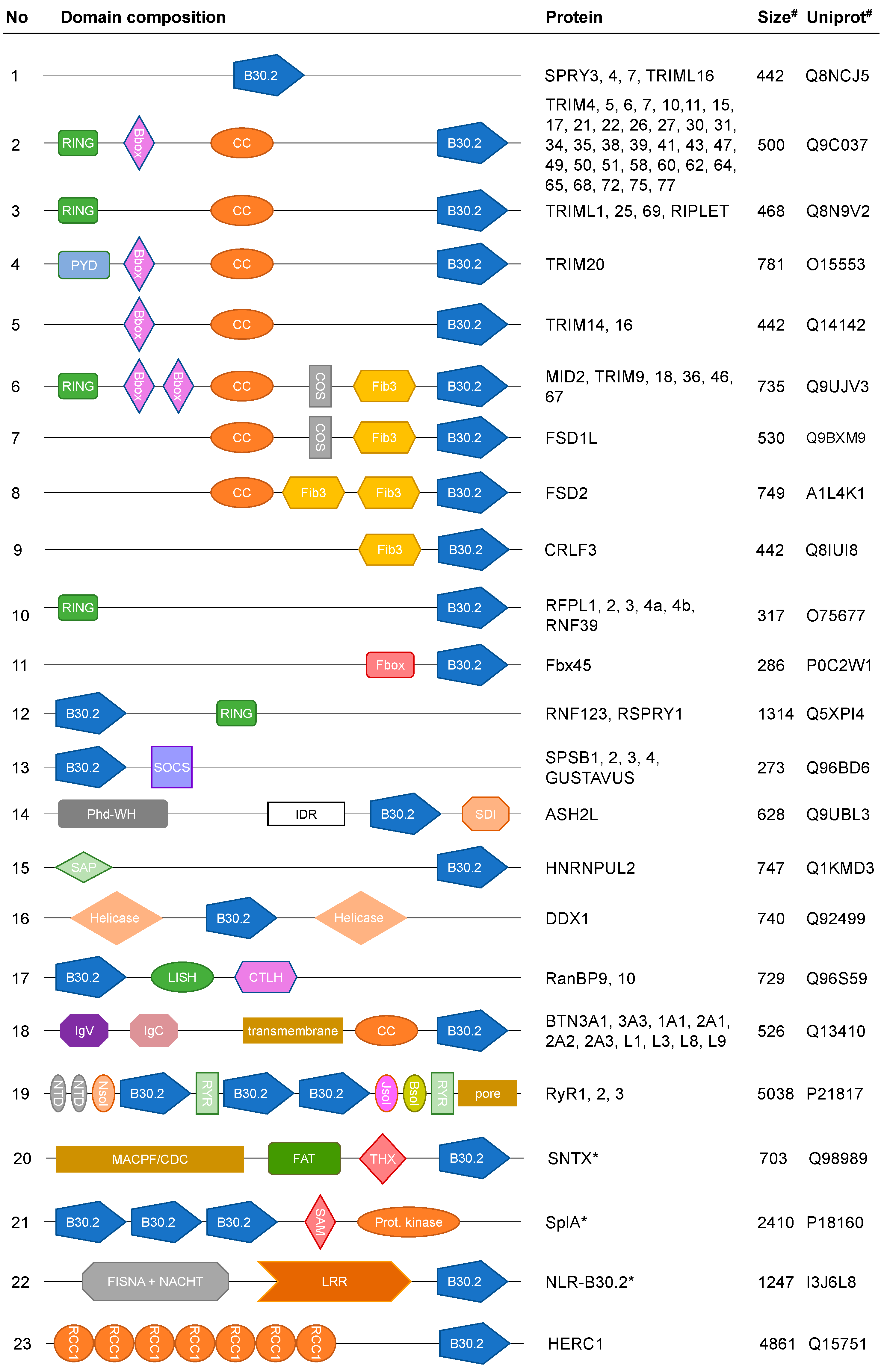
3. B30.2 Multidomain Proteins
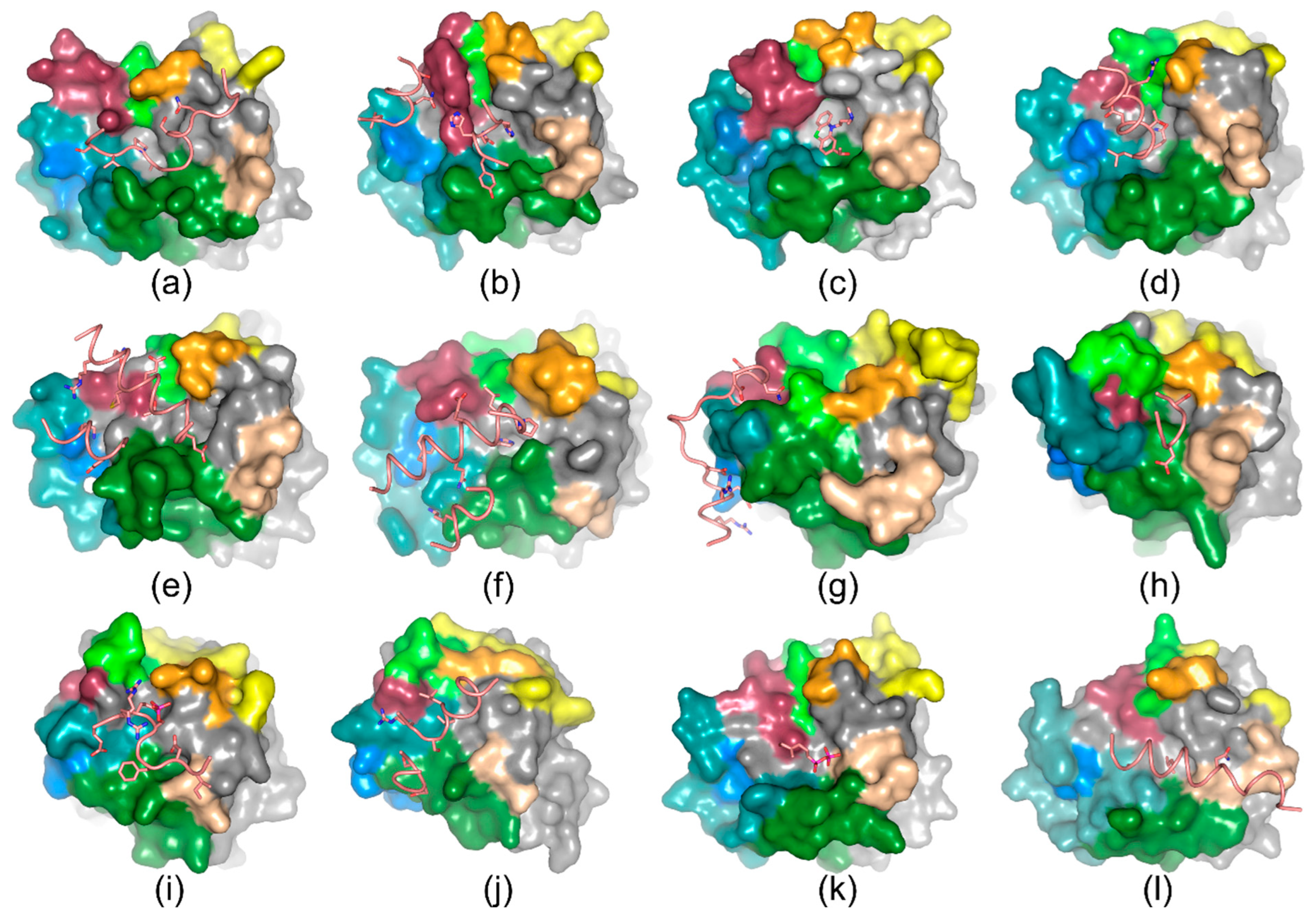
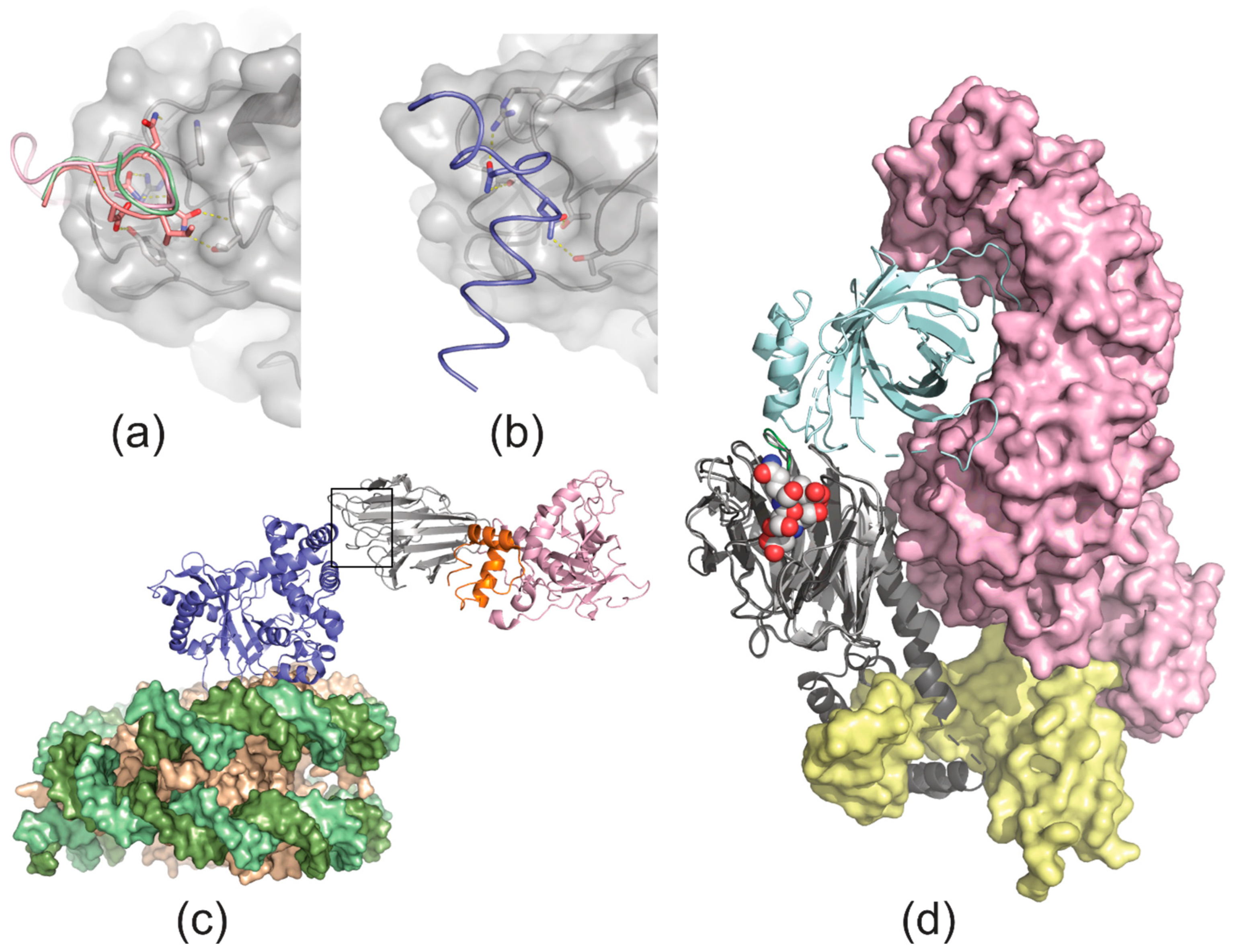
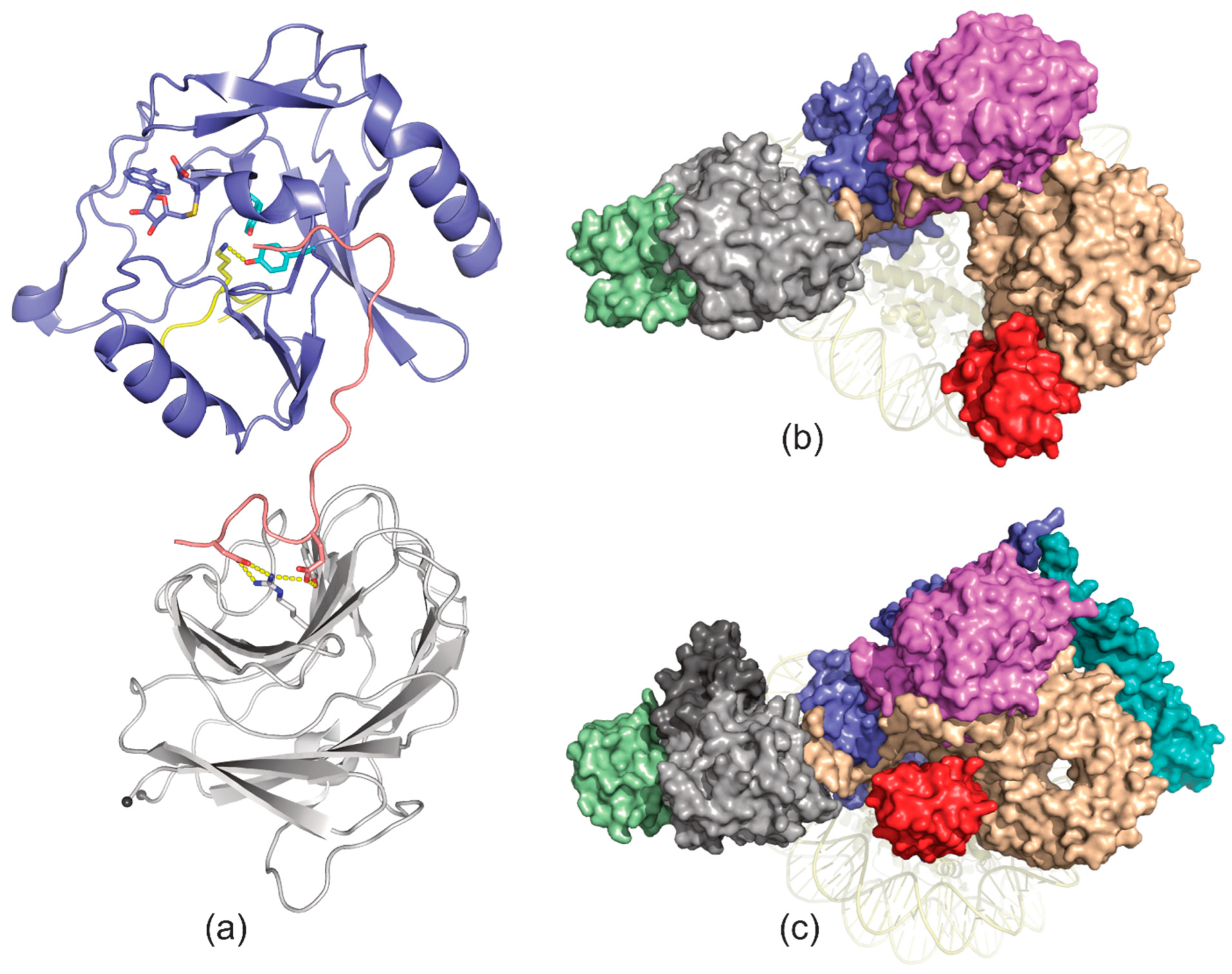
4. Ligand Recognition
5. Tripartite Motif Proteins with B30.2 Domains
6. SPRY Domain-Containing SOCS Box Proteins
7. Heterodimeric RING E3-Ligases
8. Set1/COMPASS Methyltransferase Complexes
9. Butyrophilins
10. Ryanodine Receptors
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vernet, C.; Boretto, J.; Mattei, M.G.; Takahashi, M.; Jack, L.J.; Mather, I.H.; Rouquier, S.; Pontarotti, P. Evolutionary study of multigenic families mapping close to the human MHC class I region. J. Mol. Evol. 1993, 37, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.; Schultz, J.; Bork, P. SPRY domains in ryanodine receptors (Ca2+-release channels). Trends Biochem. Sci. 1997, 22, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.A.; de Bono, B.; Trowsdale, J. Relationship between SPRY and B30.2 protein domains. Evolution of a component of immune defence? Immunology 2005, 116, 411–417. [Google Scholar] [CrossRef]
- Henry, J.; Mather, I.H.; McDermott, M.F.; Pontarotti, P. B30.2-like domain proteins: Update and new insights into a rapidly expanding family of proteins. Mol. Biol. Evol. 1998, 15, 1696–1705. [Google Scholar] [CrossRef]
- Grütter, C.; Briand, C.; Capitani, G.; Mittl, P.R.; Papin, S.; Tschopp, J.; Grütter, M.G. Structure of the PRYSPRY-domain: Implications for autoinflammatory diseases. FEBS Lett. 2006, 580, 99–106. [Google Scholar] [CrossRef]
- Woo, J.S.; Imm, J.H.; Min, C.K.; Kim, K.J.; Cha, S.S.; Oh, B.H. Structural and functional insights into the B30.2/SPRY domain. EMBO J. 2006, 25, 1353–1363. [Google Scholar] [CrossRef]
- Woo, J.S.; Suh, H.Y.; Park, S.Y.; Oh, B.H. Structural basis for protein recognition by B30.2/SPRY domains. Mol. Cell 2006, 24, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Masters, S.L.; Yao, S.; Willson, T.A.; Zhang, J.G.; Palmer, K.R.; Smith, B.J.; Babon, J.J.; Nicola, N.A.; Norton, R.S.; Nicholson, S.E. The SPRY domain of SSB-2 adopts a novel fold that presents conserved Par-4-binding residues. Nat. Struct. Mol. Biol. 2006, 13, 77–84. [Google Scholar] [CrossRef]
- Weinert, C.; Grütter, C.; Roschitzki-Voser, H.; Mittl, P.R.; Grütter, M.G. The crystal structure of human pyrin b30.2 domain: Implications for mutations associated with familial Mediterranean fever. J. Mol. Biol. 2009, 394, 226–236. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, F.; Wan, B.; Dou, Y.; Lei, M. Structure of the SPRY domain of human Ash2L and its interactions with RbBP5 and DPY30. Cell Res. 2012, 22, 598–602. [Google Scholar] [CrossRef]
- Yang, J.; Guan, X.; Zhang, D.; Zhao, P.; Guo, S.; Kuang, Z. Crystal structure of the SPRY domain-containing protein 7 reveals unique structural features. Biochem. Biophys. Res. Commun. 2020, 531, 350–356. [Google Scholar] [CrossRef]
- Bennett, C.; Lawrence, M.; Guerrero, J.A.; Stritt, S.; Waller, A.K.; Yan, Y.; Mifsud, R.W.; Ballester-Beltran, J.; Baig, A.; Mueller, A.; et al. CRLF3 plays a key role in the final stage of platelet genesis and is a potential therapeutic target for thrombocythemia. Blood 2022, 139, 2227–2239. [Google Scholar] [CrossRef] [PubMed]
- Perfetto, L.; Gherardini, P.F.; Davey, N.E.; Diella, F.; Helmer-Citterich, M.; Cesareni, G. Exploring the diversity of SPRY/B30.2-mediated interactions. Trends Biochem. Sci. 2013, 38, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Koepke, L.; Gack, M.U.; Sparrer, K.M. The antiviral activities of TRIM proteins. Curr. Opin. Microbiol. 2021, 59, 50–57. [Google Scholar] [CrossRef]
- Gushchina, L.V.; Kwiatkowski, T.A.; Bhattacharya, S.; Weisleder, N.L. Conserved structural and functional aspects of the tripartite motif gene family point towards therapeutic applications in multiple diseases. Pharmacol. Ther. 2018, 185, 12–25. [Google Scholar] [CrossRef]
- Short, K.M.; Cox, T.C. Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J. Biol. Chem. 2006, 281, 8970–8980. [Google Scholar] [CrossRef] [PubMed]
- Schnappauf, O.; Chae, J.J.; Kastner, D.L.; Aksentijevich, I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Bonnefont, J.; Laforge, T.; Plastre, O.; Beck, B.; Sorce, S.; Dehay, C.; Krause, K.H. Primate-specific RFPL1 gene controls cell-cycle progression through cyclin B1/Cdc2 degradation. Cell Death Differ. 2011, 18, 293–303. [Google Scholar] [CrossRef]
- Desbois, M.; Crawley, O.; Evans, P.R.; Baker, S.T.; Masuho, I.; Yasuda, R.; Grill, B. PAM forms an atypical SCF ubiquitin ligase complex that ubiquitinates and degrades NMNAT2. J. Biol. Chem. 2018, 293, 13897–13909. [Google Scholar] [CrossRef]
- Nguyen, K.M.; Busino, L. The Biology of F-box Proteins: The SCF Family of E3 Ubiquitin Ligases. Adv. Exp. Med. Biol. 2020, 1217, 111–122. [Google Scholar] [CrossRef]
- Kuang, Z.; Lewis, R.S.; Curtis, J.M.; Zhan, Y.; Saunders, B.M.; Babon, J.J.; Kolesnik, T.B.; Low, A.; Masters, S.L.; Willson, T.A.; et al. The SPRY domain-containing SOCS box protein SPSB2 targets iNOS for proteasomal degradation. J. Cell Biol. 2010, 190, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova-Ivantsiv, Y.; Goldhirsh, G.; Tomuleasa, C.; Pikarsky, E.; Ciechanover, A. The NF-kB p50 subunit generated by KPC1-mediated ubiquitination and limited proteasomal processing, suppresses tumor growth. Cancer Cell Int. 2023, 23, 67. [Google Scholar] [CrossRef]
- Lee, Y.T.; Ayoub, A.; Park, S.H.; Sha, L.; Xu, J.; Mao, F.; Zheng, W.; Zhang, Y.; Cho, U.S.; Dou, Y. Mechanism for DPY30 and ASH2L intrinsically disordered regions to modulate the MLL/SET1 activity on chromatin. Nat. Commun. 2021, 12, 2953. [Google Scholar] [CrossRef] [PubMed]
- Polo, S.E.; Blackford, A.N.; Chapman, J.R.; Baskcomb, L.; Gravel, S.; Rusch, A.; Thomas, A.; Blundred, R.; Smith, P.; Kzhyshkowska, J.; et al. Regulation of DNA-end resection by hnRNPU-like proteins promotes DNA double-strand break signaling and repair. Mol. Cell 2012, 45, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Linder, P.; Lasko, P.F.; Ashburner, M.; Leroy, P.; Nielsen, P.J.; Nishi, K.; Schnier, J.; Slonimski, P.P. Birth of the D-E-A-D box. Nature 1989, 337, 121–122. [Google Scholar] [CrossRef]
- Godbout, R.; Hale, M.; Bisgrove, D. A human DEAD box protein with partial homology to heterogeneous nuclear ribonucleoprotein U. Gene 1994, 138, 243–245. [Google Scholar] [CrossRef]
- Shibata, N.; Tsunekawa, N.; Okamoto-Ito, S.; Akasu, R.; Tokumasu, A.; Noce, T. Mouse RanBPM is a partner gene to a germline specific RNA helicase, mouse vasa homolog protein. Mol. Reprod. Dev. 2004, 67, 1–7. [Google Scholar] [CrossRef]
- Emes, R.D.; Ponting, C.P. A new sequence motif linking lissencephaly, Treacher Collins and oral-facial-digital type 1 syndromes, microtubule dynamics and cell migration. Hum. Mol. Genet. 2001, 10, 2813–2820. [Google Scholar] [CrossRef]
- Sherpa, D.; Chrustowicz, J.; Qiao, S.; Langlois, C.R.; Hehl, L.A.; Gottemukkala, K.V.; Hansen, F.M.; Karayel, O.; von Gronau, S.; Prabu, J.R.; et al. GID E3 ligase supramolecular chelate assembly configures multipronged ubiquitin targeting of an oligomeric metabolic enzyme. Mol. Cell 2021, 81, 2445–2459.e2413. [Google Scholar] [CrossRef]
- Abeler-Dorner, L.; Swamy, M.; Williams, G.; Hayday, A.C.; Bas, A. Butyrophilins: An emerging family of immune regulators. Trends Immunol. 2012, 33, 34–41. [Google Scholar] [CrossRef]
- Howe, K.; Schiffer, P.H.; Zielinski, J.; Wiehe, T.; Laird, G.K.; Marioni, J.C.; Soylemez, O.; Kondrashov, F.; Leptin, M. Structure and evolutionary history of a large family of NLR proteins in the zebrafish. Open Biol. 2016, 6, 160009. [Google Scholar] [CrossRef] [PubMed]
- Suurvali, J.; Garroway, C.J.; Boudinot, P. Recurrent expansions of B30.2-associated immune receptor families in fish. Immunogenetics 2022, 74, 129–147. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, A.A.; Babon, J.J.; Norton, R.S.; Nicola, N.A.; Nicholson, S.E. Structure and function of the SPRY/B30.2 domain proteins involved in innate immunity. Protein Sci. 2013, 22, 1–10. [Google Scholar] [CrossRef]
- James, L.C.; Keeble, A.H.; Khan, Z.; Rhodes, D.A.; Trowsdale, J. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc. Natl. Acad. Sci. USA 2007, 104, 6200–6205. [Google Scholar] [CrossRef]
- Hong, S.K.; Kim, K.H.; Song, E.J.; Kim, E.E. Structural Basis for the Interaction between the IUS-SPRY Domain of RanBPM and DDX-4 in Germ Cell Development. J. Mol. Biol. 2016, 428, 4330–4344. [Google Scholar] [CrossRef] [PubMed]
- Munoz Sosa, C.J.; Issoglio, F.M.; Carrizo, M.E. Crystal structure and mutational analysis of the human TRIM7 B30.2 domain provide insights into the molecular basis of its binding to glycogenin-1. J. Biol. Chem. 2021, 296, 100772. [Google Scholar] [CrossRef]
- Park, S.H.; Han, J.; Jeong, B.C.; Song, J.H.; Jang, S.H.; Jeong, H.; Kim, B.H.; Ko, Y.G.; Park, Z.Y.; Lee, K.E.; et al. Structure and activation of the RING E3 ubiquitin ligase TRIM72 on the membrane. Nat. Struct. Mol. Biol. 2023, 30, 1695–1706. [Google Scholar] [CrossRef]
- Keeble, A.H.; Khan, Z.; Forster, A.; James, L.C. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc. Natl. Acad. Sci. USA 2008, 105, 6045–6050. [Google Scholar] [CrossRef]
- Lu, P.; Cheng, Y.; Xue, L.; Ren, X.; Xu, X.; Chen, C.; Cao, L.; Li, J.; Wu, Q.; Sun, S.; et al. Selective degradation of multimeric proteins by TRIM21-based molecular glue and PROTAC degraders. Cell 2024, 187, 7126–7142.e7120. [Google Scholar] [CrossRef]
- Luptak, J.; Mallery, D.L.; Jahun, A.S.; Albecka, A.; Clift, D.; Ather, O.; Slodkowicz, G.; Goodfellow, I.; James, L.C. TRIM7 Restricts Coxsackievirus and Norovirus Infection by Detecting the C-Terminal Glutamine Generated by 3C Protease Processing. Viruses 2022, 14, 1610. [Google Scholar] [CrossRef]
- Liang, X.; Xiao, J.; Li, X.; Liu, Y.; Lu, Y.; Wen, Y.; Li, Z.; Che, X.; Ma, Y.; Zhang, X.; et al. A C-terminal glutamine recognition mechanism revealed by E3 ligase TRIM7 structures. Nat. Chem. Biol. 2022, 18, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Yan, X.; Zhang, B.; Song, L.; Feng, Q.; Ye, C.; Zhou, Z.; Yang, Z.; Li, Y.; Zhang, Z.; et al. C-terminal glutamine acts as a C-degron targeted by E3 ubiquitin ligase TRIM7. Proc. Natl. Acad. Sci. USA 2022, 119, e2203218119. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ahmad, S.; Zhu, Z.; Young, J.M.; Mu, X.; Park, S.; Malik, H.S.; Hur, S. Structural analysis of RIG-I-like receptors reveals ancient rules of engagement between diverse RNA helicases and TRIM ubiquitin ligases. Mol. Cell 2021, 81, 599–613.e598. [Google Scholar] [CrossRef] [PubMed]
- Filippakopoulos, P.; Low, A.; Sharpe, T.D.; Uppenberg, J.; Yao, S.; Kuang, Z.; Savitsky, P.; Lewis, R.S.; Nicholson, S.E.; Norton, R.S.; et al. Structural basis for Par-4 recognition by the SPRY domain- and SOCS box-containing proteins SPSB1, SPSB2, and SPSB4. J. Mol. Biol. 2010, 401, 389–402. [Google Scholar] [CrossRef]
- Kuang, Z.; Yao, S.; Xu, Y.; Lewis, R.S.; Low, A.; Masters, S.L.; Willson, T.A.; Kolesnik, T.B.; Nicholson, S.E.; Garrett, T.J.; et al. SPRY domain-containing SOCS box protein 2: Crystal structure and residues critical for protein binding. J. Mol. Biol. 2009, 386, 662–674. [Google Scholar] [CrossRef]
- You, T.; Wang, Y.; Li, K.; Zhang, D.; Wei, H.; Luo, Y.; Li, H.; Lu, Y.; Su, X.; Kuang, Z. Crystal structure of SPSB2 in complex with a rational designed RGD-containing cyclic peptide inhibitor of SPSB2-iNOS interaction. Biochem. Biophys. Res. Commun. 2017, 489, 346–352. [Google Scholar] [CrossRef]
- Sadek, M.M.; Barlow, N.; Leung, E.W.W.; Williams-Noonan, B.J.; Yap, B.K.; Shariff, F.M.; Caradoc-Davies, T.T.; Nicholson, S.E.; Chalmers, D.K.; Thompson, P.E.; et al. A Cyclic Peptide Inhibitor of the iNOS-SPSB Protein-Protein Interaction as a Potential Anti-Infective Agent. ACS Chem. Biol. 2018, 13, 2930–2938. [Google Scholar] [CrossRef]
- Li, K.; You, T.; Zhao, P.; Luo, Y.; Zhang, D.; Wei, H.; Wang, Y.; Yang, J.; Guan, X.; Kuang, Z. Structural basis for the regulation of inducible nitric oxide synthase by the SPRY domain-containing SOCS box protein SPSB2, an E3 ubiquitin ligase. Nitric Oxide 2021, 113–114, 1–6. [Google Scholar] [CrossRef]
- Suresh, B.; Ramakrishna, S.; Baek, K.H. Diverse roles of the scaffolding protein RanBPM. Drug Discov. Today 2012, 17, 379–387. [Google Scholar] [CrossRef]
- Van, H.T.; Xie, G.; Dong, P.; Liu, Z.; Ge, K. KMT2 Family of H3K4 Methyltransferases: Enzymatic Activity-dependent and -independent Functions. J. Mol. Biol. 2024, 436, 168453. [Google Scholar] [CrossRef]
- Zhang, P.; Chaturvedi, C.P.; Tremblay, V.; Cramet, M.; Brunzelle, J.S.; Skiniotis, G.; Brand, M.; Shilatifard, A.; Couture, J.F. A phosphorylation switch on RbBP5 regulates histone H3 Lys4 methylation. Genes. Dev. 2015, 29, 123–128. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.; Zhang, Y.; Cao, F.; Liu, Z.; Li, S.; Wu, J.; Hu, C.; Wang, Y.; Shuai, J.; et al. Structural basis for activity regulation of MLL family methyltransferases. Nature 2016, 530, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.L.; Li, H.; Lau, H.T.; Leonen, C.; Dhall, A.; Ong, S.E.; Chatterjee, C.; Zheng, N. Crystal Structure of the COMPASS H3K4 Methyltransferase Catalytic Module. Cell 2018, 174, 1106–1116.e1109. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Takahashi, Y.H.; Yang, Y.; Hu, H.; Zhang, Y.; Brunzelle, J.S.; Couture, J.F.; Shilatifard, A.; Skiniotis, G. Structure and Conformational Dynamics of a COMPASS Histone H3K4 Methyltransferase Complex. Cell 2018, 174, 1117–1126.e1112. [Google Scholar] [CrossRef]
- Park, S.H.; Ayoub, A.; Lee, Y.T.; Xu, J.; Kim, H.; Zheng, W.; Zhang, B.; Sha, L.; An, S.; Zhang, Y.; et al. Cryo-EM structure of the human MLL1 core complex bound to the nucleosome. Nat. Commun. 2019, 10, 5540. [Google Scholar] [CrossRef]
- Hsu, P.L.; Shi, H.; Leonen, C.; Kang, J.; Chatterjee, C.; Zheng, N. Structural Basis of H2B Ubiquitination-Dependent H3K4 Methylation by COMPASS. Mol. Cell 2019, 76, 712–723.e714. [Google Scholar] [CrossRef]
- Xue, H.; Yao, T.; Cao, M.; Zhu, G.; Li, Y.; Yuan, G.; Chen, Y.; Lei, M.; Huang, J. Structural basis of nucleosome recognition and modification by MLL methyltransferases. Nature 2019, 573, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Tian, X.; Peng, C.; Gong, F.; Chen, Y. Crystal Structure of MLL2 Complex Guides the Identification of a Methylation Site on P53 Catalyzed by KMT2 Family Methyltransferases. Structure 2020, 28, 1141–1148.e1144. [Google Scholar] [CrossRef]
- Worden, E.J.; Zhang, X.; Wolberger, C. Structural basis for COMPASS recognition of an H2B-ubiquitinated nucleosome. Elife 2020, 9, e53199. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Zhang, Y.; Wu, P.; Xu, Y.; Mencius, J.; Zheng, Y.; Wang, X.; Xu, W.; Huang, N.; et al. Structural basis for product specificities of MLL family methyltransferases. Mol. Cell 2022, 82, 3810–3825.e3818. [Google Scholar] [CrossRef]
- Ayoub, A.; Park, S.H.; Lee, Y.T.; Cho, U.S.; Dou, Y. Regulation of MLL1 Methyltransferase Activity in Two Distinct Nucleosome Binding Modes. Biochemistry 2022, 61, 1–9. [Google Scholar] [CrossRef]
- Rahman, S.; Hoffmann, N.A.; Worden, E.J.; Smith, M.L.; Namitz, K.E.W.; Knutson, B.A.; Cosgrove, M.S.; Wolberger, C. Multistate structures of the MLL1-WRAD complex bound to H2B-ubiquitinated nucleosome. Proc. Natl. Acad. Sci. USA 2022, 119, e2205691119. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, A.; Peigne, C.M.; Leger, A.; Crooks, J.E.; Konczak, F.; Gesnel, M.C.; Breathnach, R.; Bonneville, M.; Scotet, E.; Adams, E.J. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity 2014, 40, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.; Knowles, T.J.; Baker, A.T.; Davey, M.S.; Jeeves, M.; Sridhar, P.; Wilkie, J.; Willcox, C.R.; Kadri, H.; Taher, T.E.; et al. BTN3A1 Discriminates gammadelta T Cell Phosphoantigens from Nonantigenic Small Molecules via a Conformational Sensor in Its B30.2 Domain. ACS Chem. Biol. 2017, 12, 2631–2643. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Yuan, L.; Zhou, X.; Duan, J.; Xiao, H.; Cai, N.; Han, S.; Ma, X.; Liu, W.; et al. A Structural Change in Butyrophilin upon Phosphoantigen Binding Underlies Phosphoantigen-Mediated Vgamma9Vdelta2 T Cell Activation. Immunity 2019, 50, 1043–1053.e1045. [Google Scholar] [CrossRef]
- Yuan, L.; Ma, X.; Yang, Y.; Qu, Y.; Li, X.; Zhu, X.; Ma, W.; Duan, J.; Xue, J.; Yang, H.; et al. Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vgamma9Vdelta2 T cells. Nature 2023, 621, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Hoegenauer, K.; An, S.; Axford, J.; Benander, C.; Bergsdorf, C.; Botsch, J.; Chau, S.; Fernandez, C.; Gleim, S.; Hassiepen, U.; et al. Discovery of Ligands for TRIM58, a Novel Tissue-Selective E3 Ligase. ACS Med. Chem. Lett. 2023, 14, 1631–1639. [Google Scholar] [CrossRef]
- Reddy, B.A.; Kloc, M.; Etkin, L. The cloning and characterization of a maternally expressed novel zinc finger nuclear phosphoprotein (xnf7) in Xenopus laevis. Dev. Biol. 1991, 148, 107–116. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, H.; Jian, Z.; Fan, Z.; Liu, S.; Xing, J.; Li, J. Characterization of the primate TRIM gene family reveals the recent evolution in primates. Mol. Genet. Genom. 2020, 295, 1281–1294. [Google Scholar] [CrossRef]
- Morreale, F.E.; Walden, H. Types of Ubiquitin Ligases. Cell 2016, 165, 248.e241. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, G.; Lv, F.; Xiao, R.P.; Hu, X.; Chen, L. Cryo-EM structure of human MG53 homodimer. Biochem. J. 2022, 479, 1909–1916. [Google Scholar] [CrossRef]
- Napolitano, L.M.; Meroni, G. TRIM family: Pleiotropy and diversification through homomultimer and heteromultimer formation. IUBMB Life 2012, 64, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, G.A.; Benke, S.; Garcia-Sastre, A.; Rajsbaum, R. InTRIMsic immunity: Positive and negative regulation of immune signaling by tripartite motif proteins. Cytokine Growth Factor. Rev. 2014, 25, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Yang, X. SUMO E3 ligase activity of TRIM proteins. Oncogene 2011, 30, 1108–1116. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, D.E. The interferon-inducible ubiquitin-protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase. J. Biol. Chem. 2006, 281, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, L.M.; Jaffray, E.G.; Hay, R.T.; Meroni, G. Functional interactions between ubiquitin E2 enzymes and TRIM proteins. Biochem. J. 2011, 434, 309–319. [Google Scholar] [CrossRef]
- Zhai, L.; Dietrich, A.; Skurat, A.V.; Roach, P.J. Structure-function analysis of GNIP, the glycogenin-interacting protein. Arch. Biochem. Biophys. 2004, 421, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, G.A.; Rajsbaum, R.; Sanchez-Aparicio, M.T.; Maestre, A.M.; Valdiviezo, J.; Shi, M.; Inn, K.S.; Fernandez-Sesma, A.; Jung, J.; Garcia-Sastre, A. The E3-ligase TRIM family of proteins regulates signaling pathways triggered by innate immune pattern-recognition receptors. Immunity 2013, 38, 384–398. [Google Scholar] [CrossRef]
- Fan, W.; McDougal, M.B.; Schoggins, J.W. Enterovirus 3C Protease Cleaves TRIM7 To Dampen Its Antiviral Activity. J. Virol. 2022, 96, e0133222. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, O.B.; Jeong, B.C.; Yi, J.S.; Lee, C.S.; Ko, Y.G.; Song, H.K. Crystal structure of PRY-SPRY domain of human TRIM72. Proteins 2009, 78, 790–795. [Google Scholar] [CrossRef]
- Biris, N.; Yang, Y.; Taylor, A.B.; Tomashevski, A.; Guo, M.; Hart, P.J.; Diaz-Griffero, F.; Ivanov, D.N. Structure of the rhesus monkey TRIM5alpha PRYSPRY domain, the HIV capsid recognition module. Proc. Natl. Acad. Sci. USA 2012, 109, 13278–13283. [Google Scholar] [CrossRef]
- Yang, H.; Ji, X.; Zhao, G.; Ning, J.; Zhao, Q.; Aiken, C.; Gronenborn, A.M.; Zhang, P.; Xiong, Y. Structural insight into HIV-1 capsid recognition by rhesus TRIM5alpha. Proc. Natl. Acad. Sci. USA 2012, 109, 18372–18377. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, M.G.; Lethier, M.; van der Veen, A.G.; Haubrich, K.; Hennig, J.; Kowalinski, E.; Stevens, R.V.; Martin, S.R.; Reis e Sousa, C.; Cusack, S.; et al. Molecular mechanism of influenza A NS1-mediated TRIM25 recognition and inhibition. Nat. Commun. 2018, 9, 1820. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liang, L.; Jin, Y.; Yin, Y. The TRIM14 PRYSPRY domain mediates protein interaction via its basic interface. FEBS Lett. 2019, 593, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Weinert, C.; Morger, D.; Djekic, A.; Grütter, M.G.; Mittl, P.R. Crystal structure of TRIM20 C-terminal coiled-coil/B30.2 fragment: Implications for the recognition of higher order oligomers. Sci. Rep. 2015, 5, 10819. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, L.; Li, Z.; Zhou, C. Structural basis for TRIM72 oligomerization during membrane damage repair. Nat. Commun. 2023, 14, 1555. [Google Scholar] [CrossRef]
- D’Cruz, A.A.; Kershaw, N.J.; Hayman, T.J.; Linossi, E.M.; Chiang, J.J.; Wang, M.K.; Dagley, L.F.; Kolesnik, T.B.; Zhang, J.G.; Masters, S.L.; et al. Identification of a second binding site on the TRIM25 B30.2 domain. Biochem. J. 2018, 475, 429–440. [Google Scholar] [CrossRef]
- Zeng, J.; Santos, A.F.; Mukadam, A.S.; Osswald, M.; Jacques, D.A.; Dickson, C.F.; McLaughlin, S.H.; Johnson, C.M.; Kiss, L.; Luptak, J.; et al. Target-induced clustering activates Trim-Away of pathogens and proteins. Nat. Struct. Mol. Biol. 2021, 28, 278–289. [Google Scholar] [CrossRef]
- Skorupka, K.A.; Roganowicz, M.D.; Christensen, D.E.; Wan, Y.; Pornillos, O.; Ganser-Pornillos, B.K. Hierarchical assembly governs TRIM5alpha recognition of HIV-1 and retroviral capsids. Sci. Adv. 2019, 5, eaaw3631. [Google Scholar] [CrossRef]
- Reusch, J.; Franken, L.E.; Then, J.; Ringler, P.; Butzer, J.; Juroschek, T.; Klein, C.; Schlothauer, T.; Lariviere, L. TRIM21 and Fc-engineered antibodies: Decoding its complex antibody binding mode with implications for viral neutralization. Front. Immunol. 2024, 15, 1401471. [Google Scholar] [CrossRef]
- Shen, Z.; Wei, L.; Yu, Z.B.; Yao, Z.Y.; Cheng, J.; Wang, Y.T.; Song, X.T.; Li, M. The Roles of TRIMs in Antiviral Innate Immune Signaling. Front. Cell. Infect. Microbiol. 2021, 11, 628275. [Google Scholar] [CrossRef]
- Wang, H.T.; Hur, S. Substrate recognition by TRIM and TRIM-like proteins in innate immunity. Semin. Cell Dev. Biol. 2021, 111, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.; Shariq, M.; Surolia, A.; Raj, R.; Khan, M.F.; Kumar, P. Multipronged regulation of autophagy and apoptosis: Emerging role of TRIM proteins. Cell. Mol. Biol. Lett. 2024, 29, 13. [Google Scholar] [CrossRef]
- Uchil, P.D.; Quinlan, B.D.; Chan, W.T.; Luna, J.M.; Mothes, W. TRIM E3 ligases interfere with early and late stages of the retroviral life cycle. PLoS Pathog. 2008, 4, e16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, L.; Sun, X.; Song, Y.; Liu, Y.; Zhang, L. Interplay between TRIM7 and antiviral immunity. Front. Cell. Infect. Microbiol. 2023, 13, 1256882. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Nishizawa, T.; Nishitsuji, H.; Morita, H.; Yamagata, T.; Onomura, D.; Murata, K. TRIM26 positively affects hepatitis B virus replication by inhibiting proteasome-dependent degradation of viral core protein. Sci. Rep. 2023, 13, 13584. [Google Scholar] [CrossRef]
- Ganser-Pornillos, B.K.; Chandrasekaran, V.; Pornillos, O.; Sodroski, J.G.; Sundquist, W.I.; Yeager, M. Hexagonal assembly of a restricting TRIM5alpha protein. Proc. Natl. Acad. Sci. USA 2011, 108, 534–539. [Google Scholar] [CrossRef]
- Wagner, J.M.; Roganowicz, M.D.; Skorupka, K.; Alam, S.L.; Christensen, D.; Doss, G.; Wan, Y.; Frank, G.A.; Ganser-Pornillos, B.K.; Sundquist, W.I.; et al. Mechanism of B-box 2 domain-mediated higher-order assembly of the retroviral restriction factor TRIM5alpha. Elife 2016, 5, e16309. [Google Scholar] [CrossRef]
- Herkules, F.; Yu, C.H.; Taylor, A.B.; Dougherty, V.; Weintraub, S.T.; Ivanov, D.N. Structural and functional asymmetry of RING trimerization controls priming and extension events in TRIM5alpha autoubiquitylation. Nat. Commun. 2022, 13, 7104. [Google Scholar] [CrossRef]
- Park, Y.H.; Wood, G.; Kastner, D.L.; Chae, J.J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol. 2016, 17, 914–921. [Google Scholar] [CrossRef]
- Gao, W.; Yang, J.; Liu, W.; Wang, Y.; Shao, F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc. Natl. Acad. Sci. USA 2016, 113, E4857–E4866. [Google Scholar] [CrossRef] [PubMed]
- The International FMF Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell 1997, 90, 797–807. [Google Scholar] [CrossRef]
- Bernot, A.; Clepet, C.; Dasilva, C.; Devaud, C.; Petit, J.L.; Caloustian, C.; Cruaud, C.; Samson, D.; Pulcini, F.; Weissenbach, J.; et al. A candidate gene for familial Mediterranean fever. Nat. Genet. 1997, 17, 25–31. [Google Scholar] [CrossRef]
- Jamilloux, Y.; Lefeuvre, L.; Magnotti, F.; Martin, A.; Benezech, S.; Allatif, O.; Penel-Page, M.; Hentgen, V.; Seve, P.; Gerfaud-Valentin, M.; et al. Familial Mediterranean fever mutations are hypermorphic mutations that specifically decrease the activation threshold of the Pyrin inflammasome. Rheumatology 2018, 57, 100–111. [Google Scholar] [CrossRef]
- Park, Y.H.; Remmers, E.F.; Lee, W.; Ombrello, A.K.; Chung, L.K.; Shilei, Z.; Stone, D.L.; Ivanov, M.I.; Loeven, N.A.; Barron, K.S.; et al. Ancient familial Mediterranean fever mutations in human pyrin and resistance to Yersinia pestis. Nat. Immunol. 2020, 21, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Mallery, D.L.; McEwan, W.A.; Bidgood, S.R.; Towers, G.J.; Johnson, C.M.; James, L.C. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc. Natl. Acad. Sci. USA 2010, 107, 19985–19990. [Google Scholar] [CrossRef]
- Kiss, L.; Clift, D.; Renner, N.; Neuhaus, D.; James, L.C. RING domains act as both substrate and enzyme in a catalytic arrangement to drive self-anchored ubiquitination. Nat. Commun. 2021, 12, 1220. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, X.; He, Q.Y.; Liu, W. A Interacting Model: How TRIM21 Orchestrates with Proteins in Intracellular Immunity. Small Methods 2024, 8, e2301142. [Google Scholar] [CrossRef]
- Kang, D.; Hwang, H.J.; Baek, Y.; Sung, J.Y.; Kim, K.; Park, H.J.; Ko, Y.G.; Kim, Y.N.; Lee, J.S. TRIM22 induces cellular senescence by targeting PHLPP2 in hepatocellular carcinoma. Cell Death Dis. 2024, 15, 26. [Google Scholar] [CrossRef]
- Zu, S.; Li, C.; Li, L.; Deng, Y.Q.; Chen, X.; Luo, D.; Ye, Q.; Huang, Y.J.; Li, X.F.; Zhang, R.R.; et al. TRIM22 suppresses Zika virus replication by targeting NS1 and NS3 for proteasomal degradation. Cell Biosci. 2022, 12, 139. [Google Scholar] [CrossRef]
- Cai, C.; Masumiya, H.; Weisleder, N.; Matsuda, N.; Nishi, M.; Hwang, M.; Ko, J.K.; Lin, P.; Thornton, A.; Zhao, X.; et al. MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 2009, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Cadena, C.; Ahmad, S.; Xavier, A.; Willemsen, J.; Park, S.; Park, J.W.; Oh, S.W.; Fujita, T.; Hou, F.; Binder, M.; et al. Ubiquitin-Dependent and -Independent Roles of E3 Ligase RIPLET in Innate Immunity. Cell 2019, 177, 1187–1200.e1116. [Google Scholar] [CrossRef] [PubMed]
- Hayman, T.J.; Hsu, A.C.; Kolesnik, T.B.; Dagley, L.F.; Willemsen, J.; Tate, M.D.; Baker, P.J.; Kershaw, N.J.; Kedzierski, L.; Webb, A.I.; et al. RIPLET, and not TRIM25, is required for endogenous RIG-I-dependent antiviral responses. Immunol. Cell Biol. 2019, 97, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gotte, B.; Guo, R.; Pyle, A.M. The E3 ligase Riplet promotes RIG-I signaling independent of RIG-I oligomerization. Nat. Commun. 2023, 14, 7308. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, T.; Jiang, M.; Xiong, C.; Mei, C.; Nie, J.; Zhang, Q.; Zhu, Q.; Huang, X.; Zhang, X.; et al. E3 ligase TRIM65 alleviates intestinal ischemia/reperfusion injury through inhibition of TOX4-mediated apoptosis. Cell Death Dis. 2024, 15, 29. [Google Scholar] [CrossRef]
- Zhang, Z.; Kim, T.; Bao, M.; Facchinetti, V.; Jung, S.Y.; Ghaffari, A.A.; Qin, J.; Cheng, G.; Liu, Y.J. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity 2011, 34, 866–878. [Google Scholar] [CrossRef]
- Kellner, J.N.; Meinhart, A. Structure of the SPRY domain of the human RNA helicase DDX1, a putative interaction platform within a DEAD-box protein. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 1176–1188. [Google Scholar] [CrossRef]
- Kamura, T.; Maenaka, K.; Kotoshiba, S.; Matsumoto, M.; Kohda, D.; Conaway, R.C.; Conaway, J.W.; Nakayama, K.I. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004, 18, 3055–3065. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Zhu, R.; Ding, F.; Li, Y.; Cao, X.; Liu, Z. SPSB3 targets SNAIL for degradation in GSK-3beta phosphorylation-dependent manner and regulates metastasis. Oncogene 2018, 37, 768–776. [Google Scholar] [CrossRef]
- Xu, P.; Liu, Y.; Liu, C.; Guey, B.; Li, L.; Melenec, P.; Ricci, J.; Ablasser, A. The CRL5-SPSB3 ubiquitin ligase targets nuclear cGAS for degradation. Nature 2024, 627, 873–879. [Google Scholar] [CrossRef]
- Xing, Y.; Gosden, R.; Lasko, P.; Clarke, H. Murine homologues of the Drosophila gustavus gene are expressed in ovarian granulosa cells. Reproduction 2006, 131, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Carrera, P.; Johnstone, O.; Nakamura, A.; Casanova, J.; Jackle, H.; Lasko, P. VASA mediates translation through interaction with a Drosophila yIF2 homolog. Mol. Cell 2000, 5, 181–187. [Google Scholar] [CrossRef]
- Styhler, S.; Nakamura, A.; Lasko, P. VASA localization requires the SPRY-domain and SOCS-box containing protein, GUSTAVUS. Dev. Cell 2002, 3, 865–876. [Google Scholar] [CrossRef]
- Li, K.; Luo, Y.; Hu, W.; Yang, J.; Zhang, D.; Wei, H.; You, T.; Lin, H.S.; Kuang, Z. Subtle Structural Differences Affect the Inhibitory Potency of RGD-Containing Cyclic Peptide Inhibitors Targeting SPSB Proteins. Int. J. Mol. Sci. 2024, 25, 6764. [Google Scholar] [CrossRef]
- Francis, O.; Han, F.; Adams, J.C. Molecular phylogeny of a RING E3 ubiquitin ligase, conserved in eukaryotic cells and dominated by homologous components, the muskelin/RanBPM/CTLH complex. PLoS ONE 2013, 8, e75217. [Google Scholar] [CrossRef] [PubMed]
- Santt, O.; Pfirrmann, T.; Braun, B.; Juretschke, J.; Kimmig, P.; Scheel, H.; Hofmann, K.; Thumm, M.; Wolf, D.H. The yeast GID complex, a novel ubiquitin ligase (E3) involved in the regulation of carbohydrate metabolism. Mol. Biol. Cell 2008, 19, 3323–3333. [Google Scholar] [CrossRef]
- Qiao, S.; Langlois, C.R.; Chrustowicz, J.; Sherpa, D.; Karayel, O.; Hansen, F.M.; Beier, V.; von Gronau, S.; Bollschweiler, D.; Schafer, T.; et al. Interconversion between Anticipatory and Active GID E3 Ubiquitin Ligase Conformations via Metabolically Driven Substrate Receptor Assembly. Mol. Cell 2020, 77, 150–163.e159. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, J.; Myers, M.P.; Laherty, C.D.; Eisenman, R.N.; Herr, W. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 2003, 17, 896–911. [Google Scholar] [CrossRef]
- Jiang, H.; Shukla, A.; Wang, X.; Chen, W.Y.; Bernstein, B.E.; Roeder, R.G. Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains. Cell 2011, 144, 513–525. [Google Scholar] [CrossRef]
- Haddad, J.F.; Yang, Y.; Takahashi, Y.H.; Joshi, M.; Chaudhary, N.; Woodfin, A.R.; Benyoucef, A.; Yeung, S.; Brunzelle, J.S.; Skiniotis, G.; et al. Structural Analysis of the Ash2L/Dpy-30 Complex Reveals a Heterogeneity in H3K4 Methylation. Structure 2018, 26, 1594–1603.e4. [Google Scholar] [CrossRef]
- Wiemer, A.J. Structure-Activity Relationships of Butyrophilin 3 Ligands. ChemMedChem 2020, 15, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, T.; Karunakaran, M.M. Butyrophilins: Gammadelta T Cell Receptor Ligands, Immunomodulators and More. Front. Immunol. 2022, 13, 876493. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.A.; Chen, H.C.; Price, A.J.; Keeble, A.H.; Davey, M.S.; James, L.C.; Eberl, M.; Trowsdale, J. Activation of human gammadelta T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J. Immunol. 2015, 194, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Sachleben, J.R.; Boughter, C.T.; Nawrocka, W.I.; Borowska, M.T.; Tarrasch, J.T.; Skiniotis, G.; Roux, B.; Adams, E.J. Phosphoantigen-induced conformational change of butyrophilin 3A1 (BTN3A1) and its implication on Vgamma9Vdelta2 T cell activation. Proc. Natl. Acad. Sci. USA 2017, 114, E7311–E7320. [Google Scholar] [CrossRef]
- Wang, H.; Henry, O.; Distefano, M.D.; Wang, Y.C.; Raikkonen, J.; Monkkonen, J.; Tanaka, Y.; Morita, C.T. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vgamma2Vdelta2 T cells. J. Immunol. 2013, 191, 1029–1042. [Google Scholar] [CrossRef]
- Nguyen, K.; Jin, Y.; Howell, M.; Hsiao, C.C.; Wiemer, A.J.; Vinogradova, O. Mutations to the BTN2A1 Linker Region Impact Its Homodimerization and Its Cytoplasmic Interaction with Phospho-Antigen-Bound BTN3A1. J. Immunol. 2023, 211, 23–33. [Google Scholar] [CrossRef]
- Herrmann, T.; Karunakaran, M.M. Phosphoantigen recognition by Vgamma9Vdelta2 T cells. Eur. J. Immunol. 2024, 54, e2451068. [Google Scholar] [CrossRef]
- Lau, K.; Van Petegem, F. Crystal structures of wild type and disease mutant forms of the ryanodine receptor SPRY2 domain. Nat. Commun. 2014, 5, 5397. [Google Scholar] [CrossRef]
- Yuchi, Z.; Yuen, S.M.; Lau, K.; Underhill, A.Q.; Cornea, R.L.; Fessenden, J.D.; Van Petegem, F. Crystal structures of ryanodine receptor SPRY1 and tandem-repeat domains reveal a critical FKBP12 binding determinant. Nat. Commun. 2015, 6, 7947. [Google Scholar] [CrossRef]
- Alvarado, F.J.; Bos, J.M.; Yuchi, Z.; Valdivia, C.R.; Hernandez, J.J.; Zhao, Y.T.; Henderlong, D.S.; Chen, Y.; Booher, T.R.; Marcou, C.A.; et al. Cardiac hypertrophy and arrhythmia in mice induced by a mutation in ryanodine receptor 2. JCI Insight 2019, 5, e126544. [Google Scholar] [CrossRef]
- Touat-Hamici, Z.; Blancard, M.; Ma, R.; Lin, L.; Iddir, Y.; Denjoy, I.; Leenhardt, A.; Yuchi, Z.; Guicheney, P. A SPRY1 domain cardiac ryanodine receptor variant associated with short-coupled torsade de pointes. Sci. Rep. 2021, 11, 5243. [Google Scholar] [CrossRef]
- Yan, Z.; Bai, X.; Yan, C.; Wu, J.; Li, Z.; Xie, T.; Peng, W.; Yin, C.; Li, X.; Scheres, S.H.W.; et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 2015, 517, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Efremov, R.G.; Leitner, A.; Aebersold, R.; Raunser, S. Architecture and conformational switch mechanism of the ryanodine receptor. Nature 2015, 517, 39–43. [Google Scholar] [CrossRef]
- Bai, X.C.; Yan, Z.; Wu, J.; Li, Z.; Yan, N. The Central domain of RyR1 is the transducer for long-range allosteric gating of channel opening. Cell Res. 2016, 26, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- des Georges, A.; Clarke, O.B.; Zalk, R.; Yuan, Q.; Condon, K.J.; Grassucci, R.A.; Hendrickson, W.A.; Marks, A.R.; Frank, J. Structural Basis for Gating and Activation of RyR1. Cell 2016, 167, 145–157.e117. [Google Scholar] [CrossRef]
- Willegems, K.; Efremov, R.G. Influence of Lipid Mimetics on Gating of Ryanodine Receptor. Structure 2018, 26, 1303–1313.e1304. [Google Scholar] [CrossRef]
- Iyer, K.A.; Hu, Y.; Nayak, A.R.; Kurebayashi, N.; Murayama, T.; Samso, M. Structural mechanism of two gain-of-function cardiac and skeletal RyR mutations at an equivalent site by cryo-EM. Sci. Adv. 2020, 6, eabb2964. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Haji-Ghassemi, O.; Ma, D.; Jiang, H.; Lin, L.; Yao, L.; Samurkas, A.; Li, Y.; Wang, Y.; Cao, P.; et al. Structural basis for diamide modulation of ryanodine receptor. Nat. Chem. Biol. 2020, 16, 1246–1254. [Google Scholar] [CrossRef]
- Woll, K.A.; Haji-Ghassemi, O.; Van Petegem, F. Pathological conformations of disease mutant Ryanodine Receptors revealed by cryo-EM. Nat. Commun. 2021, 12, 807. [Google Scholar] [CrossRef]
- Melville, Z.; Kim, K.; Clarke, O.B.; Marks, A.R. High-resolution structure of the membrane-embedded skeletal muscle ryanodine receptor. Structure 2022, 30, 172–180.e173. [Google Scholar] [CrossRef]
- Nayak, A.R.; Samso, M. Ca2+ inactivation of the mammalian ryanodine receptor type 1 in a lipidic environment revealed by cryo-EM. Elife 2022, 11, e75568. [Google Scholar] [CrossRef] [PubMed]
- Melville, Z.; Dridi, H.; Yuan, Q.; Reiken, S.; Wronska, A.; Liu, Y.; Clarke, O.B.; Marks, A.R. A drug and ATP binding site in type 1 ryanodine receptor. Structure 2022, 30, 1025–1034.e1024. [Google Scholar] [CrossRef]
- Iyer, K.A.; Hu, Y.; Klose, T.; Murayama, T.; Samso, M. Molecular mechanism of the severe MH/CCD mutation Y522S in skeletal ryanodine receptor (RyR1) by cryo-EM. Proc. Natl. Acad. Sci. USA 2022, 119, e2122140119. [Google Scholar] [CrossRef] [PubMed]
- Cholak, S.; Saville, J.W.; Zhu, X.; Berezuk, A.M.; Tuttle, K.S.; Haji-Ghassemi, O.; Alvarado, F.J.; Van Petegem, F.; Subramaniam, S. Allosteric modulation of ryanodine receptor RyR1 by nucleotide derivatives. Structure 2023, 31, 790–800.e794. [Google Scholar] [CrossRef]
- Haji-Ghassemi, O.; Chen, Y.S.; Woll, K.; Gurrola, G.B.; Valdivia, C.R.; Cai, W.; Li, S.; Valdivia, H.H.; Van Petegem, F. Cryo-EM analysis of scorpion toxin binding to Ryanodine Receptors reveals subconductance that is abolished by PKA phosphorylation. Sci. Adv. 2023, 9, eadf4936. [Google Scholar] [CrossRef]
- Weninger, G.; Miotto, M.C.; Tchagou, C.; Reiken, S.; Dridi, H.; Brandenburg, S.; Riedemann, G.C.; Yuan, Q.; Liu, Y.; Chang, A.; et al. Structural insights into the regulation of RyR1 by S100A1. Proc. Natl. Acad. Sci. USA 2024, 121, e2400497121. [Google Scholar] [CrossRef]
- Li, C.; Willegems, K.; Uchanski, T.; Pardon, E.; Steyaert, J.; Efremov, R.G. Rapid small-scale nanobody-assisted purification of ryanodine receptors for cryo-EM. J. Biol. Chem. 2024, 300, 107734. [Google Scholar] [CrossRef]
- Lin, L.; Wang, C.; Wang, W.; Jiang, H.; Murayama, T.; Kobayashi, T.; Hadiatullah, H.; Chen, Y.S.; Wu, S.; Wang, Y.; et al. Cryo-EM structures of ryanodine receptors and diamide insecticides reveal the mechanisms of selectivity and resistance. Nat. Commun. 2024, 15, 9056. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Shen, H.; Wu, J.; Guo, W.; Pan, X.; Wang, R.; Chen, S.R.; Yan, N. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 2016, 354, aah5324. [Google Scholar] [CrossRef]
- Chi, X.; Gong, D.; Ren, K.; Zhou, G.; Huang, G.; Lei, J.; Zhou, Q.; Yan, N. Molecular basis for allosteric regulation of the type 2 ryanodine receptor channel gating by key modulators. Proc. Natl. Acad. Sci. USA 2019, 116, 25575–25582. [Google Scholar] [CrossRef]
- Gong, D.; Chi, X.; Wei, J.; Zhou, G.; Huang, G.; Zhang, L.; Wang, R.; Lei, J.; Chen, S.R.W.; Yan, N. Modulation of cardiac ryanodine receptor 2 by calmodulin. Nature 2019, 572, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Miotto, M.C.; Weninger, G.; Dridi, H.; Yuan, Q.; Liu, Y.; Wronska, A.; Melville, Z.; Sittenfeld, L.; Reiken, S.; Marks, A.R. Structural analyses of human ryanodine receptor type 2 channels reveal the mechanisms for sudden cardiac death and treatment. Sci. Adv. 2022, 8, eabo1272. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tsutsumi, A.; Kurebayashi, N.; Saito, K.; Kodama, M.; Sakurai, T.; Kikkawa, M.; Murayama, T.; Ogawa, H. Molecular basis for gating of cardiac ryanodine receptor explains the mechanisms for gain- and loss-of function mutations. Nat. Commun. 2022, 13, 2821. [Google Scholar] [CrossRef]
- Miotto, M.C.; Reiken, S.; Wronska, A.; Yuan, Q.; Dridi, H.; Liu, Y.; Weninger, G.; Tchagou, C.; Marks, A.R. Structural basis for ryanodine receptor type 2 leak in heart failure and arrhythmogenic disorders. Nat. Commun. 2024, 15, 8080. [Google Scholar] [CrossRef]
- Dhindwal, S.; Lobo, J.; Cabra, V.; Santiago, D.J.; Nayak, A.R.; Dryden, K.; Samso, M. A cryo-EM-based model of phosphorylation- and FKBP12.6-mediated allosterism of the cardiac ryanodine receptor. Sci. Signal. 2017, 10, eaai8842. [Google Scholar] [CrossRef]
- Chen, Y.S.; Garcia-Castaneda, M.; Charalambous, M.; Rossi, D.; Sorrentino, V.; Van Petegem, F. Cryo-EM investigation of ryanodine receptor type 3. Nat. Commun. 2024, 15, 8630. [Google Scholar] [CrossRef] [PubMed]
- Samso, M. A guide to the 3D structure of the ryanodine receptor type 1 by cryoEM. Protein Sci. 2017, 26, 52–68. [Google Scholar] [CrossRef]
- Cui, Y.; Tae, H.S.; Norris, N.C.; Karunasekara, Y.; Pouliquin, P.; Board, P.G.; Dulhunty, A.F.; Casarotto, M.G. A dihydropyridine receptor alpha1s loop region critical for skeletal muscle contraction is intrinsically unstructured and binds to a SPRY domain of the type 1 ryanodine receptor. Int. J. Biochem. Cell Biol. 2009, 41, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Hadiatullah, H.; He, Z.; Yuchi, Z. Structural Insight Into Ryanodine Receptor Channelopathies. Front. Pharmacol. 2022, 13, 897494. [Google Scholar] [CrossRef]
- Iyer, K.A.; Barnakov, V.; Samso, M. Three-dimensional perspective on ryanodine receptor mutations causing skeletal and cardiac muscle-related diseases. Curr. Opin. Pharmacol. 2023, 68, 102327. [Google Scholar] [CrossRef]
- Ernst, P.; Plückthun, A.; Mittl, P.R.E. Structural analysis of biological targets by host:guest crystal lattice engineering. Sci. Rep. 2019, 9, 15199. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittl, P.R.E.; Beer, H.-D. The B30.2/SPRY-Domain: A Versatile Binding Scaffold in Supramolecular Assemblies of Eukaryotes. Crystals 2025, 15, 281. https://doi.org/10.3390/cryst15030281
Mittl PRE, Beer H-D. The B30.2/SPRY-Domain: A Versatile Binding Scaffold in Supramolecular Assemblies of Eukaryotes. Crystals. 2025; 15(3):281. https://doi.org/10.3390/cryst15030281
Chicago/Turabian StyleMittl, Peer R. E., and Hans-Dietmar Beer. 2025. "The B30.2/SPRY-Domain: A Versatile Binding Scaffold in Supramolecular Assemblies of Eukaryotes" Crystals 15, no. 3: 281. https://doi.org/10.3390/cryst15030281
APA StyleMittl, P. R. E., & Beer, H.-D. (2025). The B30.2/SPRY-Domain: A Versatile Binding Scaffold in Supramolecular Assemblies of Eukaryotes. Crystals, 15(3), 281. https://doi.org/10.3390/cryst15030281








